Abstract
Transmembrane-4 L-six family member-1 (TM4SF1) is a small plasma membrane glycoprotein that regulates cell motility and proliferation. TM4SF1 is an attractive cancer target because of its high expression in both tumor cells and on the vascular endothelial cells lining tumor blood vessels. We generated mouse monoclonal antibodies against human TM4SF1 in order to evaluate their therapeutic potential; 13 of the antibodies we generated reacted with extracellular loop-2 (EL2), TM4SF1’s larger extracellular, lumen-facing domain. However, none of these antibodies reacted with mouse TM4SF1, likely because the EL2 of mouse TM4SF1 differs significantly from that of its human counterpart. Therefore, to test our antibodies in vivo, we employed an established model of engineered human vessels in which human endothelial colony-forming cells (ECFC) and human mesenchymal stem cells (MSC) are incorporated into Matrigel plugs that are implanted subcutaneously in immunodeficient nude mice. We modified the original protocol by (i) pre-culturing human ECFC on laminin, fibronectin, and collagen coated plates; and (ii) increasing the ECFC/MSC ratio. These modifications significantly increased the human vascular network in Matrigel implants. Two injections of one of our anti-TM4SF1 EL2 monoclonal antibodies, 8G4, effectively eliminated the human vascular component present in these plugs; they also abrogated human PC3 prostate cancer cells that were incorporated into the ECFC/MSC Matrigel mix. Together these studies provide a mouse model for assessing tumor xenografts that are supplied by a human vascular network and demonstrate that anti-TM4SF1 antibodies such as 8G4 hold promise for cancer therapy.
Keywords: TM4SF1, engineered human blood vessels, anti-angiogenic target, cancer target, cancer therapy
Introduction
Most tumors need to generate a new vascular supply if they are to grow beyond minimal size [1], and they do so primarily by over-expressing vascular endothelial growth factor-A (VEGF-A) [2,3]. Strategies targeting VEGF-A or its receptors were found to be highly effective in preventing the growth of rapidly growing mouse tumors [2,3], suggesting that anti-angiogenesis therapy might be a useful new approach in treating cancer. However, multiple clinical trials targeting VEGF-A or its receptors in patients with a variety of cancers have yielded only modest benefit in terms of either progression-free or overall survival [3]. Nonetheless, the strategy of targeting tumor blood vessels continues to make sense, and there is a need to identify new molecular targets on tumor blood vessels beyond the VEGF-A/VEGF-A receptor axis.
One potential target is Transmembrane-4 L-Six family member-1 (TM4SF1). TM4SF1 was discovered in 1986 as a tumor cell antigen that is also weakly expressed on normal vascular endothelium [4,5]. It is a small plasma membrane glycoprotein that has tetraspanin topology with two extracellular loops (a short EL1 and a longer EL2) [6]. We subsequently found that TM4SF1 is highly expressed in activated endothelial cells (EC), including cultured endothelial cells and the EC lining the blood vessels that supply several human cancers [7,8]. Thus, TM4SF1 is an attractive target because it is strongly represented on both tumor cells and the vasculature that supplies them.
Using cultured human endothelial cells as immunogen, we raised antibodies against human TM4SF1 with the aim of introducing them into cancer therapy. However, none of the antibodies we raised reacted with mouse TM4SF1 and therefore could not be of use in targeting the TM4SF1-overexpressing mouse blood vessels supplying human tumor xenografts.
To circumvent this difficulty, we took advantage of a well-established model for growing human blood vessels in mice [9,10]. This system makes use of a mixture of human endothelial colony-forming cells (ECFC) and human mesenchymal stem cells (MSC) that is incorporated into Matrigel and planted subcutaneously in nude mice. Implanted ECFC join host (mouse) blood vessels to form a functional vascular network that is comprised of microvessels lined by both human and mouse endothelial cells [9]. We modified this system to develop a more extensive human vascular network and showed that one of our antibodies against human TM4SF1, 8G4, effectively abrogated the human blood vessels that formed in Matrigel plugs. In addition, when human PC3 tumor cells were incorporated into the Matrigel mixture, 8G4 destroyed both the human vascular component and the tumor cells.
Materials and Methods
Preparation of monoclonal antibodies
Human umbilical vein endothelial cells (HUVEC) were acquired from Lonza (Walkersville, MD), cultured in EGM2-MV complete medium, and used at passage 3–6. HUVEC were transduced to overexpress (OE) human TM4SF1 at levels of ~400 mRNA copies/cell (~4× that of native HUVEC which express ~100 mRNA copies/cell) [7,8], and 107 TM4SF1-OE HUVEC were injected intraperitoneally into six-week old female Balb-c mice five times at biweekly intervals. Hybridomas were cultured in DMEM supplemented with 10% FBS and 2 mM glutamine. Protein-G based antibody purification, antibody isotyping and LAL endotoxin assay kits were acquired from Thermo Scientific (Waltham, MA) and were used to purify anti-TM4SF1 antibodies from the hybridoma culture medium, to identify antibody isotypes and to measure endotoxin levels, following the manufacturer’s instructions. Fifteen stable hybridoma clones were found to interact with TM4SF1. We have deposited one of these, hybridoma clone 8G4, in American Tissue Culture Collection (ATCC, PTA-120523). MS1 and HEK293 were acquired from ATCC, whereas HEMn cells were obtained from Life Technologies (Grand Island, NY).
Antibody targeting of human blood vessels and PC3 tumor cells in Matrigel plugs
We adapted the Matrigel model developed by Drs. Bischoff, Melero-Martin and colleagues [9,10]. Briefly, human endothelial cell colony forming cells (ECFC; Lonza, Walkersville, MD), were cultured in 50 ng/ml collagen-I (BD Bioscience, San Jose, CA) coated plates with EGM2-MV medium (Lonza) supplemented by 12% FBS (Sigma, St. Louis, MO), and used at passage-3 and -4. In some experiments, cells were cultured on plates coated with collagen I supplemented with 50 ng/ml fibronectin (BD Bioscience) and 10 ng/ml laminin (BD Bioscience). Human mesenchymal stem cells (MSC) were obtained from Texas A&M (Cell Distribution Center, Temple, TX), cultured in MEM-alpha medium (Life Technologies, Carlsbad, CA) supplemented with 10% FBS and 5 mM L-glutamine, and used at passage 3 to 6. ECFC and MSC were mixed in ratios of 2:3 or 3:2 so as to total 2×107 cells/ml in Hanks’ balanced salt solution (HBSS) (Life Technologies). 100 ul of the cell suspension (2×106 cells) and 50 ng basic fibroblast growth factor (R&D Systems, Minneapolis, MN) were mixed with 100 ul of Matrigel (BD Bioscience), and injected subcutaneously in the flanks of 8-week old female athymic nude mice (Charles River Laboratories, Wilmington, MA). In some experiments, 1×105 PC3 human prostate cancer cells (cultured in DMEM/10% FBS) were combined with ECFC/MSC (2×106 cells) in a final volume of 100 ul and mixed with 100 ul Matrigel before injection. Matrigel implants were harvested at different times 30 minutes after a tail vein injection of 2×106 molecular weight FITC-dextran, 100 mg/kg (Sigma).
To evaluate the effects of anti-TM4SF1 antibodies on human blood vessels and tumor cells, 8G4 or control mouse IgG1 (mIgG; Sigma) was injected intraperitoneally 10 and 14 days after implantation of Matrigel plugs. Matrigel plugs were harvested on day-18, 30 min after tail vein injection of FITC-dextran. Matrigel plugs were snap frozen in liquid nitrogen and homogenized into frozen tissue powders with Cryo-Press CP-100 (Microtech Nichion, Funabashi, Japan) for extraction of RNA (for gene expression profiling) or protein (for vascular volume measurements).
To measure vascular volumes, tissue powders were suspended in 0.5 ml RIPA lysis buffer (Tris-buffered saline, pH 7.0, 0.1% SDS, and protease/phosphatase inhibitor cocktails) to extract total protein [11]. Protein lysates were recovered after centrifugation at 15,000 rpm for 15 min, and protein concentrations were measured with the BCA protein assay kit (Pierce, Rockford, IL). FITC-dextran was measured at 515 nm in a fluorescence plate reader SpectraMax M5 (Sunnyvale, CA). Vascular volumes are presented as absorbance at 515 nm per mg protein.
Histology and immunostaining
These procedures have been described previously [7,8,12]. For immunofluorescence and H&E staining, Matrigel plugs were fixed in 4% paraformaldehyde (PFA) and embedded in OCT mounting media for preparation of frozen sections. 8 um frozen sections were blocked in PBS/2% FBS and stained with rhodamine–conjugated Ulex europaeus agglutinin I (UEA-I; Sigma) to identify human endothelial cells.
Flow Cytometry, other in vitro assays
Briefly, 1×106 cells were washed in ice cold PBS, suspended in 1 ml cold blocking buffer (PBS/1% FBS) that contained 1 ug 1st antibody (8G4 or migG), and incubated on ice for 1h with occasional agitation. Cells were centrifuged (500 × g for 5 min), washed 3× with cold PBS, incubated with 100 ng/ml 2nd antibody (Alexa-488 labeled donkey anti-mouse IgG, Life Technology), and washed 3× with cold PBS. Cell suspensions were analyzed with FACScan (Becton Dickinson, San Jose CA). 104 events were collected for each analysis.
HUVEC migration and Matrigel tube assays were performed as previously described [7,8]. Antibody affinity was determined with a binding titration assay using HUVEC and flow cytometry [13], an approach that is useful for determining affinity with cell-surface antigens and desirable in our case because, lacking recombinant TM4SF1 protein, we could not perform ELISA.
Multi-gene transcriptional profiling (MGTP)
MGTP, a form of quantitative real-time PCR, was used to determine the number of mRNA copies per cell, by normalization to 18S rRNA with the assumption that, on average, cells express ~106 18S-rRNA copies [14]. For cultured cells, RNA extraction was carried out using the RNeasy kit following the manufacturer’s instructions (Qiagen, Valencia, CA). Matrigel tissue powders were lysed in 500 ul Trizol lysis buffer (Qiagen), followed by chloroform and isopropanol treatment to recover RNA following the manufacturer’s instructions. The precipitated RNA was then transferred to an RNeasy spin column to elute total RNA. cDNA was prepared using reverse transcriptase III (Life Technologies) [7]. Mean and standard error (mean ± SD) were calculated from three cDNA samples prepared in three separate experiments. Species specific human CD31 (forward: CACCTGGCCCAGGAGTTTC; reverse: AGTACACAGCCTTGTTGCCATGT) and mouse CD31 (forward: GAGCCCAATCACGTTTCAGTTT; reverse: TCCTTCCTGCTTCTTGCTAGCT) real-time PCR primers were used to measure their expression in Matrigel samples. All real-time PCR primers and DNA were synthesized by Integrated DNA Technology (Coraville, IA)
Statistics
Statistical analysis was performed with the Student T test.
Results
Generation and specificity of mouse anti-human TM4SF1 antibodies
TM4SF1 structure is depicted in Fig. 1A; it has four transmembrane domains and two extracellular loops, EL1 and EL2. Our strategy for generating hybridomas and screening monoclonal antibodies against TM4SF1 is described in Methods. Initial screening was performed with immunocytochemistry. We identified 15 stable clones that reacted both with HUVEC and with human dermal fibroblasts transduced to overexpress human TM4SF1 (TM4SF1-OE HDF), but that did not react with native HDF which express TM4SF1 at extremely low levels (~5 mRNA copies/cell). Fig. 1B illustrates typical results for one antibody, 8G4 (IgG1 subtype), that was selected for detailed study because of its high affinity (Kd ~1 nM) for human TM4SF.
Fig. 1. Monoclonal antibodies reactive with human TM4SF1.
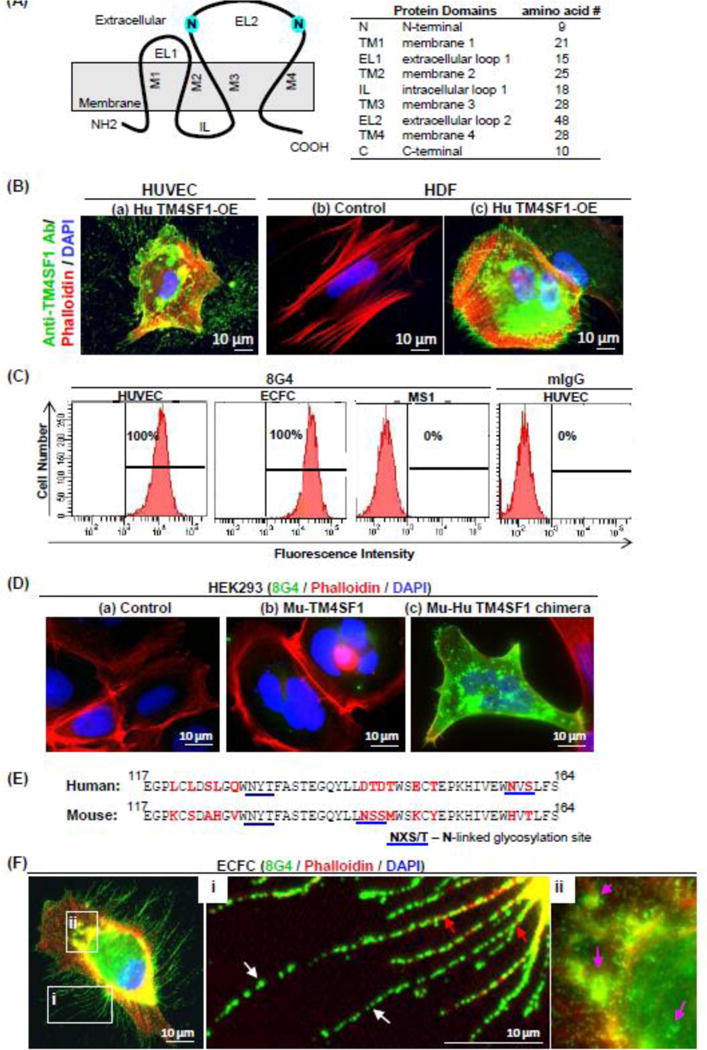
(A) Structure of human TM4SF1. (B) 8G4 staining of (a) Hu TM4SF1-OE HUVEC (b) HDF (human dermal fibroblasts) that express sub-detectable levels of TM4SF1 and (c) HDF transduced to overexpress human TM4SF1. Staining is representative of that obtained with the 15 monoclonal antibodies that reacted with TM4SF1 in fixed endothelial cells. (C) Flow cytometry demonstrates population shift of HUVEC and ECFC, but not mouse endothelial cell line MS1, with 8G4 antibody. Control mouse IgG (mIgG) did not evoke a population shift in HUVEC. (D) 8G4 immunostaining of HEK293 cells that were transfected to express (a) empty vector control, (b) murine TM4SF1 (Mu-TM4SF1), or (c) Mu-Hu TM4SF1 chimera. 8G4 recognized the Mu-Hu TM4SF1 chimera but not full length murine TM4SF1. (E) Human and mouse TM4SF1 EL2 sequence alignment. Red font indicates differences between human and mouse amino acid residues. Blue underline indicates N-glycosylation sites; N (asparagine), X (any amino acid), and S/T (serine/threonine). Both human and mouse TM4SF1 contain two potential N-glycosylation sites. The first has an identical sequence and alignment in human and mouse. However, the second differs both in sequence (NVS in human, NSS in mouse) and location (159 in human, 142 in mouse). (F) 8G4 immunofluorescence staining of ECFC localized TM4SF1 to plasma membrane, to nanopodia in an intermittent pattern (i, white arrows), and to perinuclear vesicles (ii, pink arrows). F-actin (phalloidin-staining; i red arrows) extended only into the most proximal portions of nanopodia.
Flow cytometry (Fig. 1C) demonstrated that 8G4 reacted strongly with live HUVEC and also with live human endothelial colony-forming cells (ECFC), which, like HUVEC, express high levels of TM4SF1 (149.6±37.9 mRNA copies/cell). Flow cytometry results for all 15 of our TM4SF1-reactive antibodies are shown in Supplementary Figure S1, and demonstrated that 13 of these reacted with a cell surface epitope of TM4SF1. However, none of these antibodies reacted with mouse endothelial cell line MS1 cells (Fig. 1C for 8G4 staining of MS1; data not shown for the other mouse anti-human TM4SF1 antibodies), although MS1 cells express high levels of mouse TM4SF1 (~120 mRNA copies/cell; data not shown).
To identify the epitopes with which our antibodies reacted, we generated mutant forms that expressed selected portions of human TM4SF1 (Supplementary Figure S2A). We transduced these mutant forms into neonatal human epidermal melanocytes (HEMn), cells that do not express detectable levels of TM4SF1 (Supplementary Figure S2B). 8G4 and the other 12 anti-TM4SF1 antibodies that interacted with HUVEC by flow cytometry (Fig. 1C, Supplementary Figure 1) also interacted with full length human TM4SF1 and with a mutant form that included EL2 (amino acids 67–202). However, 8G4 did not react with mutants expressing amino acids 1–94 (which includes EL1) or with a mutant (N129/159G) in which both N-glycosylation sites in EL-2 were mutated from asparagine to glycine. Further, 8G4 no longer reacted with TM4SF1 following enzymatic N-deglycosylation (data not shown).
To establish that these antibodies reacted with the EL2 of human TM4SF1, we engineered a chimeric form of mouse TM4SF1 in which human EL2 replaced mouse EL2 (Mu-Hu TM4SF1 chimera; Supplementary Figure S2A). HEK293 cells were employed for these chimeric studies because they, like HEMn cells are essentially null for TM4SF1, but are easier to maintain and transfect than HEMn cells. 8G4 strongly stained HEK293 cells that incorporated the Mu-Hu TM4SF1 chimera, thus confirming that 8G4 reacted with the EL2 portion of human TM4SF1 (Fig. 1D); the other twelve antibodies interacting with the extracellular domains of TM4SF1 gave similar results (data not shown). The failure of any of these antibodies to react with mouse TM4SF1 is not surprising in that, although human and mouse TM4SF1 are overall 89% homologous at the amino acid level, homology falls to 73% in the EL2 region which also differs importantly with regard to the location of N-glycosylation sites (Fig. 1E).
8G4 staining of ECFC was identical to that we had reported previously in HUVEC with a commercial mouse monoclonal antibody (Millipore) which, unfortunately, is no longer available [7,8,15]; that is, staining of the plasma membrane (Fig. 1F), thin, elongate nanopodia that project from the cell surface (Fig. 1Fi), and a subpopulation of intra-cytoplasmic vesicles (Fig. 1Fii). However, 8G4 did not significantly affect HUVEC migration and Matrigel tube formation at a high concentration of 100 ug/ml (data not shown).
Generation of engineered human vessels in nude mice
Our goal in preparing antibodies against TM4SF1 was to evaluate their possible efficacy in destroying angiogenic tumor blood vessels that highly expressed TM4SF1. However, because none of our antibodies cross-reacted with mouse TM4SF1, we turned to a Matrigel model in which human blood vessels can be generated in mice. In this model [9,10], ECFC that have been cultured on collagen-I-coated plastic dishes are mixed with human MSC that have been cultured on uncoated plastic in a ratio of 2:3; the cell mixture is then incorporated into Matrigel plugs that are implanted subcutaneously in nude mice.
We found that both the numbers of human blood vessels that formed, and the ratio of human to mouse endothelial cells in those blood vessels, could be improved by two modifications of the published protocol. First, we pre-adapted ECFC to Matrigel by pre-culturing them for 48h in a mixture of laminin (LN), fibronectin (FN), and collagen-I (CG), rather than on CG alone. The rationale for this change is that Matrigel contains large amounts of laminin (56%) [16], and ECFC, like cultured endothelial cells, express high levels of integrins-α5β1 (ITGA5 and ITGB1), –αvβ3 (ITGAV and ITGB3), and –αvβ5 (ITGAV and ITGB5) (Supplementary Figure S3) [7], all ligands for fibronectin. Pre-culturing ECFC in the CG/FN/LN mixture did not significantly change expression levels of these integrins (Supplementary Figure S3) but did generate a more robust human vascular network than when they were cultured on CG alone; human CD31 expression increased 2.4–fold (p<0.001) (Fig. 2Aa) and vascular volume 2.2-fold (p<0.01) at day-7 (Fig. 2Ab). Mouse CD31 continued to predominate but trended lower (Fig. 2Aa). Matrigel plug images demonstrated increased vascularity when ECFC were pre-cultured on CG/FN/LN (Fig. 2Ac).
Fig. 2. Engineered human blood vessels in Matrigel plugs implanted in mice.
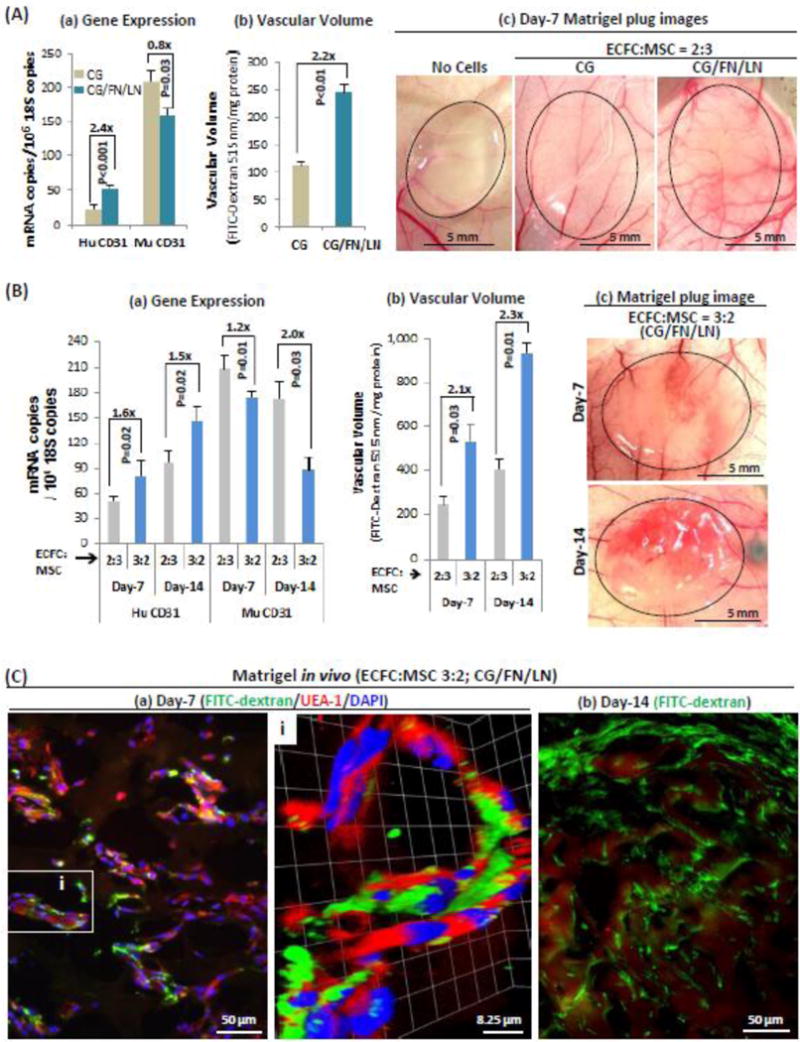
Passage 4 ECFC were: (A) pre-cultured 48h on either CG- or CG/FN/LN-coated plates and mixed with MSC at a ratio of 2:3, or (B) pre-cultured 48h on CG/FN/LN-coated plates and mixed with MSC at ratios of either 2:3 or 3:2, suspended in Matrigel, and then the mixture was implanted subcutaneously in the flanks of nude mice. Matrigel plugs were harvested on days -7 or -14, 30 min. after tail vein injection of 2000kd FITC-dextran. RNA was extracted for measurement of CD31 by MGTP, using human- and mouse-specific primers and FITC-dextran content was used to measure vascular volume. (A) Day-7 Matrigel plugs demonstrate (a) increased human CD31 expression levels, (b) increased vascular volumes, and (c) increased overall vascularity when ECFC were pre-cultured on CG/FN/LN versus CG alone. (B) Further increases in human CD31 expression and in the human CD31/mouse CD31 expression ratio (a) and vascular volume (b) occurred when the ratio of ECFC was increased from 2:3 to 3:2. (B,c) Matrigel plug images show increased vascularity at day-14 over day-7. (C) Confocal images show (a) Numerous UEA-1-positive human endothelial cells in close relation to FITC-dextran-filled vascular lumens in day-7 Matrigel plugs. Inset i, Z-stacked confocal image (42 stacks with 220 nm/stack), and (b) extensive FITC-dextran staining of day-14 Matrigel plugs. Results are representative of at least three independent experiments.
Second, we found that increasing the ratio of ECFC (pre-cultured on CG/FN/LN) to MSC from 2:3 to 3:2 resulted in an additional increase in total vascularity (Fig. 2B). On day-7, expression of human CD31 was increased 1.6-fold (Fig. 2Ba), and vascular volume 2.1-fold (p=0.03) (Fig. 2Bb). This difference persisted at 14 days with a 1.5-fold (p=0.02) increase in human CD31 expression and a 2.3-fold (p=0.01) increase in vascular volume (Figs. 2Ba,2Bb). Moreover, the ratio of human:mouse CD31 increased from 0.24 in 2:3 Matrigel plugs to 0.46 in 3:2 Matrigel plugs at day-7. Further, at day-14, the ratio of human:mouse CD31 increased from 0.56 in 2:3 Matrigel plugs to 1.65 in 3:2 Matrigel plugs, such that human endothelial cells predominated over mouse endothelial cells (Fig. 2B,a). Consistent with these findings, Matrigel plug images show greater vascularity at day-14 than at day-7 (Fig. 2Bc).
Confocal microscopy staining for FITC-dextran confirmed that the vascular structures in Matrigel plugs were connected to the host vasculature and included large numbers of human-specific (UEA-1 positive) endothelial cells (Fig. 2C).
Targeting engineered human vessels with the mouse anti-human TM4SF1 antibody 8G4
Using our modified protocol, we set out to determine whether our anti-TM4SF1 monoclonal antibodies had an effect on the human blood vessels we had engineered in Matrigel plugs. We administered 8G4 or control mIgG i.p. at a dose of 3 mg/kg, on day 10, followed by a second injection four days later, and harvested Matrigel plugs on day-18. At the time of first antibody injection, the ratio of human:mouse CD31 was 0.91 in the 3:2 (ECFC:MSC) Matrigel plugs (data not shown). Representative images show dramatic loss of blood vessels in Matrigel plugs treated with 8G4 as compared with control antibody (Fig. 3A,C). As shown in Fig. 3B, 8G4 treatment reduced human CD31 levels 52.7-fold and vascular volume 9.3-fold. Perhaps in compensation, mouse CD31 levels increased substantially. At a lower dose (1 mg/kg), 8G4 was not effective in obliterating vessels, whereas doses of 3 and 10 mg/kg were approximately equally effective (data not shown).
Fig. 3. Targeting human endothelial cells in Matrigel plugs with 8G4 or control mIgG.
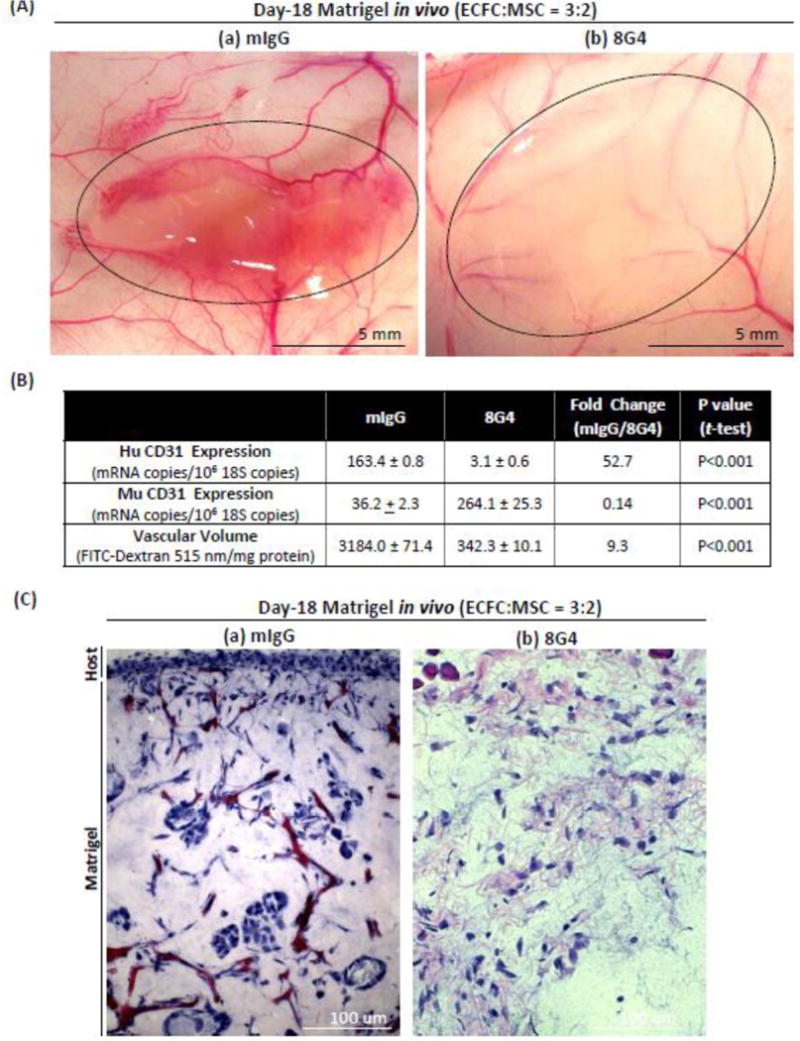
Matrigel plugs were prepared with ECFC pre-cultured on CG/FN/LN and introduced into Matrigel plugs at a ratio of 3-ECFC:2-MSC cells (total of 2×106 cells/plug). 100 ul of HBSS containing 3mg/kg (a) mIgG or (b) 8G4 was injected i.p. on days -10 and -14 after implantation. Matrigel plugs were harvested on day-18, 30 min after tail vein injection of FITC-dextran. (A) Day-18 Matrigel images (outlined) show extensive blood vessel network following mIgG treatment (a), that was largely disrupted by 8G4 treatment (b). (B) Quantification of human and mouse CD31 content (by MGTP expression) and vascular volumes (by FITC-dextran content). (C) Hematoxylin and eosin staining of Matrigel plug sections show numerous blood vessels following mIgG injection, that are absent following 8G4 injection. Results are representative of at least three separate experiments with three mice per group in each.
Targeting engineered human vessels and TM4SF1-expressing PC3 tumor cells with 8G4
Many human cancer cell lines express high levels of TM4SF1, including PC3 prostate cancer cells (65.8±16.2 TM4SF1 mRNA copies/cell). Therefore we set out to determine the effects of 8G4 on PC3 tumor cells incorporated into Matrigel without ECFC/MSC cells versus PC3 cells incorporated into Matrigel plugs that included ECFC/MSC cells. PC3 cells incorporated into Matrigel plugs without ECFC/MSC cells formed large, poorly vascularized masses whose size was reduced 4.2-fold (p<0.001) by 8G4 as compared with control mIgG (Figs. 4Aa; 4B); overall vascular volume was little affected by 8G4 (reduced 17.2% compared with tumors treated with mIgG, p=0.08) (Fig. 4D), as this antibody did not react with the TM4SF1 expressed by mouse blood vessels. In contrast, when ECFC/MSC were included along with PC3 cells, overall vascularity of Matrigel plugs was greatly increased (Fig. 4Ab; D). Further, as determined by CD31 expression, 8G4 antibody reduced the number of human endothelial cells by 117.4-fold (p<0.00001) (Fig. 4C), and vascular volume by 92.7% (p<0.0001) (Fig. 4D) compared with tumors treated with mIgG. Again mouse CD31 expression increased substantially (Fig. 4C).
Fig. 4. Targeting PC3 tumor cells in Matrigel plugs with 8G4 or control mIgG.
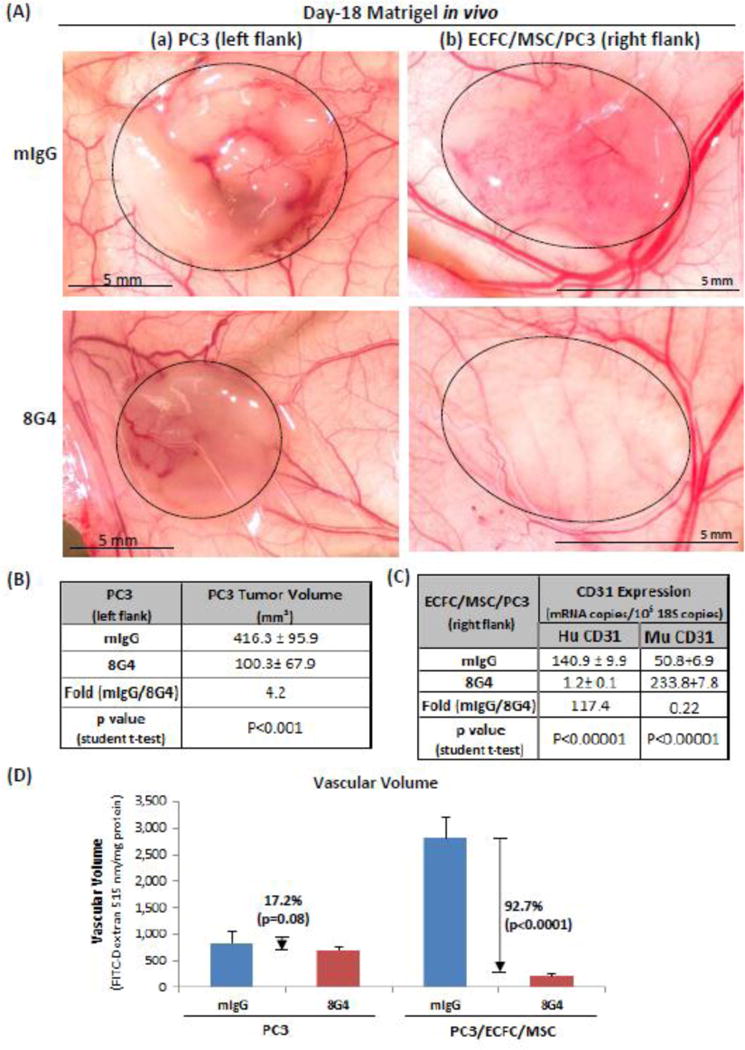
Matrigel plugs were prepared as in Fig. 3, except that PC3 tumor cells were included. Larger numbers of PC3 cells, 106, were required to induce robust tumor growth in Matrigel plugs in the absence of ECFC/MSC cells, whereas 105 PC3 cells were sufficient when they were implanted along with ECFC/MSC cells. (A) Representative macroscopic appearance of Matrigel plugs (dotted outline) in mice treated with either 3 mg/kg 8G4 or control mIgG. (B–D) Quantification of human and mouse CD31 content (by MGTP expression) and vascular volume (by FITC-dextran content) in Matrigel plugs that included PC3 cells alone or PC3 cells supplemented with ECFC/MSC cells. Results are representative of three separate experiments with three mice per group in each experiment.
Discussion
The goal of this study was twofold: 1. develop monoclonal antibodies against human TM4SF1 and 2. test their efficacy in targeting and possibly destroying human blood vessels in an in vivo setting. We raised 15 mouse monoclonal antibodies that stained TM4SF1 in fixed cells; of these, 13 also reacted with TM4SF1 in its native configuration on the surface of living cells. Further, we were able to improve an established model for growing human blood vessels in Matrigel plugs in mice, increasing both total vascularity and the fraction of blood vessels whose endothelial cells expressed human CD31. Incorporating these two technical improvements, we demonstrated that one of our antibodies, 8G4, was able to destroy the human blood vessels growing in Matrigel plug implants. Further, TM4SF1-expressing human PC3 tumor cells were also destroyed when these were incorporated in Matrigel plugs; PC3 cell death likely resulted both from direct interaction with 8G4 and, secondarily, from 8G4-mediated elimination of the human endothelial cell lined blood vessels supplying them.
8G4, and the other 12 monoclonal antibodies that reacted with human TM4SF1 in live cells, reacted with an epitope residing in TM4SF1’s EL-2 domain. This 48 amino acid sequence includes both of TM4SF1’s N-glycosylation sites (Fig. 1); these N-glycosylation sites are essential for 8G4’s reactivity in that N/G mutation of both sites resulted in loss of reactivity (Supplemetary Figure S1). Sugar groups are also likely to be important in that N-deglycosylation of TM4SF1 caused loss of 8G4 reactivity. None of the 13 monoclonal antibodies reactive with human TM4SF1 interacted with mouse TM4SF1. This is not surprising given the relative low (73%) homology of human and mouse TM4SF1 in EL2 (Fig. 1E). Of particular importance are differences in the location of the N-glycosylation sites (Fig. 1E). In human TM4SF1, the second N-glycosylation site is at amino acid 159 whereas in mouse TM4SF1 it is at amino acid 142, likely resulting in a different tertiary structure and glycosylation pattern. We did not succeed in raising antibodies against human TM4SF1’s EL-1 domain, a short 15 amino acid sequence that is largely conserved in human and mouse TM4SF1, but whose exposure may be concealed by the larger EL-2 domain. Thus, it may not be possible to raise antibodies that react with both human and mouse TM4SF1. Using different strategies for raising antibodies, Hellstrom’s group also failed to generate an antibody that reacted with both human and mouse TM4SF1 [4,5].
Melero-Martin and collaborators developed a methodology for engineering human blood vessels by mixing together ECFC and MSC in Matrigel plugs that were implanted subcutaneously in immuno-deficient mice [9,10]. The rationale for this approach was based on the expectation that ECFC and MSC could interact reciprocally with each other to generate new blood vessels. This is a likely possibility because ECFC express high levels of PDGFβ and VEGF-A receptors 1 and 2, whereas MSC express VEGF-A and PDGF receptor-β (Supplementary Figure S4). We were able to increase the number of blood vessels lined by human vis-a-vis mouse endothelial cells by pre-culturing ECFC on plates coated with collagen, laminin and fibronectin, and by increasing the ECFC/MSC ratio from 2:3 to 3:2.
8G4’s ability to destroy human blood vessels and tumor cells incorporated in Matrigel plugs likely resulted from antibody-dependent cell-mediated cytotoxicity (ADCC). ADCC is largely effected by NK cells that are fully functional in immunodeficient nude mice and is an important mechanism for the action of therapeutic monoclonal antibodies against tumors [17–19]. This is further made likely by our findings that 8G4 did not affect endothelial cell migration or tube formation in vitro.
Our findings with 8G4 (Figs. 3,4) are in accord with earlier findings with another monoclonal antibody, L6, [20,21], that also recognizes TM4SF1’s EL2 but reacts with a different epitope that requires leucine in position 122, i.e., at a site distant from the N-glycosylated regions of TM4SF1 [22]. Hellstrom’s group performed preliminary clinical studies in the 1990s with the L6 antibody and achieved partial remissions in approximately one-third of patients with metastatic breast cancer [23–25]. It was only subsequently recognized that human tumor blood vessel endothelial cells expressed high levels of TM4SF1 [7], and, in retrospect, the partial remissions they achieved may have reflected activity against tumor-associated vascular endothelial cells as well as tumor cells. Nonetheless, these studies were encouraging in that there was little toxicity, suggesting that antibodies against TM4SF1 selectively targeted the endothelial cells of tumor blood vessels, while sparing endothelial cells lining normal blood vessels. Newer approaches conjugating antibodies such as 8G4 with cytotoxic drugs may be expected to improve tumor and tumor blood vessel destruction [26].
Supplementary Material
Acknowledgments
We thank Dr. Ruei-Zeng Lin for teaching the Matrigel implantation and Andrew Zukauskas for participating hybridoma screening. This work was supported by NIH grant P01 CA92644 and by a contract from the National Foundation for Cancer Research.
References
- 1.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N. Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Semin Oncol. 2002;29(6 Suppl 16):10–14. doi: 10.1053/sonc.2002.37264. [DOI] [PubMed] [Google Scholar]
- 3.Sitohy B, Nagy JA, Dvorak HF. Anti-VEGF/VEGFR therapy for cancer: reassessing the target. Cancer Res. 2012;72(8):1909–1914. doi: 10.1158/0008-5472.CAN-11-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hellstrom I, Horn D, Linsley P, Brown JP, Brankovan V, Hellstrom KE. Monoclonal mouse antibodies raised against human lung carcinoma. Cancer Res. 1986;46(8):3917–3923. [PubMed] [Google Scholar]
- 5.O’Donnell RT, DeNardo SJ, Shi XB, Mirick GR, DeNardo GL, Kroger LA, Meyers FJ. L6 monoclonal antibody binds prostate cancer. Prostate. 1998;37(2):91–97. doi: 10.1002/(sici)1097-0045(19981001)37:2<91::aid-pros5>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 6.Marken JS, Schieven GL, Hellstrom I, Hellstrom KE, Aruffo A. Cloning and expression of the tumor-associated antigen L6. Proc Natl Acad Sci U S A. 1992;89(8):3503–3507. doi: 10.1073/pnas.89.8.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shih SC, Zukauskas A, Li D, Liu G, Ang LH, Nagy JA, Brown LF, Dvorak HF. The L6 protein TM4SF1 is critical for endothelial cell function and tumor angiogenesis. Cancer Res. 2009;69(8):3272–3277. doi: 10.1158/0008-5472.CAN-08-4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zukauskas A, Merley A, Li D, Ang LH, Sciuto TE, Salman S, Dvorak AM, Dvorak HF, Jaminet SC. TM4SF1: a tetraspanin-like protein necessary for nanopodia formation and endothelial cell migration. Angiogenesis. 2011;14(3):345–354. doi: 10.1007/s10456-011-9218-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melero-Martin JM, De Obaldia ME, Kang SY, Khan ZA, Yuan L, Oettgen P, Bischoff J. Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ Res. 2008;103(2):194–202. doi: 10.1161/CIRCRESAHA.108.178590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin RZ, Moreno-Luna R, Zhou B, Pu WT, Melero-Martin JM. Equal modulation of endothelial cell function by four distinct tissue-specific mesenchymal stem cells. Angiogenesis. 2012;15(3):443–455. doi: 10.1007/s10456-012-9272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffmann J, Schirner M, Menrad A, Schneider MR. A highly sensitive model for quantification of in vivo tumor angiogenesis induced by alginate-encapsulated tumor cells. Cancer Res. 1997;57(17):3847–3851. [PubMed] [Google Scholar]
- 12.Lin CI, Lau CY, Li D, Jaminet SC. Nanopodia – thin, fragile membrane projections with roles in cell movement and intercellular interactions. Journal of visualized experiments: JoVE. 2013 doi: 10.3791/51320. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drake AW, Klakamp SL. A rigorous multiple independent binding site model for determining cell-based equilibrium dissociation constants. J Immunol Methods. 2007;318(1–2):147–152. doi: 10.1016/j.jim.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 14.Wada Y, Li D, Merley A, Zukauskas A, Aird WC, Dvorak HF, Shih SC. A multi-gene transcriptional profiling approach to the discovery of cell signature markers. Cytotechnology. 2010;63(1):25–33. doi: 10.1007/s10616-010-9315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato K, Aoki J, Misawa N, Daikoku E, Sano K, Tanaka Y, Koyanagi Y. Modulation of human immunodeficiency virus type 1 infectivity through incorporation of tetraspanin proteins. J Virol. 2008;82(2):1021–1033. doi: 10.1128/JVI.01044-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes CS, Postovit LM, Lajoie GA. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics. 2010;10(9):1886–1890. doi: 10.1002/pmic.200900758. [DOI] [PubMed] [Google Scholar]
- 17.Lopez AF, Strath M, Sanderson CJ. Mouse immunoglobulin isotypes mediating cytotoxicity of target cells by eosinophils and neutrophils. Immunology. 1983;48(3):503–509. [PMC free article] [PubMed] [Google Scholar]
- 18.Herlyn D, Herlyn M, Steplewski Z, Koprowski H. Monoclonal anti-human tumor antibodies of six isotypes in cytotoxic reactions with human and murine effector cells. Cell Immunol. 1985;92(1):105–114. doi: 10.1016/0008-8749(85)90068-1. [DOI] [PubMed] [Google Scholar]
- 19.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6(4):443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 20.Hellstrom I, Beaumier PL, Hellstrom KE. Antitumor effects of L6, an IgG2a antibody that reacts with most human carcinomas. Proc Natl Acad Sci U S A. 1986;83(18):7059–7063. doi: 10.1073/pnas.83.18.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu AY, Robinson RR, Hellstrom KE, Murray ED, Jr, Chang CP, Hellstrom I. Chimeric mouse-human IgG1 antibody that can mediate lysis of cancer cells. Proc Natl Acad Sci U S A. 1987;84(10):3439–3443. doi: 10.1073/pnas.84.10.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fell HP, Gayle MA, Yelton D, Lipsich L, Schieven GL, Marken JS, Aruffo A, Hellstrom KE, Hellstrom I, Bajorath J. Chimeric L6 anti-tumor antibody. Genomic construction, expression, and characterization of the antigen binding site. J Biol Chem. 1992;267(22):15552–15558. [PubMed] [Google Scholar]
- 23.Goodman GE, Hellstrom I, Brodzinsky L, Nicaise C, Kulander B, Hummel D, Hellstrom KE. Phase I trial of murine monoclonal antibody L6 in breast, colon, ovarian, and lung cancer. J Clin Oncol. 1990;8(6):1083–1092. doi: 10.1200/JCO.1990.8.6.1083. [DOI] [PubMed] [Google Scholar]
- 24.Richman CM, DeNardo SJ, O’Grady LF, DeNardo GL. Radioimmunotherapy for breast cancer using escalating fractionated doses of 131I-labeled chimeric L6 antibody with peripheral blood progenitor cell transfusions. Cancer Res. 1995;55(23 Suppl):5916s–5920s. [PubMed] [Google Scholar]
- 25.DeNardo SJ, Kukis DL, Kroger LA, O’Donnell RT, Lamborn KR, Miers LA, DeNardo DG, Meares CF, DeNardo GL. Synergy of Taxol and radioimmunotherapy with yttrium-90-labeled chimeric L6 antibody: efficacy and toxicity in breast cancer xenografts. Proc Natl Acad Sci U S A. 1997;94(8):4000–4004. doi: 10.1073/pnas.94.8.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerber HP, Koehn FE, Abraham RT. The antibody-drug conjugate: an enabling modality for natural product-based cancer therapeutics. Natural product reports. 2013;30(5):625–639. doi: 10.1039/c3np20113a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


