Abstract
Whether subjects with Insomnia exhibit good sleep on some interval basis is unclear. Prior research suggests that patients with insomnia are highly variable with respect to night-to-night sleep continuity, that more than 40% of patients with insomnia exhibit temporal patterning of good sleep, and that nearly 90% of patients with insomnia exhibit better than average sleep following 1 to 3 nights of relatively poor sleep. The aim of the present study was to replicate and extend the above noted findings utilizing: 1) a large sample studied over an extended time interval; 2) absolute standards for “good” and “poor” sleep; and 3) a formal statistical methodology to assess temporal patterning and the association of Time-In-Bed with bout duration of poor or average sleep. Thirty-three subjects with insomnia and 33 good sleepers completed sleep diaries over the course of 110 days. It was found that subjects with insomnia (as compared to good sleepers) had more poor nights (e.g., about 39% vs. 7% of the assessed nights), a higher probability of a having a poor night on any given occasion (60% greater probability than good sleepers), and more consecutive nights of poor sleep between good sleep nights (median bout duration of about 3 vs. 1 night). Lastly, it was found that (as would be predicted by both the Spielman model and the Two Process model) time in bed moderated bout duration in the insomnia group. That is, longer times in bed were associated with longer bouts of poor sleep.
Keywords: Insomnia, night-to-night variability, illness severity, temporal patterning
INTRODUCTION
It has long been recognized that insomnia, even when chronic and severe, tends not to occur on a nightly basis. In recognition of this observation, the DSM-5 has adopted the long standing clinical research tradition of defining insomnia as having a frequency of 3 or more days per week. Such a criterion naturally lends itself to the question “what happens on the non-insomnia nights?”. Does the individual experience good sleep or at least better than average sleep? Further, does the incidence of good and poor sleep occur randomly or in some predictable manner? In 2005, it was suggested that insomnia symptoms may be expressed over time in a manner that is more episodic than unremitting (Perlis et al. 2005). In addition, it was suggested that 1) the waxing and waning course of insomnia may require the imposition of a typology to detect patterning over time, 2) the patterning was likely to occur over short time intervals (1-5 days), and 3) the patterning was likely to be ascribable to factors related to the homeostatic regulation of sleep. That is, it was posited that each passing night of poor sleep should increase the probability of occurrence of either a good night's sleep or (at least) a better than average night's sleep. To date, four studies have evaluated night-to-night variability, with an eye towards assessing whether temporal patterning may be observed in the incidence of insomnia in individuals with chronic insomnia.
The first empirical study was conducted by Vallières et al. (2005)
Data were available on 106 untreated subjects with insomnia who were monitored with sleep diaries for an average of 31 days (range 18-56 days). The study sample was 58% female with a mean age of 45.5 years old. Consecutive daily sleep data were conceptualized as time series data with each night being dichotomized as either a good or a poor night's sleep. A poor night was defined as Sleep Latency (SL) and/or Wake After Sleep Onset (WASO) ≥ 60 min associated with a Sleep Efficiency (SE%) ≤ 80%. Cluster analysis was used to identify subgroups who showed similar levels of conditional probabilities (likelihood of poor night's sleep). Three subgroups were identified: one group exhibited a high probability of having poor sleep on any given night (22% of subjects); the second group exhibited a low and decreasing probability of having poor sleep on any given night (42% of subjects); and the third group exhibited an unpredictable pattern (36% of subjects). It was also found that: insomnia severity was higher for Groups 1 & 3; and Group 2 showed a predictable pattern such that after three poor nights of sleep these subjects had 0% chance of experiencing a fourth consecutive night of poor sleep (p. 240). The Vallières study is invaluable in that it is the first to 1) formally assess night-to-night variability in insomnia, 2) demonstrate that high night-to-night variability is characteristic of all subjects with chronic insomnia, and 3) observe that 42% of subjects with Insomnia exhibit a temporal pattern in the incidence of insomnia. That is, after 3 nights of poor sleep, such subjects reliably exhibited a good night's sleep or (at least) a better than average night's sleep. Three limitations of this otherwise seminal study are that: 1) the threshold adopted to define poor sleep (the incidence of insomnia) was inordinately high at 60 minutes, allowing only the most severe occasions of insomnia to be classified as incidents; 2) a good night's sleep is defined as one which does not meet some or all of the criteria for a poor night's sleep, allowing moderately severe instances of insomnia (e.g., SL and/or WASO of 30-60 minutes) to be typed as good sleep; and 3) the assessment method used did not directly assay how good sleep and poor sleep temporally covary. That is, the investigators used a “3 day solution” for the data analysis (i.e., “conditional probabilities to have a poor night after 1, 2, or 3 consecutive poor nights were computed for each participant” [p.612]). Thus, it remains possible that a large percentage of the sample may have been categorized as “Cluster II”, had the period been free to vary (i.e., some subjects may require more than 3 days for there to be a 0% probability of a subsequent poor night's sleep).
The second study was conducted by Perlis et al. (2010)
In this investigation pilot data were provided on: (1) the frequency with which good sleep occurs in subjects with insomnia; and (2) whether these events occur in a predictable manner. Ten subjects with Insomnia participated in this naturalistic study. Eight of 10 subjects were female with a mean age of 45.0 years of age. All subjects completed daily sleep diaries for an average 43 days (range 20-73 days). None of the subjects received treatment for their insomnia during the monitoring period. The night-to-night data were evaluated by typing each night's sleep as Good or Bad, and then by determining the number of bad nights that occurred prior to a good night for each subject. Good and bad nights were typed in two ways: (1) using a 85% cut-off for sleep efficiency (absolute cut-off) above which was categorized as “Good” and below which was categorized as “Bad”) and (2) using a better than the individual's mean sleep efficiency (idiographic cut-off) above which was categorized as “Good” and below which was categorized as “Bad”). Subjects exhibited good sleep by absolute criteria 29% of the time and good sleep 55% of the time by idiographic criteria. The temporal patterning analysis (based on the idiographic cut-off) revealed that up to 17 days may elapse before 100% of subjects experience a better than average night's sleep. Most subjects (89%), however, experienced a better than average night's sleep following 1-3 nights of poor sleep. The primary limitations of this study are its: 1) small sample size; 2) sole reliance on idiographic criteria and/or thresholds for the frequency analysis; and 3) lack of a formal method to determine periodicity and how period (insomnia sequence duration) varies with other factors of interest (e.g., Time in Bed) or relative to other groups (e.g., good sleepers).
The third study was conducted by Buysse and colleagues (2010)
Data were gathered from 91 older adults, sixty-one of whom had chronic insomnia and thirty-one of whom were characterized as “not having insomnia”. The aims for the study were to assess between group variability and within group temporal patterning. The sample was predominantly Caucasian (96%) and female (65%), with an average age of 71 years old. All subjects wore actigraphs and completed daily sleep diaries for a 14 day period. The sleep diary results from this study were that subjects with insomnia: 1) differed from the non-insomnia group on mean values for sleep diary measures; 2) showed significantly greater variability on most sleep continuity measures than the non-insomnia group; 3) exhibited “little evidence for positive or negative correlation of sleep measures across nights” (p.609); and 4) did not exhibit (upon visual inspection) any rhythm or pattern in the time series plots for Time In Bed (TIB) or Wake After Sleep Onset (WASO). The primary limitations of this study were that the authors approach was correlative (i.e., was primarily focused on assessing inter-night coherence as opposed to patterning over time), the effort to detect patterns in group time series data was hampered by the lack of a determined start point (i.e., a biopsychosocial anchor which allow group time series data to appear as something other than white noise in group plots), and the use of only two weeks of diary data (i.e., this may have been too little data to work with to detect within subject periodicities; Vallières et al. had an average of 30 days per subject and Perlis et al., had an average of 43 days per subject. Finally, limiting the sample to older adults decreases generalizability and runs the risk that the specific population studied (subgroup) may not exhibit the phenomenon of interest.
The Fourth study was also conducted by Vallières et al. (2011)
This investigation was conducted to replicate their prior findings. The analysis was conducted on a newly aggregated sample (n=117 subjects) who were evaluated with sleep diaries for an average of 48 days (range 21-118 days). The sample was 60% female with a mean age of 51 years old. The investigators successfully replicated their prior work finding that their second sample of subjects also exhibited three patterns with respect to night-to-night variability: one group exhibited a high probability of having poor sleep on any given night (42% of subjects); the second group exhibited a low and decreasing probability of having poor sleep on any given night (26% of subjects); and the third group exhibited an unpredictable pattern (32% of subjects). As a replication study, the three limitations of the original study also apply to this investigation.
Taken together these studies suggest that patients with insomnia are highly variable with respect to night-to-night sleep continuity, that between 26%-42% of patients with insomnia exhibit “good” sleep after three poor nights of sleep, and that nearly 90% of patients with insomnia exhibit better than average sleep following 1 to 5 nights of relatively poor sleep. Further, these findings suggest that, in at least a subgroup of patients with insomnia, sleep debt may accrue across successive nights, in so doing allow for episodes of normal, or more normal sleep, and thereby account for the observation that insomnia, even when chronic and severe, tends not to be persistent and unremitting. The present study, attempts a replication of the findings of Perlis and colleagues (2010) and seeks to extend these findings by utilizing: 1) a larger sample (one comprised of both subjects with Insomnia and Good Sleepers) studied over an extended time interval; 2) absolute standards for good and poor sleep; and 3) a formal statistical methodology to assess temporal patterning and the association of Time In Bed (TIB) with bout duration of poor or average sleep(number of poor or average nights between two good nights). TIB was assessed because, according to the 3P model of insomnia (Spielman et al. 1987), increased TIB is thought to be a primary perpetuating factor for chronic insomnia. If true, longer TIB should be associated with longer bouts of insomnia in subjects with chronic insomnia.
METHODS
Data Source
This study utilized a de-identified archival dataset from a 9-month sleep diary study conducted at Loughborough University (David & Morgan, 2009). The parent study, which was IRB approved by and overseen by the Loughborough University, evaluated adherence rates for daily sleep diaries in patients with Insomnia (PI) and in Good Sleeper Controls (GS). Subjects for the parent study were recruited by advertisements and were screened using a questionnaire that was mailed to each prospective participant.
The inclusion criteria for the PI and GS subjects were they had to be between 25 and 50 years of age. Subjects with insomnia also met Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) criteria for Primary Insomnia. The exclusion criteria for both groups were: awaiting or undergoing hospital treatment; reporting a diagnosed long-term health condition; taking psychotropic medication (including hypnotics); taking medication known to affect sleep; reporting chronic pain; scoring >20 on the Beck Depression Inventory (BDI); an Epworth Sleepiness Scores score >10; reporting all 4 ‘essential diagnostic criteria for Restless Legs Syndrome’; reporting a history of or current drug or alcohol abuse; and reporting a history or a diagnosis of chronic fatigue syndrome.
All prospective subjects were informed regarding the demands of the study, encouraged to contact the project coordinator or investigators if they experienced problems, and were assured that (on completion) they would each receive a summary of the study's key findings together with a personal sleep profile. Incentive payments equivalent to approximately $60.00 were made to each participant on completion. Prior to data collection, each subject was oriented regarding how to complete the sleep diary via a 1 to 1 review of the items using the subjects previous night sleep to generate each sleep diary entry. 86 individuals served as study participants (43 with Insomnia and 43 Good Sleepers). The daily diary was comprised of 10 items including: Time to Bed (TTB), Time of Final Awakening (TFA), Time out of Bed (TOB), Sleep Latency (SL), Wake After Sleep Onset (WASO), Total Sleep Time (TST) and Sleep Quality (SQ). Two additional variables were calculated from the reported data: Time in Bed (TIB) and Sleep Efficiency (SE%). TIB represents the difference in time between Time to Bed and Time out of Bed (TOB-TTB). Sleep Efficiency was calculated as ([TST/TIB]*100). The primary results from the parent study were that: 1) a main effect was observed for adherence (compliance with completing daily sleep diaries) with a decline evident from 97.6% in the first month to 81.6% in the ninth month; and 2) the groups did not differ with respect to adherence.
Independent Variables
In the present study, both groups were evaluated for the temporal periodicity of good and poor sleep. The inclusion of good sleepers (over the course of a longitudinal study) served to allow the observation of not only occasional poor sleep and instances of acute (days), transient (weeks), and sub-chronic insomnia (months)(Ellis et al., 2012), it also allowed for an assessment of how quickly the good sleeper subjects recovered from such episodes. Further, the inclusion of the good sleeper group allowed for the statistical assessment of whether subjects with insomnia significantly differ from normal with respect to the variables of interest (e.g., number of poor sleep bouts, duration of inter-bout intervals, the effect of Time in Bed on duration of inter-bout intervals, etc.).
Group assignment was carried over from the parent study (based on the Loughborough criteria) and then was corroborated using the first two weeks of sleep diaries and quantitative criteria for the definition of insomnia. Specifically, subjects classified as having insomnia were retained in the present study sample if they met the following average profile: > 30 min. Sleep Latency (SL) or > 30 min. Wake After Sleep Onset (WASO) and a Sleep Efficiency of (SE) ≤ 85%. Good sleeper subjects were retained in the study sample if they met the following profile: < 30 min. SL and < 30 min. WASO and a SE > 85%. Given the application of these criteria, the newly aggregated dataset had 33 individuals with insomnia (18 women and 15 men) and 33 Good Sleepers (24 women and 9 men). All subjects had a minimum of 110 days of data. The data used for the temporal patterning analyses were truncated to 110 days so that all subjects had comparable data sets and were in the same “phase” of self-monitoring.
Operationalization of Outcome
For the present analysis, each night in each subject's time series data was classified as Poor (P), Average (A), or Good (G) based on absolute thresholds for sleep latency (SL) and wake after sleep onset (WASO). Nights for which both SL and WASO were less than or equal to 15 minutes were considered good nights. Nights for which either SL or WASO were greater than or equal to 30 minutes were considered poor nights. Any other nights for which SL and WASO were available were classified as average nights. Any nights that were missing SL and WASO could not be categorized and were therefore treated as missing. Nights for which SL was missing but WASO was less than 30 and nights for which SL was less than 30 but WASO was missing were also treated as missing. Absolute thresholds (as applied to SL and WASO measures) were adopted for the present analysis in order to more closely parallel the work of Vallières et al. Further, it was felt that SL and WASO values more directly map on to subject complaints (can't initiate or maintain sleep).
Within each individual's time series data, each bout of poor or average sleep was defined by its start and stop points. The start point was the last instance of a good night of sleep and the stop point was the first next instance of a good night of sleep. The duration of the bout of poor or average sleep was the number of poor or average nights between the start and stop points. For bouts with a start point of a good night of sleep but a stop point of a missing night (or end of study), the number of nights between the start and the missing night (or end of study) was counted to determine the lower bound of the number of poor or average nights within the bout of poor or average sleep. The duration of the bout was then considered censored (i.e., had missing data which disallowed the precise determination of where the bout ended). Similarly, for bouts with a start point of the beginning of the study and a stop point of a good night, the number of nights from the beginning of the study to the first good night was counted to determine the lower bound of the number of poor or average nights within the bout of poor or average sleep. The duration of the bout was then considered censored (i.e., had missing data which disallowed the precise determination of where the bout began). Bouts of poor or average sleep with a start point of a missing night were not included to avoid double-counting the same bout both before and after the missing night.
By way of example, consider Figure 1. The first three nights constitute a bout of poor or average sleep with start point of beginning of the study and stop point that is missing. In this case, the bout is assessed as lasting three nights but is designated as censored given the true start and stop points are unknown. The 5th night is excluded, since the start point is missing. Nights 7-8 constitute a bout of poor sleep with start point of a good night on night 6 and stop point at a good night on night 9, so the duration of this bout is 2 nights of poor or average sleep. Lastly, days 109-110 constitute a bout of poor or average sleep with start point at a good night on night 108 and stop point at the end of the study. In this instance, the duration of the bout is two nights though, since the true start and stop points are unknown, these data are again considered censored.
Figure 1.
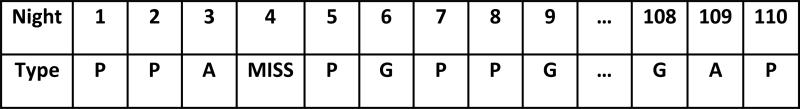
Example of a sequence of classifications.
Statistical Analysis
The mean number of poor nights, average nights, good nights, missing nights, total nights, and the mean number of poor or average sleep bouts and censored bouts were calculated for each sleep group. In addition, the mean and median duration of bouts of poor or average sleep that were not censored were calculated for each subject and then averaged across subjects within each sleep group. T-tests were used to test for significant differences in these measures across sleep groups. Differences in demographic characteristics across sleep groups were assessed using t-tests for continuous variables (age, weight, BMI) and a difference of proportions test for gender. Means and standard errors of sleep variables over the 110 day follow-up period in each sleep group were also calculated, using linear regressions. To account for the correlation of these variable values over time within each subject, regression model variances were estimated by a robust sandwich estimator (Note: this same approach was recently utilized by Klerman et al. 2013). Means across sleep groups were compared using Wald tests. Weibull survival models were used (also with variances estimated by the robust sandwich estimator to account for clustering of bouts within subjects) to measure associations between our independent variables (Group) and dependent variables (duration of bouts of poor or average sleep). The Weibull model is a standard flexible parametric survival model that estimates continuous survival functions of time to an event and can incorporate censored observations. Weibull model fit was assessed by looking for linearity and slope of 1 between the model's Cox-Snell residuals and cumulative hazard. Sensitivity analyses were conducted by fitting the models after excluding outliers. In addition, significance of the Weibull shape parameter comparing the Weibull model fit to the exponential model fit was tested. All significance tests were compared to level 0.05. The unadjusted analysis included only sleep group as a covariate in the model.
To evaluate whether the survival effects were moderated by time in bed, a second, adjusted model that included sleep group and Time-in-Bed was considered. In the adjusted model, any demographic variables that were significantly different across sleep group were adjusted for as confounders. An interaction between sleep group and Time-in-Bed was taken as an indicator that Time-in-Bed moderated the observed effects of sleep group on nights to good sleep. Using the parameter estimates from each model, the median number of nights to good sleep was estimated for each sleep group. For the adjusted model, the estimated median number of nights was calculated at the 25th, 50th, and 75th percentiles of time in bed and 50th percentiles of any demographic variables.
RESULTS
Sample Composition and Group Differences for Sleep Continuity
The study sample included 33 good sleepers (mean age 35.7±7.8 years and 27% male) and 33 patients with insomnia (40.7±7.7 years and 46% male). Table 1 (Parts A and B) show the comparison of demographic and sleep continuity variables across sleep groups. Subjects with insomnia had a significantly higher mean age than good sleepers. All other demographic variables were similar between the two groups. SL and WASO were significantly higher for those with insomnia. Total sleep time and sleep efficiency were significantly higher for the good sleepers. Time in bed did not significantly differ between groups.
TABLE 1.
Demographics and Sleep Variables by Sleep Group
| A: Demographic Variables | ||||
|---|---|---|---|---|
| Good Sleeper Mean (SD) | Insomnia Mean (SD) | T/χ2 Test Statistic | p-value | |
| Age, years | 35.7 (7.8) | 40 (7.7) | −2.25 | 0.028 |
| Weight, kg | 70.2 (14) | 73.8 (18.2) | −0.76 | 0.452 |
| BMI, kg/m2 | 24.3 (4.7) | 26 (5.1) | −1.19 | 0.240 |
| Gender, N (%) | 9 (27.3) | 15 (45.5) | 2.36 | 0.125 |
| B: Sleep Variables | ||||
|---|---|---|---|---|
| Good Sleeper Mean (SE)1 | Insomnia, Mean (SE)1 | T Test Statistic | p-value | |
| Sleep Latency, minutes | 24.4 (2.0) | 47.1 (3.2) | 6.04 | <0.001 |
| Wake After Sleep Onset, minutes | 7.7 (1.1) | 32.8 (3.4) | 6.96 | <0.001 |
| Time in Bed, hours | 8.4 (0.1) | 8.5 (0.1) | 0.36 | 0.727 |
| Total Sleep Time, hours | 7.6 (0.1) | 6.5 (0.1) | −6.88 | <0.001 |
| Sleep Efficiency | 89.3 (0.5) | 76.4 (1.3) | −9.22 | <0.001 |
| C: Sleep Types and Bouts per Subject | ||||
|---|---|---|---|---|
| Good Sleeper Mean (SD) | Insomnia, Mean (SD) | T Test Statistic | p-value | |
| Total Nights Assessed | 102.8 (17.1) | 100.9 (21.8) | 0.39 | 0.698 |
| Number of Poor Nights | 7.4 (7.0) | 39 (25.5) | −6.86 | <0.001 |
| Number of Average Nights | 12.0 (10.7) | 24.3 (12.4) | −4.33 | <0.001 |
| Number of Good Nights | 83.3 (19.7) | 37.5 (23.8) | 8.52 | <0.001 |
| Number of Bouts | 12.4 (7.4) | 17.5 (8.1) | −2.69 | 0.009 |
| Number of Missing Nights | 7.2 (17.1) | 9.1 (21.8) | −0.39 | 0.698 |
| Censored Bouts | 0.5 (0.7) | 1.6 (0.8) | −5.90 | <0.001 |
| Mean Bout Duration2 | 1.4 (0.7) | 3.9 (4.6) | −2.99 | 0.004 |
| Median Bout Duration Length2 | 1.2 (0.4) | 3.1 (4.2) | −2.64 | 0.010 |
Estimates from linear regression models with sandwich estimator for variances
Number of poor or average nights between two good nights
Descriptive Data: Bouts and Group Differences for Number of Good and Poor Nights
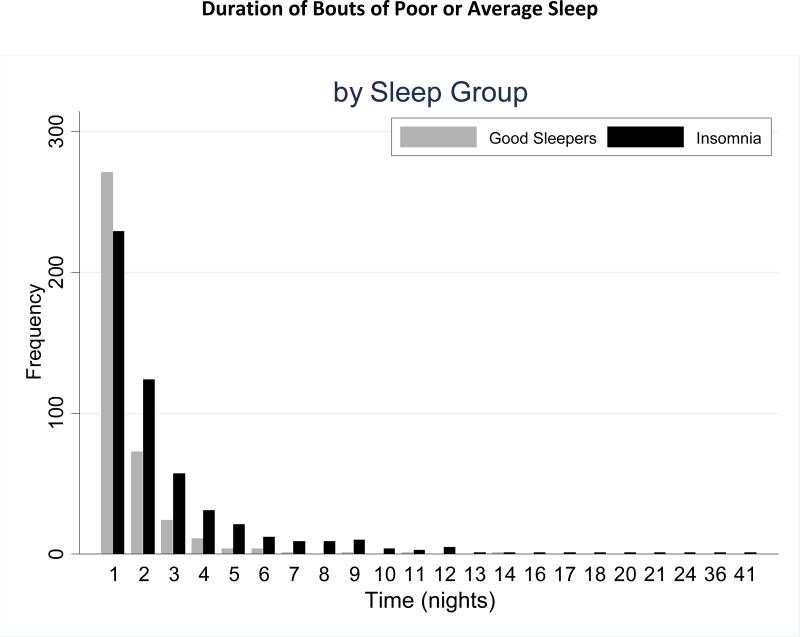
Each patient was followed for 110 days and contributed between 1 and 28 bouts of poor or average sleep. There were a total of 985 bouts included in analysis, 915 of which were “events,” meaning they began and ended with a good night. The remaining 70 bouts were censored, 52 of which were censored due to the beginning or end of the study; 17 bouts were censored due to missing values; and 1 bout was censored due to both beginning of the study and a missing sleep diary entry in the middle of the study period. Six bouts of poor or average sleep were excluded from the analysis due to starting with a missing value. Means and differences in sleep classifications and poor/ average sleep bout characteristics are shown in Table 1 (Part C). Significant differences between sleep groups were evident for number of good nights, number of average nights, number of poor nights, number of bouts, number of censored bouts, and for mean and median bout durations. Consistent with prior findings (Perlis 2009), it was also found that a good nights’ sleep (in patients with insomnia) most frequently occurred following one (43.6%), two (23.7%) or three (10.8%) nights of poor or average sleep. Figure 2 shows the frequency of the durations of bouts of poor or average sleep. There were more bouts from good sleepers than those with insomnia with just one night of poor or average sleep in the bout, whereas there were more bouts of poor sleep from those with insomnia with duration of two or more nights. The maximum duration of poor/average sleep bout was 14 nights of poor or average sleep for good sleepers, whereas the maximum duration for those with insomnia was 41 nights of poor or average sleep.
FIGURE 2.
Duration of Bouts of Poor or Average Sleep
Modeling: Bouts and Group Differences for Number of Good and Poor Nights
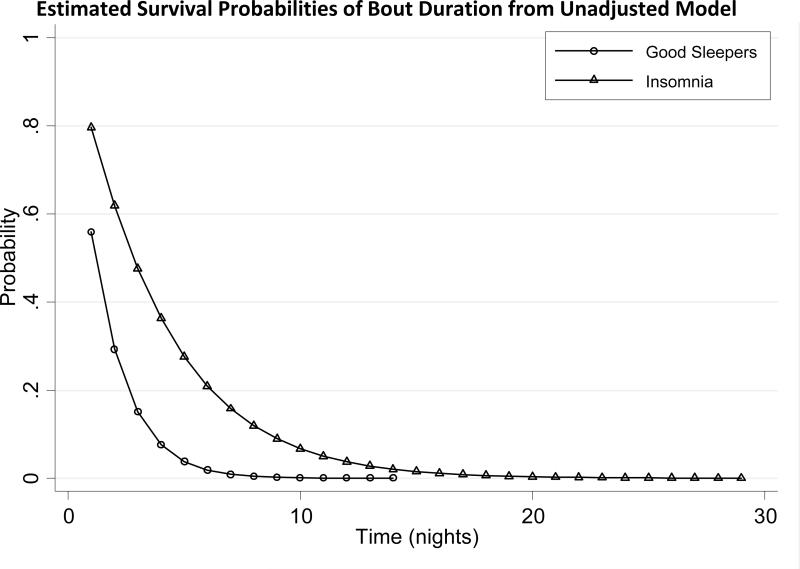
The Weibull model yielded a good fit to the data and a sensitivity analysis (which excluded 3 bouts with large durations) showed little change in results. In comparison to the exponential model, the Weibull model yielded a better fit (Z=3.77, p=0.001). In the unadjusted model of duration of poor/average sleep bout on sleep group, those with insomnia had an estimated 60.2% reduced probability of good sleep compared to good sleepers (Hazard Ratio (HR)=0.392, p<0.001). The median durations for poor/average sleep bouts, unadjusted estimates for each group from the Weibull model, are shown in Table 2. For those with good sleep, the median bout duration was 1.18 days. For those with insomnia, the median duration was 2.8 days. Thus, irrespective of the frequency of occurrence, poor/average sleep bouts were 1.6 days longer in subjects with insomnia than in good sleepers.
TABLE 2.
Estimated Median Duration of Poor or Average Sleep Bouts by Sleep Group
| Good Sleeper | Insomnia | Z Statistic | p-value1 | |
|---|---|---|---|---|
| Unadjusted Model | 1.18 | 2.82 | −5.72 | <0.001 |
| Adjusted Model by Time in Bed | ||||
| 25th percentile – 7.6 hours | 1.14 | 2.42 | ||
| 50th percentile – 8.4 hours | 1.18 | 2.77 | −3.28 | 0.001 |
| 75th percentile – 9.3 hours | 1.22 | 3.17 |
p-value is from Wald test of sleep group variable in unadjusted model and of interaction term between sleep group and covariate of interest in adjusted models
Figure 3 shows the estimated survival curves by sleep group, where survival probability is the probability of a poor/average sleep bout lasting longer than any given time. Overall, the separation between the lines illustrates the finding that the insomnia group (as compared to the good sleeper group) is more likely to have longer bouts of poor or average sleep and this is particularly evident for bout durations between 1 and 10 days. The initial difference at day 1 illustrates that patients with insomnia (as compared to the good sleeper group) are more likely to experience a bout of poor or average sleep of more than 1 night. The “tails” portion of the graph illustrates that only patients with insomnia are likely to exhibit poor/average sleep bout durations in excess of 10 days.
FIGURE 3.
Estimated Survival Probabilities of Bout Duration from Unadjusted Model
Modeling: Bouts & Group Differences for Number of Good and Poor Nights taking into account TIB
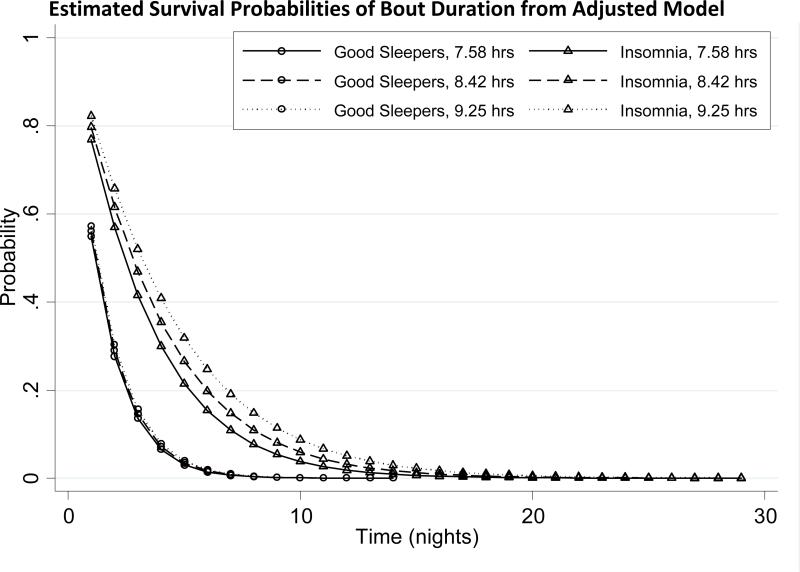
In the adjusted model of poor/average sleep bout duration on sleep group and Time-in-Bed (TIB), age was included since it was significantly different across the two sleep groups. In these analyses it was found that there was a significant interaction between sleep group and Time-in-Bed (p=0.001). For any given sleep bout duration, increasing time in bed by one hour was associated with a 13.4% reduced probability of ending the poor/average sleep bout for subjects with insomnia and a 2.6% decrease for good sleepers. The median durations of poor or average sleep bout estimated from this adjusted model for each group are shown in Table 2 at the 25th, 50th, and 75th percentiles of time in bed. Subjects with insomnia had bouts of poor or average sleep that were 1.3 to 2 nights longer than those of good sleepers, given a time in bed between 7.6 and 9.3 hours. Longer times in bed were associated with longer bouts of poor or average sleep for those with insomnia, whereas there was only a marginal increase in bout duration with longer time in bed for good sleepers. The estimated survival curves by percentiles of time in bed and by sleep group are shown in Figure 4. In good sleepers, the curves did not vary with time in bed, meaning that Time-in-Bed did not shorten or extend the duration of poor or average sleep bouts. In contrast, those with insomnia exhibit three distinct probability curves when taking into account time in bed, such that longer times in bed were associated with longer bouts of poor or average sleep.
FIGURE 4.
Estimated Survival Probabilities of Bout Duration from Adjusted Model
DISCUSSION
Summary of Findings
In the present study, 110 days of sleep diary data per subject were evaluated in sixty-six subjects (33 subjects with insomnia and 33 Good Sleepers). Each night's data per subject was classified using an absolute standard for “poor”, “average”, or a “good” night's sleep. Each subjects’ data were also classified into bouts of poor or average sleep. The basic data were evaluated for group differences as were the bout data. It was found that subjects with insomnia (as compared to good sleepers) had more poor nights (e.g., about 39% vs. 7% of the assessed nights), a higher probability of a having a poor night on any given occasion (60% greater probability than good sleepers), and more consecutive nights of poor sleep between good sleep nights (median bout duration of 3 vs. 1 night). Further, as would be predicted by both the Spielman model (sleep extension [Spielman et al. 1987]) and the Two Process model (curtailed time awake [Borbely, 1982]), Time-in-Bed moderated the group effect: longer times in bed were associated with longer bouts of poor or average sleep in the patients with insomnia. No such association was evident in the good sleeper group.
Relevance
The present findings suggest that as a group, patients with insomnia show a temporal patterning with respect to average and poor sleep (bouts of poor or average sleep range from 1 to 41 nights with a median bout duration of 3 nights). This finding, it should be noted, lends empirical weight to the seemingly arbitrary but precise choice to have a 3 day per week minimum criteria for the diagnosis of insomnia.
The patterning of good sleep over time suggests that sleep debt accrues across successive nights and in so doing allows for episodes of good sleep following bouts of insomnia. Taken in context, good sleepers suffering an “acute” bout of insomnia experience a good night's sleep in about 1 days’ time where subjects with insomnia require about 3 days’ time before experiencing a good night's sleep. This suggests that greater than normal levels of sleep debt may be required to produce good sleep in patients with insomnia. As noted in our previous study, this may be true for one or more of several reasons. First, in keeping with the hyperarousal perspective (Riemann et al., 2010), additional sleep drive may be required to attenuate arousal to a level that is compatible with sleep. Second, in keeping with behavioral theory and practice (Spielman et al., 1987; Spielman et al., 1987b) increased sleep pressure may be required to compensate for the tendency of patients to expand their sleep period in order to increase sleep opportunity (creating a fundamental mismatch between sleep opportunity and ability). Third, the functioning of “the” sleep homeostat (Borbely, 1982) may be altered such that more sustained wakefulness (or sleep debt) is required to produce good sleep and that this alone, or interaction with arousal and / or behavioral factors, may modulate the incidence and severity of insomnia.
The finding with respect to TIB moderating the group effect is particularly important. That is, longer times in bed for the insomnia subjects [but not GS subjects] were associated with longer durations of poor/average sleep bouts. This suggests, as would be predicted by the Spielman model, that the mismatch between sleep opportunity (time in bed) and sleep ability (inherent capacity to initiate and maintain sleep) may indeed moderate the incidence of poor sleep (the occurrence of insomnia). Further, this suggests (as would be predicted by the Psychobiological Inhibition model [Espie et al., 2002; Sanchez-Ortuno et al., 2011]) there may be less plasticity within the sleep-wake system in patients with insomnia. That is, the good sleeper may have sufficient sleep ability to accommodate variable increases in Time-in-Bed whereas this capacity is attenuated in patients with insomnia.
Finally, the present findings appear to be to be at odds with the two studies conducted by Vallières et al. (2005; 2011). They did not observe an overall group pattern and we did not observe subtypes with respect to variability. This discordance may simply reflect that the groups used different approaches. We suspect that if the investigators adopted one another's methodologies, it is possible (if not likely) that both sets of findings would cross replicate. More important, the cross replication would likely yield a complete picture. That is, there is a detectable overall pattern and it is also the case that some subgroup of subjects shows the patterning, some less so, and some not at all (i.e., exhibit unrelentingly poor sleep). If this turns out to be the case, the critical questions at that juncture will be: 1) “do the insomnia frequency types exhibit distinct etiologies or pathophysiologies and 2) “do the insomnia frequency types map onto any of the given ICSD-2 classifications (insomnia types), the insomnia subtypes (initial, middle, late insomnia), clinical history variables (age of onset, illness chronicity, illness severity), and/or onto treatment outcome.
Limitations
The present study suffers from several limitations
First, the investigation does not account for total sleep time across the 24 hour day. Thus, a major potential confound has gone unassessed: “napping”. Second, and as with many studies, the observed effects are not assessed for the relevance of insomnia type (e.g., Psychophysiological, Paradoxical and Idiopathic Insomnia) and/or insomnia subtype (e.g., initial, middle, late and mixed insomnia); nor is clinical history accounted for (i.e., age of onset, illness duration, index episode severity. etc.). Third, the use of only subjective measures obviously begs the question: would such variability be observed with extended objective monitoring. Fourth, as with most self-report studies, the lack of a PSG assessment and a formal diagnostic interview leaves open the possibility that the present sample was partially populated by subjects with other intrinsic sleep disorders and that this may account for some of the observed findings.
Future Directions
Sample Aggregation
Future studies would benefit from the use of large clinically heterogeneous samples where each subjects’ clinical profile for insomnia is well characterized regarding insomnia type, subtype and clinical history. Each of these factors should be tested for whether they moderate / mediate the observed periodicity.
Assessment Methods (Actigraphy)
Studies with actigraphy would allow for extended objective monitoring and the assessment of whether the insomnia severity variability observed night-to-night exists within both the subjective and objective domains. Further, the use of actigraphy would allow investigators to account for napping, duration of diurnal wakefulness, and level of activity during wakefulness. These measures will allow, in turn, for a more comprehensive assessment of sleep homeostasis effects in patients with insomnia.
Assessment Methods (Polysomnography)
While most would agree that extended day to day PSG assessment is not feasible, a fruitful approach might be to assess the implications of the present observational findings. For example, if one implication of the present work is that patients with insomnia require an exceptionally high “homeostatic prime” to produce good (or better than average sleep), then a polysomnographic study could be conducted to assess this possibility. That is, a polysomnographic assessment prior-to-and following sleep restriction (1–2 hours), partial sleep deprivation (2–4 hours) or true sleep deprivation (4–8 hours) would be expected to produce differences (PI vs. GS) not only sleep continuity and architecture, but also in QEEG measures of delta activity. To date, this possibility has been evaluated only a few times using traditional PSG measures. The findings were mixed. For a review of the literature with respect to insomnia and sleep homeostasis, the reader is referred to a paper by Pigeon and Perlis (2006).
Statistical Methods
As noted above, it would be useful to see if adoption of the two methodological approaches espoused by Vallières et al. and Perlis et al. cross replicate in the two complementary data sets or in an indepedent data set. Further, it has been suggested that a modeling approach would be valuable regarding the issue of randomness. That is, given that patients with insomnia exhibit poor sleep on about 40% of nights, average sleep on 24% of nights, and good sleep on about 37% of nights, if such data were randomized over 110 nights, would the sequencing frequencies and bout durations differ from those observed? Such an exercise would allow for 1) a definitive statement regarding the non-randomness of good sleep in the context of chronic insomnia, 2) further evidence that the incidence of insomnia over time is predictable, and 3) additional data implicating behavioral and homeostatic processes regarding the incidence of insomnia over time.
Correlates with Outcomes
If it is indeed the case that each passing night of poor sleep should increase the probability of occurrence of either a good night's sleep or (at least) a better than average night's sleep, and this is related to the accrual of sleep debt across nights, a similar pattern should be observed for day time performance. For example, “recovery sleep (an instance of good sleep) should result in recovery of daytime functioning”. Such an assessment would be informative even if full recovery was not observed. For a discussion regarding neuropsychological deficits and insomnia, See Drummond et al. 2013 and Orff et al. 2007.
Concluding Remarks / Clinical Implications
The present findings allow the following clinical recommendations: 1) by way of acute insomnia (Ellis et al., 2012): “the best thing to do when experiencing poor sleep is nothing, and within a 1-3 days the ship should right itself... If one needs to expand sleep opportunity to counter fatigue/sleepiness, one must balance the books (reduce TIB by an equal or greater amount of time than was spent as extra time in bed in the AM, napping, or as extra time in bed in the PM])”; 2) by way of transient and sub-chronic insomnia (Ellis et al., 2012), it may be best to reduce time in bed to an amount equal to or less than one's present sleep ability (average total sleep time); and 3) by way of chronic insomnia: while it is probably past time to seek treatment, the patient should “take heart as it is unlikely that the insomnia will persist unabated for weeks or months and that a good night's sleep is likely to be less than 5 nights away”. Specifically with respect to Cognitive Therapy, the data from the present study can be presented to patients while they are evaluating beliefs about sleep, such as “I'll never get another good night of sleep”. That is, the data from the present study can be used (for many if not most patients) to provide counter evidence to the common belief that that they will “never” have another night of good sleep (Perlis and Gehrman, 2011).
Acknowledgement
1) While Drs. David and Morgan have been active investigators on this project, both for the present paper and on the dissertation that preceded this work (C. Swinkels), we wish to express our deep gratitude for their openness to collaboration and data sharing. It's been informative and fun. 2) We are grateful for the reviewers’ feedback and ideas. It was a stimulating exchange and the work here (and related work in the future) is better for their input
Grant Support
MP : R01MH077900;R01AT003332
JZ: NIMH T32MH065218
REFERENCES
- Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1(3):195–204. [PubMed] [Google Scholar]
- Buysse DJ, Cheng Y, Germain A, et al. Night-to-night sleep variability in older adults with and without chronic insomnia. Sleep Med. 2010 Jan;11(1):56–64. doi: 10.1016/j.sleep.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David BM, Morgan K. Adherence to the completion of a daily sleep diary over 9 months (252). Sleep. 2009;32(A377):1154. Abstract Supplement, 2009. [Google Scholar]
- Drummond SP, Walker M, Almklov E, Campos M, Anderson DE, Straus LD. Neural correlates of working memory performance in primary insomnia. Sleep. 2013 Sep 1;36(9):1307–16. doi: 10.5665/sleep.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis JG, Gehrman P, Espie CA, et al. Acute insomnia: current conceptualizations and future directions. Sleep Med Rev. 2012 Feb;16(1):5–14. doi: 10.1016/j.smrv.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Espie CA. Insomnia: conceptual issues in the development, persistence, and treatment of sleep disorder in adults. Annu Rev Psychol. 2002;53:215–43. doi: 10.1146/annurev.psych.53.100901.135243. Review. [DOI] [PubMed] [Google Scholar]
- Klerman EB, Wang W, Duffy JF, Dijk DJ, Czeisler CA, Kronauer RE. Survival analysis indicates that age-related decline in sleep continuity occurs exclusively during NREM sleep. Neurobiol Aging. 2013 Jan;34(1):309–18. doi: 10.1016/j.neurobiolaging.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichstein KL, Durrence HH, Taylor DJ, et al. Quantitative criteria for insomnia. Behav Res Ther. 2003 Apr;41(4):427–45. doi: 10.1016/s0005-7967(02)00023-2. [DOI] [PubMed] [Google Scholar]
- Orff HJ, Drummond SP, Nowakowski S, Perils ML. Discrepancy between subjective symptomatology and objective neuropsychological performance in insomnia. Sleep. 2007 Sep;30(9):1205–11. doi: 10.1093/sleep/30.9.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis ML, McCall WV, Jungquist CR. Placebo effects in primary insomnia. Sleep Med Rev. 2005 Oct;9(5):381–9. doi: 10.1016/j.smrv.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Perlis ML, Swinkels CM, Gehrman PR, et al. The incidence and temporal patterning of insomnia: a pilot study. J Sleep Res. 2010 Mar;19(1 Pt 1):31–5. doi: 10.1111/j.1365-2869.2009.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis ML, Gehrman PR. Cognitive Restructuing: Cognitive Therapy for Catastrophic Beliefs. In: Perlis Michael, Aloia Mark, Kuhn Brett., editors. Behavioral Treatments for Sleep Disorders. Chapter 12. Academic Press; 2011. pp. 119–126. [Google Scholar]
- Pigeon WR, Perlis ML. Sleep homeostasis in primary insomnia. Sleep Med Rev. 2006 Aug;10(4):247–54. doi: 10.1016/j.smrv.2005.09.002. Epub 2006 Mar 24. Review. [DOI] [PubMed] [Google Scholar]
- Sánchez-Ortuño MM, Carney CE, Edinger JD, et al. Moving beyond average values: assessing the night-to-night instability of sleep and arousal in DSM-IV-TR insomnia subtypes. Sleep. 2011 Apr 1;34(4):531–9. doi: 10.1093/sleep/34.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielman AJ, Caruso LS, Glovinsky PB. A behavioral perspective on insomnia treatment. Psychiatr Clin North Am. 1987a Dec;10(4):541–53. [PubMed] [Google Scholar]
- Spielman AJ, Saskin P, Thorpy MJ. Treatment of chronic insomnia by restriction of time in bed. Sleep. 1987b Feb;10(1):45–56. [PubMed] [Google Scholar]
- Riemann D, Spiegelhalder K, Feige B, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010 Feb;14(1):19–31. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Vallières A, Ivers H, Bastien CH, et al. Variability and predictability in sleep patterns of chronic insomniacs. J Sleep Res. 2005 Dec;14(4):447–53. doi: 10.1111/j.1365-2869.2005.00480.x. [DOI] [PubMed] [Google Scholar]
- Vallières A, Ivers H, eaulieu-Bonneau S, Morin CM. Predictability of sleep in patients with insomnia. Sleep. 2011 May 1;34(5):609–17. doi: 10.1093/sleep/34.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]