Abstract
Objective
To determine the pharmacokinetics (PK) and placental transfer of intravenous (IV) N-acetylcysteine (NAC) in mothers with a clinical diagnosis of chorioamnionitis and determine the PK of IV NAC in their infants.
Study design
In this prospective, double blind study IV NAC 100 mg/kg/dose or saline was administered within 4 hours of CA diagnosis to pregnant women ≥24 weeks gestation and then every 6 hours until delivery. Maternal PK and placental transfer were determined with maternal blood and matched maternal and cord venous blood. Neonatal PK estimates were determined from IV NAC (12.5 – 25 mg/kg/dose) administered every 12 hours for 5 doses. Noncompartmental analyses were performed for maternal and neonatal PK estimates.
Results
Eleven mothers (5 preterm, 6 near-term) and 12 infants (1 set of twins) received NAC. Maternal clearance (CL) of NAC was faster than in non-pregnant adults, with a t1/2 of 1.2 ± 0.2 hours. The NAC cord to maternal ratio was 1.4 ± 0.8 suggesting rapid placental transfer and slower rate of fetal CL. Neonatal PK estimates for near-term compared with preterm infants showed a significantly shorter t1/2 (5.1 vs.7.5 hours, respectively) and higher CL (53.7 vs. 45.0 mL/hr/kg, respectively).
Conclusions
Maternal CL and placental transfer of NAC was rapid with umbilical cord concentrations frequently exceeding maternal concentrations. NAC administration to mothers with CA achieves predictable NAC plasma concentrations in the fetus, indicating that antenatal neuroprotection may be possible for these newborns at high risk for neuroinflammation.
Keywords: Transplacental, neonate, maternal, neuroprotective drugs, brain injuries, N-acetylcysteine, fetal diseases, pregnancy complications
Chorioamnionitis (CA) is an acute inflammation of the amniotic and chorionic membranes, typically due to an ascending bacterial infection in the setting of ruptured membranes. CA is a common complication of pregnancy, affecting 1–4% of all births in the US, and is associated with significant maternal and neonatal morbidity, regardless of gestational age.1–3 Maternal inflammation with immune activation results in an increase in proinflammatory mediators such as interleukins 1 and 6, and TNF-alpha which may stimulate the fetal inflammatory response syndrome (FIRS). Aside from direct fetal infection and sepsis, FIRS may result in cerebral white matter injury leading to the development of short and long-term neurological sequelae.1, 4 CA is associated with cystic periventricular leukomalacia, intraventricular hemorrhage (IVH) in preterm infants and cerebral palsy in both preterm and term infants.3, 5–7 No accepted therapy for prevention or treatment of the inflammatory brain injury is available to the developing fetus or neonate exposed to CA.
Protecting the brain from injury during the fetal, peripartum and neonatal period is critical to improving short and long-term outcomes.8 Considering the inflammatory cytokine link in fetal brain injury and the associated release of reactive oxygen species9, N-acetylcysteine (NAC) has potential therapeutic use in this setting.10–12 Investigations of NAC in an animal model of CA and in hypoxic- ischemic brain injury have shown consistent neuroprotection.11–15 Prophylactic maternal NAC administration in rats significantly reduces diffuse cerebral injury in offspring with maternal lipopolysaccharide induced inflammation.16 However, for optimal neuroprotection, NAC must be administered before the potentially hypoxic ischemic process of birth.14, 17
NAC, a precursor of reduced glutathione (GSH), has been in clinical use for more than thirty years in the management of acetaminophen overdose and as a mucolytic agent. The beneficial effects of NAC are thought to be mediated by scavenging free-radicals, reducing oxidative stress by restoring intracellular GSH, modulating apoptosis, decreasing cytokine expression and anti-inflammatory activity.18 Both pediatric and adult studies show a favorable NAC safety profile with limited adverse effects. However, the antenatal administration of a fetal neuroprotectant poses significant challenges when considering drug safety, pharmacokinetics (PK) and efficacy in the maternal-fetal-neonatal setting.
Animal models of CA and available evidence in humans suggest NAC is a strong candidate for further investigation as a potential neuroprotective agent for the fetus and newborn.19 Our aim was to assess the PK of IV NAC administered to mothers with CA prior to delivery, the placental transfer of NAC during a significant inflammatory process, and the neonatal PK immediately after delivery.
Methods
This prospective, double blind, pilot study was approved by the Medical University of South Carolina’s Institutional Review Board, with written informed consent obtained for pregnant women at gestational ages ≥24 completed weeks, who presented with clinical diagnosis of CA. Clinical CA was defined as maternal fever ≥38°C in the presence of rupture of membranes or two of the following: uterine tenderness, maternal white blood cell count >15,000 cells/mm, fetal tachycardia >160 bpm, or malodorous amniotic fluid. Exclusion criteria included: medication dependent asthma, sepsis, seizure disorder, suspected major congenital abnormalities, fetal weight less than the 10th % for gestational age (GA), indication for immediate delivery, or participation in another clinical trial. Exit criteria were designated as bronchospasm requiring β-agonist treatment, refractory bleeding, refractory hypotension or seizures.
NAC Dosing
Pregnant mothers were recruited concurrently in 2 parallel cohorts based on GA at presentation: near-term/ term (term, ≥33 completed weeks of gestation) and preterm (preterm, 24–32 completed weeks gestation). Gestational age cohorts were chosen based on the expected decreased nephron numbers in preterm infants <32 completed weeks gestation and a previous NAC PK study in preterm infants up to 31 weeks gestation.20, 21 Mothers and their infants were randomized as a dyad to saline or NAC for the first 10 patients in each gestational cohort for evaluation of safety, and then solely into the NAC group thereafter. Maternal PK estimates were determined from IV NAC (100 mg/kg/dose) administered within 4 hours of clinical diagnosis of CA. NAC was infused over 60 minutes every 6 hours up to a total of 4 doses before delivery. Maternal dosing was determined from non-pregnant adult studies recognizing the likelihood that PK estimates would be altered during pregnancy.22, 23 Neonatal PK data were generated from IV NAC (term, 25 mg/kg/dose; preterm, 12.5 mg/kg/dose) administered over 60 minutes every 12 hours for 5 doses. Neonatal NAC dosing was determined from a previous PK study in preterm infants with a goal range for NAC plasma concentrations of 100 to 300 μmol/L.21, 24 No cysteine supplementation was added to neonatal parenteral nutrition solutions during the two days of NAC administration. Only Investigational Pharmacy Services was aware of the randomization status.
NAC Sampling and Analysis
Maternal venous blood for NAC plasma concentrations was drawn just prior to and at 30, 60, 120 and 180 minutes after the first NAC infusion. Two mothers had more than one dose of NAC prior to delivery with trough levels measured prior to the second dose. Simultaneous venous cord and maternal NAC venous levels were measured at delivery. Neonatal venous NAC plasma levels were drawn just prior to and at 30 minutes, 8 hours and 12 hours after the first dose and just prior to the 3rd and 4th dose. Blood samples (0.8 mL/ sample) were collected in sodium EDTA tubes, immediately centrifuged and plasma stored in polypropylene tubes at −70°C until analysis.
Total NAC (ie, oxidized, reduced and protein-bound drug) plasma concentrations were determined using a modified, reverse phase high performance liquid chromatography method25 with penicillamine as the internal standard. Plasma samples were initially treated with dithiothreitol to reduce available oxidized NAC (NAC2) and then derivatized with N-(1-pyrenyl)maleimide (NPM). The NAC-NPM adduct was then analyzed by fluorescence detection. The limit of sensitivity for the assay was 6.0 μmol/L. The five-point standard curve was linear and reproducible over the range of 60 to 3000 μmol/L (R2 > 0.99). No sample was used that was below the assay limit of sensitivity. The coefficients of variation for the within-run and between-run precision were all less than 10%.
Pharmacokinetic Analyses
The NAC plasma concentration-time data were analyzed using noncompartmental PK methods (PK Solutions, version 2.0, Summit Research Services, Montrose, CO 81401). We evaluated terminal elimination half-life (t½), volume of distribution (Vd), and total body clearance (CL). The terminal elimination rate constant (kel) was determined by linear regression of the final linear portion of the natural log of the concentration-time curve for concentrations in plasma. Venous umbilical cord to maternal venous blood NAC concentration ratios at the time of delivery were used to characterize placental transfer. To further examine placental transfer, the relationship between NAC cord concentrations and the time from the end of maternal NAC infusion were compared. Only cord concentrations from mothers that delivered after completing the first dose of NAC were used for this analysis. The variables of interest from all patients were used to generate mean and standard deviation (SD) values. Statistical comparisons were conducted using unpaired two-sided Student’s T-test (GraphPad Prism 6, La Jolla, CA). A P value less than 0.05 was considered significant.
Results
A total of 22 mothers (12 preterm, 10 term) and 24 infants, including 2 sets of twins were enrolled. There were no significant differences in demographic data between NAC and control groups (Table I; available at www.jpeds.com). Two withdrawals occurred: one for mother’s comfort level and one by study personnel for the presence of IVH on first cranial ultrasound, prior to the first infant study dose. No participant met exit criteria. Eleven mothers (6 preterm, 5 term) and 12 infants (1 set of preterm twins) received NAC (Table I). Seven patients delivered after completing the first NAC dose. Two patients delivered during the first NAC infusion. One patient delivered 30 minutes into the second NAC dose. One patient delivered after the third NAC dose. No adverse effects were observed during NAC administration to the mothers or their infants.
Table I.
Patient characteristics
| NAC | Control | Total | |
|---|---|---|---|
| Entry Strata: | |||
| Preterm mothers (n) | 6 | 6 | 12 |
| Preterm infants (n) | 7 | 7 | 14 |
| Gestational Age at Birth (wks) | 28.1 ± 1.8 | 29.4 ±3.3 | NS |
| Birth Weight (grams) | 1207 ± 235 | 1339 ± 462 | NS |
| Term mothers (n) | 5 | 5 | 10 |
| Term infants (n) | 5 | 5 | 10 |
| Gestational Age at Birth (wks) | 38.6 ± 2.4 | 38.4± 2.8 | NS |
| Birth Weight (grams) | 3451 ± 415 | 3150 ± 582 | NS |
| Sex infants (male) | 7 | 6 | 54% |
| Race of mothers: | |||
| African-American | 8 | 3 | 50% |
| Caucasian | 3 | 8 | 50% |
| Inclusion Criteria: | |||
| Fever ≥100°F | 3 | 6 | 9 |
| Uterine Tenderness | 4 | 5 | 9 |
| WBC >15,000 cells/mm | 5 | 3 | 8 |
| Fetal tachycardia >160bpm | 7 | 8 | 15 |
| Malodorous amniotic fluid | 3 | 2 | 5 |
| Labor and Delivery: | |||
| Maternal BMI | 35.4± 9.0 | 29.7± 5.2 | NS |
| Antenatal Steroids | 6 | 6 | NS |
| Maternal antibiotic therapy | 10 | 11 | NS |
| Rupture of membranes (hrs) | 69 (0–229) | 95 (0–322) | NS |
| >18 hours | 5 | 5 | 10 |
| Mean hrs 1st maternal Dose to delivery (range) | 2.9 ± 2.2 (0–7) |
1.8 ± 1.8 (0–5) |
NS# |
excludes 2 NAC mothers who labored 17 & 32 hrs
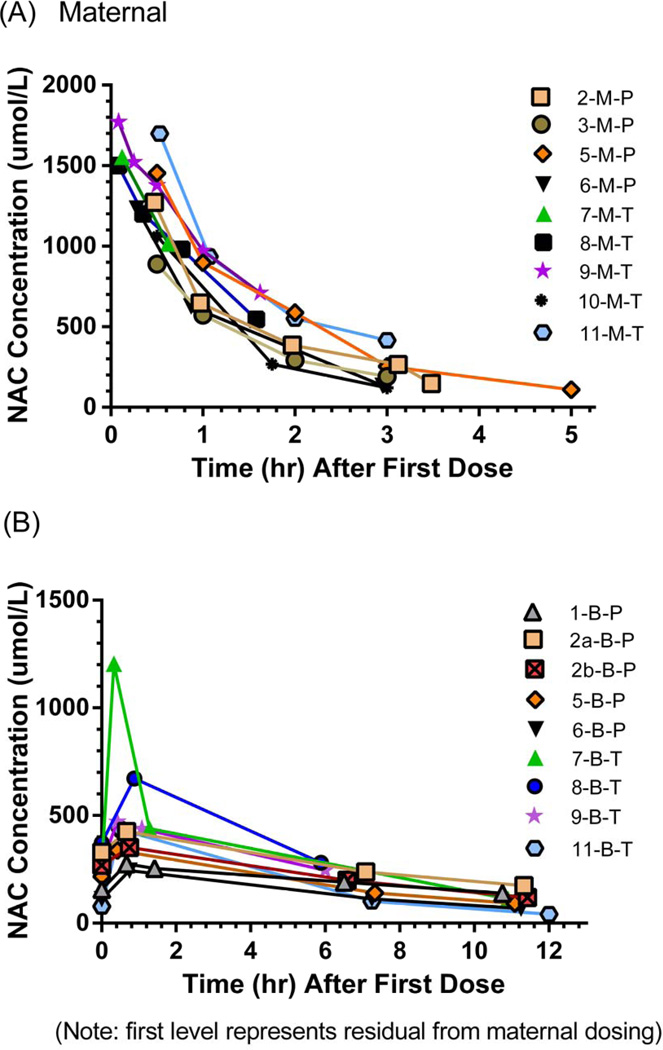
Maternal NAC plasma concentrations versus time profiles are shown in Figure 1, A and individual PK estimates reported in Tables II (available at www.jpeds.com) and IV. The mean NAC concentration 30 min post infusion NAC was 1222 ± 415 μmol/L. No significant differences were observed in PK estimates between the maternal-P and maternal-T patients with the exception of Vd being larger in the maternal-P group (P = 0.03, R2 = 0.57).
Figure 1.
A, Maternal NAC concentrations. B, Neonatal NAC concentrations.
Table II.
Maternal Pharmacokinetic Parameter Estimates Following 100 mg/kg NAC Dose
| ID† | Weight (kg) |
NAC dose (gm) |
t1/2 (hrs) |
Vd (L/kg) |
CL (mL/hr/kg ) |
NAC Cp# @delivery μmol/L |
|---|---|---|---|---|---|---|
| 1-M-P | 129.0 | 8.60 | NA | NA | NA | NA†† |
| 2-M-P | 108.0 | 10.8 | 1.1 | 0.43 | 264 | 147.0 |
| 3-M-P | 63.0 | 6.30 | 1.1 | 0.54 | 331 | 572.9 |
| 4-M-P | 95.0 | 1.40 | NA | NA | NA | NA* |
| 5-M-P | 106.5 | 10.65 | 1.7 | 0.49 | 198 | 109.7 |
| 6-M-P | 87.3 | 8.73 | 0.9 | 0.39 | 290 | 1234.6 |
| P:Mean (SD) | 98.1(22.2) | 1.2(0.31) | 0.46(0.07) | 271(56) | 442.2(482.1) | |
| 7-M-T | 73.2 | 6.30 | NA | NA | NA‡‡ | 1016.5 |
| 8-M-T | 84.0 | 8.40 | 1.1 | 0.39 | 256 | 546.5 |
| 9-M-T | 89.1 | 8.91 | 1.2 | 0.34 | 193 | 711.3 |
| 10-M-T | 90.5 | 9.05 | 0.8 | 0.38 | 331 | NA∞ |
| 11-M-T | 149.1 | 14.91 | 1.4 | 0.34 | 175 | 219.3** |
| T:Mean (SD) P+T Mean (SD) P value |
97(30) 98(25) 0.95 |
1.1(0.2) 1.2(0.2) 0.60 |
0.36(0.03) 0.41(0.07) 0.03‡ |
239(71) 255(61) 0.53 |
623(332) 523(408) 0.57 |
M, maternal; P, preterm; T, term
NAC plasma concentration
Delivered 40 minutes into NAC infusion
Stopped collecting NAC samples at delivery
Delivered 9 minutes into NAC infusion
Delivered after 3rd NAC dose. Sample mistakenly obtained from NAC infusion line
Cp after receiving ½ of second NAC dose
P vs. T Student t-test
Neonatal NAC plasma concentrations versus time profiles are shown in Figure 1, B, with individual PK estimates reported in Tables III (available at www.jpeds.com) and IV. Significant differences between preterm and term groups were found for GA, weight, t1/2, and CL. The PK estimates for term and preterm infants indicate that steady-state NAC concentrations were achieved after 1 day and 2 days, respectively. NAC trough concentrations at 48 hours for both the preterm and term groups (mean: 66.2 and 61.2 μmol/L, respectively) were below the target trough of 100 μmol/L.
Table III.
Neonatal Pharmacokinetic Parameter Estimates
| ID† | GA (wks) |
Weight (gm) |
NAC dose (mg) |
t1/2 (hrs) |
Vd (L/kg) |
CL (mL/hr/kg) |
NAC Cord Cp* μmol/L |
NAC @ SS(48hrs)## μmol/L |
|---|---|---|---|---|---|---|---|---|
| 1-B-P | 29.3 | 1450 | 18.1 | 10.7 | 0.52 | 33.6 | NA∞ | 68.0 |
| 2a-B-P | 27.6 | 1095 | 13.7 | 8.2 | 0.47 | 39.6 | 369.5 | 83.9 |
| 2b-B-P | 27.6 | 1090 | 13.6 | 6.9 | 0.47 | 47.2 | 357.8 | 60.7 |
| 3-B-P | 28.0 | 1310 | NA | NA | NA | NA | 465.0** | NA** |
| 4-B-P | 26.0 | 900 | 12.5 | 6.0 | 0.45 | 52.0 | 107.2†† | 60.0 |
| 5-B-P | 31.5 | 1550 | 19.4 | 5.6 | 0.42 | 51.9 | 235.3 | 57.6 |
| 6-B-P | 27.0 | 1055 | 13.2 | 6.0 | 0.48 | 52.6 | 689.3 | 66.8 |
| P Mean (SD) | 28.1(1.8) | 1207(235) | 7.5(2.0) | 0.47(0.04) | 45.0(8.2) | 370.7(199.2) | 66.2(9.5) | |
| 7-B-T | 38.3 | 3326 | 83.2 | 5.3 | 0.39 | 50.7 | 1183.7 | 63.1 |
| 8-B-T | 40.2 | 3940 | 98.5 | 4.0 | 0.30 | 52.2 | 586.4 | NA∞ |
| 9-B-T | 40.1 | 3705 | 92.6 | 5.9 | 0.53 | 62.0 | 598.6 | 102.9 |
| 10-B-T | 34.6 | 2842 | 45.0 | 3.9 | 0.38 | 73.0 | NA∞ | 49.0 |
| 11-B-T | 40.0 | 3440 | 85.0 | 3.5 | 0.34 | 66.3 | 189.9‡ | 29.4 |
| T Mean(SD) P |
39(2.4) <0.0001# |
3451(415) <0.0001# |
5.1(1.3) 0.02# |
0.38(0.09) .096 |
53.7(11.3) 0.021# |
639.7(409.4) 0.20 |
61.2(31.1) 0.71 |
P, preterm; T, term
NAC venous cord concentration at delivery
Missed NAC sample collection
Withdrawn due to IVH
Delivered 9 min into NAC infusion
Mother received ½ of second NAC dose
P vs. T Student t-test
Estimated NAC trough concentration at steady-state
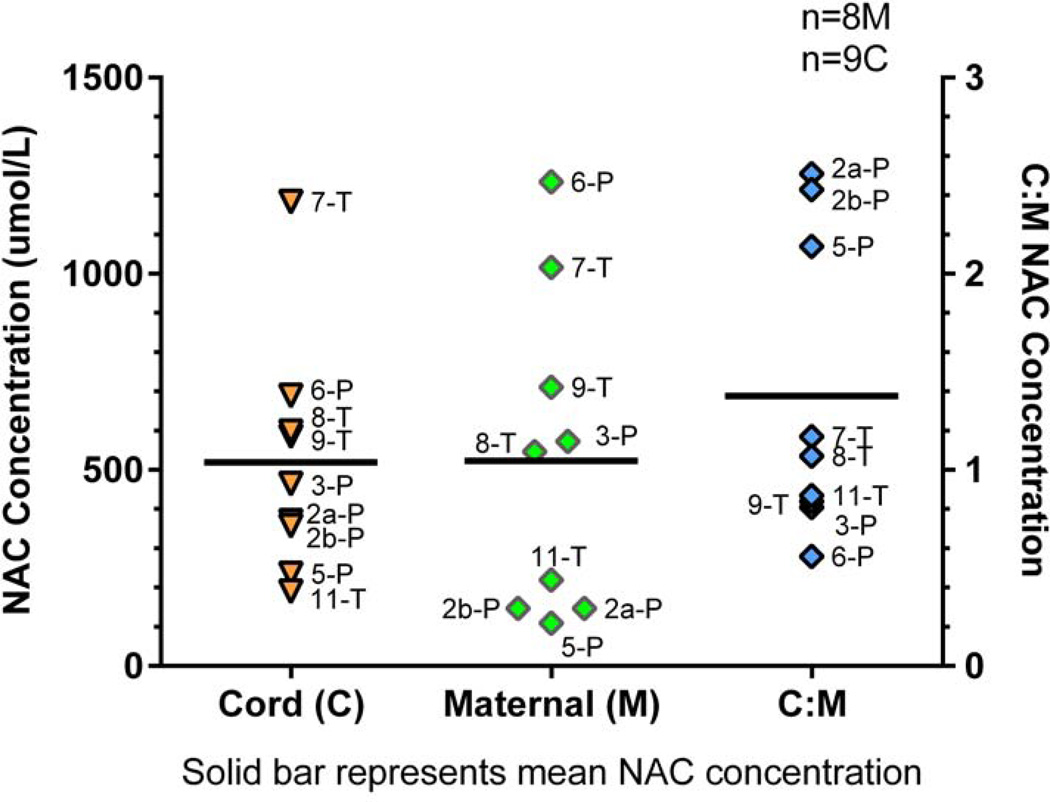
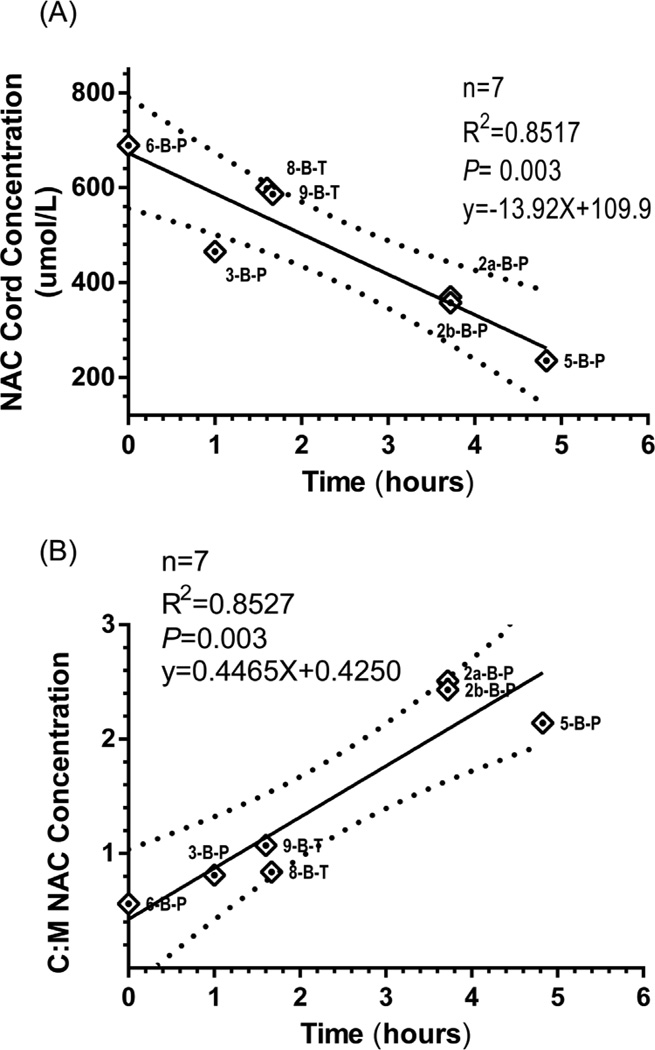
Nine NAC cord plasma concentrations matched to their respective maternal plasma concentration (n=8, 1 pair twins) were measured (Figure 2). The mean maternal NAC plasma concentration at time of delivery was 523 ± 409 μmol/L (range: 110 to 1235 μmol/L) which was similar to the mean cord concentration of 520 ± 300 μmol/L (range: 190 to 1184 μmol/L). The mean NAC cord to maternal ratio was 1.4 ± 0.8. NAC cord concentration was significantly associated (P = 0.0005, R2 = 0.8829) with the time from the end of maternal NAC infusion providing an estimate on the level and duration of fetal NAC exposure from a single maternal dose (Figure 3, A). An appreciation for the difference in NAC disposition between the mother and the fetus is provided by the association between C: M and end of maternal NAC infusion (Figure 3, B) showing an increasing C: M ratio with time.
Figure 2.
NAC cord and maternal levels at delivery.
Figure 3.
A, Relationship between NAC cord concentration and time to end of maternal infusion. B, Relationship between C:M NAC concentrations and time to end of maternal infusion. 95% CI bands.
Discussion
This pilot trial characterizes the disposition of IV NAC in mothers antenatally and their infants postnatally. NAC maternal and venous cord concentrations at time of delivery provide new insight into placental transfer, fetal exposure and possibly fetal metabolism. The only previous investigation examining fetal exposure of NAC was in 4 pregnant women receiving NAC as a treatment for acetaminophen toxicity. NAC was detected in cord blood of three viable infants and cardiac blood from a fourth infant at autopsy. The paired maternal-fetal total NAC concentrations were almost identical, documenting placental transfer of NAC.26 Our study affirms placental transfer of NAC after maternal dosing. Fetal NAC exposure occurs rapidly and was substantial after one maternal dose (Figure 3, A). The difference in maternal and fetal disposition is evident with the increasing C: M NAC concentration associated with time from end of maternal infusion (Figure 3, B). A decreased fetal CL as compared with maternal CL supports the increasing C: M NAC concentration with time. Many factors influence drug transfer across the placenta including uteroplacental and umbilical blood flow, surface area for exchange, placental permeability in relation to physicochemical properties of a drug (e.g., molecular weight, pKa, protein binding, lipid solubility) and activity of specific transporter proteins in the placenta. Amino acids cross the human placenta by one of twenty known active transport mechanisms present in the maternal-facing apical membrane and fetal-facing basal membrane of the syncytiotrophoblast.27 NAC, a derivative of naturally occurring amino acid L-cysteine, is postulated to be transported by the L-system of amino acid transporters. Selective placental transport of amino acids against a concentration gradient via the L-amino acid transporters has been reported and is well characterized for some amino acids.28 Umbilical blood flow has also been shown to directly influence the fetal to maternal enrichment of the essential amino-acid leucine in normal pregnancies.29 However, we measured umbilical blood flow in several participants prior to delivery and found no correlation with umbilical cord NAC levels. Also important in the antenatal dosing of NAC and placental transfer is the unique physiology of umbilical blood flow. Drugs delivered by the umbilical vein are not subject to first pass metabolism, as most blood from the ductus venosus flows through the patent foramen ovale into the left atrium and shunts into the systemic circulation. This provides an advantage in dosing the fetus in utero, as the highest concentrations of NAC will be directed toward the cerebral circulation similar to intra-arterial delivery.30 PK variables, such as Vd and CL, should be considered in characterizing placental transport in both the maternal and fetal compartments both of which would influence the extent of permeation of a substance across the placenta.27, 31, 32
The PK of NAC during pregnancy has not been previously reported. In 17 non- pregnant adults receiving IV NAC for acetaminophen overdose the t1/2 , CL and Vd of total NAC was 5.7 hours , 190 mL/hr/kg and 0.54 L/kg, respectively.22 Similar findings were reported for 6 healthy adult volunteers receiving IV NAC with a t1/2 of 5.6 hours, CL of 110 mL/hr/kg and Vd of 0.47 L/kg for total NAC.23 Although the maternal Vd for IV NAC appears comparable with previously reported values, the estimate of the mean terminal t1/2 was much shorter (mean: 1.2 vs. 6.02 hrs, respectively) and mean CL was double previously reported values.23 Physiologic changes during pregnancy such as increased cardiac output, blood volume, renal perfusion, hepatic blood flow, glomerular filtration, alterations in protein binding and the presence of the fetal-placental unit may lead to pronounced alterations in drug disposition.33, 34 Race was also considered as a possible factor that influenced the PK with 8 of 11 mothers being African-American (Table I). However, our sample size was not sufficient to answer this question. Our finding of a more rapid CL suggests that maternal metabolism is increased and/or the fetal-placental unit is significantly involved in the CL of maternal NAC.
Neonatal PK estimates for term infants showed a significantly shorter t1/2 and higher CL than the preterm infants. The estimates for t1/2 reported for preterm patients were shorter (mean: 7.5 vs. 11 hr) and CL rates higher (mean: 45 vs. 37 mL/hr/kg) than previously reported in preterm infants of similar GA (27.7±2.0 wks) who received NAC by continuous IV infusion.21 The longer t1/2 in preterm patients is possibly due to immature deacetylation activity and/ or reduced renal clearance.23 The PK estimates for term and preterm infants indicate that steady-state NAC concentrations were achieved after 1 day and 2 days, respectively. Using the dosing scheme applied in this study, steady-state trough concentrations will be approximately 60 μmol/L which is below the target goal of 100 to 300 μmol/L for antioxidant treatment in a neonatal clinical trial for bronchopulmonary dysplasia.24 This target concentration range was based on glutathione restoration in adult myocardial infarction patients.35
Preclinical studies show consistent neuroprotection with NAC in animal models of CA, however, target plasma concentrations are not defined. NAC’s effect on redox regulation can be measured to some extent in circulating leukocytes or even blood itself, but circulating cysteine and total GSH levels are tightly regulated and show little difference in animal or clinical studies of NAC.13 NAC’s restoration of the intracellular redox status in neurons, astrocytes, oligodendroglia and their precursors, determines the extent of fetal and neonatal neuroprotection, which is perhaps best assessed in human infants by magnetic resonance imaging techniques.
This was a pilot trial with a small sample size. Neonatal PK analyses were limited by the amount of blood samples, particularly from preterm infants. Although we estimated total NAC, this probably does not represent its true disposition in vivo. Because NAC is a precursor to cysteine and glutathione biosynthesis, the plasma concentration of total NAC at any time may not be a reliable index of its (or any substrates) biological activity at that time. Before making dosing recommendations (maternal or neonatal) targeted NAC concentrations have to be determined for fetal and infant neuroprotection. We are currently engaged in these studies (animal and human), which are needed for successful clinical translation.
Table IV.
Maternal and Neonatal NAC PK estimates
| ID† Maternal |
Weight (kg) |
t1/2 (hrs) |
Vd (L/kg) |
CL (mL/hr/kg) |
|---|---|---|---|---|
| 1-M-P | 129.0 | NA | NA | NA†† |
| 2-M-P | 108.0 | 1.1 | 0.43 | 264 |
| 3-M-P | 63.0 | 1.1 | 0.54 | 331 |
| 4-M-P | 95.0 | NA | NA | NA* |
| 5-M-P | 106.5 | 1.7 | 0.49 | 198 |
| 6-M-P | 87.3 | 0.9 | 0.39 | 290 |
| P:Mean (SD) | 98.1(22.2) | 1.2(0.31) | 0.46(0.07) | 271(56) |
| 7-M-T | 73.2 | NA | NA | NA‡ |
| 8-M-T | 84.0 | 1.1 | 0.39 | 256 |
| 9-M-T | 89.1 | 1.2 | 0.34 | 193 |
| 10-M-T | 90.5 | 0.8 | 0.38 | 331 |
| 11-M-T | 149.1 | 1.4 | 0.34 | 175 |
| T:Mean (SD) P+T Mean (SD) P value |
97(30) 98(25) 0.95 |
1.1(0.2) 1.2(0.2) 0.60 |
0.36(0.03) 0.41(0.07) 0.03‡‡ |
239(71) 255(61) 0.53 |
| ID Neonate |
Weight (gm) |
t1/2 (hrs) |
Vd (L/kg) |
CL (mL/hr/kg) |
| 1-B-P | 1450 | 10.7 | 0.52 | 33.6 |
| 2a-B-P | 1095 | 8.2 | 0.47 | 39.6 |
| 2b-B-P | 1090 | 6.9 | 0.47 | 47.2 |
| 3-B-P | 1310 | NA | NA | NA** |
| 4-B-P | 900 | 6.0 | 0.45 | 52.0 |
| 5-B-P | 1550 | 5.6 | 0.42 | 51.9 |
| 6-B-P | 1055 | 6.0 | 0.48 | 52.6 |
| P: Mean (SD) | 1207(235) | 7.5(2.0) | 0.47(0.04) | 45.0(8.2) |
| 7-B-T | 3326 | 5.3 | 0.39 | 50.7 |
| 8-B-T | 3940 | 4.0 | 0.30 | 52.2 |
| 9-B-T | 3705 | 5.9 | 0.53 | 62.0 |
| 10-B-T | 2842 | 3.9 | 0.38 | 73.0 |
| 11-B-T | 3440 | 3.5 | 0.34 | 66.3 |
| T Mean(SD) P |
3451(415) <0.0001‡‡ |
5.1(1.3) 0.02‡‡ |
0.38(0.09) .096 |
53.7(11.3) 0.021‡‡ |
M, maternal; P, preterm; T, term
Delivered 40 minutes into NAC infusion
Delivered 9 minutes into NAC infusion
Stopped collecting NAC samples at delivery
P vs. T Student t-test
Withdrawn due to IVH
Acknowledgments
Supported by the National Institute of Neurological Disorders and Stroke (RO1NSO52448).
Abbreviations
- PK
Pharmacokinetic
- IV
intravenous
- NAC
N-acetylcysteine
- CA
chorioamnionitis
- CL
clearance
- GSH
glutathione
- C: M
cord: maternal
- GA
gestational age
- IVH
intraventricular hemorrhage
- FIRS
fetal inflammatory response syndrome
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Tita AT, Andrews WW. Diagnosis and management of clinical chorioamnionitis. Clin Perinatol. 2010;37:339–354. doi: 10.1016/j.clp.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaudet LM, Flavin M, Islam O, Smith GN. Diffusion MRI brain findings in neonates exposed to chorioamnionitis: a case series. J Obstet Gynaecol Can. 2009;31:497–503. doi: 10.1016/s1701-2163(16)34211-6. [DOI] [PubMed] [Google Scholar]
- 3.Wu YW, Escobar GJ, Grether JK, Croen LA, Greene JD, Newman TB. Chorioamnionitis and cerebral palsy in term and near-term infants. Jama. 2003;290:2677–2684. doi: 10.1001/jama.290.20.2677. [DOI] [PubMed] [Google Scholar]
- 4.Burd I, Balakrishnan B, Kannan S. Models of fetal brain injury, intrauterine inflammation, and preterm birth. Am J Reprod Immunol. 2012;67:287–294. doi: 10.1111/j.1600-0897.2012.01110.x. [DOI] [PubMed] [Google Scholar]
- 5.Bastek JA, Gomez LM, Elovitz MA. The role of inflammation and infection in preterm birth. Clin Perinatol. 2011;38:385–406. doi: 10.1016/j.clp.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Wu YW, Colford JM., Jr Chorioamnionitis as a risk factor for cerebral palsy: A meta-analysis. Jama. 2000;284:1417–1424. doi: 10.1001/jama.284.11.1417. [DOI] [PubMed] [Google Scholar]
- 7.Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, Choi JH, et al. Fetal exposure to an intraamniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol. 2000;182:675–681. doi: 10.1067/mob.2000.104207. [DOI] [PubMed] [Google Scholar]
- 8.Rees S, Harding R, Walker D. The biological basis of injury and neuroprotection in the fetal and neonatal brain. Int J Dev Neurosci. 2011;29:551–563. doi: 10.1016/j.ijdevneu.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoon BH, Jun JK, Romero R, Park KH, Gomez R, Choi JH, et al. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol. 1997;177:19–26. doi: 10.1016/s0002-9378(97)70432-0. [DOI] [PubMed] [Google Scholar]
- 10.Paintlia MK, Paintlia AS, Singh AK, Singh I. Attenuation of lipopolysaccharide-induced inflammatory response and phospholipids metabolism at the feto-maternal interface by N-acetyl cysteine. Pediatr Res. 2008;64:334–339. doi: 10.1203/PDR.0b013e318181e07c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paintlia MK, Paintlia AS, Contreras MA, Singh I, Singh AK. Lipopolysaccharide-induced peroxisomal dysfunction exacerbates cerebral white matter injury: attenuation by N-acetyl cysteine. Exp Neurol. 2008;210:560–576. doi: 10.1016/j.expneurol.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beloosesky R, Gayle DA, Amidi F, Nunez SE, Babu J, Desai M, et al. N-acetyl-cysteine suppresses amniotic fluid and placenta inflammatory cytokine responses to lipopolysaccharide in rats. Am J Obstet Gynecol. 2006;194:268–273. doi: 10.1016/j.ajog.2005.06.082. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Svedin P, Nie C, Lapatto R, Zhu C, Gustavsson M, et al. N-acetylcysteine reduces lipopolysaccharide-sensitized hypoxic-ischemic brain injury. Annals of neurology. 2007;61:263–271. doi: 10.1002/ana.21066. [DOI] [PubMed] [Google Scholar]
- 14.Paintlia MK, Paintlia AS, Barbosa E, Singh I, Singh AK. N-acetylcysteine prevents endotoxin-induced degeneration of oligodendrocyte progenitors and hypomyelination in developing rat brain. J Neurosci Res. 2004;78:347–361. doi: 10.1002/jnr.20261. [DOI] [PubMed] [Google Scholar]
- 15.Jatana M, Singh I, Singh AK, Jenkins D. Combination of systemic hypothermia and N-acetylcysteine attenuates hypoxic-ischemic brain injury in neonatal rats. Pediatric research. 2006;59:684–689. doi: 10.1203/01.pdr.0000215045.91122.44. [DOI] [PubMed] [Google Scholar]
- 16.Beloosesky R, Ginsberg Y, Khatib N, Maravi N, Ross MG, Itskovitz-Eldor J, et al. Prophylactic maternal N-acetylcysteine in rats prevents maternal inflammation-induced offspring cerebral injury shown on magnetic resonance imaging. Am J Obstet Gynecol. 2013;208:213, e1–e6. doi: 10.1016/j.ajog.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 17.Beloosesky R, Weiner Z, Ginsberg Y, Ross MG. Maternal N-acetyl-cysteine (NAC) protects the rat fetal brain from inflammatory cytokine responses to lipopolysaccharide (LPS) J Matern Fetal Neona. 2012;25:1324–1328. doi: 10.3109/14767058.2011.632793. [DOI] [PubMed] [Google Scholar]
- 18.Kelen D, Robertson NJ. Experimental treatments for hypoxic ischaemic encephalopathy. Early Hum Dev. 2010;86:369–377. doi: 10.1016/j.earlhumdev.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Robertson NJ, Tan S, Groenendaal F, van Bel F, Juul SE, Bennet L, et al. Which neuroprotective agents are ready for bench to bedside translation in the newborn infant? J Pediatr. 2012;160:544–552. e4. doi: 10.1016/j.jpeds.2011.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez MM, Gomez AH, Abitbol CL, Chandar JJ, Duara S, Zilleruelo GE. Histomorphometric analysis of postnatal glomerulogenesis in extremely preterm infants. Pediatr Dev Pathol. 2004;7:17–25. doi: 10.1007/s10024-003-3029-2. [DOI] [PubMed] [Google Scholar]
- 21.Ahola T, Fellman V, Laaksonen R, Laitila J, Lapatto R, Neuvonen PJ, et al. Pharmacokinetics of intravenous N-acetylcysteine in pre-term new-born infants. European journal of clinical pharmacology. 1999;55:645–650. doi: 10.1007/s002280050687. [DOI] [PubMed] [Google Scholar]
- 22.Prescott LF, Donovan JW, Jarvie DR, Proudfoot AT. The disposition and kinetics of intravenous N-acetylcysteine in patients with paracetamol overdosage. Eur J Clin Pharmacol. 1989;37:501–506. doi: 10.1007/BF00558131. [DOI] [PubMed] [Google Scholar]
- 23.Olsson B, Johansson M, Gabrielsson J, Bolme P. Pharmacokinetics and bioavailability of reduced and oxidized N-acetylcysteine. Eur J Clin Pharmacol. 1988;34:77–82. doi: 10.1007/BF01061422. [DOI] [PubMed] [Google Scholar]
- 24.Ahola T, Lapatto R, Raivio KO, Selander B, Stigson L, Jonsson B, et al. N-acetylcysteine does not prevent bronchopulmonary dysplasia in immature infants: a randomized controlled trial. J Pediatr. 2003;143:713–719. doi: 10.1067/S0022-3476(03)00419-0. [DOI] [PubMed] [Google Scholar]
- 25.Ercal N, Oztezcan S, Hammond TC, Matthews RH, Spitz DR. High-performance liquid chromatography assay for N-acetylcysteine in biological samples following derivatization with N-(1-pyrenyl)maleimide. J Chromatogr B Biomed Appl. 1996;685:329–334. doi: 10.1016/s0378-4347(96)00196-x. [DOI] [PubMed] [Google Scholar]
- 26.Horowitz RS, Dart RC, Jarvie DR, Bearer CF, Gupta U. Placental transfer of N-acetylcysteine following human maternal acetaminophen toxicity. J Toxicol Clin Toxicol. 1997;35:447–451. doi: 10.3109/15563659709001226. [DOI] [PubMed] [Google Scholar]
- 27.Lager S, Powell TL. Regulation of nutrient transport across the placenta. J Pregnancy. 2012;2012:179827. doi: 10.1155/2012/179827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Regnault TR, de Vrijer B, Galan HL, Wilkening RB, Battaglia FC, Meschia G. Umbilical uptakes and transplacental concentration ratios of amino acids in severe fetal growth restriction. Pediatr Res. 2013;73:602–611. doi: 10.1038/pr.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galan HL, Marconi AM, Paolini CL, Cheung A, Battaglia FC. The transplacental transport of essential amino acids in uncomplicated human pregnancies. Am J Obstet Gynecol. 2009;200:291, e1–e7. doi: 10.1016/j.ajog.2008.06.054. [DOI] [PubMed] [Google Scholar]
- 30.Neuwelt EA, Pagel MA, Hasler BP, Deloughery TG, Muldoon LL. Therapeutic efficacy of aortic administration of N-acetylcysteine as a chemoprotectant against bone marrow toxicity after intracarotid administration of alkylators, with or without glutathione depletion in a rat model. Cancer Res. 2001;61:7868–7874. [PubMed] [Google Scholar]
- 31.Syme MR, Paxton JW, Keelan JA. Drug transfer and metabolism by the human placenta. Clin Pharmacokinet. 2004;43:487–514. doi: 10.2165/00003088-200443080-00001. [DOI] [PubMed] [Google Scholar]
- 32.Cleal JK, Lewis RM. The mechanisms and regulation of placental amino acid transport to the human foetus. J Neuroendocrinol. 2008;20:419–426. doi: 10.1111/j.1365-2826.2008.01662.x. [DOI] [PubMed] [Google Scholar]
- 33.Pavek P, Ceckova M, Staud F. Variation of drug kinetics in pregnancy. Curr Drug Metab. 2009;10:520–529. doi: 10.2174/138920009788897993. [DOI] [PubMed] [Google Scholar]
- 34.Matsui DM. Therapeutic drug monitoring in pregnancy. Ther Drug Monit. 2012;34:507–511. doi: 10.1097/FTD.0b013e318261c372. [DOI] [PubMed] [Google Scholar]
- 35.Arstall MA, Yang J, Stafford I, Betts WH, Horowitz JD. N-acetylcysteine in combination with nitroglycerin and streptokinase for the treatment of evolving acute myocardial infarction. Safety and biochemical effects. Circulation. 1995;92:2855–2862. doi: 10.1161/01.cir.92.10.2855. [DOI] [PubMed] [Google Scholar]