Abstract
Background
Serum albumin concentrations are a strong predictor of mortality and cardiovascular disease in HIV-infected individuals. We studied the longitudinal associations between serum albumin levels and kidney function decline in a population of HIV-infected women.
Study Design
Retrospective cohort analysis.
Setting & Participants
The study participants were recruited from the Women’s Interagency HIV Study (WIHS), a large observational study designed to understand risk factors for the progression of HIV infection in women living in urban communities. 908 participants had baseline assessment of kidney function and two follow-up measures over an average of 8 years.
Predictor
The primary predictor was serum albumin concentration.
Outcomes
We examined annual change in kidney function. Secondary outcomes included rapid kidney function decline and incident reduced estimated glomerular filtration rate (eGFR).
Measurements
Kidney function decline was determined by cystatin C–based (eGFRcys) and creatinine-based eGFR (eGFRcr) at baseline and follow up. Each model was adjusted for kidney disease and HIV-related risk factors using linear and relative risk regression.
Results
After multivariate adjustment, each 0.5-g/dL decrement in baseline serum albumin concentration was associated with a 0.56-mL/min faster annual decline in eGFRcys (P<0.001), which was only slightly attenuated to 0.55-mL/min/1.73 m2 after adjustment for albuminuria. Results were similar whether using eGFRcys or eGFRcr. In adjusted analyses, each 0.5-g/dL lower baseline serum albumin was associated with a 1.71-fold greater risk of rapid kidney function decline (p<0.001) and a 1.72-fold greater risk of incident reduced eGFR (p<0.001).
Limitations
The cohort is composed of only female participants from urban communities within the United States.
Conclusions
Lower levels of serum albumin were strongly associated with kidney function decline and incident reduced eGFR in HIV-infected women, independent of HIV disease status, BMI and albuminuria.
Keywords: albumin, kidney function, HIV, incident reduced eGFR, albuminuria, disease trajectory, chronic kidney disease (CKD) progression
Advances over the past 20 years in the treatment of HIV infection have led to increased life expectancy; yet adjusted mortality rates remain significantly elevated when compared to non-infected individuals 1. Chronic kidney disease (CKD) is one of the most important morbidities in this population, accounting for 17% of mortality risk 2,3,4.
Risk factors for the onset of CKD and progression to end-stage renal disease (ESRD) in the setting of HIV appear to be both infection-related (CD4 lymphocyte count, HIV viral load, hepatitis C virus [HCV] co-infection) and non–infection related (hypertension, diabetes, cardiovascular disease) 4. Serum albumin is a widely available, routine clinical test and serves as a marker of acute and chronic disease 5,6,7. Decreased serum albumin has been strongly associated with mortality and cardiovascular disease in HIV-infected populations 8,9,10. A previous study in an elderly population demonstrated that decreased albumin concentrations were more strongly associated with declining kidney function than several inflammatory markers, including C-reactive protein (CRP), interleukin 6 and D-dimer 11. However, to our knowledge, no study has examined the associations of serum albumin concentrations with kidney function decline in the setting of HIV infection.
We hypothesized that lower concentrations of serum albumin would be associated with faster decline in kidney function in HIV-infected individuals, independent of markers of HIV viral load, albuminuria, and inflammation. To investigate this hypothesis, we conducted a longitudinal study nested within a nationally representative cohort of ethnically diverse, HIV-infected women.
METHODS
Study Population
We included 908 HIV-infected women participating in the Women’s Interagency HIV Study (WIHS), a large observational study designed to understand risk factors for the progression of HIV infection in women. The WIHS design and methods have been described previously 12. Women were recruited from 6 US urban communities (Bronx, Brooklyn, Chicago, Los Angeles, San Francisco, and Washington, DC) to be representative of the HIV-infected population. Participants are interviewed and examined every six months. Serum specimens are stored at −80°C until biomarker measurement.
The WIHS HIV Kidney Aging study was designed to investigate the onset of kidney disease in the setting of HIV, utilizing stored urine and serum specimens. The baseline visit for this ancillary study was conducted from October 1999 through March 2000. Follow up lasted an average of eight (interquartile range, 7.5–8.1) years. One thousand HIV-infected and 250 uninfected women were included. Of these women 450 were sampled from the WIHS bone sub-study and 800 were selected at random including age-/race-matched uninfected controls. There were no exclusions based on race or ethnicity. For this study 908 HIV-infected women who had stored urine available and at least one follow up visit were included. The WIHS was approved by the institutional review boards at all study sites. The WIHS HIV Kidney Aging study was also approved by the University of California, San Francisco, San Francisco Veterans Affairs Medical Center, and Yale committees on human research.
Predictors
The primary predictor in this study was the serum albumin concentration. Albumin measurements were conducted at each clinical site using albumin assays from CLIA-certified labs. We evaluated serum albumin as a continuous variable and dichotomized at 3.8 g/dL. The cut-point of 3.8 g/dL was chosen because it represented the lowest quartile of serum albumin concentrations in the study population. Serum albumin concentrations were measured at six-month intervals as part of the WIHS Core examination. We evaluated baseline serum albumin concentrations as well as changes in albumin, calculated from the baseline visit of the study to the last follow up visit. Additional analyses included measures of the average albumin concentration over the entire study.
Secondary predictors included baseline cystatin C–based estimated glomerular filtration rate (eGFRcys), urine albumin-creatinine ratio (ACR), CD4 count, and HIV RNA. Baseline eGFRcys was calculated as a continuous and dichotomous (< or ≥ 60 mL/min/1.73 m2) predictor. Similarly, ACR was modeled as a continuous and dichotomous (< or ≥ 30 mg/g) predictor. The HIV RNA was log-transformed for analysis and was also dichotomized (< or ≥1000 copies/mL, < or ≥10,000 copies/mL). The CD4 cell count was also log-transformed and discretized (<200, 200–350, 350–500, >500 cells/mm3).
Outcomes
Estimated glomerular filtration rate was determined using the 2012 CKD-EPI (CKD Epidemiology Collaboration) cystatin C equation (eGFRcys). Cystatin C was measured at the baseline visit, and year four and eight of follow-up. Cystatin C was chosen because it is less biased by muscle mass and health status than creatinine and thus may be more reliable in the setting of HIV infection; it has also been shown to predict mortality better than creatinine in the setting of HIV 2,13. Additional analyses evaluated GFR using the CKD-EPI creatinine equation (eGFRcr) and a combined creatinine–cystatin C equation. We analyzed eGFR as a continuous outcome, expressed as annual change in eGFR in mL/min/1.73 m2 over the eight years of follow up. Secondary outcomes included the following: rapid kidney function decline, defined as a decrease in kidney function of greater than 5% per year, using baseline and final eGFRcys; and incident reduced eGFR, defined as a final eGFR less than 60 mL/min/1.73 m2.
Covariates
Candidate covariates included demographic characteristics, traditional risk factors for kidney disease, markers of inflammation, and HIV-related risk factors. The following characteristics were tested as candidate covariates in all multivariate models: age, race/ethnicity, antihypertensive drug use, baseline eGFR, diabetes (defined by any of the following confirmed criteria: fasting glucose ≥126 mg/dL, self-reported diabetes, self-reported diabetes medication use, or hemoglobin A1C ≥6.5%), cigarette smoking status (current, former, never), systolic and diastolic blood pressure, low-density lipoprotein and high-density lipoprotein cholesterol, triglycerides, body mass index (BMI), urine albumin-creatinine ratio (albuminuria), waist circumference, HCV infection (confirmed by detectable HCV RNA following a positive HCV antibody result), and current heroin use. The HIV-related risk factors included CD4 lymphocyte count, history of AIDS diagnosis, HIV viral load, and anti-retroviral therapy. We modeled anti-retroviral therapy use based on end of study treatment status (never, past, current, or new user of antiretroviral therapy). The anti-retroviral therapies included current combination antiretroviral therapy (cART) use, current nucleoside reverse transcriptase inhibitor use, current non-nucleoside reverse transcriptase inhibitor use, and current protease inhibitor use. Additionally, all models were adjusted for study site in order to account for the possibility of differences in laboratory assays at each location.
Statistical Analyses
We compared clinical characteristics of dichotomized, baseline serum albumin concentrations using the Kruskal-Wallis test for continuous variables and Fisher’s exact test for categorical variables. We evaluated unadjusted and adjusted associations of serum albumin with the continuous outcome of annual mean eGFRcys change by using linear mixed models with random intercepts and slopes to account for repeated measures for each participant. We used Huber-White standard errors which are designed to be robust to non-normally distributed residuals. We estimated the incidence rate ratio for binary outcomes (rapid kidney function decline and incident reduced eGFR) using relative risk regression. A Poisson working model was employed, using a robust variance estimator, which has fewer convergence problems than the log binomial model, and gives an unbiased estimate of the relative risk when the response variable is binary rather than Poisson 14. We performed additional analyses testing interactions of albumin with ACR and viral load. Lastly, figures were created to examine whether these relationships were additive or multiplicative. Interaction tests and figures were included to verify that the association of serum albumin with kidney function decline was not conditional on the presence or absence of albuminuria or the patient’s level of HIV viral load control.
We initially used stepwise backward selection with a significance level of α=0.05 to remove candidate covariates that were not associated with linear kidney function decline. Factors forced into the full model included age, race/ethnicity, systolic and diastolic blood pressure, BMI, antiretroviral therapy use, HCV infection, and study site. The final model for each outcome included age, race/ethnicity, systolic and diastolic blood pressure, BMI, baseline eGFR, log HIV RNA, CD4 count, antiretroviral therapy use, and study site.
All analyses were conducted using the SAS system, version 9.2 (SAS Institute Inc., Cary, North Carolina).
RESULTS
At baseline, median age was 41 years and 58% of participants were African American. The median follow up time was 7.9 (interquartile range, 7.5–8.1) years, and the median eGFRcys was 89 mL/min/1.73 m2. Individuals with lower serum albumin concentrations (<3.8 g/dL) were more likely to be African American, current smokers, hypertensive, and have lower eGFRcys as well as lower low-density lipoprotein and high-density lipoprotein cholesterol levels compared with participants with higher serum albumin (Table 1). Comparisons among WIHS participants who are included and excluded from this study are shown in Table S1 (provided as online supplementary material). Lower serum albumin concentrations were also associated with worse HIV disease status (as indicated by lower CD4 counts and higher HIV viral loads), less antiretroviral use, and a higher prevalence of HCV co-infection. Rates of incident reduced eGFR and rapid kidney function decline were higher for low serum albumin concentration (< 3.8 g/dL) than normal levels. Similarly the annual change for these two groups differed by approximately 0.6 mL/min. The overall annual change in eGFR for the entire cohort was −1.54 mL/min/1.73 m2 per year (95% confidence interval [CI], −1.74 to −1.34).
Table 1.
Baseline characteristics of HIV-infected WIHS participants, stratified by serum albumin
| <3.8 g/dL (n = 214) | ≥3.8 g/dL (n = 691) | P-value | |
|---|---|---|---|
| Parameter | |||
| Baseline Age | 42 (37–47) | 41 (36–45) | 0.02 |
| <30 y | 12 (6%) | 43 (6%) | |
| 30–40 y | 68 (32%) | 280 (41%) | |
| 40–50 y | 114 (53%) | 295 (43%) | |
| >50 y | 20 (9%) | 73 (11%) | |
| Race | <0.001 | ||
| African American | 143 (67%) | 379 (55%) | |
| Caucasian | 21 (10%) | 153 (22%) | |
| Other | 50 (23%) | 159 (23%) | |
| Menopause | 55 (26%) | 130 (19%) | 0.03 |
| Cigarette smoking | 0.001 | ||
| Current | 132 (62%) | 331 (48%) | |
| Past | 37 (17%) | 186 (27%) | |
| Never | 45 (21%) | 174 (25%) | |
| Diabetes mellitus | 20 (9%) | 66 (10%) | 0.9 |
| Systolic BP (mmHg) | 120 (108–130) | 118 (108–128) | 0.08 |
| Diastolic BP (mmHg) | 72 (66–80) | 71 (65–80) | 0.2 |
| Hypertension | 76 (36%) | 151 (22%) | <0.001 |
| Antihypertensive use | 32 (15%) | 66 (10%) | 0.03 |
| LDL cholesterol (mg/dL) | 95 (74–119) | 107 (82–135) | <0.001 |
| HDL cholesterol (mg/dL) | 41 (32–50) | 45 (36–57) | <0.001 |
| Trigylcerides (mg/dL) | 131 (95–182) | 134 (92–201) | 0.6 |
| BMI (kg/m2) | 27 (23–32) | 26 (23–31) | 0.5 |
| Waist Circumference (cm) | 88 (79–101) | 88 (80–99) | 0.8 |
| Current cART use | 112 (52%) | 419 (61%) | 0.03 |
| Current NRTI use | 123 (57%) | 480 (69%) | 0.002 |
| Current NNRTI use | 50 (23%) | 194 (28%) | 0.2 |
| Current PI use | 80 (37%) | 299 (43%) | 0.1 |
| Current CD4 | 337 (197–517) | 414 (254–587) | 0.001 |
| Nadir CD4 | 200 (100–330) | 214 (113–324) | 0.4 |
| History of AIDS (CD4 or OI) | 122 (57%) | 322 (47%) | 0.008 |
| Plasma HIV RNA category | 0.005 | ||
| ≤80 copies/mL | 48 (23%) | 226 (33%) | |
| 81–1999 copies/mL | 45 (21%) | 159 (23%) | |
| 2000–9999 copies/mL | 37 (17%) | 109 (16%) | |
| ≥10,000 copies/mL | 83 (39%) | 192 (28%) | |
| Hepatitis C Infection | 89 (42%) | 192 (28%) | <0.001 |
| Current heroin use | 13 (6%) | 30 (4%) | 0.4 |
| eGFRcys (mL/min/1.73 m2) | 81 (68–96) | 92 (77–106) | <0.001 |
| eGFRcr (mL/min/1.73 m2) | 104 (82–120) | 96 (81–112) | 0.01 |
| Urine ACR(mg/g) | 11.7 (6.0–32.3) | 9.7 (6.0–20.9) | 0.1 |
| Outcome | |||
| Incident Reduced eGFR* | 46/174 (26%) | 101/646 (16%) | 0.002 |
| Rapid kidney function decline^ | 50/214 (23%) | 73/691 (11%) | <0.001 |
| Annual Change in eGFR (mL/min/1.73 m2) | −2.01 (−2.50, −1.51) | −1.41 (−1.63, −1.20) | 0.005 |
Note: Values for categorical variables are given as number or n/N (percentage); values for continuous variables, as median [interquartile range].
Abbreviations: ACR, albumin-creatinine ratio; BMI, body mass index; BP, blood pressure; cART, combination antiretroviral therapy; eGFR, estimated glomerular filtration rate; eGFRcr, creatinine-based estimated glomerular filtration rate; eGFRcys, cystatin C–based estimated glomerular filtration rate; HD, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; Women’s Interagency HIV Study (WIHS),
Incident Reduced eGFR defined as eGFR< 60 mL/min/1.73 m2
Defined as 5% or more per year, using baseline and final eGFRcys.
As a linear variable, serum albumin concentrations were strongly associated with declining kidney function (Table 2). The finding was minimally attenuated by albuminuria. Baseline urine ACR was also associated with declining kidney function. Furthermore, there was no significant interaction of serum albumin concentration with HIV RNA levels or ACR (p=0.2 and p=0.08, respectively).
TABLE 2.
Associations of serum albumin concentration with annual change in eGFRcys in HIV-infected WIHS participants.
| Unadjusted Estimate (95% CI) | P Value | Adjusted without ACR** Estimate (95% CI) | P Value | Adjusted with ACR** Estimate (95% CI) | P Value | |
|---|---|---|---|---|---|---|
| Continuous Predictors | ||||||
| Serum Albumin, per −0.5 g/dL less | −0.44 (−0.59, −0.28) | <0.001 | −0.56 (−0.72, −0.39) | <0.001 | −0.55 (−0.71, −0.39) | <0.001 |
| ACR, per doubling | −0.22 (−0.31, −0.14) | <0.001 | −0.28 (−0.36, −0.20) | <0.001 | ||
| Baseline eGFRcys, per 10- ml/min/1.73 m2 greater | −0.28 (−0.37, −0.20) | <0.001 | −0.47 (−0.56, −0.39) | <0.001 | −0.53 (−0.62, −0.45) | <.0001 |
| Dichotomized Predictors | ||||||
| Serum Albumin < 3.8 g/dL | −0.43 (−0.74, −0.13) | 0.005 | −0.43 (−0.75, −0.12) | 0.007 | −0.42 (−0.73, −0.10) | 0.009 |
| ACR ≥ 30 mg/g | −0.50 (−0.96, −0.047) | 0.03 | −0.61 (−1.08, −0.15) | 0.009 | ||
| Baseline eGFRcys <60 ml/min/1.73 m2 | 1.15 (0.54, 1.76) | <0.001 | 1.53 (0.91, 2.1) | <0.001 | 1.73 (1.09, 2.4) | <0.001 |
ACR, albumin-creatinine ratio; CI, confidence interval; eGFRcys, cystatin C–based estimated glomerular filtration rate; Women’s Interagency HIV Study (WIHS),
Note: Results reported as estimated annual change (95% confidence interval) in eGFRcys calculated from multivariable linear mixed models
Adjusted models control for age, race, HIV RNA, CD4, HCV, diabetes mellitus, anti-retroviral therapy use, baseline estimated glomerular filtration rate, systolic and diastolic blood pressures, body mass index, and study site. For ACR as a predictor instead of adjusting for ACR the full model adjusts for serum albumin concentration. Anti-retroviral therapy included current combination antiretroviral therapy use, current nucleoside reverse transcriptase inhibitor use, current non-nucleoside reverse transcriptase inhibitor use, and current protease inhibitor use.
Dichotomized serum albumin concentrations (<3.8 vs ≥ 3.8 g/dL) produced similar results as linear declines (Table 2). Results also remained equivalent using both the creatinine-based CKD-EPI equation (Table S2) and a combined creatinine–cystatin C eGFR equation to define the outcomes. Dichotomized ACR (≤30 vs ≥30 mg/g) also predicted declining kidney function after adjustment for serum albumin concentration (Table 2).
Lower levels of serum albumin were associated with both rapid kidney function decline and incident reduced eGFR (Table 3). Continuous declines in ACR were weakly associated with both rapid kidney function decline and incident reduced eGFR; however, dichotomized ACR levels were the strongest predictor of both rapid kidney function decline and incident reduced eGFR.
TABLE 3.
Multivariable-adjusted associations of serum albumin with rapid kidney function decline and incident reduced eGFR in HIV-infected WIHS participants.
| Rapid Kidney Function Decline | Incident Reduced eGFRcys | |||
|---|---|---|---|---|
| Adjusted IRR (95% CI) | P Value | Adjusted IRR (95% CI) | P Value | |
| Continuous Predictors | ||||
| Serum Albumin, per −0.5 g/dL less | 1.71 (1.29, 2.26) | <0.001 | 1.72 (1.31, 2.25) | <0.001 |
| ACR, per doubling | 1.19 (1.09, 1.31) | <0.001 | 1.13 (1.00, 1.27) | 0.04 |
| Baseline eGFRcys, per 10 ml/min/1.73 m2 greater | 0.98 (0.90, 1.07) | 0.7 | 1.41 (1.22, 1.64) | <0.001 |
| Dichotomized Predictors | ||||
| Serum Albumin < 3.8 g/dL | 2.13 (1.30, 3.49) | 0.003 | 1.71 (1.05, 2.79) | 0.03 |
| ACR ≥ 30 mg/g | 2.03 (1.28, 3.20) | 0.002 | 1.95 (1.19, 3.20) | 0.008 |
| Baseline eGFRcys <60 ml/min/1.73 m2 | 1.05 (0.62, 1.77) | 0.9 | na*** | |
Note: Rapid kidney function decline defined as 5% or more per year, using baseline and final eGFRcys. Results reported as relative risk of rapid kidney function decline or incident reduced eGFRcys (95% CI). Estimates are calculated from generalized estimating equation relative risk regression models. Models control for age, race, HIV RNA, CD4, HCV, DM, anti-retroviral therapy use, baseline eGFR, systolic and diastolic blood pressures, body mass index, ACR, and study site. For ACR as a predictor instead of adjusting for ACR the full model adjusts for serum albumin concentration.
Anti-retroviral therapy included current combination antiretroviral therapy use, current nucleoside reverse transcriptase inhibitor use, current non-nucleoside reverse transcriptase inhibitor use, and current protease inhibitor use.
Analysis of incident reduced eGFR excludes those with reduced eGFR at baseline. Model instead controls for continuous eGFR.
ACR, albumin-creatinine ratio; CI, confidence interval; eGFR, estimated glomerular filtration rate; eGFRcys, cystatin C–based estimated glomerular filtration rate; IRR, incidence rate ratio; Women’s Interagency HIV Study (WIHS),
Baseline HIV viremia was also independently associated with rapid kidney function decline (Table 4). After multivariable adjustment excluding serum albumin, each ten-fold higher level of the baseline HIV RNA was associated with a 56% increased risk of rapid kidney function decline. However, when serum albumin was included as a covariate in the same model, the effect size of HIV RNA was attenuated to 39%. In analyses of change in HIV RNA level and average HIV RNA level, serum albumin attenuated the point estimates by a similar proportion. We also evaluated associations of CD4 count with kidney function decline and found a non-significant association in adjusted analyses (−0.026 [95% CI, −0.20 to 0.15] mL/min/1.73 m2 per year, per doubling of CD4 count).
TABLE 4.
Multivariable-adjusted associations of HIV viral load with rapid kidney function decline by eGFRcys in HIV-infected WIHS participants.
| Unadjusted IRR (95% CI) | P Value | Adjusted without serum albumin** IRR (95% CI) | P Value | Adjusted with serum albumin** IRR (95% CI) | P Value | |
|---|---|---|---|---|---|---|
| Baseline HIV RNA, per 10-fold greater | 1.38 (1.06–1.79) | 0.02 | 1.56 (1.19–2.06) | 0.001 | 1.39 (1.11–1.73) | 0.003 |
| Mean HIV RNA, per 10-fold greater | 1.44 (1.02–2.01) | 0.04 | 1.46 (1.10–1.94) | 0.009 | 1.31 (1.02–1.67) | 0.03 |
Note: Rapid kidney function decline defined as 5% or more per year, using baseline and final eGFRcys Results reported as relative risk of rapid kidney function decline (95% CI)). Estimates are calculated from generalized estimating equation relative risk regression models.
Models control for age, race, HIV RNA, CD4, HCV, DM, anti-retroviral therapy use, baseline estimated glomerular filtration rate, systolic and diastolic blood pressures, body mass indexI, ACR, and study site. For ACR as a predictor instead of adjusting for ACR the full model adjusts for serum albumin concentration. Anti-retroviral therapy included current combination antiretroviral therapy use, current nucleoside reverse transcriptase inhibitor use, current non-nucleoside reverse transcriptase inhibitor use, and current protease inhibitor use
ACR, albumin-creatinine ratio; CI, confidence interval; eGFRcys, cystatin C–based estimated glomerular filtration rate; IRR, incidence rate ratio; Women’s Interagency HIV Study (WIHS),
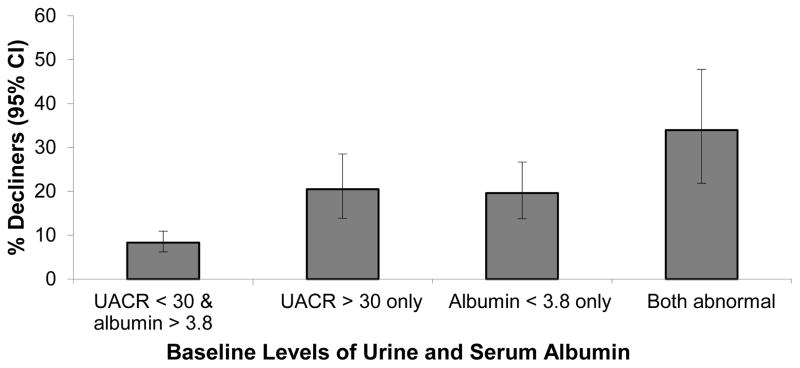
Joint associations of baseline HIV RNA (<1000 vs ≥1000 copies/mL) and serum albumin (<3.8 vs. ≥3.8 g/dL) with rapid decline were also examined (Figure 1). The prevalence of rapid decline was highest in those who had both low albumin and high HIV RNA (26%), intermediate in those with low albumin alone or high HIV RNA (20% and 13%, respectively), and lowest in those with neither low albumin nor high HIV RNA (8%). We performed a similar analysis for serum albumin concentrations (<3.8) and ACR (>30 mg/g) (Figure 2), which demonstrated additive risk for rapid kidney function decline.
FIGURE 1.
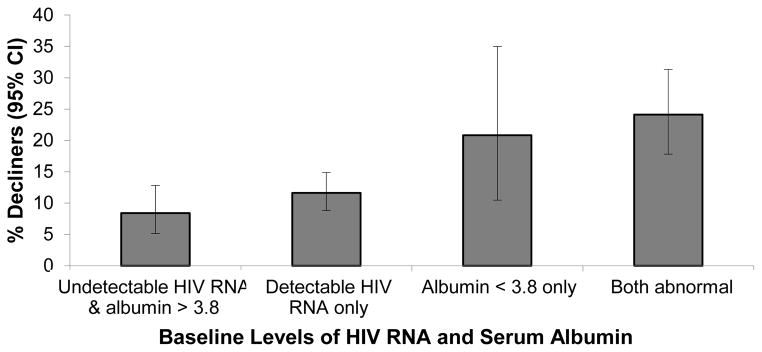
Association of Serum Albumin and HIV RNA with Rapid kidney function decline.
Bars denote percentage of subjects in each category with rapid kidney function decline (determined by eGFRcys), with 95% confidence intervals. CI, confidence interval.
FIGURE 2.
Association of Serum Albumin and Urine Albumin-creatinine ratio (ACR) with Rapid kidney function Decline. Bars denote percentage of subjects in each category with rapid kidney function decline (determined by eGFRcys), with 95% confidence intervals. UACR, urine albumin to creatinine ratio; CI, confidence interval.
DISCUSSION
We found that serum albumin concentrations were a strong and independent risk factor for kidney function decline in this cohort of HIV-infected women. These findings remained robust in the analyses of serum albumin as a linear or dichotomous outcome, and using outcomes based on eGFRcys, eGFRcr, or combined creatinine–cystatin C eGFR. For the clinical endpoints of rapid kidney function decline and incident reduced eGFR, each 0.5-g/dL lower level of baseline serum albumin concentration was associated with an approximately 60% increased risk. Furthermore, not only was serum albumin a stronger risk factor than viral load, but it also appeared to attenuate the association of HIV viral load with rapid kidney function decline. Serum albumin concentrations less than 3.8 g/dL had similar strength of association with kidney function decline as urine ACR of 30 mg/g or greater.
Numerous studies have examined the associations of serum albumin with adverse events in HIV-infected individuals 8,9,10,15,16. However, no previous studies, to our knowledge, have evaluated the relationship between serum albumin and longitudinal kidney function in an HIV-infected population, and few have studied serum albumin and kidney disease in the general population 11,17. In the Cardiovascular Health Study, each 0.4-g/dL lower level of baseline serum albumin was associated with a 1.14 higher odds of rapid kidney function decline. Other studies have found serum albumin to be an important risk factor for ESRD 18,19.
Despite the surprisingly strong associations in this study, the underlying mechanisms are still unclear. As far as we know, there is not a direct physiologic link between lower concentrations of serum albumin and kidney function decline. Serum albumin levels may be depressed for one of several reasons, including extreme cases of poor nutrition, liver disease, albuminuria, and both acute and chronic inflammation. It is unlikely that decreased serum albumin concentrations reflect poor nutrition, since the only condition of malnourishment exhibiting consistently decreased levels of serum albumin is Kwashiorkor, a rare disease in the United States 7. Significant albuminuria can also contribute to decreased albumin, but is unlikely to be a major cause in this population. Few participants in this study had high levels of albuminuria, with most having normal to moderately increased albuminuria. Furthermore, the median ACR was not much higher in those with low vs. high serum albumin. Finally, the effects of serum albumin and albuminuria are additive with regard to change in kidney function.
It is likely that several biological mechanisms contribute to serum albumin concentration reflecting a state of ill health. Liver disease may explain lower levels of serum albumin, though our analyses adjust for rates of both hepatitis B and C virus infection. One other possible biological mechanism linking serum albumin concentration and kidney function is systemic inflammation. A number of studies have linked inflammatory markers to kidney disease 17,20,21. Individuals infected with HIV, even those with a well-controlled viral load, are known to exhibit elevated concentrations of various inflammatory markers 22,23. Moreover, in a study of eight inflammatory markers and serum albumin and kidney function in an elderly population, serum albumin was the only factor associated with kidney function decline 17. In summary, serum albumin likely reflects chronic illness through multiple mechanisms that remain unclear.
Our study had a large sample size, with rigorously ascertained clinical data collected over eight years in a well-characterized cohort, and repeated measurements of both cystatin C and creatinine. Our study also had several limitations. The WIHS is comprised entirely of women, and thus our findings may not be generalizable to men with HIV infection, women outside of the United States, or the uninfected population. We also cannot fully explain the physiological basis of the relationship between serum albumin concentrations and kidney function decline.
In summary, we found that lower levels of serum albumin were strongly associated with kidney function decline and incident reduced eGFR in HIV-infected women, independent of HIV disease status, and albuminuria. Lower serum albumin concentration, a ubiquitously available laboratory test, may help clinicians plan future treatment or prevention efforts in the setting of HIV infection. Already studies in the general population and HIV-infected populations document serum albumin as an important covariate in determining risk for ESRD 4,24, including one widely used ESRD prediction tool 25. Furthermore, laboratory experiments and future studies examining risk factors for serum albumin concentration declines may illuminate the biological link between HIV infection and kidney disease.
Supplementary Material
Acknowledgments
Support: This study was supported by the National Institute on Aging (grants 1R03AG034871-01 [PI, Shlipak] and 5R01AG034853-04 [PI, Shlipak]).
Footnotes
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Contributions: Research area and study design: JL, MGS; data acquisition: PCT, KA, AA, AS, MHC, AWB, MN, MME; data analysis/interpretation: JL, MGS, CG, CRP, RS, PCT, KA, AA, AS, MHC, AWB, MN, MME; statistical analysis: RS; supervision and mentorship: MGS. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. MGS takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Table S1: Baseline characteristics, stratified by inclusion/exclusion status.
Table S2: Associations of serum albumin concentration with annual change in eGFRcr.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
Descriptive Text for Online Delivery of Supplementary Material
Supplementary Table S1 (PDF)
Baseline characteristics, stratified by inclusion/exclusion status.
Supplementary Table S2 (PDF)
Associations of serum albumin concentration with annual change in eGFRcr.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cockerham L, Scherzer R, Zolopa A, Rimland D, Lewis CE, Bacchetti P, Grunfeld C, Shlipak M, Tien PC. Association of HIV Infection, Demographic and Cardiovascular Risk Factors With All-Cause Mortality in the Recent HAART Era. J Acquir Immune Defic Syndr. 2010;53(1):102–106. doi: 10.1097/QAI.0b013e3181b79d22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi A, Scherzer R, Bacchetti P, Tien PC, Saag MS, Gibert CL, Szczech LA, Grunfeld C, Shlipak MG. Cystatin C, Albuminuria, and 5-year All-Cause Mortality in HIV-infected Persons. AJKD. 2010;56(5):872–882. doi: 10.1053/j.ajkd.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Odden MC, Scherzer R, Bacchetti P, Szczech LA, Sidney S, Grunfeld C, Shlipak MG. Cystatin C level as a marker of kidney function in human immunodeficiency virus infection: the FRAM study. Arch Intern Med. 2007;167(20):2213. doi: 10.1001/archinte.167.20.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jotwani V, Li Y, Grunfeld C, Choi AI, Shlipak MG. Risk Factors for ESRD in HIV-Infected Individuals: Traditional and HIV-Related Factors. American Journal of Kidney Diseases. 2012;59(5):628–635. doi: 10.1053/j.ajkd.2011.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 6.Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial. 2004;17(6):432–437. doi: 10.1111/j.0894-0959.2004.17603.x. [DOI] [PubMed] [Google Scholar]
- 7.Friedman AN, Fadem SZ. Reassessment of Albumin as a Nutritional Marker in Kidney Disease. Journal of the American Society of Nephrology. 2010;21(2):223–230. doi: 10.1681/ASN.2009020213. [DOI] [PubMed] [Google Scholar]
- 8.Lang J, Scherzer R, Weekley CC, Tien PC, Grunfeld C, Shlipak MG. Serum Albumin and Short-Term Risk for Mortality and Cardiovascular Disease among HIV-Infected Veterans. AIDS. 2013;27(8):1339–1343. doi: 10.1097/QAD.0b013e32835f1dd6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldman JG, Burns DN, Gange SJ, Bacchetti P, Cohen M, Anastos K, Nowicki M, Delapena R, Miotti P. Serum albumin as a predictor of survival in HIV-infected women in the Women’s Interagency HIV Study. AIDS. 2000;14(7):863–870. doi: 10.1097/00002030-200005050-00013. [DOI] [PubMed] [Google Scholar]
- 10.Feldman JG, Gange SJ, Bacchetti P, Cohen M, Young M, Squires KE, Williams C, Goldwasser P, Anastos K. Serum albumin is a powerful predictor of survival among HIV-1-infected women. J Acquir Immune Defic Syndr. 2003;33(1):66–73. doi: 10.1097/00126334-200305010-00010. [DOI] [PubMed] [Google Scholar]
- 11.Keller C, Katz R, Sarnak MJ, Fried LF, Kestenbaum B, Cushman M, Shlipak MG for the CHS study. Inflammatory biomarkers and decline in kidney function in the elderly: the Cardiovascular Health Study. Nephrology Dialysis Transplantation. 2009;25(1):119–124. doi: 10.1093/ndt/gfp429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bacon MC, Wyl von V, Alden C, Sharp G, Robison E, Hessol N, Gange S, Barranday Y, Holman S, Weber K, Young MA. The Women’s Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12(9):1013–1019. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Driver TH, Scherzer R, Peralta CA, Tien PC, Estrella MM, Parikh CR, Butch AW, Anastos K, Cohen MH, Nowicki M, Sharma A, Young MA, Abraham A, Shlipak MG. Comparisons of creatinine and cystatin C for detection of kidney disease and prediction of all-cause mortality in HIV-infected women. AIDS. 2013;1 doi: 10.1097/QAD.0b013e328362e874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zou G. A Modified Poisson Regression Approach to Prospective Studies with Binary Data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 15.Dao CN, Peters PJ, Kiarie JN, Zulu I, Muiruri P, Ong’ech J, Mutsotso W, Potter D, Njobvu L, Stringer JSA, Borkowf CB, Bolu O, Weidle PJ. Hyponatremia, hypochloremia, and hypoalbuminemia predict an increased risk of mortality during the first year of antiretroviral therapy among HIV-infected Zambian and Kenyan women. AIDS Res Hum Retroviruses. 2011;27(11):1149–1155. doi: 10.1089/AID.2010.0345. [DOI] [PubMed] [Google Scholar]
- 16.Mehta SH, Astemborski J, Sterling TR, Thomas DL, Vlahov D. Serum albumin as a prognostic indicator for HIV disease progression. AIDS Res Hum Retroviruses. 2006;22(1):14–21. doi: 10.1089/aid.2006.22.14. [DOI] [PubMed] [Google Scholar]
- 17.Fried L, Solomon C, Shlipak M, Seliger S, Stehman-Breen C, Bleyer AJ, Chaves P, Furberg C, Kuller L, Newman A. Inflammatory and prothrombotic markers and the progression of renal disease in elderly individuals. J Am Soc Nephrol. 2004;15(12):3184–3191. doi: 10.1097/01.ASN.0000146422.45434.35. [DOI] [PubMed] [Google Scholar]
- 18.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE. Hypoalbuminemia, cardiac morbidity, and mortality in end-stage renal disease. J Am Soc Nephrol. 1996;7(5):728–736. doi: 10.1681/ASN.V75728. [DOI] [PubMed] [Google Scholar]
- 19.Phelan PJ, O’Kelly P, Walshe JJ, Conlon PJ. The Importance of Serum Albumin and Phosphorous as Predictors of Mortality in ESRD Patients. Ren Fail. 2008;30(4):423–429. doi: 10.1080/08860220801964236. [DOI] [PubMed] [Google Scholar]
- 20.Oberg BP, McMenamin E, Lucas FL, McMonagle E, Morrow J, Ikizler TA, Himmelfarb J. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004;65(3):1009–1016. doi: 10.1111/j.1523-1755.2004.00465.x. [DOI] [PubMed] [Google Scholar]
- 21.de Mutsert R, Grootendorst DC, Indemans F. Association between serum albumin and mortality in dialysis patients is partly explained by inflammation, and not by malnutrition. Journal of Renal Nutrition. 2009;19(2):127–135. doi: 10.1053/j.jrn.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunt PW. HIV and Inflammation: Mechanisms and Consequences. Curr HIV/AIDS Rep. 2012;9(2):139–147. doi: 10.1007/s11904-012-0118-8. [DOI] [PubMed] [Google Scholar]
- 24.Wagner M, Ansell D, Kent DM, Griffith JL, Naimark D, Wanner C, Tangri N. esrd albumin 2. YAJKD. 2011;57(6):894–902. doi: 10.1053/j.ajkd.2010.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tangri N. A Predictive Model for Progression of Chronic Kidney Disease to Kidney Failure. JAMA. 2011;305(15):1553. doi: 10.1001/jama.2011.451. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.