Abstract
Objective
To examine the dose-dependent and time-specific relationships of prenatal smoking with neonatal body mass, fat mass (FM), fat-free mass (FFM) and fat to fat-free mass ratio (F:FFM), as measured by air displacement plethysmography (PEA POD).
Study design
We analyzed 916 mother-neonate pairs participating in the longitudinal pre-birth cohort study, Healthy Start. Maternal prenatal smoking information was collected in early-, mid- and late-pregnancy by self-report. Neonatal body composition was measured by PEA POD following delivery. Multiple general linear regression models were adjusted for maternal and neonatal characteristics.
Results
For each additional pack smoked during pregnancy, neonatal body mass was significantly reduced (adjusted mean difference: −2.8 grams; 95% confidence interval [CI]: −3.9 to −1.8; P<0.001), FM (−0.7 g [−1.1 to −0.3]; P<0.001) and FFM (−2.1 g [−2.9 to −1.3]; P<0.001). Neonates exposed to prenatal smoking throughout pregnancy had significantly less body mass (P<0.001), FM (P<0.001) and FFM (P <0.001) compared with those unexposed. However, neonates of mothers who only smoked before late-pregnancy had no significant differences in body mass (P = 0.47), FM (P = 0.43) or FFM (P = 0.59) compared with unexposed offspring.
Conclusions
Exposure to prenatal smoking leads to systematic growth restriction. Smoking cessation before late-pregnancy may reduce the body composition consequences of exposure to prenatal smoking. Follow-up of this cohort is needed to determine the influence of catch-up growth on early-life body composition and the risk of childhood obesity.
Keywords: Adiposity, ADP, developmental origins, fat mass, fat-free mass, fetal programming, obesity, PEA POD, pregnancy, tobacco
From 2000 to 2010, the prevalence of prenatal smoking has only decreased from 13.3% to 12.3%.1 Exposure to prenatal smoking is associated with numerous adverse outcomes, such as growth restriction2–4, medically indicated and spontaneous pre-term birth5, 6 and abortion.7 There also are associations between intrauterine tobacco exposure and later-life morbidities, including asthma8 and childhood overweight and obesity.9–12 Fetal growth restriction leads to compensatory acceleration in the rate of growth early in life, also known as catch-up growth.13 Offspring that demonstrate postnatal catch-up growth have an increased risk for later chronic diseases.14, 15 It has been theorized that catch-up growth, as a result of exposure to prenatal smoking, may be responsible for these long-term adverse effects.
The current knowledge base is limited regarding the relationships between the quantity and timing of prenatal smoking and measures of neonatal body composition: fat mass (FM) and fat-free mass (FFM) and their ratio, fat to fat-free mass (F:FFM). In general, previous studies of prenatal smoking16–21 analyzed growth restriction using birth weight and indirect measures (e.g. skinfolds) for neonatal body composition. Only one study2 directly measured neonatal body composition, albeit using a less accurate measure relative to more novel body composition systems.22 Using birth weight or indirect measures of body composition may be a biased representation of body composition. Understanding how neonatal body composition is affected by prenatal smoking may lead to a better understanding of the fetal programming effects. Our aims were to assess the dose-dependent and time-specific associations of intrauterine tobacco exposure and neonatal body composition. We hypothesized that exposure to prenatal smoking would be associated with an overall reduction in neonatal body mass, primarily accounted for by a reduction in FFM, and a comparable proportionate reduction in FM resulting in similar F:FFM. Further, we hypothesized that these relationships would be dose-dependent and time-specific with growth restriction primarily being attributable to late-pregnancy smoking.
Methods
Healthy Start is an ongoing prospective pre-birth cohort study in Colorado that enrolls ethnically-diverse pregnant women and follows them until delivery. The study was approved by the Colorado Multiple Institutional Review Board. Participants are primarily recruited at the prenatal obstetrics clinics located at the University of Colorado Hospital (UCH) Outpatient Pavilion within the Anschutz Medical Campus of the University of Colorado - Denver. Women were not eligible if multiple births were expected or if they had a previous stillbirth, were less than 16-years of age at consent or had a gestational age at the time of baseline research visit greater than 24-weeks. Participants were excluded from analyses if they withdrew consent before delivery or if their index pregnancy resulted in fetal death or a very preterm birth (i.e. less than 32-weeks). A total of 1,092 mother-neonate pairs who were enrolled in the study and delivered between March 19, 2010 and November 1, 2013 were eligible to participate in this study. The women were invited to participate in three research visits. The first visit occurred during early-pregnancy (median = 17-weeks), followed by a second visit during mid-pregnancy (median = 27-weeks) and a third visit following delivery (median = 1-day).
Exposure - Prenatal Smoking
Information about prenatal smoking was ascertained through interview-administered questionnaires at each of the three prenatal research visits. Data were collected on the quantity and duration of early-, mid- and late-pregnancy smoking. The duration of exposure per week to secondhand smoke during these periods was also assessed. Subjects reported the average number of cigarettes per day that they generally smoked. An interviewer recorded the value within a pre-specified range (eg, < 1 cigarette/day; 1–4 cigarettes/day; 5–14 cigarettes/day; 15–24 cigarettes/day or 25 cigarettes/day or more). For analytic purposes, the center of each range was taken and used to estimate total packs smoked during pregnancy. Duration of prenatal smoking was calculated based on the sum of the differences between dates of a) conception and the first prenatal research visit (i.e. early-pregnancy); b) the first and second prenatal research visits (i.e. mid-pregnancy); and c) the second prenatal research visit until date of delivery (i.e. late-pregnancy). The quantity of prenatal smoking reported at a specific research visit was used in conjunction with the duration between visits. Among smokers who missed their mid-pregnancy visit (n = 21), we took the mean number of cigarettes smoked per day during their two completed visits to estimate the quantity of prenatal smoking, and the duration by using the observed median time (65-days) from our cohort between early- and mid-pregnancy visits.
We separately tested the associations of total packs smoked during pregnancy and time-specific relationships on neonatal body mass, FM, FFM and F:FFM. Time-specific analyses compared: (1) neonates of mothers who smoked throughout pregnancy relative to non-smokers; (2) neonates of mothers who smoked before late-pregnancy to non-smokers; and (3) an exploratory analysis of neonates of mothers who smoked throughout pregnancy to before late-pregnancy.
Outcomes – Neonatal Body Mass and Composition
PEA POD (COSMED, Rome, Italy) is a 2-compartment model that measures neonatal body mass, FM (i.e. adipose tissue) and FFM (ie, water, bone, and non-bone mineral and protein) in both absolute and proportionate terms. We took the ratio of absolute measures for FM and FFM to calculate F:FFM. To measure these variables, the PEA POD system uses a densitometric technique based on air displacement plethysmography (ADP).22 This technique is reliable and valid for measuring neonatal body composition.22–25 Trained clinical personnel measured each neonate by PEA POD twice, and if %FM differed by >2%, then a third exam was conducted. Neonatal body composition was generally measured within 24 hours following delivery (median = 1-day). To reduce measurement error for each outcome, we took the mean of the two closest measures.
Covariates
Covariate information was collected on mother-neonate pairs during research visits and through medical record abstraction. Maternal age at delivery was calculated based on offspring delivery date and maternal date of birth. Data on education, gravidity, household income and race/ethnicity were collected through research questionnaires. Maternal pre-pregnancy weight from research visits and medical records and maternal height measured at the baseline research visit were used to calculate pre-pregnancy body mass index (BMI). Weight during pregnancy was measured at research visits and also abstracted from medical records (median = 12 measurements per participant). Total gestational weight gain was estimated using mixed models predicting gestational weight gain at 39-weeks of gestation (mean gestational age of the cohort). Maternal physical activity levels were ascertained through a validated26, 27 pregnancy physical activity questionnaire (PPAQ) during each research visit. Gestational age at delivery was estimated using ultrasound data, self-reported last menstrual period or both. Neonatal birth weight and length were obtained during the delivery visit and from medical records. Using United States national reference data,28 small-for-gestational age (SGA) was indicated as a birth weight below the 10th percentile for gestational age, given sex of the offspring.
Data Analyses
All statistical analyses were conducted in SAS 9.3 (SAS Institute, Cary, NC). Relationships between prenatal smoking and continuous and categorical maternal and neonatal characteristics were analyzed by t-tests and χ2 tests, respectively. Simple linear models were first tested. Multiple linear regression models (PROC GLM) were then constructed. Standard confounders of the relationship between prenatal smoking and offspring growth restriction including maternal age, education, household income and race/ethnicity, and gestational age at delivery and chronological age at PEA POD were initially entered into models. Next, independent variables were individually entered into the models. A variable remained in the model if a Partial F-test showed that the covariate meaningfully contributed to predicting the outcome of interest (p-value <0.10) or if the adjusted estimate of prenatal smoking was meaningfully altered (i.e. ≥10% change). Pre-pregnancy BMI and race/ethnicity were tested as potential effect modifiers of the relationship between prenatal smoking and neonatal body composition.
The dose-dependent associations of prenatal smoking were shown by plotting predicted neonatal FM and FFM as a function of packs smoked during pregnancy with 95% confidence limits. For the plots, categorical predictors were set at the reference group, and continuous covariates were set to their mean values.
Results
Of the 1,092 mothers who were eligible, 916 mother-neonate pairs had complete data on exposures and outcomes of interest and were analyzed. Prenatal smoking status (smokers vs. non-smokers) was not significantly different between those with complete compared with missing data (χ2 = 0.10; P = 0.75). Further, there were no clinically relevant differences in main variables of interest, including maternal age (27.7 vs. 27.7 years), gravidity (1.4 vs. 1.4), pre-pregnant BMI (25.8 vs. 25.8 kg/m2), gestational weight gain (14.4 vs. 14.4 kg), gestational age (275 vs. 276 days), birth length (49.2 vs. 49.2 cm), birth weight (3,227 vs. 3,255 g), offspring sex, racial/ethnic distribution, physical activity, educational attainment and household income between the eligible cohort and those used in final analyses.
In our cohort, 9.2% (n = 100) of mothers reported prenatal smoking. Of the 100 prenatal smokers, 46 smoked throughout pregnancy and 30 smoked only during early- and/or mid-pregnancy. The other 24 prenatal smokers reported smoking during early- and late-pregnancy (n = 3); mid- and late-pregnancy (n = 12); and only late-pregnancy (n = 9). Among prenatal smokers, the median estimated number of packs smoked during pregnancy was 29 (range: 3 to 257).
On average, mothers who smoked during pregnancy relative to non-smokers were significantly younger and had more previous pregnancies (Table I). Compared with non-smokers, prenatal smoking mothers were significantly more likely to have educational attainment of high school or less (P<0.001) and lower household income (P<0.001), and were less likely to be Hispanic (P<0.001). No significant differences were found between the two groups for pre-pregnancy BMI, gestational weight gain or chronological age at PEA POD exam (Table I).
Table 1.
Characteristics of Healthy Start mother-neonate pairs used in complete case analyses by prenatal smoking status (N = 916)
| Characteristics | Prenatal Smoking† | ||
|---|---|---|---|
| Yes | No | ||
|
| |||
| n = 85 (9.3%) Mean (95% CI) |
n = 831 (90.7%) Mean (95% CI) |
P | |
| Maternal age (years) | 24.8 (23.7, 25.9) | 28.0 (27.6, 28.4) | <0.001 |
| Gravidity | 2.1 (1.6, 2.5) | 1.3 (1.2, 1.4) | <0.001 |
| Pre-pregnancy BMI (kg/m2) | 26.6 (25.3, 28.0) | 25.7 (25.2, 26.1) | 0.17 |
| Gestational weight gain (kg)a | 14.5 (14.0, 15.0) | 14.4 (14.3, 14.5) | 0.76 |
| Gestational age (days) | 274 (272, 276) | 276 (276, 277) | 0.04 |
| Age at PEA POD exam (days) | 1.8 (1.3, 2.3) | 1.5 (1.3, 1.6) | 0.16 |
| Birth length (cm) | 48.3 (47.8, 48.7) | 49.3 (49.2, 49.4) | <0.001 |
| Birth weight (g) | 3,037 (2,947, 3,128) | 3,277 (3,246, 3,308) | <0.001 |
| Birth weight z-score | −0.8 (−1.0, −0.7) | −0.4 (−0.4, −0.3) | <0.001 |
| Total body mass (g) | 2,924 (2,835, 3,014) | 3,141 (3,111, 3,171) | <0.001 |
| Neonatal fat mass (g) | 261 (233, 290) | 295 (285, 306) | 0.05 |
| Neonatal fat-free mass (g) | 2,663 (2,590, 2,735) | 2,845 (2,822, 2,869) | <0.001 |
| Neonatal fat mass (%) | 8.7 (7.9, 9.5) | 9.1 (8.8, 9.4) | 0.34 |
| Neonatal fat-free mass (%) | 91.3 (90.5, 92.1) | 90.9 (90.6, 91.2) | 0.34 |
| Fat to fat-free mass (F:FFM) (g) | 0.10 (0.09, 0.11) | 0.10 (0.10, 0.11) | 0.32 |
| Characteristics | n (%) | n (%) | P |
|---|---|---|---|
| Small-for-gestational ageb | 0.08 | ||
| Yes | 17 (20.0) | 109 (13.1) | |
| No | 68 (80.0) | 722 (86.9) | |
| Sex | 0.66 | ||
| Male | 46 (54.1) | 429 (51.6) | |
| Female | 39 (45.9) | 402 (48.4) | |
| Race/ethnicity | <0.001 | ||
| Non-Hispanic black | 29 (34.1) | 126 (15.2) | |
| Hispanic | 13 (15.3) | 202 (24.3) | |
| Non-Hispanic white | 38 (44.7) | 451 (54.3) | |
| Other | 5 (5.9) | 52 (6.3) | |
| Vigorous activity (> 6.0 METs)c | 0.13 | ||
| Yes | 3 (3.5) | 68 (8.2) | |
| No | 82 (96.5) | 763 (91.8) | |
| Education | <0.001 | ||
| High school degree/GED or less | 49 (57.6) | 252 (30.3) | |
| More than high school | 36 (42.4) | 579 (69.7) | |
| Household income | <0.001 | ||
| ≤$20,000 | 33 (38.8) | 109 (13.1) | |
| $20,001 to $40,000 | 11 (12.9) | 121 (14.6) | |
| >$40,001 to $70,000 | 18 (21.2) | 444 (53.4) | |
| Don’t Know | 23 (27.1) | 157 (18.9) |
Self-reported smoking at any study specific research visit
Predicted gestational weight gain at 39-weeks of gestation
Birth weight less than 10th percentile given gestational age and sex
Self-reported vigorous physical activity (>6.0 METs) at two or more study specific research visits
Dose-dependent associations
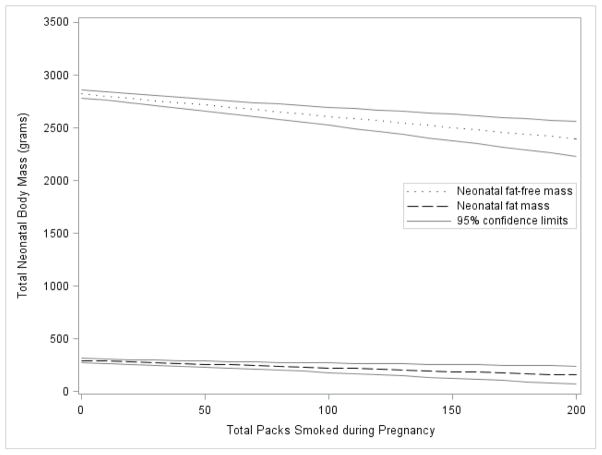
Following adjustment for gestational age and chronological age at PEA POD, offspring sex, gravidity, maternal age, race/ethnicity, educational status, household income, gestational weight gain, pre-pregnancy BMI and physical activity, for each additional pack smoked during pregnancy, there was a 2.8 grams (95% confidence interval [CI]: −3.9 to −1.8; P<0.001) decrease in neonatal body mass. This decrease was accounted for by a 0.7 g ([−1.1 to −0.3]; P = 0.001) decrease in FM and a 2.1 g ([−3.0 to −1.3]; P<0.001) reduction in FFM (Figure). There was a statistically significant relationship, per pack smoked, with the F:FFM ratio (adjusted mean difference: −0.0002 [−0.0003 to −0.00002]; P = 0.02), suggesting a proportionally greater dose-dependent reduction in FM relative to FFM. Pre-pregnancy BMI and race/ethnicity were not statistically significant effect modifiers of these associations.
Figure.
Adjusted mean neonatal fat-free mass and fat mass by total packs smoked during pregnancy with 95% confidence limits
Time-specific associations
Smoking throughout pregnancy compared with non-smokers
Following statistical adjustment for the previously mentioned maternal and offspring characteristics, neonates of mothers who smoked throughout pregnancy had significantly less body mass (P<0.001), FM (P<0.001) and FFM (P<0.001) compared with neonates of mothers who were non-smokers. Similar direction and significance of the association as the dose-dependent model was observed in F:FFM (P = 0.008; Table II).
Table 2.
Summary effects of time specific exposure to prenatal smoking on neonatal body mass and composition
| Models and Outcomes | Effect Size [β] (95% CI)† | p-value |
|---|---|---|
| Model 1: Smoked throughout pregnancy vs. non-smokers | ||
| Body mass (g) | −296 (−411, −180) | <0.001 |
| Fat mass (g) | −81.2 (−127, −35.3) | <0.001 |
| Fat-free mass (g) | −215 (−303, −126) | <0.001 |
| F:FFM (g) | −0.02 (−0.04, −0.005) | 0.008 |
| Model 2: Smoked before late-pregnancy vs. non-smokers | ||
| Body mass (g) | 59.9 (−102, 222) | 0.47 |
| Fat mass (g) | 25.9 (−38.8, 90.6) | 0.43 |
| Fat-free mass (g) | 34.0 (−89.6, 158) | 0.59 |
| F:FFM (g) | 0.008 (−0.01, 0.03) | 0.43 |
| Model 3: Smoked throughout vs. before late-pregnancy | ||
| Body mass (g) | −371 (−559, −183) | <0.001 |
| Fat mass (g) | −103 (−170, −36.1) | 0.003 |
| Fat-free mass (g) | −268 (−423, −113) | 0.001 |
| F:FFM (g) | −0.03 (−0.05, −0.002) | 0.03 |
Adjusted by gestational and chronological age, offspring sex, gravidity, maternal age, race/ethnicity, educational status, household income, gestational weight gain, pre-pregnancy BMI and physical activity.
Smoking before late-pregnancy compared with non-smokers
After adjustment, neonates of mothers who smoked before late-pregnancy had no significant differences in neonatal body mass (P = 0.47), FM (P = 0.43), FFM (P = 0.59) or F:FFM (P = 0.43), compared with neonates of mothers who did not smoke during pregnancy (Table II).
Smoking throughout pregnancy compared with before late-pregnancy
In exploratory analyses, we compared neonates of mothers who smoked throughout pregnancy with neonates of mothers who smoked during early- and/or mid-pregnancy. Following adjustment, neonates who were exposed to smoking throughout pregnancy had significantly less body mass, FM and FFM. F:FFM was also significantly reduced (Table II).
Exclusion of mothers exposed to secondhand smoke during late-pregnancy
In both dose-dependent and time-specific models where sensitivity analyses removed non-smoking mothers who were exposed to secondhand smoke more than 1 hour per week during late-pregnancy (n = 61), greater reductions in neonatal body mass, FM, FFM and F:FFM among neonates exposed to prenatal smoking were observed.
Discussion
We found that associations between prenatal smoking and neonatal body mass and composition were dose-dependent and time-specific. The dose-dependent models showed significant reductions in neonatal FM and FFM. Time-specific analyses showed that mothers who smoked throughout pregnancy compared to those who stopped before late-pregnancy had neonates with significantly reduced FM and FFM. Our data provide novel evidence that prenatal smoking results in overall neonatal growth restriction. However, if smoking cessation occurs before late-pregnancy, neonates will be phenotypically similar to those who were not exposed during pregnancy, possibly reducing the likelihood of postnatal catch-up growth.
Using indirect measures of body composition, studies have suggested that prenatal smoking significantly reduces neonatal body mass through reductions in FFM.16–21 Harrison et al16 analyzed 285 term Caucasian neonates and found that prenatal smoking significantly reduces birth weight. Using skinfold measurements, they found no relationship between prenatal smoking and subcutaneous fat between exposed and unexposed neonates. In a subsequent study by Samper et al21, 1216 Caucasian mothers and their term singleton births were analyzed. Using sum of skinfolds as an indirect measure of adiposity, neonates of mothers who smoked had lower values than unexposed neonates, but subcutaneous fat distribution was again not significantly different between groups. Analyzing 129 term neonates, Lindsay et al2 directly measured neonatal body composition using total body electrical conductivity and found significant differences in FFM among exposed relative to unexposed neonates (2,799 g ± 292 g vs. 2,965 g ± 359 g; P = 0.02), but did not find significant differences in FM (343 g ± 164 g vs. 387 g ± 216 g; P = 0.32). This study may have been limited by comparing mothers who smoked at any point during pregnancy to those who were non-smokers, and only having 30 prenatal smokers. Our findings in a larger cohort, using more precise measures of neonatal body composition, suggest a greater proportionate reduction in FM relative to FFM. This finding was consistent between dose-dependent and time-specific analyses. Moreover, sensitivity analyses removing mothers who were non-smokers, but exposed to secondhand smoke during late-pregnancy, indicate that our findings from our main analyses, across outcomes, may be conservative. In unadjusted analyses, there were no statistically significant differences between neonates exposed and unexposed to prenatal smoking in %FM or %FFM in unadjusted analyses (Table I). However, following adjustment, F:FFM was statistically significantly different in dose-dependent and time-specific models.
Our time-specific findings have strong biological support, because both FM and FFM primarily develop during late-pregnancy, and are consistent with previous studies focused on birth weight. MacArthur et al studied 4,341 pregnant women, 1,235 of which were prenatal smokers, and found that smoking cessation before 16-weeks of gestation resulted in neonates with comparable anthropometric measures to those who were never exposed to prenatal smoking.29 In a large study of 11,177 women with term singleton births, Lieberman et al demonstrated that the risk of SGA was similar between mothers who began smoking during late second or third trimester relative to those who smoked throughout pregnancy.30 The researchers also found that the risk of SGA was attenuated among mothers who quit smoking before the third trimester.30 In a prospective study with 160 prenatal smokers, Bernstein et al showed that third trimester cigarette consumption was the strongest predictor of birth weight percentile.20
Our study has some limitations. Prenatal smoking was assessed by self-report. However, several studies that compared self-reported prenatal smoking with exhaled carbon monoxide31 and plasma32, 33 and urine34 cotinine levels found that self-reported smoking is a valid marker of tobacco exposure. Nevertheless, if mothers who smoked during pregnancy reported as non-smokers, this bias would likely underestimate the true association. Given total body mass changes after birth, by design, we tried to measure all newborns with 24-hours of delivery, to avoid confounding by weight loss in the first days of life. The difference in postnatal age at PEA POD exam between neonates exposed to prenatal smoking compared with those unexposed was not significantly different (Table I). As a result, any bias due to age when measured by PEA POD would likely result in attenuated associations.
In summary, our findings suggest that both the dose and timing of exposure to prenatal smoking is associated with systematic growth restriction, which has significant public health implications. Given the fairly static prevalence of prenatal smoking1, the public health benefits of smoking cessation before or during early stages of pregnancy may be vast, including decreasing the offspring’s future risk for several chronic diseases. Based on our findings, smoking cessation efforts should not be lessened as pregnancy progresses. Follow-up data on anthropometric and body composition changes during postnatal life will lead to a better understanding of the relationship between exposure to prenatal smoking and catch-up growth and development of childhood obesity.
Acknowledgments
Supported by the National Institutes of Health (DK076648 to D.D.).
We gratefully acknowledge the Healthy Start Study Project Coordinator, Mercedes Martinez, and the study investigators and personnel. We would also like to thank the Healthy Start Study participants for their time and participation.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- FM
fat mass
- FFM
fat-free mass
- F:FFM
fat to fat free mass ratio
- PPAQ
pregnancy physical activity questionnaire
- SGA
small-for-gestational age
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tong VTDP, Morrow B, D’Angelo DV, Farr SL, Rockhill KM, England LJ. Trends in Smoking Before, During, and After Pregnancy — Pregnancy Risk Assessment Monitoring System, United States, 40 Sites, 2000–2010. MMWR - Surveillance Summaries. 2013;62:1–19. [PubMed] [Google Scholar]
- 2.Lindsay CA, Thomas AJ, Catalano PM. The effect of smoking tobacco on neonatal body composition. American Journal of Obstetrics and Gynecology. 1997;177:1124–8. doi: 10.1016/s0002-9378(97)70027-9. [DOI] [PubMed] [Google Scholar]
- 3.Andersen MR, Uldbjerg N, Stender S, Sandager P, Aalkjcr C. Maternal Smoking and Smoking Cessation in Early Pregnancy and Endothelium-Dependent Bradykinin-Mediated Relaxation of Uterine Small Arteries. Circulation. 2009;120:S1109–S. [Google Scholar]
- 4.La Merrill M, Stein CR, Landrigan P, Engel SM, Savitz DA. Prepregnancy Body Mass Index, Smoking During Pregnancy, and Infant Birth Weight. Annals of Epidemiology. 2011;21:413–20. doi: 10.1016/j.annepidem.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aliyu MH, Lynch O, Saidu R, Alio AP, Marty PJ, Salihu HM. Intrauterine Exposure to Tobacco and Risk of Medically Indicated and Spontaneous Preterm Birth. American Journal of Perinatology. 2010;27:405–10. doi: 10.1055/s-0029-1243316. [DOI] [PubMed] [Google Scholar]
- 6.Lee KA, Chang MH, Park MH, Park H, Ha EH, Park EA, et al. A Model for Prediction of Spontaneous Preterm Birth in Asymptomatic Women. J Womens Health. 2011;20:1825–31. doi: 10.1089/jwh.2011.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nielsen A, Hannibal CG, Lindekilde BE, Tolstrup J, Frederiksen K, Munk C, et al. Maternal smoking predicts the risk of spontaneous abortion. Acta Obstet Gynecol Scand. 2006;85:1057–65. doi: 10.1080/00016340600589560. [DOI] [PubMed] [Google Scholar]
- 8.Kanoh M, Kaneita Y, Hara M, Harada S, Gon Y, Kanamaru H, et al. Longitudinal study of parental smoking habits and development of asthma in early childhood. Prev Med. 2012;54:94–6. doi: 10.1016/j.ypmed.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 9.von Kries R, Toschke AM, Koletzko B, Slikker W. Maternal smoking during pregnancy and childhood obesity. American Journal of Epidemiology. 2002;156:954–61. doi: 10.1093/aje/kwf128. [DOI] [PubMed] [Google Scholar]
- 10.Al Mamun A, Lawlor DA, Alati R, O’Callaghan MJ, Williams GM, Najman JM. Does maternal smoking during pregnancy have a direct effect on future offspring obesity? Evidence from a prospective birth cohort study. American Journal of Epidemiology. 2006;164:317–25. doi: 10.1093/aje/kwj209. [DOI] [PubMed] [Google Scholar]
- 11.Oken E, Levitan EB, Gillman MW. Maternal smoking during pregnancy and child overweight: systematic review and meta-analysis. International Journal of Obesity. 2008;32:201–10. doi: 10.1038/sj.ijo.0803760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorog K, Pattenden S, Antova T, Niciu E, Rudnai P, Scholtens S, et al. Maternal Smoking During Pregnancy and Childhood Obesity: Results from the CESAR Study. Maternal and Child Health Journal. 2011;15:985–92. doi: 10.1007/s10995-009-0543-5. [DOI] [PubMed] [Google Scholar]
- 13.Ong KKL, Preece MA, Emmett PM, Ahmed ML, Dunger DB, Team AS. Size at birth and early childhood growth in relation to maternal smoking, parity and infant breast-feeding: Longitudinal birth cohort study and analysis. Pediatric Research. 2002;52:863–7. doi: 10.1203/00006450-200212000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Adair LS. Size at birth and growth trajectories to young adulthood. American Journal of Human Biology. 2007;19:327–37. doi: 10.1002/ajhb.20587. [DOI] [PubMed] [Google Scholar]
- 15.Rueda-Clausen CF, Morton JS, Davidge ST. The Early Origins of Cardiovascular Health and Disease: Who, When, and How. New York, NY, ETATS-UNIS: Thieme; 2011. [DOI] [PubMed] [Google Scholar]
- 16.Harrison GG, Branson RS, Vaucher YE. Association of maternal smoking with body-composition of the newborn. American Journal of Clinical Nutrition. 1983;38:757–62. doi: 10.1093/ajcn/38.5.757. [DOI] [PubMed] [Google Scholar]
- 17.Cliver SP, Goldenberg RL, Cutter GR, Hoffman HJ, Davis RO, Nelson KG. The effect of cigarette smoking on neonatal anthropometric measurements. Obstet Gynecol. 1995;85:625–30. doi: 10.1016/0029-7844(94)00437-I. [DOI] [PubMed] [Google Scholar]
- 18.Zaren B, Lindmark G, GebreMedhin M. Maternal smoking and body composition of the newborn. Acta Paediatrica. 1996;85:213–9. doi: 10.1111/j.1651-2227.1996.tb13995.x. [DOI] [PubMed] [Google Scholar]
- 19.Godfrey K, Walker-Bone K, Robinson S, Taylor P, Shore S, Wheeler T, et al. Neonatal bone mass: Influence of parental birthweight, maternal smoking, body composition, and activity during pregnancy. Journal of Bone and Mineral Research. 2001;16:1694–703. doi: 10.1359/jbmr.2001.16.9.1694. [DOI] [PubMed] [Google Scholar]
- 20.Bernstein IM, Mongeon JA, Badger GJ, Solomon L, Heil SH, Higgins ST. Maternal smoking and its association with birth weight. Obstet Gynecol. 2005;106:986–91. doi: 10.1097/01.AOG.0000182580.78402.d2. [DOI] [PubMed] [Google Scholar]
- 21.Samper MP, Jimenez-Muro A, Nerin I, Marqueta A, Ventura P, Rodriguez G. Maternal active smoking and newborn body composition. Early Human Development. 2012;88:141–5. doi: 10.1016/j.earlhumdev.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Ma GS, Yao MJ, Liu Y, Lin AW, Zou H, Urlando A, et al. Validation of a new pediatric air-displacement plethysmograph for assessing body composition in infants. American Journal of Clinical Nutrition. 2004;79:653–60. doi: 10.1093/ajcn/79.4.653. [DOI] [PubMed] [Google Scholar]
- 23.Urlando A, Dempster P, Aitkens S. A new air displacement plethysmograph for the measurement of body composition in infants. Pediatric Research. 2003;53:486–92. doi: 10.1203/01.PDR.0000049669.74793.E3. [DOI] [PubMed] [Google Scholar]
- 24.Yao M, Nommsen-Rivers L, Dewey K, Urlando A. Preliminary evaluation of a new pediatric air displacement plethysmograph for body composition assessment in infants. Acta Diabetologica. 2003;40:S55–S8. doi: 10.1007/s00592-003-0027-9. [DOI] [PubMed] [Google Scholar]
- 25.Ellis KJ, Yao M, Shypailo RJ, Urlando A, Wong WW, Heird WC. Body-composition assessment in infancy: air-displacement plethysmography compared with a reference 4-compartment model. The American Journal of Clinical Nutrition. 2007;85:90–5. doi: 10.1093/ajcn/85.1.90. [DOI] [PubMed] [Google Scholar]
- 26.Chasan-Taber L, Schmidt MD, Roberts DE, Hosmer D, Markenson G, Freedson PS. Development and validation of a pregnancy physical activity questionnaire. Medicine and Science in Sports and Exercise. 2004;36:1750–60. doi: 10.1249/01.mss.0000142303.49306.0d. [DOI] [PubMed] [Google Scholar]
- 27.Ota E, Haruna M, Yanai H, Suzuki M, Anh DD, Matsuzaki M, et al. Reliability and validity of the Vietnamese version of the Pregnancy Physical Activity Questionnaire (PPAQ) Southeast Asian J Trop Med Public Health. 2008;39:562–70. [PubMed] [Google Scholar]
- 28.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:8. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macarthur C, Knox EG. Smoking in pregnancy - effects of stopping at different stages. British Journal of Obstetrics and Gynaecology. 1988;95:551–5. doi: 10.1111/j.1471-0528.1988.tb09481.x. [DOI] [PubMed] [Google Scholar]
- 30.Lieberman E, Gremy I, Lang JM, Cohen AP. Low-birth-weight at term and the timing of fetal exposure to maternal smoking. American Journal of Public Health. 1994;84:1127–31. doi: 10.2105/ajph.84.7.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christensen AE, Tobiassen M, Jensen TK, Wielandt H, Bakketeig L, Host A. Repeated validation of parental self-reported smoking during pregnancy and infancy: a prospective cohort study of infants at high risk for allergy development. Paediatric and Perinatal Epidemiology. 2004;18:73–9. doi: 10.1111/j.1365-3016.2003.00520.x. [DOI] [PubMed] [Google Scholar]
- 32.Lindqvist R, Lendahls L, Tollbom O, Aberg H, Hakansson A. Smoking during pregnancy: comparison of self-reports and cotinine levels in 496 women. Acta Obstet Gynecol Scand. 2002;81:240–4. doi: 10.1034/j.1600-0412.2002.810309.x. [DOI] [PubMed] [Google Scholar]
- 33.Kvalvik LG, Nilsen RM, Skjaerven R, Vollset SE, Midttun O, Ueland PM, et al. Self-reported smoking status and plasma cotinine concentrations among pregnant women in the Norwegian Mother and Child Cohort Study. Pediatr Res. 2012;72:101–7. doi: 10.1038/pr.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swamy GK, Reddick KL, Brouwer RJ, Pollak KI, Myers ER. Smoking prevalence in early pregnancy: comparison of self-report and anonymous urine cotinine testing. J Matern Fetal Neonatal Med. 2011;24:86–90. doi: 10.3109/14767051003758887. [DOI] [PMC free article] [PubMed] [Google Scholar]