Abstract
Motivated by evidence that the dentate gyrus differentially mediates the pattern separation (PS) component of declarative memory function and that dentate gyrus harbors molecular and cellular pathology in schizophrenia, we examined whether PS performance is altered in volunteers with schizophrenia (SZV) relative to healthy volunteers (HV). In groups of well-characterized SZV (n=14) and HV (n=15), we contrasted performance on the Behavioral Pattern Separation (BPS) Task, acquiring two outcome measures, a PS parameter and a Recognition Memory (RM) parameter, as well as specific recognition data by stimulus type. The SZVs showed a significant decrement in PS performance relative to HV (mean±SE, SZV: 3.1%±4.8; HV: 17.1%±4.7; p=0.039, d=0.86); whereas SZV and HV did not significantly differ in RM performance (SZV: 50.1%±4.8; HV: 59.3%±4.7; p=0.350, d=0.36). Moreover, the SZVs showed a selective defect in correctly identifying similar lure items (SZV: 24.0%±4.1; HV: 41.2%±4.1; p<0.05), but demonstrated no impairment in identifying targets and novel foils. These data suggest that dentate gyrus is dysfunctional in schizophrenia, a feature that could contribute to declarative memory impairment in the disorder and possibly to psychosis, a conclusion consistent with the considerable molecular pathology in dentate gyrus in schizophrenia.
Introduction
Human studies have consistently supported an involvement of hippocampal dysfunction in schizophrenia (SZ) based on functional (Medoff et al., 2001;Heckers et al., 1998;Schobel et al., 2013), molecular (Harrison, 2004;Gao et al., 2000) and cellular (Wang et al., 2011) outcomes. These hippocampal alterations could underlie declarative memory dysfunction in the syndrome and mediate some manifestations of psychosis (Tamminga et al., 2010). We have begun to examine the role of subfield-specific hippocampal alterations in SZ, persuaded by evidence of distinct subfield functions in declarative memory formation that is emerging from basic investigations (Leutgeb et al., 2007;Schobel et al., 2013;Rangel and Eichenbaum, 2013;Deng et al., 2013). The dentate gyrus (DG) appears to be differentially affected in SZ compared to other hippocampal subfields, based on the distinctive molecular and cellular changes in DG tissue from SZ cases (Gao et al., 2000;Knable et al., 2004). Reductions in GluN1 protein have been observed in SZ in the hippocampus; moreover, this GluN1 change is expressed selectively in DG (Stan et al., 2013). Because GluN1 is the critical subunit in NMDA receptor signaling, it raises the possibility that excitatory signaling in DG is reduced in SZ. Together these findings support the model that pathologically reduced DG signaling onto CA3 pyramidal neurons can alter plasticity dynamics in CA3, potentially increasing neuronal activity there, generating hyper-associations and mistakes in conjunctive encoding which could create false memories with psychotic content (Fig 1) (Tamminga et al., 2010). It is important to establish the presence of meaningful alterations in DG function in living individuals with SZ, to corroborate this hippocampal model of psychosis.
Figure 1. MODEL OF HIPPOCAMPAL DYSFUNCTION IN SCHIZOPHRENIA.
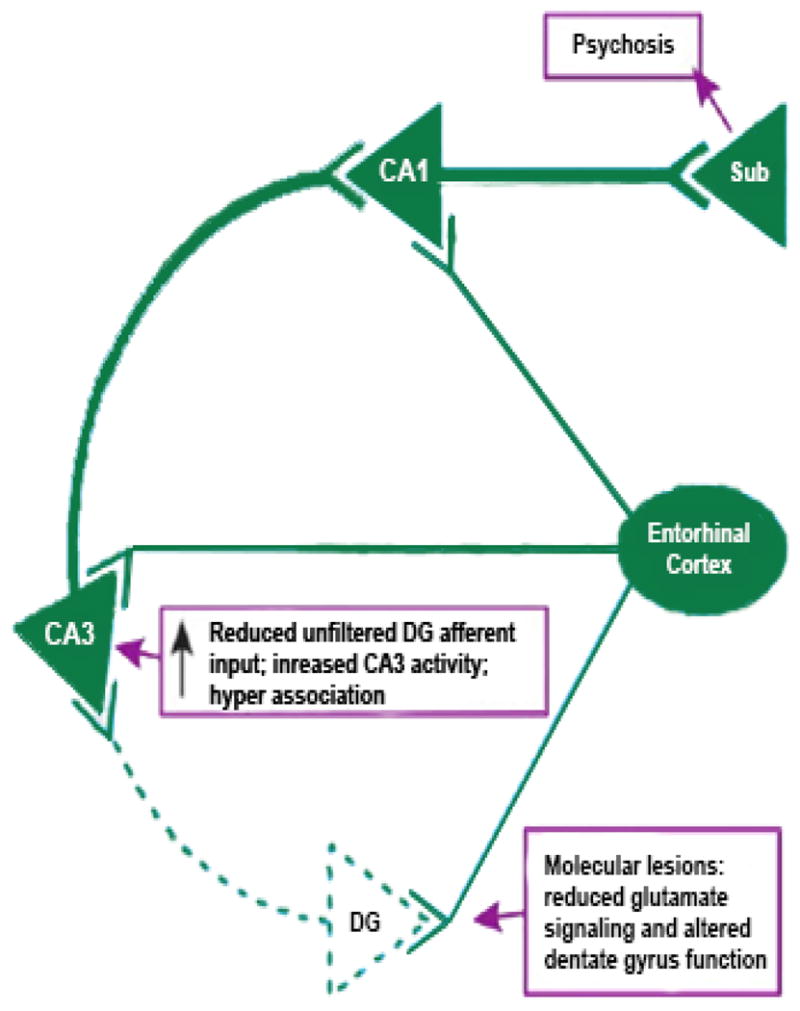
This illustration is a theoretical model of psychosis in schizophrenia developed from human in vivo imaging and postmortem molecular observations. The model includes a defect in dentate gyrus function which starts with reduced GluN1-containing NMDA receptors in DG and reduced mossy fiber afferent stimulation in CA3. While reduced GluN1 protein and mRNA have been reported, dentate gyrus pathology associated with reduced NMDA signaling would not have to be limited to this single pathology, but could include (for example) decreased neurogenesis (Reif et al., 2006). The outcome of reduced Mossy Fiber tract glutamatergic signaling in CA3 is to sensitize the pyramidal neuron to excitatory stimulation, a situation that (unless it can naturally reverse itself) may lead to hyper-excitability, hyper-associations and the creation of false memories with psychotic content.
A computational component of declarative memory thought to differentially reflect DG function is pattern separation (Yassa and Stark, 2011). Pattern separation is the process of establishing independent non-overlapping representations, often thought to be critical for rapidly forming new memories (O’Reilly and McClelland, 1994;LaRocque et al., 2013). This function is essential for sound memory formation in that it establishes whether or not a particular stimulus is new and needs to be held in memory or already exists as a memory trace and merely needs to be recalled. Pattern separation processes rely critically on DG function (Leutgeb et al., 2007;Kesner, 2013;Bakker et al., 2008;Rolls, 2013;Marr, 1971;Kumaran and McClelland, 2012). Stark, et al have developed the Behavioral Pattern Separation Task (BPS) (Stark et al., 2013), which aims to indirectly evaluate DG-mediated function in living humans by assaying behavioral expressions that putatively reflect pattern separation function. The task has been normed in healthy humans; and, until now, it has been applied mostly in age-related hippocampal decline, where it signals memory impairment (Stark et al., 2010). The BPS task further distinguishes ‘pattern separation’ from ‘recognition memory’ function, potentially offering a means to determine whether pattern separation is differentially impaired relative to other aspects of declarative memory function.
To test whether the apparent DG tissue changes previously documented in SZ have a functional fingerprint, we examined performance on the BPS task in SZ volunteers. We hypothesized that DG-dependent performance is reduced in SZ due to molecular deficiencies in DG, and as such, we a priori predicted that the ability of persons with schizophrenia to distinguish subtle target differences would be reduced and that the ‘pattern separation’ parameter calculated from the BPS task (as explained below) would be reduced in individuals with SZ compared to healthy volunteers.
Methods
Participants
Fourteen SZ volunteers (SZV) and 15 healthy volunteers (HV) were recruited for the study through advertising and referrals from community out-patient centers. Individuals with a history of major neurological or decompensated medical illness, mental retardation, traumatic brain injury, substance abuse within the last month, or substance dependence within the last three months were excluded from the study. The study was approved by the UT Southwestern Medical Center IRB and all volunteers provided written informed consent before participating.
The SZV psychiatric diagnoses were based on the Structured Clinical Interview for DSM-IV-TR Diagnosis (SCID-I/P) (First et al., 1996). The Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987) was used to evaluate active symptoms and their severity. All volunteers completed the Brief Assessment of Cognition in Schizophrenia (BACS) scale (Keefe et al., 2004); subscale and total z-scores were calculated. The Behavioral Pattern Separation (BPS) Task was done with all volunteers.
The BPS task consists of two phases, initially, an incidental ‘encoding’ phase (runs 1–2) and subsequently, a ‘test’ phase (runs 3–4). In runs 1–2, volunteers were exposed to 128 pictures of everyday objects in each run, with each object shown for 2 sec followed by a 0.5 sec inter-stimulus interval (ISI); volunteers identified each object as either belonging “indoors” or “outdoors”. Total run time for the encoding phase was 320 sec. In the test phase, runs 3–4, volunteers were exposed to 192 pictures in each run, with a picture exposure time, as before, of 2 sec and an ISI of 0.5 sec. Sixty-four of these pictures were exact repetitions of objects from runs 1–2; 64 pictures were completely new objects, and 64 were similar objects (lures) to pictures shown in runs 1–2. Each test run lasted 480 sec. Volunteers were instructed to identify each object as a repeated picture (“old”), as a new picture (“new”), or as a similar picture (“similar”).
Analysis
Outcomes from test phase/runs 3–4 were calculated as percent response on each of the three stimuli types, ‘target (old)’, ‘foil (new)’, and ‘lure (similar)’. These data were used to calculate the ‘Pattern Separation’ (PS) parameter [‘similar’ responses to lures minus ‘similar’ responses to new] and the ‘Recognition Memory’ (RM) parameter [‘old’ responses to targets minus ‘old’ responses to new (Stark et al., 2013)]. To test the a priori hypothesis postulating a reduction in the PS parameter in the SZV group with no change in RM, the primary analysis was a two-tailed t test directly contrasting SZV vs NV on the two key task parameters, PS and RM. In the exploratory analysis, to examine between-group differences in response accuracy to each of the task conditions underlying the PS and RM parameters, we conducted a three-way repeated measure ANOVA with two within-subject factors [stimulus type (‘target’, ‘lure’ and ‘new’) and response (‘old’, ‘similar’, and ‘new’)] and group as the between-subject factor, followed by nine post hoc pair-wise comparisons using adjusted ANOVA mean square error terms and using the Bonferroni correction for multiple comparisons (Bailey, 1977;Winer et al., 1991). Finally, Pearson correlations between the PS and RM parameters and the PANSS total and positive subscale scores, as well as BACS total and verbal memory subscale scores were carried out. Two-tailed t test and chi-square test were used, as appropriate, for demographic and cognitive variables. Alpha was set at 0.05 for all analyses. The analyses were carried out using the NCSS-8 (Hintze, 2012).
Results
Both HV and SZV were evaluated with the diagnostic, cognition and symptom data, as described; these outcomes are shown in Table 1. No between-group differences were found in any demographic characteristics, including age, sex, race, and handedness. SZV showed a trend toward lower total BACS scores compared to HV (p=0.093). The majority of SZV were treated with antipsychotic plus other medications. Only one SZV was off any medications while active in the study; 11/15 SZV (73%) were taking more than one medication. All were clinically stable and optimally medicated.
Table 1.
Demographic and Clinical Characteristics of Study Sample
| Demographic and clinical characteristics | SZV (n=14) | HV (n=15) | Test statistic | p value |
|---|---|---|---|---|
| Age, yrs; Mean (SD) | 38.2 (10.26) | 43.56(10.99) | t (22) = 1.21 | 0.24 |
| Sex/Male; n (%) | 11 (73.33) | 5 (33.33) | χ2 (1) = 0.20 | 0.65 |
| Handedness; n (%)a | χ2 (2) = 1.43 | 0.49 | ||
| Right-handed | 9 (60.0) | 5 (33.33) | ||
| Left-handed | 4 (26.67) | 1 (6.67) | ||
| Ambidextrous | 1 (6.67) | 0 (0.00) | ||
| Race; n (%)b | χ2 (2) = 0.88 | 0.64 | ||
| White | 6 (40.0) | 4 (26.67) | ||
| Black | 6 (40.0) | 2 (13.33) | ||
| Other | 2 (13.33) | 0 (0.00) | ||
| PANSS Positive; Mean (SD) | 20.8 (4.92) | -- | -- | -- |
| PANSS Negative; Mean (SD) | 20.62 (6.69) | -- | -- | -- |
| PANSS Total; Mean (SD) | 83.92 (16.06) | -- | -- | -- |
| BACS Total (z-score); Mean (SD) c | −1.82 (1.3) | −.69 (.83) | t (16) = 1.78 | 0.093 |
|
| ||||
|
Concomitant medications; n (%)
d
| ||||
| Off medications | 1 (6.67) | 14 (93.33) | -- | -- |
| Antipsychotics | 10 (66.67) | 0 (0.00) | -- | -- |
| Mood stabilizers | 5 (33.33) | 0 (0.00) | -- | -- |
| Antidepressants | 6 (40.0) | 0 (0.00) | -- | -- |
| Anxiolytics/Hypnotics | 1 (6.67) | 1 (6.67) | -- | -- |
| Anticholinergic | 4 (26.67) | 0 (0.00) | -- | -- |
| Combined medications | 11 (73.33) | 0 (0.00) | -- | -- |
SZV – volunteers with schizophrenia/schizoaffective disorder, HV – healthy volunteers, SD – standard deviation, PANSS – The Positive and Negative Syndrome Scale, BACS –Brief Assessment of Cognition in Schizophrenia scale
Handedness data are avaliable in 14 SZV and 6 HV;
Race data are avaliable in 14 SZV and 6 HV,
BACS data are avaliable in 13 SZV and 5 HV,
Medication data are avaliable in 12 SZV
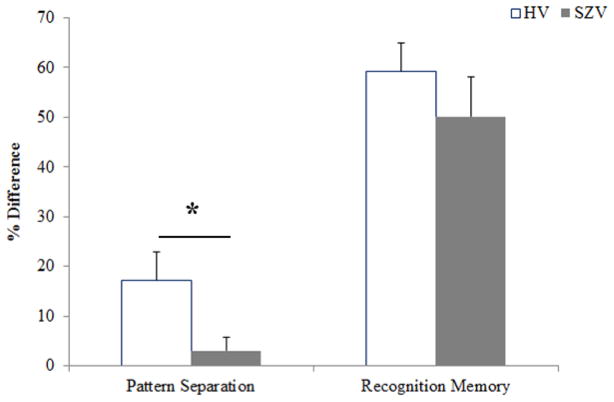
Based on the assessed accuracies of volunteer responses on the BPS task, two primary constructs were calculated, the PS parameter and the RM parameter. Pairwise comparisons directly contrasting SZV with HV on each of the BPS task parameters (PS and RM) revealed a significantly lower PS parameter in SZV (mean±SE, 3.1%±4.8) than in HV (17.1%±4.7) [t(20)=2.20; p=0.039; Cohen’s d = 0.86], showing that the SZ group has a significantly lower rate of correctly detecting objects similar to, but not exactly the same, as previously seen objects (‘lures’). By contrast, the RM parameter did not significantly differ between the groups: SZV (50.1%±4.8); HV (59.3%±4.7) [t(27)=0.95; p=0.35; Cohen’s d = 0.36], showing that the SZV can correctly distinguish distinct familiar and new objects (Fig 2).
Figure 2. PATTERN SEPARATION PARAMETER AND RECOGNITION MEMORY PARAMETER (Mean +/− SEM).
The graph shows the differences in percent response rate (mean±SE) on the elements of the Behavioral Pattern Separation Task in the SZV (grey) and HV (white) groups. On the Pattern Separation parameter, the SZV group is significantly impaired compared to the HV group (p=0.039; d′ =0.86). However, in the Recognition Memory Parameter, the SZV group performance does not significantly differ from that of HV (p=0.35; d′=0.36).
The response accuracies underlying the PS alterations in the SZV are reported in Fig 3. A three-way repeated measures ANOVA showed no effect of group [F(1,28) = 0.59, p=0.45], no effect of stimulus type [F(2,56) = 2.85, p=0.54)], an effect of response [F(2,56) = 8.78, p<0.001)], and a significant group by stimulus type by response interaction [F(4,112) = 2.54, p=0.04)]. Critically, post hoc between-group comparisons revealed significantly lower accuracy in identifying the lure items as ‘similar’ in SZV (mean±SE, 24.0%±4.1) compared to HV (41.2%±4.1) (p<0.05, Bonferroni adjusted). Accuracy in identifying the lure as ‘old’ increased by 9.1% in SZV (from 38.0% [NV] to 47.1% [SZV]) and in identifying the lure as ‘new’ increased by 11.3% in SZV (from 13.4% [NV] to 24.7% [SZV]), neither change being significant (all p>0.05, Bonferroni adjusted). No differences in response accuracies to ‘target’ or ‘new’ stimuli were observed in SZV vs. HV (all p>0.05, adjusted for multiple comparisons).
Figure 3. PERCENT ACCURACY (Mean +/− SEM) IN THE TEST PHASE.
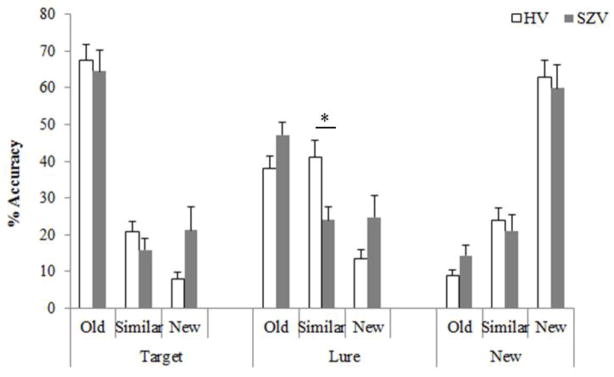
This graph shows the percent response rate (mean±SE) of the SZV (grey) and the HV (white) on identifying ‘target’, ‘lure’ and ‘new’ stimuli in the second part of the BPS task as old, similar, or new. The SZV and HV performed similarly with the exception of the SZV performing significantly lower than HV on classifying the ‘lure’ as ‘similar’ (p<0.05).
To explore any relationship between patient characteristics and these memory alterations, we correlated selected demographic characteristics, symptom and cognition variables and the PS and the RM parameters in the SZV group. There were no significant correlations between any demographic variable or clinical characteristic (PANSS total, PANSS positive subscale; BACS total, BACS verbal memory subscale scores) and the PS or RM scores, albeit the power to detect correlations was low.
DISCUSSION
These data show a significant reduction in ‘pattern separation’ performance in individuals with schizophrenia compared to HV. Our data reveal a specific reduction in the SZ group of recognizing the ‘lure’ stimuli as ‘similar’, specifically a reduction in the ability to detect subtle differences between objects. The hallmark of a pattern separation impairment in the BPS is argued to be just such a reduction, combined with an increase in the probability of calling ‘lure’ stimuli ‘old’. Qualitatively, we observed that the SZ group’s decline in recognizing ‘lures’ as ‘similar’ was manifest as a non-significant increase both in calling these items ‘old’ (i.e., increased generalization) and in calling them ‘new’ (i.e., increased forgetting) (Fig. 3). Because the patient numbers are not robust, additional follow-up studies with more volunteers are planned to resolve these outcomes further.
The identification of this behavioral manifestation of hippocampal dysfunction, even of specific DG→CA3 impairment, could be a correlate of the molecular and cellular alterations that have been previously reported in postmortem hippocampal subfields in SZ (Stan et al., 2014;Gao et al., 2001). Therefore, we posit that the presently suggested functional impairment in PS performance is related to the reported regional DG pathology in SZ, including the reduction in the GluN1 subunit of the NMDA receptor (Stan et al., 2014), and in reported reductions in neurogenesis (Reif et al., 2006). The dramatic reduction in pattern separation performance (i.e., ‘lure’ recognition) in SZV to 3% (which represents their low recognition of subtle object differences) suggests this as a vitally impaired function in these individuals. This loss of pattern separation function is not accounted for by age or other demographic differences between groups.
The impairment in the PS parameter of the BPS task observed here shows that SZV, when presented with an object similar to, but not exactly the same as, a previously seen object, have reduced (or almost no) capacity to identify it as “similar” to something previously seen, compared to HV. Deserving additional study in the SZV is whether they mistakenly identify ‘similar’ stimuli as both ‘old’ and ‘new’, or only ‘old’. These results help to parse which components of declarative memory function are altered in individuals with SZ psychosis, which may ultimately help direct attention to discrete hippocampal regions and cellular systems and, perhaps, to treatment targets.
This functional outcome was predicted a priori based on previously identified deficiencies demonstrated in vivo and in vitro in DG in SZV and in SZ case tissue (Tamminga et al., 2010). The functional alterations reported here serve to associate the already described subfield-specific tissue pathology with specific and predicted functional cognitive impairment in persons with the illness.
There are several limitations of this study that will be mitigated by research already underway. Here, the number of patients and controls is modest, resulting in limited power to detect differences; study power will improve with additional subject volunteers. All individuals with schizophrenia were treated with antipsychotic medication, which could have confounded these outcomes; studies in drug-free patient volunteers are important in the future. A general population of research volunteers was sampled without attention to a specialized sample, and we need to quantify these specific memory parameters in other psychiatric disease groups, including individuals with bipolar disorder and depression.
Acknowledgments
This work was supported by the NIMH (MH083957, Tamminga), a NARSAD Young Investigator Award (2011, Ivleva) and a NARSAD Established Investigator Award (2009, Tamminga). Neither NIMH nor NARSAD had a role in study design or in the collection, analysis and interpretation of data. We would like to thank John Bartko, Ph.D. for conducting statistical analyses, Valerie Carr, Ph.D. for insightful comments on earlier versions of the manuscript, and Morgan McConkey, B.A. for help with the manuscript preparation. We also express appreciation to all clinicians for SZVs’ referral, and, most importantly, to the individuals themselves that took part in this study.
Role of Funding Source
This work was supported by the NIMH (MH083957, Tamminga) and NARSAD Young Investigator Award (2011, Ivleva). Neither NIMH nor NARSAD had a role in study design or in the collection, analysis and interpretation of data.
Footnotes
Contributors
Tanusree Das, Ph.D., enrolled participants, conducted study procedures, worked on data analysis and substantially contributed to writing the manuscript.
Elena Ivleva, M.D., Ph.D. contributed to the data analysis, the interpretation and contributed in writing the manuscript.
Anthony D. Wagner Ph.D. gave insight into study design and assisted in writing and editing the manuscript.
Craig E. Stark Ph.D. gave insight into the study design, his lab assisted in the development of the behavior pattern separation task, the interpretation of the data and Dr. Stark assisted in the writing and editing of the manuscript.
Carol A. Tamminga, MD was the Principle Investigator for the study she gathered the funds to do the study and substantially contributed to the writing and editing of the manuscript.
All Authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Bailey JR. Tables of the Bonferroni t Statistic. J Am Stat Assoc. 1977;72:469–478. [Google Scholar]
- Bakker A, Kirwan CB, Miller M, et al. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Mayford M, Gage FH. Selection of distinct populations of dentate granule cells in response to inputs as a mechanism for pattern separation in mice. Elife. 2013;2:e00312. doi: 10.7554/eLife.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis I Disorders/Patient Edition (SCID-I/P) New York State Psychiatric Institute, Biometrics Research Department; New York, NY: 1996. [Google Scholar]
- Gao WJ, Krimer LS, Goldman-Rakic PS. Presynaptic regulation of recurrent excitation by D1 receptors in prefrontal circuits. Proc Natl Acad Sci U S A. 2001;98:295–300. doi: 10.1073/pnas.011524298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao XM, Sakai K, Roberts RC, et al. Ionotropic glutamate receptors and expression of N-methyl-D-aspartate receptor subunits in subregions of human hippocampus: effects of schizophrenia. Am J Psychiatry. 2000;157:1141–1149. doi: 10.1176/appi.ajp.157.7.1141. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology (Berl) 2004;174:151–162. doi: 10.1007/s00213-003-1761-y. [DOI] [PubMed] [Google Scholar]
- Heckers S, Rauch SL, Goff D, et al. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nature. 1998;1:318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- Hintze J. NCSS 8. Kaysville, Utah: NCSS, LLC; Jan 3, 2012. [Google Scholar]
- Kay SR, Fiszbein A, Opler L. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Goldberg TE, Harvey PD, et al. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68:283–297. doi: 10.1016/j.schres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Kesner RP. An analysis of the dentate gyrus function. Behav Brain Res. 2013;254:1–7. doi: 10.1016/j.bbr.2013.01.012. [DOI] [PubMed] [Google Scholar]
- Knable MB, Barci BM, Webster MJ, et al. Molecular abnormalities of the hippocampus in severe psychiatric illness: postmortem findings from the Stanley Neuropathology Consortium. Mol Psychiatry. 2004;9:609–20. 544. doi: 10.1038/sj.mp.4001471. [DOI] [PubMed] [Google Scholar]
- Kumaran D, McClelland JL. Generalization through the recurrent interaction of episodic memories: a model of the hippocampal system. Psychol Rev. 2012;119:573–616. doi: 10.1037/a0028681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRocque KF, Smith ME, Carr VA, et al. Global similarity and pattern separation in the human medial temporal lobe predict subsequent memory. J Neurosci. 2013;33:5466–5474. doi: 10.1523/JNEUROSCI.4293-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, et al. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Marr D. Simple memory: a theory for archicortex. Philos Trans R Soc Lond B Biol Sci. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- Medoff DR, Holcomb HH, Lahti AC, et al. Probing the human hippocampus using rCBF: contrasts in schizophrenia. Hippocampus. 2001:543–550. doi: 10.1002/hipo.1070. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC, McClelland JL. Hippocampal conjunctive encoding, storage, and recall: avoiding a trade-off. Hippocampus. 1994;4:661–682. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- Rangel LM, Eichenbaum H. What’s new is older. Elife. 2013;2:e00605. doi: 10.7554/eLife.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif A, Fritzen S, Finger M, et al. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry. 2006;11:514–522. doi: 10.1038/sj.mp.4001791. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The mechanisms for pattern completion and pattern separation in the hippocampus. Front Syst Neurosci. 2013;7:74. doi: 10.3389/fnsys.2013.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobel SA, Chaudhury NH, Khan UA, et al. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013;78:81–93. doi: 10.1016/j.neuron.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stan AD, Ghose S, Husley K, et al. Magnetic resonance spectroscopy and tissue protein concentrations together suggest glutamate signaling in dentate gyrus in schizophrenia. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.54. [DOI] [PubMed] [Google Scholar]
- Stan AD, Ghose S, Yanagi M, et al. Magnetic Resonanace Spectroscopy and Tissue Protein Concentrations Together Suggest Reduced Glutamate Signaling in Dentate Gyrus (DG) in Schizophrenia. 2013. Submitted. [DOI] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Lacy JW, et al. A task to assess behavioral pattern separation (BPS) in humans: Data from healthy aging and mild cognitive impairment. Neuropsychologia. 2013;51:2442–2449. doi: 10.1016/j.neuropsychologia.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Stark CE. Individual differences in spatial pattern separation performance associated with healthy aging in humans. Learn Mem. 2010;17:284–288. doi: 10.1101/lm.1768110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga CA, Stan AD, Wagner AD. The hippocampal formation in schizophrenia. Am J Psychiatry. 2010;167:1178–1193. doi: 10.1176/appi.ajp.2010.09081187. [DOI] [PubMed] [Google Scholar]
- Wang AY, Lohmann KM, Yang CK, et al. Bipolar disorder type 1 and schizophrenia are accompanied by decreased density of parvalbumin- and somatostatin-positive interneurons in the parahippocampal region. Acta Neuropathologica. 2011;122:615–626. doi: 10.1007/s00401-011-0881-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer BJ, Brown DR, Michels KM. Statistical Principles in Experimental Design. 3. McGraw Hill; New York: 1991. p. 535. [Google Scholar]
- Yassa MA, Stark CE. Pattern separation in the hippocampus. Trends Neurosci. 2011;34:515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]