Abstract
The DSM-5 includes depression as a dimension of psychosis. We tested whether persistent experience of depression, called ‘trait depression’, is a clinical feature separate from psychosis and several well-known, trait-like deficits of schizophrenia. 126 individuals with schizophrenia and 151 control participants completed the Maryland Trait and State Depression questionnaire, with a subset completing measures of cognition and functional capacity, and diffusion tensor imaging (n=73 patients and 102 controls for imaging analysis). Subjectively experienced, longitudinal trait depression is significantly higher in patients with schizophrenia compared with controls. Higher trait depression scores were associated with more severe psychosis. Surprisingly, individuals with higher trait depression manifested less cognitive and global functioning deficits. In addition, trait depression scores were positively associated with fractional anisotropy of white matter. Trait depression appears to be a highly relevant clinical domain in the care of patients with schizophrenia that also has distinct relationships with some other known traits of the disease. Trait depression may be an important contributor to the clinical heterogeneity of schizophrenia.
Keywords: Depression, Schizophrenia, White Matter, Cognition
Introduction
Depression is common during the course of schizophrenia, reported in prodromal state (Häfner et al., 2013), first episode psychosis (Sönmez et al., 2013), and during and following acute episodes (McGlashan and Carpenter, 1976). Depressive symptoms are associated with higher risk of suicidal attempts and ideation in schizophrenia (Andriopoulos et al., 2011; Upthegrove et al., 2010). Despite the clinical importance of depression in the care of schizophrenia patients, depressive symptoms are often not considered core pathology in schizophrenia. Instead, depression has been viewed from a categorical perspective and used to delineate the boundary between affective and non-affective psychoses. The DSM-5 includes depression as a dimension of psychosis, inviting a re-examination of its role in schizophrenia using modern phenomenological concepts and technology. Previous studies compared current depressive symptoms to other features in schizophrenia and typically found that depression showed no significant association with cognitive deficits in patients. For example, a study by Bowie and colleagues employed Beck's Depression Inventory and showed that the depressive symptoms had no relationship with neuropsychological performance or laboratory assessment of functional capacity (Bowie et al., 2006). Similar results were observed when the Calgary Depression Scale for Schizophrenia was used (Tanaka et al., 2012). Several more studies linked depressive symptoms to awareness of negative symptoms and functional deficits but not to the deficits themselves (Liddle et al., 1993; Maggini and Raballo, 2006). The common interpretation is that better insight into the illness and the associated deficits and stigma contributes to depression formation in schizophrenia patients (Crumlish et al., 2005; Delaney et al., 2012; Drake et al., 2004; Moore et al., 1999; Staring et al., 2009). However, this argument considers only the current experience of depression.
Indeed, most if not all studies in schizophrenia have employed instruments that measure only current or very recent depressive symptoms, and neglect the longitudinally experienced, ‘trait’ aspects of depression. We used the Maryland Trait and State Depression (MTSD) Scale that was developed to provide a tool to overcome this limitation (Chiappelli et al., 2014). This is a self-rated scale performed in an interview setting, with some items assessing the frequency of depressive symptoms experienced over the course of adult life, and other items asking specifically about depressive symptoms experienced in the past week. Factor analysis revealed that depression symptoms captured by MTSD in both schizophrenia patients and controls were segregated into two domains: trait (symptoms experienced frequently throughout adult life) and state (current symptoms) depression. Trait depression reported by the patients through MTSD was distinct from their negative symptoms evaluated during the same session; depression and negative symptoms may overlap in observable behaviors, such as psychomotor retardation, but trait depression involves the tendency to experience negative emotions and distressing thoughts, whereas negative symptoms are characterized by apparent deficits in motivation and affect regardless of negative inner experience (Chiappelli et al., 2014). Most importantly, the initial work on the MTSD taught us that trait depression is a prominent feature in the presentation of schizophrenia patients – when we ask our patients. The immediate question then becomes whether trait depression is a distinct clinical domain or if it is biologically linked to other core or trait-like features of schizophrenia.
If trait depression is measuring a stable feature in some patients with schizophrenia, we hypothesized that it should either associate with other trait-like features of schizophrenia, or represent a separate, trait-like clinical domain. Here, we examined the relationship between trait depression and three well-replicated, relatively persistent behavioral and brain structure abnormalities in schizophrenia: 1) cognitive deficits, particularly in processing and speed and working memory, are among the most prominent, well-replicated, trait-like clinical deficits in schizophrenia (Barder et al., 2013; Bonner-Jackson et al., 2010; Hoff et al., 2005; Roalf et al., 2013); 2) functional capacity impairment as measured with the UCSD Performance Based Skills Assessment, which is a well-recognized feature of schizophrenia that is relatively stable over time (Harvey et al., 2012; Light et al., 2012) and 3) white matter integrity as measured by the fractional anisotropy (FA) derived from diffusion tensor imaging (DTI); one of the most replicated brain structural abnormalities in schizophrenia (Ellison-Wright and Bullmore, 2009; Yao et al., 2013). We expected that this effort would yield clues on the possible biological and functional underpinnings of trait depression, paving the way to use this novel phenomenological approach to parse some of the clinical heterogeneity of the disorder.
Methods
Participants
Individuals with schizophrenia were recruited from the Maryland Psychiatric Research Center and the neighboring community mental health clinics. Community comparison participants were recruited from advertisements in local media. A total of 277 participants (126 patients and 151 controls) participated in the study and completed MTSD assessment. Among them, 118 patients and 151 controls completed cognitive and functional assessments; within which 73 patients and 102 controls completed a MRI scan for DTI. All participants were assessed with the Structured Clinical Interview for DSM Disorders (SCID). Among the patient sample, 22 met criteria for schizoaffective disorder, and an additional 19 had a past history of at least one episode of major depressive disorder.
Of the 277 participants in this study, 197 (71.4%) were previously included in the initial psychometric and factorial analysis of the MTSD (Chiappelli et al., 2014). For the patients, 9 were not on antipsychotic medication at time of the study; 15 were taking clozapine, 16 were taking a typical antipsychotic, 49 were taking an atypical antipsychotic, and the rest were on two or more antipsychotic medications. There were also 44 patients who were taking an antidepressant medication at time of study. Current psychosis severity was assessed using the psychosis subscale in the Brief Psychiatric Rating Scale (BPRS), which includes clinician ratings for conceptual disorganization, suspiciousness, hallucinatory behavior, and unusual thought content. The relative independence between trait depression and negative symptoms was previously reported (Chiappelli et al., 2014) and is not repeated here.
MTSD assessments
The Maryland Trait and State Depression (MTSD) questionnaire consists of 36 items asking participants to rate frequency of depressive symptoms over the past week (‘State’ questions) or throughout their adult lives excluding the past week (‘Trait’ questions). This questionnaire has been demonstrated to have suitable psychometric properties and construct validity, in particular in its ability to distinguish depressive from negative symptoms (Chiappelli et al., 2014). The scale and user instructions are available to download at http://www.mdbrain.org/MTSD_instructions_and_scale.pdf.
Assessment of cognition and functional capacity
Cognition testing included the Digit Symbol Coding task of the WAIS-3 (Wechsler, 1997) and the Digit Sequencing task from the Brief Assessment of Cognition in Schizophrenia (Keefe et al., 2004), to assess processing speed and working memory, respectively. While the cognitive deficit in schizophrenia encompasses multiple domains, deficits in processing speed (Dickinson et al., 2007; Dickinson et al., 2008; Knowles et al., 2010) and working memory (Forbes et al., 2009; Keefe et al 2004) are particularly severe. Scores were calculated as t scores according to population norms based on age for the Digit Symbol Coding task, and age and sex for the Digit Sequencing task (Keefe et al., 2008). The UCSD Performance-based Skills Assessment (UPSA-2) was used to measure functional capacity. UPSA-2 uses role-play to assess functioning across five domains: organization/planning, financial skills, communication skills, transportation, and household skills, and is a validated tool to assess community functional capacity in schizophrenia patients (Bowie et al., 2006). The UPSA-2 also shows considerable stability over time, indicating it measures a trait-like characteristic in schizophrenia patients (Leifker et al., 2010). The total score was used as a measure of community functional capacity.
Assessment of cerebral white matter integrity
Diffusion tensor data were collected at the University of Maryland Center for Brain Imaging Research using a Siemens 3T TRIO MRI (Erlangen, Germany) system equipped with a 32-channel phase array head coil. The high-angular resolution diffusion imaging (HARDI) DTI data were collected using a single-shot, echo-planar, single refocusing spin-echo, T2-weighted sequence with a spatial resolution of 1.7×1.7×3.0 mm. The sequence parameters were: TE/TR=87/8000ms, FOV=200mm, axial slice orientation with 50 slices and no gaps, five b=0 images and 64 isotropically distributed diffusion weighted directions with b= 700 s/mm2. These parameters maximized the contrast to noise ratio for FA measurements (Kochunov et al., 2012). A tract-based spatial statistics (TBSS) method, distributed as a part of FMRIB Software Library (FSL) package, was used for tract-based analysis of diffusion anisotropy (Smith et al 2006). The population-based, 3D, DTI cerebral white matter tract atlas developed in Johns Hopkins University and distributed with the FSL package (Wakana et al., 2004) was used to calculate population average FA values along the spatial course of major white matter tracts. First, fractional anisotropy (FA) images were created by fitting the diffusion tensor to the motion and eddy current diffusion data. RMSDIFF (Smith et al., 2006) was used to estimate the root mean square (RMS) movement distance between diffusion sensitized and b=0 images. All data passed QA control of <3mm accumulated motion during the scan. There were no difference in the average motion per TR between patients and controls (0.42±0.21 vs. 0.43±0.20, for patients and controls, respectively). In the next step, all FA images were globally spatially normalized to the Johns Hopkins University (JHU) (Wakana et al., 2004) and then nonlinearly aligned to a group-wise, minimal-deformation target (MDT) brain using the FLIRT method (Kochunov et al., 2001; Smith et al., 2006). Next, individual FA images were averaged to produce a group-average anisotropy image. This image was used to create a group-wise skeleton of white matter tracts. The skeletonization procedure was a morphological operation, which extracts the medial axis of an object. Finally, FA images were thresholded at FA=0.20 level to eliminate non-white matter voxels and FA values were projected onto the group-wise skeleton of white matter structures. This step accounts for residual misalignment among individual white matter tracts. FA values were assigned to each point along a skeleton using the peak value found within a designated range perpendicular to the skeleton. This processing was performed under two constraints. A distance map was used to establish search borders for individual tracts. The borders were created by equally dividing the distance between two nearby tracts. Secondly, a multiplicative 20mm full width at half-max Gaussian weighting was applied during the search to limit maximum projection distance from the skeleton. The average FA from whole brain white matter was used as the primary measure. The FA from the fourteen major tracts were used for exploratory analyses.
Data analysis
One-way ANOVA was used for group comparisons of clinical and trait measures that had a normal distribution, and Kruskal-Wallis test used for measures with non-normal distribution. MTSD means had a non-normal distribution as determined by the one-sample Kolmogorov-Smirnov test, therefore Spearman's correlations were used to compare depression severity with other trait measures. Correction for multiple comparisons was done using the method of Benjamini and Hochberg (1995). All tests were two-tailed.
Results
Relationship of Trait and State Depression to Psychosis, Cognition and Functional Capacity Schizophrenia patients and controls were matched in age but there were significantly more smokers (p=0.007) and lower education levels in patients compared with controls (Table 1). Patients had significantly higher MTSD trait (χ2=16.48, p<0.001; Cohen's d=0.49) and state (χ2=33.49, p<0.001; Cohen's d=0.68) scores compared with controls (Table 1). Patients also showed significantly impaired cognition (processing speed and working memory) and functional capacity (UPSA-2; Table 1). Patients with schizoaffective disorder and/or a history of at least one episode of MDD had significantly higher MTSD trait and state scores compared to schizophrenia patients without a history of MDD; however, these subgroups did not significantly differ on measures of cognition, functional capacity, or whole brain average FA (Supplementary Table 1). The following analyses include all patients combined in one group.
Table 1.
Group differences in demographic, clinical and neuropsychological variables.
| Schizophrenia | Control | Test statistic | p value | |
|---|---|---|---|---|
| Total N | 126 | 151 | n/a | n/a |
| Age [years](±sd) | 37.9 (13.3) | 35.8(13.8) | t=1.25 | .214 |
| %Male | 55.5 | 34.4 | χ2=12.5 | .002 |
| %Smoker | 43.7 | 27.8 | χ2=7.36 | .007 |
| CPZ [mg] | 542 | n/a | n/a | n/a |
| MTSD-Trait | 1.11 | 0.70 | χ2=16.48 | <.001 |
| MTSD-State | 0.92 | 0.43 | χ2=33.49 | <.001 |
| Working memory | 36.6 | 45.4 | t=5.67 | <.001 |
| Processing speed | 7.4 | 10.5 | t=8.01 | <.001 |
| Functional capacity | 86.7 | 101.5 | t=8.31 | <.001 |
CPZ = chlorpromazine dose equivalent of antipsychotic medication. Working memory was assessed using the Digit Sequencing task from the Brief Assessment of Cognition in Schizophrenia, processing speed was assessed with the Digit Symbol Coding task (WAIS-3), and functional capacity was measured using the UPSA-2.
We found that the severity of trait and state depression measures were significantly and positively associated with BPRS psychosis score (rho=.246, p=.005, rho=.312, p<.001 respectively) in patients with schizophrenia. However, there were no indications that more severe trait depression was related to more severe deficits in any cognitive measures. Instead, the MTSD trait score had positive and significant correlations with working memory (rho=0.186, p=.044), processing speed (rho=0.206, p=.024), and functional capacity (rho=0.220, p=.017) in patients. The MTSD state score did not show any significant associations with cognition or functional capacity measurements. There were no significant correlations between depression state and trait scores and cognition or functional capacity in controls (Table 2).
Table 2.
Correlation coefficients between MTSD Trait and State scales and measure of working memory, processing speed, and functional capacity (UPSA-2 Total).
| Controls (n=151) | Patients (n=118) | |||
|---|---|---|---|---|
| MTSD-Trait | MTSD-State | MTSD-Trait | MTSD-State | |
| BPRS Psychosis | n/a | n/a | .246* | .312* |
| Working memory | −.016 | −.069 | .186* | .090 |
| Processing speed | −.081 | −.110 | .200* | .084 |
| Functional capacity | .033 | .010 | .217* | .080 |
significant after Benjamini and Hochberg correction for multiple comparisons (p<.05)
Relationship of depressive symptoms to white matter integrity
Whole brain average FA was greater in healthy controls than schizophrenia patients (F=11.86, p=.001). The same trend was observed in individual tracts, with FA being statistically greater in controls than patients in all tracts (p=.001 to .024) except corticospinal tract, cingulum and inferior fronto-occipital tract (p=.051 to .711).
In patients, greater trait depression was significantly and positively associated with the whole brain average FA values and FA values for four white matter tracts – the corona radiata, thalamic radiation, superior longitudinal fasciculus, and superior fronto-occipital tract (Table 3). Significantly (p<0.05) positive associations were found between MTSD trait scores and FA of four additional tracts, including body of corpus callosum, fornix, internal capsule and sagittal striatum, although these associations were not significant after correcting for multiple tests. MTSD state scores showed no significant association with FA in any tracts after correction for multiple comparisons. In contrast, FA values were not associated with psychosis severity (r=-.130 to .008, all p>.290) (Table 4). In controls, the whole-brain average FA value was not significantly associated with MTSD trait (rho=-.041, p=.667) or MTSD state (rho=.010, p=.914). However, the average FA value for the cingulum was negatively correlated with trait depression in normal controls (rho=-.278, p=.003) and this was the only relationship that was significant after correction for multiple comparisons. None of the other 14 tracts including the whole brain FA was significantly associated with trait (rho=-.174 to .022, all p>.065) or state depression (rho=-.117 to .184, all p>.051) in controls.
Table 3.
Correlations of MTSD scores with FA of white matter tracts in individuals with schizophrenia (n=73).
| White matter tract | MTSD-Trait | MTSD-State |
|---|---|---|
| Average FA | .241* | .228 |
| Genu of CC | .176 | .189 |
| Body of CC | .241* | .275* |
| Splenium of CC | .124 | .138 |
| Fornix | .249* | .288* |
| Corticospinal | .052 | −.005 |
| Internal capsule | .262* | .183 |
| External capsule | .219 | .167 |
| Corona radiata | .340*† | .320* |
| Thalamic radiation | .341*† | .258* |
| Sagittal striatum | .303* | .311* |
| Cingulum | .195 | .181 |
| SLF | .367*† | .275* |
| SFO | .346*† | .276* |
| IFO | .064 | .017 |
CC=corpus callosum; SLF=superior longitudinal fasciculus; SFO=superior fronto-occipital fasciculus; IFO=inferior fronto-occipital fasciculus.
p<.05 – nominally significant
significant after correction for multiple comparisons using method of Benjamini and Hochberg (1995)
Table 4.
Correlation coefficients between symptom and cognitive measures and FA values in individuals with schizophrenia.
| White matter tract | BPRS-Psychosis | Processing speed | Working memory | Functional capacity |
|---|---|---|---|---|
| Average FA | −.112 | .443* | .261* | .333* |
| Genu of CC | .008 | .272* | .176 | .241 |
| Body of CC | −.014 | .427*† | .289* | .328* |
| Splenium of CC | −.004 | .407*† | .216 | .177 |
| Fornix | −.024 | .363*† | .075 | .206 |
| Corticospinal | −.130 | .310*† | .208 | .238 |
| Internal capsule | −.125 | .412*† | .303* | .387*† |
| External capsule | −.086 | .339*† | .263* | .314* |
| Corona radiata | −.069 | .363*† | .203 | .375*† |
| Thalamic radiation | −.036 | .304*† | .252* | .383*† |
| Sagittal striatum | −.049 | .436*† | .289* | .417*† |
| Cingulum | −.111 | .383*† | .300* | .270* |
| SLF | −.034 | .384*† | .266* | .410*† |
| SFO | .019 | .313*† | .159 | .327* |
| IFO | −.013 | .166 | .114 | .038 |
p<.05 – nominally significant
significant after correction for multiple comparisons using method of Benjamini and Hochberg (1995)
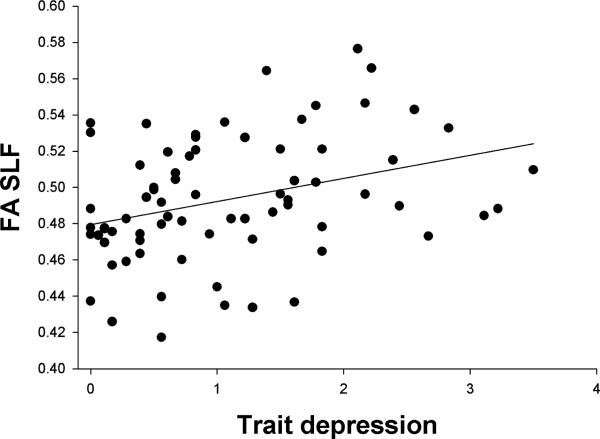
Using the strongest correlation between trait depression and FA, for that of the SLF (Figure 1), partial correlations were calculated to examine if the relationship is mediated by cognition (mean of the z scores of working memory and processing speed). The partial correlation between MTSD-Trait and SLF FA controlling for the combined cognitive score was .234 (p=.063), suggesting that part of the relationship between trait depression and white matter integrity is mediated by cognition. In contrast, partial correlations between whole brain average FA and cognition remained significant even controlling for state (r=.356, p=.004) or trait depression (r=.359, p=.004).
Figure 1.
Scatterplot of relationship between trait depression and fractional anisotropy (FA) of the superior longitudinal fasciculus (SLF) in schizophrenia patients.
Potential clinical confounding factors
We considered the potential impact of medications by examining the association between chlorpromazine dose equivalents (CPZ) of antipsychotic medication, depression scores, and FA values for patients. This revealed only one significant correlation surviving correction for multiple comparisons: a negative correlation between CPZ and FA of the genu of the corpus callosum (rho=-.396, p=.001). CPZ was not associated with trait depression (rho=-.093, p=327) nor state depression (rho=-.109, p=.251). We also examined the potential confounding effect of antidepressant use. Patients taking antidepressants had higher trait depression (χ2=5.34, p=.021) but not state depression (χ2=2.77, p=.096) than patients not on antidepressants. However, patients taking antidepressants did not have higher whole-brain average FA values (t=.674, p=.502). Patients who smoke did not differ from patients who were nonsmokers in trait depression (F=.010, p=.922) nor state depression (F=.183, p=.670). Finally, we also examined the correlations of FA with psychosis, working memory, processing speed, and UPSA-2 (Table 4). Processing speed showed the most robust correlations with FA of multiple tracts in the white matter even after corrections for multiple comparisons.
Discussion
We found that subjectively reported trait depression is prominent in patients with schizophrenia, but greater trait depression did not correspond to more severe deficits in several cognitive and biological markers associated with schizophrenia. If anything, trait depression was associated with slightly higher cognitive and functional capacity, and greater white matter integrity in individuals with schizophrenia.
These findings appear paradoxical, especially when examined in comparison to major depressive disorder (MDD). In MDD, severity of depression is associated with worse cognition (Lee et al., 2012; Snyder, 2013), and modest cognitive deficits are still apparent in euthymic periods (Bora et al., 2013). In mood disorders trait depression is more closely linked to cognitive impairment than state depression (Sarapas et al., 2012). In comparison, our results indicate that trait depression is associated with less cognitive impairment in schizophrenia. Individuals with MDD also tend to exhibit decreased FA in white matter tracts compared to individuals without MDD (Liao et al., 2013), particularly in the SLF, where greater severity of depressive symptoms correlates with more reduction in FA values (Murphy and Frodl, 2011). In our study the results show an opposite trend as trait depression in schizophrenia is associated with higher FA, with the trend strongest in the SLF (Figure 1). Therefore, there appeared consistently “paradoxical” findings on how trait depression in schizophrenia is related to cognition, function, and white matter integrity, as compared with how depression in MDD is related to these measures. We believe these observations raise an interesting question on whether trait depression is a relatively separate clinical domain compared to depression in MDD or these other trait-like clinical and biological measures in schizophrenia. That the FA of the SLF in particular showed the strongest relationship to trait depression is intriguing, as this tract exhibits delayed maturation relative to other tracts in the brain, remaining plastic through adolescence (Lebel and Beaulieu, 2011); additionally, SLF FA may be influenced by the same genetic factors contributing to working memory (Karlsgodt et al., 2010).
Our observations are in fact consistent with research done in deficit vs. nondeficit syndrome schizophrenia. Individuals with deficit syndrome are characterized by persistent negative symptoms and more severe cognitive deficits, and tend to have less depression when compared with patients without deficit syndrome, who tend to have high levels of depression and better cognitive functioning (Cohen et al., 2007; Kirkpatrick et al., 2001). Longitudinal studies have also found that presence of depression is a good prognostic indicator for persons with chronic schizophrenia (McGlashan, 1988). Additionally, in individuals with chronic schizophrenia with overall very poor functioning, depression was found to be associated with higher social and cognitive functioning (Rieckmann et al., 2005). These previous data suggest that, after all, it may not be surprising that trait depression in schizophrenia is associated with relatively preserved cognitive ability and functional capacity, despite the fact that depression in schizophrenia is associated with other significantly negative social and clinical consequences (Conley et al., 2007).
Our data may support the ‘affective pathway’ to psychosis as conceptualized by Myin-Germeys and van Os (2007). This theory predicts that heightened affective reactivity to stress contributes to expression of positive symptoms of schizophrenia in a pathway independent of cognitive deficits, and may in fact be associated with a good-outcome type of psychosis (Myin-Germeys and van Os 2007). Given the clear association between stress exposure and risk of depression (Vinkers et al., 2014), it is possible that trait depression reflects chronically heightened affective reactivity, and in this context the association between trait depression and positive symptoms reported here is consistent with the affective pathway theory. Alternatively, our results can be interpreted as representing the influence of insight, in that preserved cognitive ability may be associated with better recognition of the limitations posed by severe mental illness, which may in turn induce symptoms of depression. This hypothesis is not incompatible with the affective pathway theory, but further work is necessary to clarify the complex interactions of clinical insight, cognition, depression, and positive symptoms of psychosis.
Despite the associations of trait depression with relatively preserved neuropsychological functioning and white matter integrity, depression remains a clinically grave phenomenon in schizophrenia, as greater levels of depression predict lower psychological feelings of well-being (Strauss et al., 2012), worse subjective quality of life (Narvaez et al., 2008), and suicidal ideation (Upthegrove et al., 2010), and must be closely monitored and treated. Within the chronic course of schizophrenia, capacity for understanding of self, insight into illness, and recognition of stigma surrounding the disorder, may be markers of preserved cognitive function, but also a risk factor for longitudinally experienced depression. In this context, further study of the neurobiology of trait depression as compared with state depression, and the neurobiology of depression in schizophrenia versus depression in MDD, may offer new ways to reduce clinical heterogeneity and improve individualized treatment planning.
An important limitation of this study is a potential recall bias inherent in our measure of trait depression. As the MTSD-Trait scale requires a retrospective assessment of experience of depressive symptoms, individuals with better memory may be more able to recall such symptoms. However, we would expect this bias to be present in the community sample used as a control group in our study, but within that sample there was no association between trait depression and cognitive performance (Table 2). Another limitation is that some of the individuals with schizophrenia who participated in this study were taking antidepressants, and these patients had higher levels of trait depression. However, there was no evidence that patients taking antidepressants had higher levels of cognitive performance or white matter FA, arguing against the possibility that the associations of these measures with trait depression could be attributed to antidepressant use.
To summarize, the longitudinal experience of depression is related to more severe psychosis yet less deficit in several trait-like clinical features of schizophrenia. Furthermore, trait depression in schizophrenia appeared to be distinct from how trait depression is related to cognition in patients with major depression. We also replicated previous findings that state depression has essentially no relationship with cognitive impairment in schizophrenia. However, the neurobiology of trait depression, and its biological convergence and divergence from state depression require further investigation. These results may also have implications for the ongoing efforts to identify domains of psychopathology that cut across traditional diagnostic boundaries (Insel et al., 2010). Schizophrenia and affective disorders overlap in several symptom domains, including depressive symptoms and cognitive deficits (Harvey, 2011); however, there appear to be distinct patterns in how trait depression is related to cognition and white matter integrity in these disorders. This underscores the importance of developing constructs that account for different pathophysiological pathways to similar symptom domains. The current analysis lends validity to the proposal that trait depression could be a clinical domain unlike other clinical or biological measures commonly included in the discourse of schizophrenia.
Supplementary Material
Acknowledgments
None
Role of the funding source
This research was supported by National Institute of Health grants R01MH085646, P50MH103222, R01DA027680, T32MH067533, and R01EB015611.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Joshua Chiappelli contributed to data analysis and prepared the first draft of the manuscript. Peter Kochunov contributed to study design and data analysis. Katherine DeRiso, Kavita Thangavelu, Hemalatha Sampath, and Florian Muellerklein oversaw protocol implementation and data management. Katie Nugent, Teodor Postolache, and William Carpenter Jr. contributed to data analysis, writing and editing. Elliot Hong served as principal investigator and assisted in all stages of design, analysis and writing. All authors contributed to and have approved the final manuscript.
Conflict of interest
All authors declare no conflict of interest.
References
- Andriopoulos I, Ellul J, Skokou M, Beratis S. Suicidality in the “prodromal” phase of schizophrenia. Compr Psychiatry. 2011;52(5):479–85. doi: 10.1016/j.comppsych.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Barder HE, Sundet K, Rund BR, Evensen J, Haahr U, Ten Velden Hegelstad W, et al. Ten year neurocognitive trajectories in first-episode psychosis. Front Hum Neurosci. 2013;7(7):643. doi: 10.3389/fnhum.2013.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B. 1995;57(1):289–300. [Google Scholar]
- Bonner-Jackson A, Grossman LS, Harrow M, Rosen C. Neurocognition in schizophrenia: a 20-year multi-follow-up of the course of processing speed and stored knowledge. Compr Psychiatry. 2010;51(5):471–9. doi: 10.1016/j.comppsych.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Harrison BJ, Yücel M, Pantelis C. Cognitive impairment in euthymic major depressive disorder: a meta-analysis. Psychol Med. 2013;43(10):2017–26. doi: 10.1017/S0033291712002085. [DOI] [PubMed] [Google Scholar]
- Bowie CR, Reichenberg A, Patterson TL, Heaton RK, Harvey PD. Determinants of real-world functional performance in schizophrenia subjects: correlations with cognition, functional capacity, and symptoms. Am J Psychiatry. 2006;163(3):418–25. doi: 10.1176/appi.ajp.163.3.418. [DOI] [PubMed] [Google Scholar]
- Chiappelli J, Nugent KL, Thangavelu K, Searcy K, Hong LE. Assessment of Trait and State Aspects of Depression in Schizophrenia. Schizophr Bull. 2014;40(1):132–42. doi: 10.1093/schbul/sbt069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, Saperstein AM, Gold JM, Kirkpatrick B, Carpenter WT, Jr, Buchanan RW. Neuropsychology of the deficit syndrome: new data and meta-analysis of findings to date. Schizophr Bull. 2007;33(5):1201–12. doi: 10.1093/schbul/sbl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley RR, Ascher-Svanum H, Zhu B, Faries DE, Kinon BJ. The burden of depressive symptoms in the long-term treatment of patients with schizophrenia. Schizophr Res. 2007;90(1-3):186–97. doi: 10.1016/j.schres.2006.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumlish N, Whitty P, Kamali M, Clarke M, Browne S, McTigue O, et al. Early insight predicts depression and attempted suicide after 4 years in first-episode schizophrenia and schizophreniform disorder. Acta Psychiatr Scand. 2005;112(6):449–55. doi: 10.1111/j.1600-0447.2005.00620.x. [DOI] [PubMed] [Google Scholar]
- Delaney C, McGrane J, Cummings E, Morris DW, Tropea D, Gill M, et al. Preserved cognitive function is associated with suicidal ideation and single suicide attempts in schizophrenia. Schizophr Res. 2012;140(1-3):232–6. doi: 10.1016/j.schres.2012.06.017. [DOI] [PubMed] [Google Scholar]
- Dickinson D, Ramsey ME, Gold JM. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry. 2007;64(5):532–42. doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]
- Dickinson D, Ragland JD, Gold JM, Gur RC. General and specific cognitive deficits in schizophrenia: Goliath defeats David? Biol Psychiatry. 2008;64(9):823–7. doi: 10.1016/j.biopsych.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake RJ, Pickles A, Bentall RP, Kinderman P, Haddock G, Tarrier N, et al. The evolution of insight, paranoia and depression during early schizophrenia. Psychol Med. 2004;34(2):285–92. doi: 10.1017/s0033291703008821. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res. 2009;108(1-3):3–10. doi: 10.1016/j.schres.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Forbes NF, Carrick LA, McIntosh AM, Lawrie SM. Working memory in schizophrenia: a meta-analysis. Psychol Med. 2009;39(6):889–905. doi: 10.1017/S0033291708004558. [DOI] [PubMed] [Google Scholar]
- Häfner H, Maurer K, an der Heiden W. ABC Schizophrenia study: an overview of results since 1996. Soc Psychiatry Psychiatr Epidemiol. 2013;48(7):1021–31. doi: 10.1007/s00127-013-0700-4. [DOI] [PubMed] [Google Scholar]
- Harvey PD. Mood symptoms, cognition, and everyday functioning: in major depression, bipolar disorder, and schizophrenia. InnovClinNeurosci. 2011;8(10):14–8. [PMC free article] [PubMed] [Google Scholar]
- Harvey PD, McClure MM, Patterson TL, McGrath JA, Pulver AE, Bowie CR, et al. Impairment in functional capacity as an endophenotype candidate in severe mental illness. Schizophr Bull. 2012;38(6):1318–26. doi: 10.1093/schbul/sbr046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff AL, Svetina C, Shields G, Stewart J, DeLisi LE. Ten year longitudinal study of neuropsychological functioning subsequent to a first episode of schizophrenia. Schizophr Res. 2005;78(1):27–34. doi: 10.1016/j.schres.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748–51. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, Kochunov P, Winkler AM, Laird AR, Almasy L, Duggirala R, et al. A multimodal assessment of the genetic control over working memory. J Neurosci. 2010;30(24):8197–202. doi: 10.1523/JNEUROSCI.0359-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68(2-3):283–97. doi: 10.1016/j.schres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Harvey PD, Goldberg TE, Gold JM, Walker TM, Kennel C, et al. Norms and standardization of the Brief Assessment of Cognition in Schizophrenia (BACS). Schizophr Res. 2008;102(1-3):108–15. doi: 10.1016/j.schres.2008.03.024. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Buchanan RW, Ross DE, Carpenter WT., Jr. A separate disease within the syndrome of schizophrenia. Arch Gen Psychiatry. 2001;58(2):165–71. doi: 10.1001/archpsyc.58.2.165. [DOI] [PubMed] [Google Scholar]
- Knowles EE, David AS, Reichenberg A. Processing speed deficits in schizophrenia: reexamining the evidence. Am J Psychiatry. 2010;167(7):828–35. doi: 10.1176/appi.ajp.2010.09070937. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Lancaster JL, Thompson P, Woods R, Mazziotta J, Hardies J, et al. Regional spatial normalization: toward an optimal target. J Comput Assist Tomogr. 2001;25(5):805–16. doi: 10.1097/00004728-200109000-00023. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Williamson DE, Lancaster J, Fox P, Cornell J, Blangero J, et al. Fractional anisotropy of water diffusion in cerebral white matter across the lifespan. Neurobiol Aging. 2012;33:9–20. doi: 10.1016/j.neurobiolaging.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. J Neurosci. 2011;31(30):10937–47. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RS, Hermens DF, Porter MA, Redoblado-Hodge MA. A meta-analysis of cognitive deficits in first-episode Major Depressive Disorder. J Affect Disord. 2012;140(2):113–24. doi: 10.1016/j.jad.2011.10.023. [DOI] [PubMed] [Google Scholar]
- Leifker FR, Patterson TL, Bowie CR, Mausbach BT, Harvey PD. Psychometric properties of performance-based measurements of functional capacity: test-retest reliability, practice effects, and potential sensitivity to change. Schizophr Res. 2010;119(1-3):246–52. doi: 10.1016/j.schres.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Huang X, Wu Q, Yang C, Kuang W, Du M, et al. Is depression a disconnection syndrome? Meta-analysis of diffusion tensor imaging studies in patients with MDD. J Psychiatry Neurosci. 2013;38(1):49–56. doi: 10.1503/jpn.110180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle PF, Barnes TR, Curson DA, Patel M. Depression and the experience of psychological deficits in schizophrenia. Acta Psychiatr Scand. 1993;88(4):243–7. doi: 10.1111/j.1600-0447.1993.tb03450.x. [DOI] [PubMed] [Google Scholar]
- Light GA, Swerdlow NR, Rissling AJ, Radant A, Sugar CA, Sprock J, et al. Characterization of neurophysiologic and neurocognitive biomarkers for use in genomic and clinical outcome studies of schizophrenia. PLoS One. 2012;7(7):e39434. doi: 10.1371/journal.pone.0039434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggini C, Raballo A. Exploring depression in schizophrenia. Eur Psychiatry. 2006;21(4):227–32. doi: 10.1016/j.eurpsy.2005.07.001. [DOI] [PubMed] [Google Scholar]
- McGlashan TH. The prediction of outcome in chronic schizophrenia. IV. The Chestnut Lodge follow-up study. Arch Gen Psychiatry. 1986;43(2):167–76. doi: 10.1001/archpsyc.1986.01800020077010. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Carpenter WT., Jr. Postpsychotic depression in schizophrenia. Arch Gen Psychiatry. 1976;33(2):231–9. doi: 10.1001/archpsyc.1976.01770020065011. [DOI] [PubMed] [Google Scholar]
- Moore O, Cassidy E, Carr A, O'Callaghan E. Unawareness of illness and its relationship with depression and self-deception in schizophrenia. Eur Psychiatry. 1999;14(5):264–9. doi: 10.1016/s0924-9338(99)00172-8. [DOI] [PubMed] [Google Scholar]
- Murphy ML, Frodl T. Meta-analysis of diffusion tensor imaging studies shows altered fractional anisotropy occurring in distinct brain areas in association with depression. Biol Mood Anxiety Disord. 2011;1(1):3. doi: 10.1186/2045-5380-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myin-Germeys I, van Os J. Stress-reactivity in psychosis: evidence for an affective pathway to psychosis. Clin Psychol Rev. 2007;27(4):409–24. doi: 10.1016/j.cpr.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Narvaez JM, Twamley EW, McKibbin CL, Heaton RK, Patterson TL. Subjective and objective quality of life in schizophrenia. Schizophr Res. 2008;98(1-3):201–8. doi: 10.1016/j.schres.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieckmann N, Reichenberg A, Bowie CR, Parrella M, White L, Friedman JI, et al. Depressed mood and its functional correlates in institutionalized schizophrenia patients. Schizophr Res. 2005;77(2-3):179–87. doi: 10.1016/j.schres.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Roalf DR, Gur RC, Almasy L, Richard J, Gallagher RS, Prasad K, et al. Neurocognitive performance stability in a multiplex multigenerational study of schizophrenia. Schizophr Bull. 2013;39(5):1008–17. doi: 10.1093/schbul/sbs078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarapas C, Shankman SA, Harrow M, Goldberg JF. Parsing trait and state effects of depression severity on neurocognition: Evidence from a 26-year longitudinal study. J Abnorm Psychol. 2012;121(4):830–7. doi: 10.1037/a0028141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol Bull. 2013;139(1):81–132. doi: 10.1037/a0028727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sönmez N, Romm KL, Andreasssen OA, Melle I, Røssberg JI. Depressive symptoms in first episode psychosis: a one-year follow-up study. BMC Psychiatry. 2013;13(1):106. doi: 10.1186/1471-244X-13-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staring AB, Van der Gaag M, Van den Berge M, Duivenvoorden HJ, Mulder CL. Stigma moderates the associations of insight with depressed mood, low self-esteem, and low quality of life in patients with schizophrenia spectrum disorders. Schizophr Res. 2009;115(2-3):363–9. doi: 10.1016/j.schres.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Sandt AR, Catalano LT, Allen DN. Negative symptoms and depression predict lower psychological well-being in individuals with schizophrenia. Compr Psychiatry. 2012;53(8):1137–44. doi: 10.1016/j.comppsych.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Tomotake M, Ueoka Y, Kaneda Y, Taniguchi K, Nakataki M, et al. Clinical correlates associated with cognitive dysfunction in people with schizophrenia. Psychiatry Clin Neurosci. 2012;66(6):491–8. doi: 10.1111/j.1440-1819.2012.02390.x. [DOI] [PubMed] [Google Scholar]
- Upthegrove R, Birchwood M, Ross K, Brunett K, McCollum R, Jones L. The evolution of depression and suicidality in first episode psychosis. Acta Psychiatr Scand. 2010;122(3):211–8. doi: 10.1111/j.1600-0447.2009.01506.x. [DOI] [PubMed] [Google Scholar]
- Vinkers CH, Joëls M, Milaneschi Y, Kahn RS, Penninx BW, Boks MP. Stress exposure across the life span cumulatively increases depression risk and is moderated by neuroticism. Depress Anxiety. 2014 Apr 17; doi: 10.1002/da.22262. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale – Third Edition (WAIS-III) Harcourt Assessment; San Antonio, TX: 1997. [Google Scholar]
- Yao L, Lui S, Liao Y, Du MY, Hu N, Thomas JA, et al. White matter deficits in first episode schizophrenia: an activation likelihood estimation meta-analysis. ProgNeuropsychopharmacolBiol Psychiatry. 2013;45:100–6. doi: 10.1016/j.pnpbp.2013.04.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.