Abstract
Purpose
Intratumoral vascular shunting within liver metastases may provide a conduit for circulating tumor cells (CTCs) to traverse capillary beds and access distant sites. We assessed how intratumoral shunting relates to metastasis and to clinical outcome.
Materials and Methods
Lung shunt fraction (LSF) was calculated from macroaggregated albumin scan following transcatheter injection of radioactive particles in 62 colorectal cancer patients with liver metastases evaluated for selective internal radiation therapy (SIRT) from 5/07 to 8/12. We assessed how LSF, liver tumor burden, and systemic chemotherapy relate to survival and the presence of lung metastases. LSF and tumor burden were also assessed in a subset of patients who underwent genetic profiling with SNaPshot analysis.
Results
Patients with higher LSF were more likely to have lung metastases and decreased survival, whereas tumor burden was not associated. Patients with genetic mutations had significantly higher LSF than those with no mutations. Patients who received chemotherapy prior to SIRT and had low LSF had the longest post-SIRT survival.
Conclusion
LSF may be a more robust marker of metastasis than tumor size. Increased LSF due to vascular shunting within liver metastasis is an indicator of distant lesions and is associated with decreased survival after SIRT. Intratumoral shunting may provide a conduit for CTCs to access more remote organs, bypassing filtration by liver parenchyma and may be an important factor in metastatis from colorectal cancer.
Introduction
The mechanisms underlying metastasis of colorectal carcinoma and their associated genetic mutations are poorly understood (1-3). Primary tumors shed circulating tumor cells (CTCs), typically 20—30 microns in diameter (4). It is unclear how these large cells traverse capillary beds 8—10 microns in diameter to establish distant lesions. One possibility is that intratumoral vascular shunting provides a conduit for CTCs to access distant sites (1). Arteriovenous shunt formation within tumors could allow these cells to bypass capillaries. For example, CTCs emerging from colorectal cancer could form lung metastases by bypassing normal hepatic capillary beds through shunts within hepatic metastases.
Endovascular hepatic arterial procedures have shown that intratumoral vascular shunting is common (5, 6). Assessment of the degree of arteriovenous shunting in liver tumors is required prior to selective internal radiation therapy (SIRT) (7). After transarterial injection of technetium labeled macroaggregated albumin (99mTc-MAA) particles measuring 30—150 microns in the left or right hepatic artery, the fraction of radioactivity detected in the lungs is calculated and expressed as the lung shunt fraction (LSF) (7). LSF, representing the extent of intratumoral shunting within liver lesions, determines the radiation dose that may be safely administered without causing radiation pneumonitis.
To determine whether intratumoral shunting is associated with metastasis, the relationship of tumor shunting to the presence of disseminated disease and clinical outcome in colorectal cancer was assessed. Specifically, how LSF, compared with liver tumor burden, related to the presence of distant metastases in the lungs and to overall survival was characterized. The association of genetic mutations to shunting and metastasis was analyzed. Additionally, how the use of chemotherapy impacted the degree of intratumoral shunting was studied.
Materials and Methods
All patients evaluated for SIRT (May 2007—August 2012) were included in this Institutional Review Board approved retrospective study (Table 1). Before SIRT, workup included chest, abdomen and pelvis CT followed by evaluation in interventional radiology (pre-SIRT), as previously described (7). Briefly, this included hepatic angiography, embolization of the gastroduodenal and other arterial branches to prevent nontarget radioembolization and transcatheter injection of radioactive particles (99mTc-MAA) into the artery planned for treatment, usually the right or left lobar hepatic artery. Then, planar and SPECT imaging of the head, chest, and abdomen was performed to calculate LSF, reflecting the amount of intratumoral shunting (8) (Figure 1). No patients were excluded.
Table 1.
Summary of patient demographics; data is presented as mean (standard error) or percentage of study population.
| Age (years) | 62.9 (1.5) |
| Gender | |
| Male | 50% |
| Female | 50% |
| Performance Status* | |
| 0 | 43% |
| 1 | 50% |
| 2 | 7% |
| Laboratory | |
| Total bilirubin (mg/dL) | 1.7 (1.0) |
| AST (U/L) | 46.9 (4.1) |
| ALT (U/L) | 40.6 (4.4) |
| CEA level (ng/mL) | 411.7 (132.7) |
| Tumor Location | |
| Bilobar | 84% |
| Right Lobe | 16% |
| Liver Tumor burden | |
| Diameter of index lesion (cm) | 7.2 (1.0) |
| Overall tumor volume (mL) | 280.3 (46.0) |
| Prior Chemotherapy | 60% |
| Prior Invasive Intervention | |
| Radiofrequency ablation | 2% |
| Partial hepatectomy | 8% |
| Hepatic arterial pump | 2% |
Eastern Cooperative Oncology Group performance status.
Figure 1. Examples of 99mTc-MAA Scans during Pre-SIRT Evaluation.
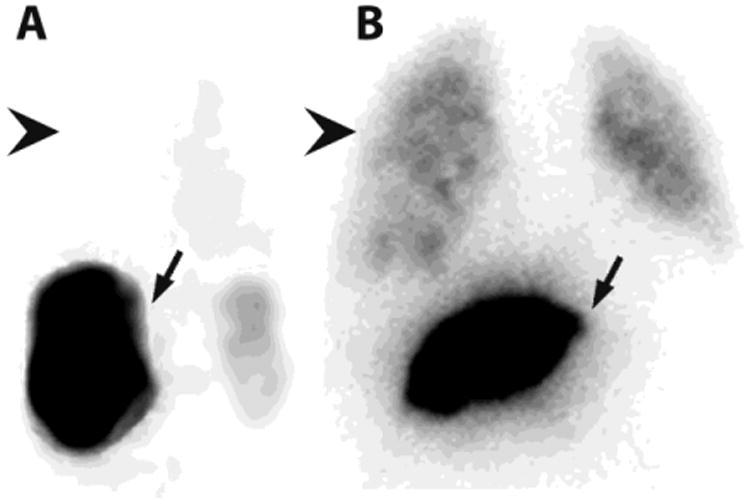
After 99mTc-MAA is injected into the hepatic artery to be treated during SIRT, the patient is transferred to nuclear medicine for SPECT and planar imaging to calculate the lung shunt fraction (LSF). A. In patients with little intratumoral vascular shunting (4%), activity predominates in the liver (arrow), with little or no activity in the lung (arrowhead). B. In patients with a great deal of shunting (12%), lung activity (arrowhead) is quantified and compared to liver activity (arrow) to calculate the LSF, which will determine the safe dose of radioembolization particles that may be administered during SIRT.
Cross-sectional imaging was reviewed for the presence of liver lesions and other metastases before and after SIRT. The greatest diameter of the largest liver lesion on cross-sectional axial images was measured. Total hepatic tumor volume was assessed using three-dimensional software, TeraRecon (Foster city, CA, USA), that allowed for multiple regions of interest to be created for all tumors, with summation of the total tumor volume. Regions of interest and measurements were performed by a board-certified vascular radiology fellow (AD) and reviewed by a board- and CAQ-certified interventional radiologist (RO).
The time between SIRT and disease progression and survival after SIRT was recorded. The medical charts were reviewed for cancer staging, baseline laboratory values, and chemotherapy agents administered before, during and after pre-SIRT evaluation. Survival was determined by chart review; when there was no record of death, the last note in the chart was used as an estimate of survival. Additionally, a research database was reviewed for the presence of genetic mutations determined by SNaPshot analysis of pathology specimens obtained by percutaneous biopsy (9).
The relationship of LSF to the presence of lung metastases was assessed with Kaplan Meier survival analysis by dividing patients into two groups by the median LSF (7.3); those with lower (<7.3%; n=31) and higher (>7.3%; n=31) LSF. Similarly, the relationship of LSF to patient survival after SIRT, progression of liver disease, and time to next new distant metastasis were also characterized. The relationship of tumor burden, assessed by both greatest diameter of dominant lesion and the overall hepatic tumor volume, and the presence of lung metastasis and survival were then assessed by Cox regression analysis. The impact of receiving chemotherapy before, during and after pre-SIRT evaluation were evaluated using Wilcoxon signed rank sum test. Finally, whether tumor burden or LSF differed among patients with and without genetic mutations was assessed using Mann-Whitney U-tests.
Results
A total of 62 patients with liver metastases from colorectal cancer (31F, 31M; mean age 63 years, SD 12) were included (Table 1). Thirty-three patients had documented lung metastases in addition to liver metastases. Patients with higher LSF were significantly more likely to have lung metastases at the time of pre-SIRT (p=0.0008). This relationship was not true for index lesion size or overall liver tumor volume. Higher LSF was not associated with hepatic tumor volume (p=0.40) or with increased index tumor lesion size (p=0.72).
Kaplan Meier analysis demonstrated that patients with higher LSF had significantly decreased survival compared with those with lower LSF (p=0.03). After initial overlap in the two groups' survival curves, over time those with lower LSF lived significantly longer (Figure 2). Patients with lower hepatic tumor volume did not have longer survival by Cox regression analysis (p>0.05). Higher LSF did not predict time to the next new documented metastasis or to the next documented liver lesion (p>0.05).
Figure 2. Kaplan Meier Survival Curve for Survival after SIRT.
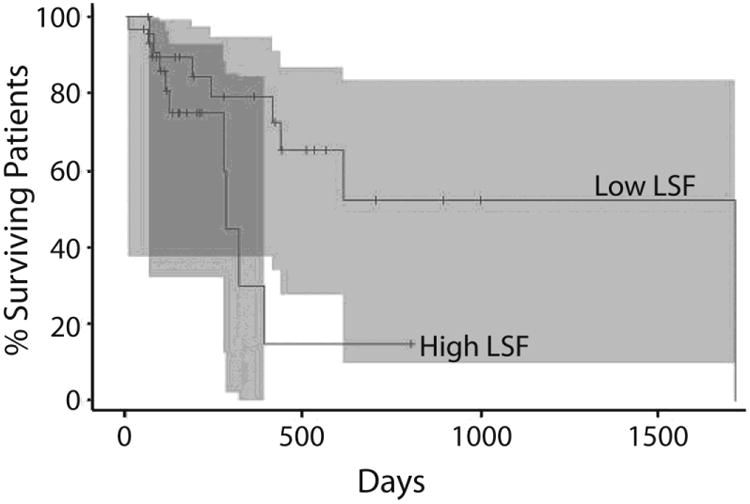
After initial overlap, patients with lower LSF survive significantly longer than patients with higher LSF.
Nineteen patients received chemotherapy before pre-SIRT evaluation; one patient received chemotherapy before and at the time of pre-SIRT; 12 patients received chemotherapy before and after pre-SIRT; 3 patients received chemotherapy after pre-SIRT; 5 patients received chemotherapy before, during and after pre-SIRT and 22 patients had no chemotherapy. Patients that received chemotherapy, had multiple drug regimens. Of the 37 patients who received chemotherapy before SIRT, 20 received cetuximab, 28 received irinotecan, 29 received bevacizumab, 22 received oxaliplatin, 34 received 5-FU, 21 received leucovorin, 7 received panitumumab, 10 received a study drug, and 1 received carboplatin, paclitaxel, and cisplatin. Of the 6 patients on chemotherapy concurrent with SIRT, 2 received cetuximab, 3 received irinotecan, 2 received bevacizumab, 4 received 5-FU, 1 received cisplatin, and 1 received panitumumab. Of the 20 patients who received chemotherapy after SIRT, 8 received cetuximab, 9 received irinotecan, 10 received bevacizumab, 5 received oxaliplatin, 7 received 5-FU, 1 received leucovorin, 5 received panitumumab, 5 received a study drug, 1 received gemcitabine, and 2 received mitomycin-C. Kaplan Meier Survival analysis demonstrated that any chemotherapy during and after had no impact on survival. Patients who received any chemotherapy before pre-SIRT evaluation tended to have lower LSF (p=0.08). There was a significant interaction, whereby patients who received chemotherapy prior to pre-SIRT and had low LSF had the longest post-SIRT survival (p=0.02).
Sixteen patients underwent SNaPshot analysis of liver biopsy specimens. Nine had cancer mutations (APC, BRAF, EGFR, IDH1, KRAS, TP53), whereas 7 had none identified. Patients with mutations had significantly higher LSF by Mann-Whitney U-test (mean 11.0; SD 3.8) compared with those without mutations (mean 7.3; SD 0.9) (U=7; Z=2.54; p=0.01). Patients with genetic mutations did not have increased hepatic tumor volume or index lesion size (p>0.05).
Discussion
Though metastasis is the leading cause of cancer-related deaths, its pathogenesis is poorly understood (2). The findings of this study suggest that intratumoral vascular shunting, quantified as LSF, is associated with distant metastasis and decreased survival. Whereas LSF predicted these outcomes, hepatic tumor volume and index lesion size did not. It is possible that specific tumoral features including shunting may better predict metastatic potential than the burden of malignant disease as indicated by overall tumor volume or index lesion size. Correlation between LSF and tumor size has been shown previously (10). However, this study suggests that LSF may be a novel marker of metastasis and survival.
Patients with cancer mutations demonstrated more intratumoral shunting than those without mutations. This study implicated a variety of genes including APC, a tumor suppressor protein; BRAF, a protein kinase that regulates cell division and differentiation; EGFR, a cell surface receptor that regulates cell proliferation; IDH1, an isocitrate dehydrogenase, an enzyme involved in cellular metabolism; KRAS, an oncogene, that promotes tumor cell invasiveness and TP53, a tumor suppressor protein, that regulates cell cycle arrest, apoptosis, DNA repair, and metabolism (11).
It remains unclear how these mutations relate to intratumoral shunting, which could partly be due to proangiogenic tumor microenvironment (12). Some of the pathways highlighted in our study possibly relate to angiogenesis, mediated by multiple mechanisms. Arteriovenous shunts result in poor delivery of nutrients to capillaries supplying tumors, inducing further angiogenesis (13). Such shunts could interfere with delivery of therapies including chemotherapy, SIRT and drug eluting beads in transarterial chemoembolization (14). Therefore, understanding the etiology of shunts has important implications for targeted cancer therapy. Further research is necessary to delineate the role that these mutations play in the creation of intratumoral shunts; studying other vascular tumors and arteriovenous malformations could provide insight into the pathogenesis of metastasis and possibly therapeutic targets.
There was a significant interaction whereby patients who received chemotherapy prior to pre-SIRT evaluation and had low LSF had significantly longer survival. Furthermore, there was a trend for chemotherapy before pre-SIRT to be associated with lower LSF. It is possible that chemotherapy may reduce the amount of intratumoral vascular shunting. One theory is that certain chemotherapeutic agents, such as VEGF inhibitors, may improve outcomes by “normalizing” tumor vascularity (15). Vessels supplying tumors are heterogeneous and abnormal, characterized by a wide variety of subtypes including leaky sinusoids, capillaries, and arteriovenous shunts, leading to hypoperfusion of tumor parenchyma, which in turn drives disorderly angiogenesis (15). While counterintuitive, normalizing such vasculature and hence improving blood flow to tumors may hypothetically decrease the pro-angiogenic drive and improve perfusion and efficacy of chemotherapies and other treatments. In one study, HCC patients with excessive pre-SIRT intratumoral shunting precluding SIRT received Sorafinib, a small molecular inhibitor of several tyrosine protein kinases (e.g., VEGF and PDGF receptors). Sorafinib treatment reduced intratumoral shunting and LSF, allowing for eventual treatment with SIRT (16). Theoretically, adjuvant chemotherapy could normalize tumor vasculature, decreasing vascular shunting, and possibly promoting efficacy of SIRT and other liver-targeted therapies.
The primary limitation of the study is its retrospective nature, precluding the assessment of the impact of increased LSF on progression of metastatic disease. Future studies could assess the relationship between high LSF and the development of distant metastases. Another limitation of the study is that patients were treated with multiple chemotherapeutic agents, precluding our ability to evaluate the relative contribution specific chemotherapies might make. Furthermore, because of the retrospective nature of the study, it is possible that there is selection bias in patients who did and did not receive chemotherapy, which could confound the relationship of chemotherapy, LSF and survival. Finally, the relatively small number of patients who underwent SNaPshot analysis precludes assessment of the relationship of specific cancer genes to LSF. Future work may focus on the impact of particular pathways.
In conclusion, LSF may be a novel marker of metastasis and cancer survival, and may be more predictive than tumor size. Increased LSF due to vascular shunting within liver metastases indicated the presence of distant lesions. Intratumoral shunting could theoretically provide a conduit for circulating tumor cells to access more remote organs, bypassing filtration by liver parenchyma, and could be an important factor in metastatic potential of colorectal cancer. Certain genetic mutations may promote the formation of these vascular shunts, and could represent possible therapeutic targets.
Acknowledgments
This study was supported by NIH grant RO3 CA172738 (RO) and the Department of Radiology, Massachusetts General Hospital.
Footnotes
A portion of this data was presented at the SIR 2014 meeting.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sheth RA, Hesketh R, Deipolyi AR, Oklu R. Circulating tumor cells: personalized medicine in interventional oncology? J Vasc Interv Radiol. 2012;24:221–228. doi: 10.1016/j.jvir.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 2.Stein U, Schlag PM. Clinical, biological, and molecular aspects of metastasis in colorectal cancer. Cancer Res. 2007;176:61–80. doi: 10.1007/978-3-540-46091-6_7. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen DX, Massague J. Genetic determinants of cancer metastasis. Nat Rev Genet. 2007;8:341–352. doi: 10.1038/nrg2101. [DOI] [PubMed] [Google Scholar]
- 4.Yu M, Stott S, Toner M, Maheswaran S, Haber DA. Circulating tumor cells: approaches to isolation and characterization. J Cell Biology. 2011;192:373–382. doi: 10.1083/jcb.201010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bookstein JJ, Cho KJ, Davis GB, Dail D. Arterioportal communications: observations and hypotheses concerning transsinusoidal and transvasal types. Radiology. 1982;142:581–590. doi: 10.1148/radiology.142.3.7063671. [DOI] [PubMed] [Google Scholar]
- 6.Ngan H, Peh WC. Arteriovenous shunting in hepatocellular carcinoma: its prevalence and clinical significance. Clin Radiol. 1997;52:36–40. doi: 10.1016/s0009-9260(97)80303-0. [DOI] [PubMed] [Google Scholar]
- 7.Salem R, Thurston KG. Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: Technical and methodologic considerations. J Vasc Interv Radiol. 2006;17:1251–1278. doi: 10.1097/01.RVI.0000233785.75257.9A. [DOI] [PubMed] [Google Scholar]
- 8.Ahmadzadehfar H, Sabet A, Biermann K, et al. The significance of 99mTc-MAA SPECT/CT liver perfusion imaging in treatment planning for 90Y-microsphere selective internal radiation treatment. J Nucl Med. 2010;51:1206–1212. doi: 10.2967/jnumed.109.074559. [DOI] [PubMed] [Google Scholar]
- 9.Dias-Santagata D, Akhavanfard S, David SS, et al. Rapid targeted mutational analysis of human tumours: a clinical platform to guide personalized cancer medicine. EMBO Mol Med. 2010;2:146–158. doi: 10.1002/emmm.201000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho S, Lau WY, Leung WT, et al. Arteriovenous shunts in patients with hepatic tumors. J Nucl Med. 1997;38:1201–1205. [PubMed] [Google Scholar]
- 11.Available at http://www.ncbi.nlm.nih.gov/gene. Accessed June 15, 2014.
- 12.Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17:1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- 13.Pries AR, Hopfner M, le Noble F, Dewhirst MW, Secomb TW. The shunt problem: control of functional shunting in normal and tumour vasculature. Nat Rev Cancer. 2010;10:587–593. doi: 10.1038/nrc2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheth RA, Hesketh R, Kong DS, Wicky S, Oklu R. Barriers to drug delivery in interventional oncology. J Vasc Interv Radiol. 2013;24:1201–1207. doi: 10.1016/j.jvir.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 15.Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10:417–427. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- 16.Theysohn JM, Schlaak JF, Muller S, et al. Selective internal radiation therapy of hepatocellular carcinoma: potential hepatopulmonary shunt reduction after sorafenib administration. J Vasc Interv Radiol. 2012;23:949–952. doi: 10.1016/j.jvir.2012.04.007. [DOI] [PubMed] [Google Scholar]


