Abstract
While accentuated kyphosis is associated with osteoporosis, it is unknown whether it increases risk of future fractures, independent of bone mineral density (BMD) and vertebral fractures. We examined the associations of baseline Cobb angle kyphosis and 15 year change in kyphosis with incident non-spine fractures using data from the Study of Osteoporotic Fractures. A total of 994 predominantly white women, aged 65 or older, were randomly sampled from 9,704 original participants to have repeated Cobb angle measurements of kyphosis measured from lateral spine radiographs at baseline and an average of 15 years later. Non-spine fractures, confirmed by radiographic report, were assessed every four months for up to 21.3 years. Compared with women in the lower three quartiles of kyphosis, women with kyphosis greater than 53 degrees (top quartile) had a 50% increased risk of non-spine fracture (95% CI, 1.10 –2.06 after adjusting for BMD, prevalent vertebral fractures, prior history of fractures, and other fracture risk factors. Cobb angle kyphosis progressed an average of 7 degrees (SD = 6.8) over 15 years. Per 1 SD increase in kyphosis change, there was a multivariable adjusted 28% increased risk of fracture (95% CI, 1.06 – 1.55) that was attenuated by further adjustment for baseline BMD (HR per SD increase in kyphosis change, 1.19; 95% CI 0.99 –1.44). Greater kyphosis is associated with an elevated non-spine fracture risk independent of traditional fracture risk factors in older women. Furthermore, worsening kyphosis is also associated with increased fracture risk that is partially mediated by low baseline BMD that itself is a risk factor for kyphosis progression. These results suggest that randomized controlled fracture intervention trials should consider implementing kyphosis measures to: 1) further study kyphosis and kyphosis change as an additional fracture risk factor; and 2) test whether therapies may improve or delay its progression.
Keywords: kyphosis, hyperkyphosis, fracture
INTRODUCTION
Hyperkyphosis, or accentuated thoracic spinal curvature, is a common condition estimated to affect about 30% of the older population (1). It occurs as a result of multifactorial causes and is associated with increased health vulnerability; thus, hyperkyphosis has been defined as a new geriatric syndrome (1–3). Besides vertebral fractures, low bone mineral density (BMD) and BMD loss, low body weight and weight loss are other important determinants of greater kyphosis progression in older women (3). While vertebral fractures, BMD, and weight status are known fracture risk factors, it is not definitively known whether hyperkyphosis itself also carries an independent risk for future non-spine fracture.
One prospective study of older women reported that those who had kyphotic posture were at increased risk for non-spine fractures after adjusting for age, prior fracture, and BMD, but no adjustment was made for underlying vertebral fractures (4). Besides fractures, other adverse health outcomes associated with hyperkyphosis include worsening physical function (5–6), falls (7) and earlier mortality (8–9). Given the growing older population and the high prevalence of age-related hyperkyphosis, better delineation of associated ill-health outcomes will help inform the development and testing of effective kyphosis treatments.
Having a previous vertebral fracture is a strong non-spine fracture risk factor (10), but the effects of kyphosis independent of vertebral fractures on fracture risk are less clear. Furthermore, the effect of kyphosis progression on non-spine fracture risk is unknown. Thus, using data from the Study of Osteoporotic Fractures, we sought to determine whether: 1) greater degrees of thoracic kyphosis were associated with increased short and long-term non-spine fracture risk independent of prevalent and incident vertebral fractures; and whether 2) progressive thoracic kyphosis measured over 15 years was associated with subsequent increase in non-spine fracture risk in women aged 65 years and older.
METHODS
Study Population
From 1986 to 1988, 9,704 ambulatory women aged 65 years or older (>99% non-Hispanic white) were recruited for participation in the Study of Osteoporotic Fractures (SOF), the details of which have been published (11). Population-based listings were used to recruit women in Baltimore, MD; Minneapolis, MN; Portland, OR; and the Mononghela Valley near Pittsburgh, PA. Women who reported a bilateral hip replacement were excluded. Additionally, black women were initially excluded due to their low incidence of hip fracture.
In this study we randomly sampled 1,000 women with spine radiographs done at baseline (Visit 1), an average of 3.7 (Visit 3) and 15 years later (Visit 8). To account for survivor bias in selecting only women with all three films available for Cobb angle calculation, we also included a random sample of about 200 women who had at least one, but not all three radiographs available for study. Of the 1,196 women sampled, 119 were excluded due to poor scan quality, 3 due to incomplete data, and 80 were missing incident fracture data, leaving an analytic sample of 994 women. The women were followed for incident fractures for an average of 14.0 years after the baseline examination.
Kyphotic posture measurement
The degree of kyphosis was calculated at baseline and a follow-up visit an average of 15 years later using digitized Cobb angle derived from supine lateral thoracic spine radiographs centered at T8 (T4–T12) (12). The modified Cobb method uses the fixed cutoff of T4 and T12 to determine the superior and inferior margins for line placement. Using a translucent digitizer (GTCO, Rockville, MD) and cursor, a technician marked points corresponding to the four corners of the vertebral body at T4 and T12. From the superior surface of T4 and the inferior surface of T12, a digitization program erected perpendicular lines, the intersection of which is the kyphotic angle. The intra-rater reliability ICC for repeated digitized Cobb angle readings was 0.984 (12).
Incident non-spine fractures
Incident non-spine fractures were assessed from postcard follow-up every 4 months for the study duration, and participant reported fractures were confirmed by centrally reviewed radiographic report. Fracture types included: 1) wrist, 2) humerus, 3) pelvis, 4) rib, 5) leg, 6) finger and hand, 7) toe and foot, 8) skull and face, 9) chest, 10) ankle, 11) elbow, 12) clavicle, 13) scapula, 14) knee, 15) heel, 16) hip, femoral neck, inter-trochanter, 17) neck, and 18) tailbone. Fractures not confirmed by radiographic report were excluded.
Prevalent vertebral fractures
Baseline spine x-rays were performed on all participants. X-rays with any possible or uncertain vertebral deformity were digitized. Quantitative vertebral morphometry was performed on digitized films to calculate anterior (Ha), middle (Hm), and posterior (Hp) height for each vertebral body from T4 to L4 (detailed description has been published) (13). A prevalent vertebral fracture was recorded if any of the following ratios were greater than 3 SDs below the normal mean for that vertebral level: 1) Ha/Hp, 2)Hm/Hp, 3)Hp/Hp (above and below), 4)Ha/Ha (above and below).
Incident Vertebral Fractures
Follow-up spine x-rays were performed on average at 3.7 years and 15 years after baseline. An incident vertebral fracture was diagnosed if, compared to baseline, any of the 3 heights from baseline decreased by ≥ 20%, and at least 4mm from the baseline to the follow-up x-ray (14).
Other Measurements
Self-administered questionnaires were used to collect baseline data for previous non-spine fractures (fractures that occurred after age 50 but prior to baseline), history of falls in the past year, physical activity (total kilocalories burned per week), smoking (lifetime pack years), drinking (average number of drinks per week), and self-rated health (range: excellent for my age, good, fair, poor, and very poor for my age). Body mass index (BMI) was calculated from baseline standard height and weight measurements (15). If participants were obese or had kyphotic posture, detailed instructions were given to ensure that the legs were close together as possible, with the buttocks, and if possible, the scapula, coming in contact with the wall-plate. The clinic staff would then verify that after positioning, the participant maintain maximal erect posture in the Frankfort horizontal plane (16). Tests of physical function included grip strength measured by a hand held Jamar dynamometer and walking speed (time in seconds to walk 6 meters at usual pace). Calcaneal bone mineral density (BMD, g/cm2) was measured using single-photon absorptiometry (OsteoAnalyzers-Siemens-Osteon, Wahiawa, HI, U.S.A.). The mean CV between centers was 1.2% for in vivo measurements of the calcaneus. A description of measurement and quality control procedures has been published (17).
Frailty status
Frailty status was assessed using similar criteria to those incorporated in previously published Study of Osteoporotic Fracture studies (18). Five components were assessed: 1) shrinking/sarcopenia defined as weight loss of greater than 5% between the baseline and the third follow-up visit; 2) weakness classified by grip strength in the lowest quintile stratified by BMI (quartiles); 3) Exhaustion determined by an answer of “no” to the question “Do you feel full of energy” from the Geriatric Depression Scale (19); 4) slowness defined by walking speed in the lowest quintile stratified by standing height (median); and 5) low physical activity level as identified by kilocalories per week from walking in the lowest quintile. Women with none of the above were considered normal, those with one or two components were defined as intermediate stage, and those with three to five components considered frail.
Statistical Analyses
After confirming model assumptions of proportionality, Cox proportional hazard models were used to assess the effect of baseline Cobb angle on future non-spine fracture risk. To assess for thresholds, quartile, quintile, and deciles of kyphosis were plotted against non-spine fracture rates and visual inspection used to identify plausible risk thresholds. Cobb angle kyphosis was examined in two ways: 1) as a continuous predictor; and 2) as a dichotomous indicator for hyperkyphosis, defined as the top quartile for Cobb angle (> 53 degrees) versus other three quartiles. Initial models controlled for clinical site and age. A secondary model added controls for factors known to influence future fractures, including prevalent and incident vertebral fractures, prior history of non-spine fracture, BMI, BMD, height and weight. The final model added self-rated health, maximum grip strength, a history of falls in the past year and health behaviors that could influence fracture risk such as smoking, drinking, and physical activity.
Because the effects of fracture risk factors tend to wane over time and our current study included long-term follow up of up to 21.3 years, we ran hazard models that included two versions of follow-up time (20–22). Initial models investigated the effect of baseline Cobb angle on non-spine fractures within the first 5 years of the study, and subsequent models used the full follow-up time (mean 14.0 years, maximum 21.3 years) (Figure 1).
Figure 1.
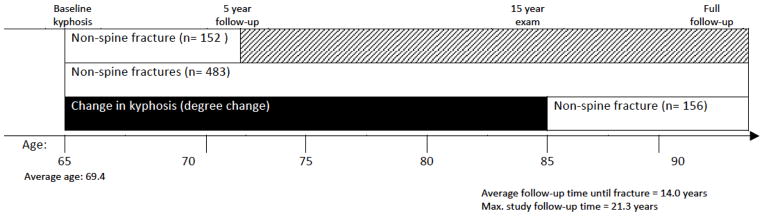
Study timeline.
Since vertebral fractures are a major fracture risk factor (10), we tested for an interaction between kyphosis and prevalent vertebral fracture status for prediction of future non-spine fracture. We also ran stratified models of women with and without prevalent vertebral fractures as a separate method to assess whether kyphosis and the risk for fracture might differ significantly between these groups. In addition, because BMD can impact kyphosis, we tested for an interaction between kyphosis and BMD for prediction of risk for incident fractures.
We examined the effect of long-term change in kyphosis (calculated over 15 years) and subsequent 5-year non-spine fracture risk (Figure 1). In addition to the covariates mentioned above, these models controlled for baseline Cobb angle to see if the effect of kyphosis progression on fractures is independent of initial level of kyphosis. In addition, as persons with worsening kyphosis may also more likely be frail and at increased fracture risk, we adjusted for frailty status. Finally, because our aim was to assess the effect of kyphosis on fracture risk, we calculated a Harrell’s C statistic to investigate whether adding kyphosis to well-accepted fracture risk factors would improve non-spine fracture risk prediction. We used SAS software to conduct all statistical analyses (SAS Institute, version 9.1, Cary, NC, USA).
RESULTS
The study sample included 994 women with a mean age of 69.4 years (range 65 – 88 years), of whom 792 had measures of 15 year change in kyphosis. At baseline, the mean Cobb angle was 44.9 degrees (range, 6.45 – 85.5), and average BMI was 26.3 gm/cm2. The majority of study participants reported high self-rated health, about 15% had a prevalent vertebral fracture, and slightly more than 32% reported a previous non-spine fracture (Table 1). A total of 483 women suffered an incident non-spine fracture after an average of 14 years of follow-up of whom 152 women fractured within the first five years of study. The most common types of fractures reported included wrist fractures (n = 151), hip fractures (n = 132), and ankle or foot fractures, not including the toe (n = 118).
Table 1. Baseline characteristics, Full Sample and Stratified by Top Quartile of Kyphosis [Mean, (SD) or %].
Baseline characteristics, full sample and stratified by top quartile of kyphosis
| Baseline characteristics | Full sample (n=994) | Top Quartile of Cobb angle (n=254) | Bottom 3 quartiles of Cobb angle (n=740) | p-value |
|---|---|---|---|---|
| Cobb angle (degrees) | 44.9 (11.8) | 60.6 (6.2) | 39.5 (7.7) | < .0001 |
| Age, years | 69.4 (4.0) | 69.7 (4.1) | 69.2 (4.0) | .08 |
| BMI (kg/m2) | 26.3 (4.4) | 26.5 (4.2) | 26.3 (4.4) | .42 |
| Height (cm) | 160.1 (6.0) | 160 (6.2) | 160 (5.9) | .87 |
| Weight (kg) | 67.5 (11.6) | 68.0 (11.3) | 67.3 (11.7) | .39 |
| Calcaneal BMD (gm/cm2) | 0.42 (.09) | 0.40 (.09) | 0.42 (.08) | .0001 |
| Maximum grip strength (kg) | 23.2 (4.0) | 22.7 (4.1) | 23.3 (4.0) | 0.03 |
| Prevalent vertebral fractures (yes/no) | 14.7 | 27.6 | 10.3 | < .0001 |
| Previous non-spine fracture (yes/no) | 32.2 | 36.8 | 30.7 | .07 |
| Any falls in past 12 months (yes/no) | 27.6 | 32.0 | 26.2 | 0.08 |
| Self-reported health (% good/excellent) | 89.8 | 89.9 | 89.8 | .96 |
| Physical activity (calories burned/week) | 1921 (1826) | 1855 (1765) | 1943 (1847) | .51 |
| Pack years smoking | 8.64 (16.4) | 7.4 (16.2) | 9.1 (16.5) | .16 |
| Alcohol use (# drinks/week) | 2.05 (4.2) | 1.45 (2.89) | 2.25 (4.57) | .001 |
Women with hyperkyphosis (defined as quartile 4 of Cobb angle (>53 degrees)) compared with those with lesser kyphosis had lower BMD, were more likely to have a prevalent vertebral fracture, lower grip strength, and report fewer drinks per week. These hyperkyphotic women had an age-clinic-adjusted 65% five year increased risk of fracture (95% CI, 1.22 – 2.25) that decreased to 50 (95% CI, 1.10 – 2.06) after adjusting for prevalent vertebral fracture, prior history of non-spine fracture, height, weight, BMI, calcaneal BMD, maximum grip strength, any falls in the past year, self-reported health, smoking, drinking, and self-reported physical activity. With extended follow-up time, the effects were slightly weaker: women with hyperkyphosis had a 39% increased fracture risk (95% CI, 1.10 – 1.77) that decreased to 26% (95% CI, 0.99 – 1.62) with full adjustment.
Similar results were obtained examining kyphosis as a continuous predictor of future non-spine fractures. With follow-up time of 5 years in the fully adjusted model, with each standard deviation increase in kyphosis, there was a 29% increased risk of future non-spine fracture (95% CI: 1.10 –1.52). Besides kyphosis, calcaneal BMD (HR 0.78, 95% CI: 0.64 – 0.95 per 1 SD decrease), previous non-spine fracture (HR 1.64; 95% CI: 1.17 – 2.30), grip strength (HR 0.95; 95% CI: 0.91 – 0.99), and smoking (HR 1.16; 95% CI: 1.02 – 1.33) were the factors that remained the independent predictors of future non-spine fracture. Using the full follow-up period of 14 instead of 5 years, the results were slightly weaker, but remained statistically significant (HR 1.13: 95% CI: 1.02 – 1.24). The Harrell’s C statistic that included Cobb angle kyphosis in addition to age, clinic, height, weight, BMI and BMD did not demonstrate substantially improved fracture risk prediction (Harrell’s C increased from 0.602 to 0.623).
Because of the strong correlation between vertebral fractures and hyperkyphosis, we performed stratified analyses to assess the effect of kyphosis on 5-year fracture risk among those with and without prevalent vertebral fractures (Table 2). Both groups had a significantly increased fracture risk. Women with prevalent vertebral fractures were at 42% and women without prevalent vertebral fractures were at 23% increased risk, with a p value for interaction = 0.47.
Table 2.
Adjusted hazard ratios for non-spine fracture over 5 years in women stratified by prevalent vertebral fracture.
| Kyphosis | Vertebral fracture status | Model 1* | Model 2† | Model3‡ |
|---|---|---|---|---|
| Hazard Ratio (95% CI) | ||||
| Cobb angle (per SD unit change) | No prevalent vertebral fracture | 1.28 (1.08 – 1.53) | 1.25 (1.04 – 1.49) | 1.21 (1.02 – 1.45) |
| Cobb angle (per SD unit change) | One or more prevalent vertebral fractures | 1.39 (1.05 – 1.85) | 1.48 (1.09 – 2.01) | 1.53 (1.11 –2.11) |
Model 1 adjusted for clinical site and age.
Model 2 = Model 1 + prior history of fracture, BMI, BMD, height and weight.
Model 3 = Model 2 + maximum grip strength, any falls in the past year, self-reported health, smoking, drinking and physical activity.
Finally, we calculated the change in kyphosis over 15-years and examined non-spine fracture risk over the subsequent 4.0-years (maximum of 5.91 years). Kyphosis progressed an average of 7.1 degrees (SD = 6.8). Adjusting for age, clinical site, and baseline kyphosis, each standard deviation increase in Cobb angle was associated with a 46% greater non-spine fracture risk (95% CI: 1.15 – 1.85). With additional adjustment for BMI, height, weight, maximum grip strength, frailty status, self-reported health, previous non-spine fracture, history of falls in the past year, drinking, exercise, and smoking, Cobb angle change remained a predictor of fracture risk (Table 3, model 1). Further adjustment for prevalent or incident vertebral fractures (occurring simultaneously with Cobb angle change) (Table 3, models 2 and 3) did not substantially alter this association even when both prevalent and incident vertebral fractures were considered together (HR = 1.24; 95% CI: 1.0 – 1.52). However, while kyphosis change in a model that included age, clinic, and BMD remained significant (HR = 1.31; 95% CI: 1.03 – 1.67), addition of BMD to the fully adjusted model diminished the effect of Cobb change on fracture risk (Table 3, model 4).
Table 3. Fully adjusted models of change in kyphosis over 15 years and relative risk of non-spine fracture in models adjusted either for prevalent vertebral fracture, incident vertebral fracture, or calcaneal BMD*.
Fully adjusted models of change in kyphosis over 15 years and relative risk of non-spine fracture in models adjusted either for prevalent vertebral fracture, incident vertebral fracture, calcaneal bone mineral density or simultaneously including all covariates.
| Variables | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| Hazards Ratio (95% Confidence Interval) | ||||
| Change in Cobb (per SD) | 1.27 (1.06 – 1.55) | 1.28 (1.06 – 1.55) | 1.24 (1.01 – 1.52) | 1.19 (0.99 – 1.44) |
| Baseline Cobb angle (per SD) | 1.22 (1.01 – 1.46) | 1.21 (1.00 – 1.47) | 1.22 (1.01 – 1.46) | 1.13 (0.94 – 1.36) |
| Prevalent vertebral fracture | 1.03 (0.60 – 1.76) | |||
| Incident vertebral fracture | 1.25 (0.77 – 2.05) | |||
| Calcaneal BMD (gm/cm2, per SD) | 0.59 (0.47 – 0.75) | |||
Models 1 –4 each represent a different model adjusted age, clinical site, previous non-spine fracture, BMI, height, weight, maximum grip strength, any falls in the past year, self-reported health, drinking, exercise, smoking, frailty status, and each listed variable within that column.
DISCUSSION
We found that older women with greater degrees of thoracic kyphosis are at increased risk for future non-spine fractures independent of both BMD and vertebral fractures. Furthermore, this increased non-spine fracture risk is present even among hyperkyphotic women without underlying vertebral fractures. Finally, our findings suggest that women who experience greater kyphosis progression have an increased long-term risk of future non-spine fracture that is at least as strong as that with underlying prevalent and incident vertebral fractures. Though formal calculation of the Harrell’s C statistic did not indicate a significant improvement in fracture risk prediction with the addition of kyphosis to the model of traditional risk factors, it should be noted that other well-accepted clinical risk factors for cardiovascular disease such lipids and smoking also only have a marginal impact on the C-statistic (23). Thus, these study findings suggest that thoracic curvature should be considered in the clinical setting when assessing a patient’s fracture risk. Compared with other fracture risk assessments such as DXA and FRAX® (WHO Fracture Risk Assessment Tool), hyperkyphosis is immediately apparent on clinical exam making it a potentially attractive modality for risk assessment.
A unique aspect of this study was the extremely long follow-up time of up to 21.3 years. Past studies have shown that over time, fracture risk factors tend to lose their predictive power (20–22). Therefore, in evaluating kyphosis as a possible fracture risk factor, we assessed the risk of fracture within a 5-year window and then over the entire length of follow-up. We found that with shorter versus longer follow-up time, the effect of kyphosis on fracture risk was strongest within the first 5 years. Survival bias may be at least in part responsible for the waning effects. Older persons with greater degrees of kyphosis suffer from earlier mortality (8–9). Likewise, women with low BMD and faster rates of bone loss are also more likely to die sooner (24–25). Thus, those who were likely at greatest risk for non-spine fracture were also those who were more likely to experience earlier death. In fact, those who survived the full follow-up time and included in our study sample were more robust and had fewer fracture risk factors at baseline than all women enrolled in the parent cohort (3).
The term “kyphosis” is often misused. Technically, “kyphosis” refers to the concave curvature of the thoracic spine, whether normal or abnormal. If a patient is described as “kyphotic,” the implication is that the degree of kyphosis is abnormal. To date, there is not a set cut-point that distinguishes an older person from having hyperkyphosis versus normal kyphosis. Previous studies done in younger populations suggest that the normal range of kyphosis is somewhere between 20 and 40 degrees (26–27). Since all persons have kyphosis by definition, we propose that “hyperkyphosis” be used to describe the magnitude of kyphosis associated with poorer health.
Considering hyperkyphosis as a predictor of ill health, there is a continuous gradient of increased risk without an apparent threshold effect (5, 9). Thus, the degree of kyphosis that may be considered “hyperkyphosis” depends on perceived acceptable levels of increased risk of specific health outcomes. Examination of the kyphosis angles in older women in the Study of Osteoporotic Fractures (n = 944), the Fracture Intervention Trial (n = 6,459), and the Rancho Bernardo Study (n = 854), reveals that kyphosis is normally distributed with a mean angle of 44 – 49 degrees (3, 28–29). In each of these studies, women in the top quartile of kyphosis had Cobb angle that ranged from 53 to 55.5 degrees. If we use 53 degrees as a cut-off definition of hyperkyphosis, the relative hazards ratio is 1.5 for incident non-spine fracture. Thus, a woman with kyphosis of 53 degrees (Figure 2) could be easily identified in clinic by simple observation of her standing posture and it could be inferred that she would have a 50% relative increase fracture risk over a woman with less kyphosis (95% confidence limits 10 –106%).
Figure 2.
A woman with thoracic kyphosis of 53 degrees.
Our study results point to two very strong both short and long-term fracture risk factors: hyperkyphosis and BMD. While BMD is a well-established risk factor with long-range predictability (30), little attention has been paid to the potential effects of hyperkyphosis, particularly, non-osteoporotic hyperkyphosis. One prospective study suggested that older women with accentuated kyphotic posture had a 1.7-fold increased fracture risk that was independent of age, BMD and other risk factors, but could not account for the possible confounding influence of silent vertebral fractures (4). As two thirds of vertebral fractures remain clinically undiagnosed (31), most have assumed that the increased fracture risk observed in women with hyperkyphosis was explained by vertebral fractures. Our study results refute this possibility and indicate that it is not only vertebral fracture related kyphosis that confers the excess risk.
Hyperkyphosis is a multifactorial health condition, largely influenced by bone health, but not synonymous with vertebral fractures. Approximately two thirds of older persons affected with severe kyphosis do not have vertebral fractures (32). Other factors associated with hyperkyphosis include a family history of hyperkyphosis, degenerative disc disease, low BMD, lower paraspinal muscle density, greater weight, and smoking (3, 33). Each incident vertebral fracture is estimated to increase kyphosis by about 3.8 degrees, and other strong predictors of kyphosis progression include bone density and weight loss (3). Our findings suggest that kyphosis progression is associated with increased fracture risk in large part due to lower BMD among those with progression. Based upon these results and knowledge of what may lead to worse kyphosis, targeted interventions to prevent vertebral fractures, maintain bone density and weight may not only forestall kyphosis progression, but also help prevent non-spine fractures.
A major strength of our study includes that it is the largest prospective cohort study of kyphosis in older women that has standardized assessments of prevalent and incident vertebral fractures and meticulous fracture follow-up information. It also has a measure of kyphosis progression assessed over 15 years to assess the effect of kyphosis change on fracture risk. Study limitations include that we evaluated predominantly older white women, so that our results may not be generalizable to older men or other ethnic groups. Also, hip DXAs that are currently considered the gold standard assessment for BMD were not included in these analyses because they were not measured at the same time as kyphosis and vertebral fractures. Nonetheless, the calcaneal BMD measurement proved to be a robust predictor of fractures. Morever, adjustment for hip BMD measured at visit 2 instead of calcaneal BMD made no difference in our study results. A third limitation is possible misclassification of prevalent vertebral status; if such misclassification differed by kyphosis, this may have biased the results. However, any of such misclassification is likely to be small and thus have a small effect. Additional adjustment for prevalent fracture status strongly influenced the association between kyphosis status and risk of non-spine fracture, and it is unlikely that this attenuation of the effect was due to solely to misclassification of prevalent vertebral status. Fourth, we did not assess for other underlying medical conditions that are associated with increased thoracic kyphosis such as Scheurman’s kyphosis, ankylosing spondylitis, cystic fibrosis, Ehlers Danlos syndrome and Marfan’s syndrome that might also affect fracture risk. Fifth, although adjusting for a history of falls did not alter our results, we did not collect information on the exact timing and circumstances of falls that could further affect the association between kyphosis and fracture risk. Finally, because one of the main aims of our original study was to assess kyphosis progression, our study selected for survivors, so it may be that the effects would be even stronger for the non-survivors, especially since kyphosis is associated with earlier mortality.
In conclusion, women with hyperkyphosis are at increased risk for non-spine fractures regardless of underlying vertebral fracture status. Furthermore, women with kyphosis progression experience greater non-spine fracture rates, suggesting that slowing progression may attenuate non-spine fracture risk. Based upon these observational study results and the ease of kyphosis measurement from digitized radiographs, multi-center randomized controlled fracture intervention trials should evaluate hyperkyphosis and kyphosis progression as additional fracture risk factors as well as whether therapies may improve or delay its progression.
Acknowledgments
This work was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Aging Public Service Health Grants R01 AG24246, R56AG024246, AR060828, AG02754, AG005407, AG02756, AG00507, AG005394, AR35583, AR35582, and AR35584. The funding agencies had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
References
- 1.Kado DM, Prenovost K, Crandall C. Narrative review: hyperkyphosis in older persons. Ann Intern Med. 2007;147:330–8. doi: 10.7326/0003-4819-147-5-200709040-00008. [DOI] [PubMed] [Google Scholar]
- 2.Tinetti ME, Williams CS, Gill TM. Dizziness among older adults: a possible geriatric syndrome. Ann Intern Med. 2000;132:337–44. doi: 10.7326/0003-4819-132-5-200003070-00002. [DOI] [PubMed] [Google Scholar]
- 3.Kado DM, Huang MH, Karlamangla AS, Cawthon P, Katzman W, Hillier TA, Ensrud K, Cummings SR. Factors associated with kyphosis progression in older women: 15 years’ experience in the study of osteoporotic fractures. J Bone Miner Res. 2013;28:179–87. doi: 10.1002/jbmr.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang MH, Barrett-Connor E, Greendale GA, Kado DM. Hyperkyphotic posture and risk of future osteoporotic fractures: The Rancho Bernardo Study. J Bone Miner Res. 2006;21:419–423. doi: 10.1359/JBMR.051201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katzman WB, Vittinghoff E, Ensrud K, Black DM, Kado DM. Increasing kyphosis predicts worsening mobility in older community-dwelling women: a prospective study. J Am Geriatr Soc. 2011;59:96–100. doi: 10.1111/j.1532-5415.2010.03214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katzman WB, Huang MH, Lane NE, Ensrud KE, Kado DM. Kyphosis and decline in physical function over 15 years in older community-dwelling women: the Study of Osteoporotic Fractures. J Gerontol A Biol Med Sci. 2013;68:976–83. doi: 10.1093/gerona/glt009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kado DM, Huang MH, Nguyen CB, Barrett-Connor E, Greendale GA. Hyperkyphotic posture and risk of injurious falls in older persons: The Rancho Bernardo Study. J Gerontol Med Sci. 2007;62A:652–657. doi: 10.1093/gerona/62.6.652. [DOI] [PubMed] [Google Scholar]
- 8.Kado DM, Huang MH, Karlamangla AS, Barrett-Connor E, Greendale GA. Hyperkyphotic posture predicts mortality in older community-dwelling men and women: a prospective study. J Am Geriatr Soc. 2004;52:1662–67. doi: 10.1111/j.1532-5415.2004.52458.x. [DOI] [PubMed] [Google Scholar]
- 9.Kado DM, Lui LY, Ensrud KE, Fink HA, Karlamangla AS, Cummings SR for the Study of Osteoporotic Fractures. Hyperkyphosis predicts mortality independent of vertebral osteoporosis in older women. Ann Intern Med. 2009;150:681–87. doi: 10.7326/0003-4819-150-10-200905190-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15:721–739. doi: 10.1359/jbmr.2000.15.4.721. [DOI] [PubMed] [Google Scholar]
- 11.Cummings SR, Nevitt MC, Browner WS, Stone K, Fox KM, Ensrud KE, Cauley J, Black D, Vogt TM. Risk Factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332:767–773. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 12.Kado DM, Christianson L, Palermo L, Smith-Bindman R, Cummings SR, Greendale GA. Comparing a supine radiologic versus standing clinical measurement of kyphosis in older women: the Fracture Intervention Trial. Spine. 2006;31:463–67. doi: 10.1097/01.brs.0000200131.01313.a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Black DM, Palermo L, Nevitt MC, Genant HK, Epstein R, SanValentin R, Cummings SR. Comparison of methods for defining prevalent vertebral fractures: the Study of Osteoporotic Fractures. J Bone Miner Res. 1995;10:890–902. doi: 10.1002/jbmr.5650100610. [DOI] [PubMed] [Google Scholar]
- 14.Black D, Palermo L, Nevitt MC, Genant HK, Christianson L, Cummings SR. Defining incident vertebral deformity: a prospective comparison of several approaches. J Bone Miner Res. 1999;14:90–101. doi: 10.1359/jbmr.1999.14.1.90. [DOI] [PubMed] [Google Scholar]
- 15.Lohman T, Roche A, Martorell R. Anthropometric Standardization Reference Manual. Human Kinetics Books; Champaign, Champaign, IL: 1988. pp. 39–54. [Google Scholar]
- 16.Hillier TA, Lui LY, Kado DM, LeBlanc ES, Vesco KK, Bauer DC, Cauley JA, Ensrud KE, Black DM, Hochberg MC, Cummings SR. Height loss in older women: risk of hip fracture and mortality independent of vertebral fractures. J Bone Miner Res. 2012;27:153–59. doi: 10.1002/jbmr.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steiger P, Cummings SR, Black DM, Spencer NE, Genant HK. Age-related decrements in bone mineral density in women over 65. J Bone Min Res. 1992;7:625–632. doi: 10.1002/jbmr.5650070606. [DOI] [PubMed] [Google Scholar]
- 18.Ensrud KE, Ewing SK, Fredman L, Hochberg MC, Cauley JA, Hillier TA, Cummings SR, Yaffe K, Cawthon PM. Circulating 25-hydroxyvitamin D levels and frailty status in older women. J Clin Endocrinol Metab. 2010;95:5266–73. doi: 10.1210/jc.2010-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheikh JI, Yesavage JA. Geriatric depression scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–73. [Google Scholar]
- 20.Stone KL, Seeley DB, Lui LY, Cauley JA, Ensrud K, Browner WS, Nevitt MC, Cummings SR. BMD at multiple sites and risk of facture of multiple types: long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res. 2003;18:1947–1954. doi: 10.1359/jbmr.2003.18.11.1947. [DOI] [PubMed] [Google Scholar]
- 21.Johnell O, Kanis JA, Oden A, Sernbo I, Redlund-Hohnell, Petterson C, De Laet C, Jonsson B. Fracture risk following an osteoporotic fracture. Osteoporos Int. 2004;15:175–79. doi: 10.1007/s00198-003-1514-0. [DOI] [PubMed] [Google Scholar]
- 22.Schousboe JT, Fink HA, Lui L-Y, Taylor BC, Ensrud KE. Association between prior non-spine non-hip fractures or prevalent radiographic vertebral deformities known to be at least 10 years old and incident hip fracture. J Bone Miner Res. 2006;21:1557–1564. doi: 10.1359/jbmr.060711. [DOI] [PubMed] [Google Scholar]
- 23.Cook NR. Use and misuse of receiver operating characteristic curve in risk prediction. Circulation. 2007;115:928–35. doi: 10.1161/CIRCULATIONAHA.106.672402. [DOI] [PubMed] [Google Scholar]
- 24.Browner WB, Seeley DG, Vogt TM, Cummings SR. Non-trauma mortality in elderly women with low bone mineral density. Study of Osteoporotic Fractures Research Group. Lancet. 1991;338:355–8. doi: 10.1016/0140-6736(91)90489-c. [DOI] [PubMed] [Google Scholar]
- 25.Kado DM, Brown WS, Blackwell T, Gore R, Cummings SR. Rate of bone loss is associated with mortality in older women: a prospective study. J Bone Miner Res. 2000;15:1974–80. doi: 10.1359/jbmr.2000.15.10.1974. [DOI] [PubMed] [Google Scholar]
- 26.Fon GT, Pitt MJ, Thies AC., JR Thoracic kyphosis: range in normal subjects. AJR Am J Roentgenol. 1980;134:979–83. doi: 10.2214/ajr.134.5.979. [DOI] [PubMed] [Google Scholar]
- 27.Durmala J, Detko E, Krawcyzk K. Values of thoracic kyphosis and lumbar lordosis in adolescents from Czestochowa. Scoliosis. 2009;4(Suppl 1):053. [Google Scholar]
- 28.Ensrud KE, Black DM, Harris F, Ettinger B, Cummings SR. Correlates of kyphosis in older women. J Am Geriatr Soc. 1997;45:682–87. doi: 10.1111/j.1532-5415.1997.tb01470.x. [DOI] [PubMed] [Google Scholar]
- 29.Schneider DL, von Muhlen D, Barrett-Connor E, Sartoris DJ. Kyphosis does not equal vertebral fractures: The Rancho Bernardo Study. J Rheumatol. 2004;31:747–52. [PubMed] [Google Scholar]
- 30.Cauley JA, Hochberg MC, Lui LY, Palermo L, Ensrud KE, Hillier TA, Nevitt MC, Cummings SR. Long-term risk of incident vertebral fractures. JAMA. 2007;23:2761–7. doi: 10.1001/jama.298.23.2761. [DOI] [PubMed] [Google Scholar]
- 31.Cooper C, Atkinson EJ, O’Fallon WM, Melton LJ. Incidence of clinically diagnosed vertebral fractures: a population-based study. J Bone Miner Res. 1992;7:221–7. doi: 10.1002/jbmr.5650070214. [DOI] [PubMed] [Google Scholar]
- 32.Kado DM, Browner WS, Palermo L, Nevitt MC, Genant HK, Cummings SR. Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1999;159:1215–20. doi: 10.1001/archinte.159.11.1215. [DOI] [PubMed] [Google Scholar]
- 33.Katzman W, Cawthon P, Hicks GE, Vittinghoff E, Shepherd J, Cauley JA, Harris T, Simonsick EM, Strotmeyer E, Womack C, Kado DM. Association of spinal muscle composition and prevalence of hyperkyphosis in healthy community-dwelling older men and women. J Gerontol A Biol Sci Med Sci. 2012;67:191–5. doi: 10.1093/gerona/glr160. [DOI] [PMC free article] [PubMed] [Google Scholar]


