Abstract
The deleterious effects of cannabis use in schizophrenia have been linked, in part, to underlying disturbances in endogenous cannabinoid signaling in the prefrontal cortex. However, while receptor autoradiography studies of the primary cannabinoid receptor (CB1R) have consistently found higher CB1R binding in the prefrontal cortex in schizophrenia, deficits in CB1R mRNA levels and protein immunoreactivity have also been reported in the illness. To investigate this apparent discrepancy, we quantified CB1R binding using receptor autoradiography with the selective CB1R ligand [3H]-OMAR in the prefrontal cortex of 21 subjects with schizophrenia that were previously found to have lower levels of both CB1R mRNA using in situ hybridization and CB1R protein using radioimmunocytochemistry relative to matched healthy comparison subjects. We observed higher levels of [3H]-OMAR binding in the prefrontal cortex of schizophrenia subjects that did not appear to be attributable to psychotropic medications or substance abuse. The combination of lower levels of CB1R mRNA and immunoreactivity with higher CB1R receptor binding may reflect either altered trafficking of the receptor resulting in higher levels of membrane-bound CB1R or higher CB1R affinity. In either case, greater CB1R receptor availability may contribute to the increased susceptibility of schizophrenia subjects to the deleterious effects of cannabis use.
Keywords: CB1R, cannabis, cannabinoid, marijuana, PET, rimonabant, radioligand, prefrontal cortex, schizophrenia
1. Introduction
Cannabis use during early adolescence has been linked to a higher risk of developing schizophrenia, an earlier age of onset, and greater illness severity (D'Souza et al. 2005; Compton et al. 2009; Foti et al. 2010; Casadio et al. 2011; Galvez-Buccollini et al. 2012). These associations may indicate that the disease process of schizophrenia involves disturbances in endogenous cannabinoid signaling which may in turn predispose at-risk adolescents to greater debilitating effects of cannabis use. However, at the present time, studies of the primary endogenous cannabinoid receptor (CB1R) in the brains of individuals with schizophrenia have yielded apparently conflicting results. Receptor autoradiography studies using a CB1R agonist ([3H]-CP55940) or antagonists/inverse agonists ([3H]-SR141716 and [3H]-MePPEP) have consistently found higher CB1R binding to receptor protein across multiple brain regions, including the prefrontal cortex, in schizophrenia subjects (Dean et al. 2001; Zavitsanou et al. 2004; Newell et al. 2006; Dalton et al. 2011; Jenko et al. 2012). In contrast, we previously reported lower CB1R mRNA levels by in situ hybridization and CB1R protein levels using radioimmunocytochemistry in the prefrontal cortex of subjects with schizophrenia (Eggan et al. 2008; Eggan et al. 2010b). Furthermore, other groups have reported either lower or unchanged levels of CB1R immunoreactivity and mRNA in prefrontal cortex and anterior cingulate cortex (Koethe et al. 2007; Uriguen et al. 2009). The reason for this discrepancy in CB1R measures in schizophrenia has remained unclear.
Interestingly, binding of an allosteric modulation site has been reported to induce a conformational change in CB1R, which in turn increases the affinity of ligands such as [3H]-CP55940 for the orthosteric binding site on CB1R (Price et al. 2005). Thus, reports of higher CB1R binding in the prefrontal cortex in schizophrenia may reflect greater receptor affinity, perhaps even in the presence of fewer CB1Rs. The recent development of a novel analog of the selective CB1R inverse agonist rimonabant, OMAR (JHU75528; 4-cyano-1-(2,4-dichlorophenyl)-5-(4-methoxyphenyl)-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide), that displays a high binding affinity for CB1R (Fan et al. 2006) provides an opportunity to examine this idea. Indeed, a pilot PET study employing radiolabeled [11C]-OMAR reported evidence of higher brain CB1R receptor binding in some subjects with schizophrenia (Wong et al. 2010) and of an inverse correlation between CB1R receptor binding and negative symptoms assessed using the Brief Psychiatric Rating Scale (Wong et al. 2012). Consequently, we conducted a receptor autoradiography study using [3H]-OMAR in the same schizophrenia subjects in whom we had previously found lower CB1R mRNA levels by in situ hybridization and protein levels by radioimmunocytochemistry. Employing a highly similar film-based quantification approach across studies, we sought to determine the relationship between CB1R binding and CB1R mRNA and immunoreactivity levels in nearby tissue sections from the same subjects with schizophrenia.
2. Materials and methods
2.1. [3H]-OMAR ([Methoxy-3H]JHU75528)
The radiotracer was purchased from PerkinElmer (Shelton, Connecticut). The specific radioactivity was > 80 Ci/mmol and radiochemical purity was 97.0%.
2.2. Human subjects
Brain specimens were obtained during routine autopsies conducted at the Allegheny County Office of the Medical Examiner (Pittsburgh, PA) after consent was obtained from next-of-kin. An independent committee of experienced research clinicians made consensus DSMIV (American Psychiatric Association 1994) diagnoses for each subject using structured interviews with family members and review of medical records, and the absence of a psychiatric diagnosis was confirmed in healthy comparison subjects (Volk et al. 2011; Volk et al. 2012). To control for experimental variance, subjects with schizophrenia or schizoaffective disorder (n=21) were matched individually to one healthy comparison subject for sex and as closely as possible for age and postmortem interval (Table 1; Supplemental Table S1) as previously described (Eggan et al. 2008), and samples from subjects in a pair were processed together throughout all stages of the study. The mean age, postmortem interval, brain pH, and tissue freezer storage time did not differ between subject groups (t(40) ≤0.67, p ≥0.51) (Table 1). In the right hemisphere of all subject pairs, CB1R mRNA and protein levels were previously quantified by in situ hybridization and radioimmunocytochemistry, respectively (Eggan et al. 2008). (Two subject pairs from this previous study of CB1R mRNA and protein levels were not included in the present study due to lack of tissue availability). All procedures were approved by the University of Pittsburgh's Committee for the Oversight of Research Involving the Dead and Institutional Review Board for Biomedical Research.
2.3. Tissue processing
For each subject, coronal blocks through the right prefrontal cortex were frozen and stored at −80°C. Cryostat sections (20 μm) from the middle portion of the superior frontal sulcus were thaw mounted on Superfrost slides (VWR Scientific, West Chester, Pennsylvania) and stored at −80°C. Cytoarchitectonic criteria (Rajkowska and Goldman-Rakic 1995) were used to identify the location of area 9 in Nissl-stained sections (Volk et al. 2000). For each subject, 3 sections separated by at least 320 μm and at a similar rostral-caudal level to the matched subject within the pair were processed for quantitative receptor autoradiography. The selected sections were located within the same tissue block as the tissue sections previously studied for CB1R mRNA using in situ hybridization and protein using radioimmunocytochemistry.
2.4. CB1R autoradiography with [3H]-OMAR
To our knowledge, [3H]-OMAR has not been employed previously in radiolabeled ligand binding studies of human prefrontal cortex tissue. Consequently, we tested a wide range of concentrations of [3H]-OMAR (1.25 nM - 140 nM) in a series of pilot studies including prefrontal cortex tissue sections from 3-4 healthy comparison subjects. We found that 10 nM [3H]-OMAR provided the clearest and most specific signal as indicated by a sharp and clearly demarcated gray matter-white matter border (Figure 1A-B), and this specific signal was greatly reduced by competition with 1000× fold excess cold CP55940 (a CB1R agonist), cold SR141716 (a CB1R antagonist/inverse agonist), and cold OMAR in separate pilot studies. Consequently, a concentration of 10 nM [3H]-OMAR was used in the final study.
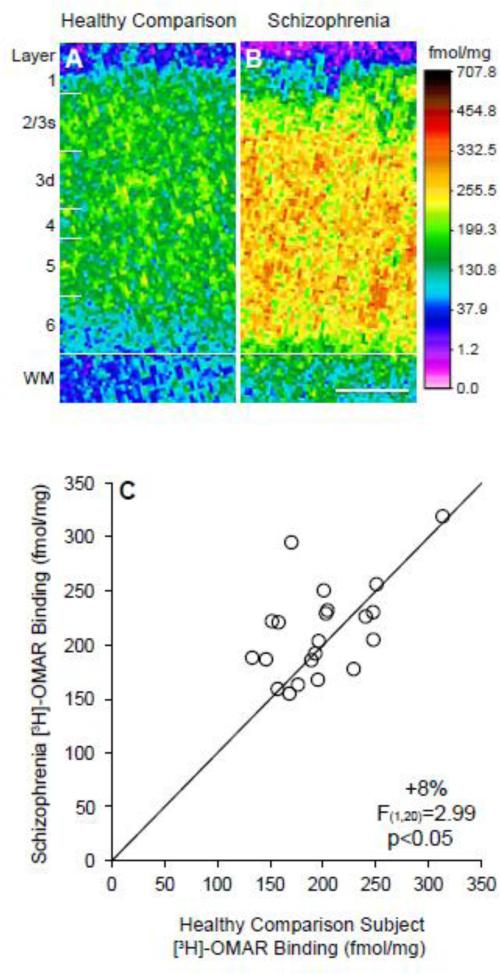
Figure 1. Receptor autoradiography for [3H]-OMAR binding in the prefrontal cortex in schizophrenia.
A-B. Pseudocolored film autoradiographs of prefrontal cortical sections processed by receptor autoradiography demonstrate higher [3H]-OMAR binding in a schizophrenia subject (B) relative to the matched comparison subject (A). Solid white line indicates the layer 6/white matter (WM) border; white distance calibration bar = 1 mm. C. Average [3H]-OMAR binding levels across gray matter of prefrontal cortical area 9 for schizophrenia subjects relative to matched healthy comparison subjects in a pair are indicated by open circles. Data points to the left of the unity line indicate higher [3H]-OMAR binding levels in the schizophrenia subject relative to the healthy comparison subject and vice versa. Mean [3H]-OMAR binding was 8% higher in schizophrenia subjects relative to matched healthy comparison subjects.
Glass slides containing tissue sections were removed from the freezer and allowed to thaw and dry at room temperature. Slides were placed in a pre-incubation buffer containing 50 mM Tris-HCl (pH 7.4) for 30 min at room temperature, then incubated in 10 nM [3H]-OMAR (specific activity 81.8 Ci/mmol) in 50 mM Tris-HCl containing 1% bovine serum albumin (pH 7.4) for one hour at room temperature. Slides were then placed through a series of post-incubation rinses in 50 mM Tris-HCl/1% bovine serum albumin (pH 7.4) at 4°C, rinsed briefly in ice-cold ultrapure water, and air dried in front of a fan. Tissue sections were then exposed to BioMax MR film (Kodak, Sigma-Aldrich, St. Louis, Missouri) along with tritium standards (μCi/g) on glass slides (American Radiolabeled Chemicals, St. Louis, Missouri) for 4.5 weeks. All three tissue sections from each of the 21 schizophrenia and 21 healthy comparison subjects were incubated in [3H]-OMAR on the same day and exposed to film on the same day, and later the films were developed on the same day.
2.5. Quantification of [3H]-OMAR binding
[3H]-OMAR binding was quantified using a Microcomputer Imaging Device system (Imaging Research Inc, London, Ontario, Canada) without knowledge of diagnosis or subject number by random coding of film autoradiograms in a manner highly similar to our previous studies of CB1R mRNA using in situ hybridization and protein using radioimmunocytochemistry conducted in nearby tissue sections from the same subjects (Eggan et al. 2008). Briefly, prefrontal cortex area 9 gray matter optical density was measured in each tissue section by drawing contours of the full thickness of the cortex exclusively in the zones where the cortex was cut perpendicular to the pial surface. The mean (SD) total area of gray matter sampled in each subject was 277 (109) mm2 for control subjects and 291 (141) mm2 for subjects with schizophrenia. Standardization curves derived from the tritium standards exposed on the same film were used to convert optical density measures of the autoradiograms into fmol [3H]-OMAR/mg estimated tissue equivalents using the specific activity of [3H]-OMAR (81.8 Ci/mmol). Next, to determine differences in [3H]-OMAR binding across lamina, optical density was measured in approximately 1 mm-wide cortical traverses extending from the pial surface to the white matter (Eggan et al. 2008). Three cortical traverses per section (9 traverses per subject) were placed in locations where the tissue section was cut perpendicular to the pial surface as determined by the presence of pyramidal neurons with vertically oriented apical dendrites in adjacent Nissl-stained sections. Within each traverse, the optical density in each layer was determined by dividing the total cortical thickness from the pial surface to white matter into zones of 1% to 10%, 10% to 30%, 30% to 50%, 50% to 60%, 60% to 80%, and 80% to 100% approximating layers 1, 2 to superficial 3, deep 3, 4, 5, and 6, respectively (Pierri et al. 1999; Eggan et al. 2008).
2.6. Statistical analysis
Analyses of covariance (ANCOVA) were first conducted to determine whether [3H]-OMAR binding measures were related to sex, race, age at death, postmortem interval, brain pH, and/or freezer storage time. No effect of these covariates was found on [3H]-OMAR binding (all F≤2.3; p≥0.14), and Pearson correlation analyses also found no relationship between the continuous covariates and [3H]-OMAR binding (all r≤|.24|, p≥.13) Consequently, these covariates were not included in the final analysis. However, subject pairing, which accounts for the parallel processing of tissue samples from a pair and balances diagnostic groups for sex, age, and postmortem interval, had a significant effect on [3H]-OMAR binding (F(1,20)=3.30; p=0.005). The significant effect of pair may be thought of as validating the paired approach as the matching of subjects as pairs captured a substantial portion of the variance across all subjects due to factors other than the effect of diagnosis. Therefore, the ANCOVA model we report includes [3H]-OMAR binding as the dependent variable, diagnostic group as the main effect, and subject pair as a blocking factor. The reported p values for comparisons of [3H]-OMAR binding are one-tailed because CB1R receptor binding has been repeatedly shown to be higher in the prefrontal cortex in schizophrenia (Dean et al. 2001; Zavitsanou et al. 2004; Newell et al. 2006; Dalton et al. 2011; Jenko et al. 2012).
3. Results
3.1. [3H]-OMAR binding in the prefrontal cortex in schizophrenia
Horti, Wong, and colleagues previously demonstrated a high degree of specificity of [11C]-OMAR binding for CB1R including: 1) mouse, baboon, and human studies that found a regional distribution of OMAR binding (e.g., high levels in cerebral cortex gray matter, globus pallidus, hippocampus and cerebellum and low levels in cerebral cortex white matter and thalamus) (Horti et al. 2006; Wong et al. 2010) consistent with the reported distribution of CB1R by radioimmunocytochemistry (Eggan and Lewis 2007); and 2) blocking of specific OMAR binding by a selective CB1R inverse agonist rimonabant (SR141716) (Horti et al. 2006). Consistent with this evidence of specific binding to CB1R, we also found that [3H]-OMAR binding is much greater in prefrontal cortex gray matter than in white matter (Figure 1).
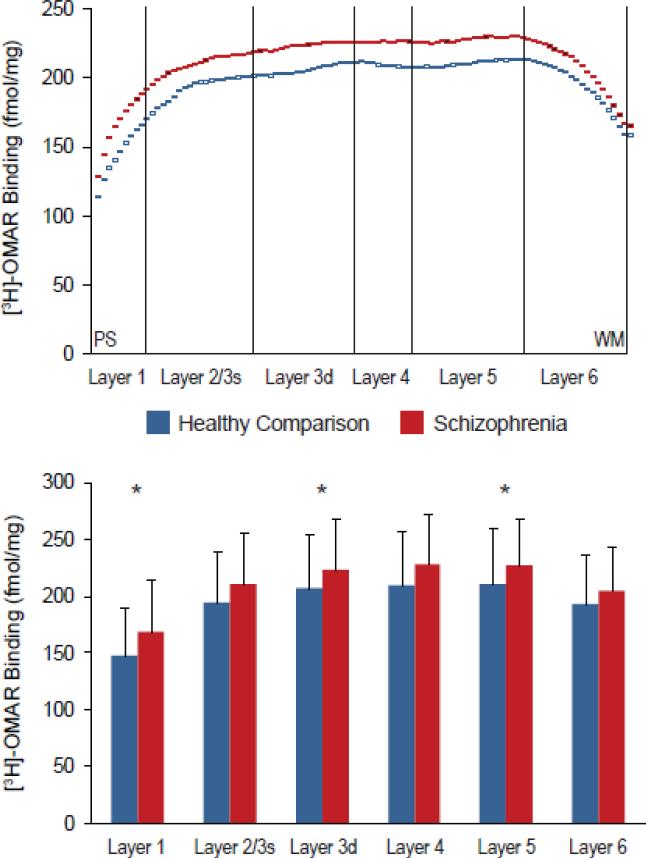
Quantitative receptor autoradiography with the highly selective CB1R ligand [3H]-OMAR revealed higher binding (+8%; F(1,20)=2.99, p<.05) in the gray matter of schizophrenia subjects (213.3 ± 43.1 fmol/mg) relative to healthy comparison subjects (197.5 ± 43.8 fmol/mg). Laminar analysis revealed that [3H]-OMAR binding was increased in all cortical layers in schizophrenia subjects relative to healthy comparison subjects (Figure 2). These differences were statistically significant in layer 1 (+14.5%; F(1,20)=3.90, p=0.031), layer deep 3 (+8.2%; F(1,20)=3.01, p=0.049), and layer 5 (+8.2%; F(1,20)=2.97, p=0.05), but did not reach significance in layer 2/superficial 3 (+8.8%; F(1,20)=2.57, p=0.063), layer 4 (+8.2%; F(1,20)=2.85, p=0.054), or layer 6 (+6.2%; F(1,20)=1.98, p=0.088). Furthermore, we found that [3H]-OMAR binding did not differ in schizophrenia subjects with versus without a history of suicide as manner of death, cannabis use, smoking or use of antipsychotic, antidepressant, or benzodiazepine or anticonvulsant medications at time of death (all F≤2.39, p≥0.148).
Figure 2. Laminar analysis of [3H]-OMAR binding in the prefrontal cortex of schizophrenia subjects.
A. Mean [3H]-OMAR binding levels from all schizophrenia subjects (red) and healthy comparison subjects (blue) measured in cortical traverses from the pial surface (PS) to the white matter (WM) border. B. Mean [3H]-OMAR binding levels from all schizophrenia and healthy comparison subjects were determined in each cortical layer. Error bars indicate standard deviations in each cortical layer, and asterisks indicate statistically significant differences between schizophrenia and healthy comparison subjects.
3.2. Relationship between [3H]-OMAR binding and mRNAs for CB1R and endogenous cannabinoid synthesizing and metabolizing enzymes
In the prefrontal cortex of the present cohort of schizophrenia subjects, we previously reported lower CB1R mRNA levels and lower levels of CB1R radioimmunoreactivity (Eggan et al. 2008). In the same schizophrenia subjects, we also previously reported higher mRNA levels for serine hydrolase α-β-hydrolase domain 6 (ABHD6) (Volk et al. 2013), which metabolizes the major cortical endocannabinoid 2-arachidonoylglycerol and tightly regulates 2-arachidonoylglycerol signaling in the prefrontal cortex (Marrs et al. 2010). In contrast, mRNA levels for the synthesizing (diacylglycerol lipase α) and for other metabolizing (monoglyceride lipase) enzymes for 2-arachidonoylglycerol were not altered in these subjects with schizophrenia (Volk et al. 2010).
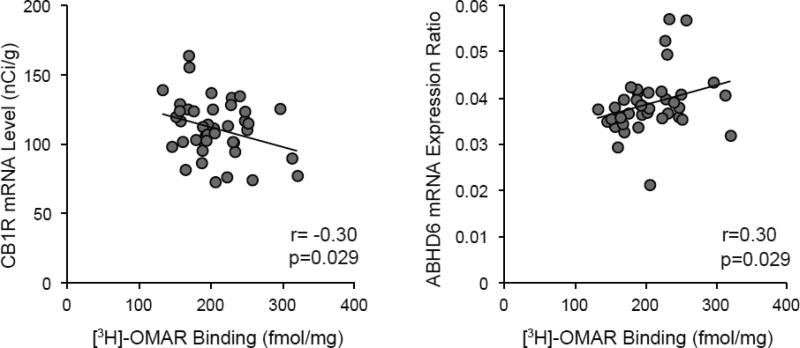
Therefore, we sought to determine whether [3H]-OMAR binding is associated with CB1R mRNA levels, CB1R radioimmunoreactivity levels, or mRNA levels for 2-arachidonoylglycerol synthesizing and metabolizing enzymes in the same subjects. Interestingly, as shown in Figure 3, [3H]-OMAR binding was negatively correlated with mRNA levels for CB1R (r=-0.30 [95% confidence interval -0.60, 0.01], p=0.029) and positively correlated with mRNA levels for ABHD6 (r=0.30 [95% confidence interval -0.01, 0.60], p=0.029). In contrast, no relationship was found between [3H]-OMAR binding and CB1R radioimmunoreactivity levels (r=.003; p=.49) or mRNA levels for diacylglycerol lipase α (r=-0.16, p=0.16) or monoglyceride lipase (r=0.04, p=0.41).
Figure 3. Relationship between [3H]-OMAR binding and mRNA levels for CB1R and an endogenous endocannabinoid synthesizing enzyme.
In prefrontal cortex gray matter from the same cohort of subjects, [3H]-OMAR binding is negatively correlated with mRNA levels for CB1R (r=-0.30, p=0.029) that were previously quantified using in situ hybridization and a highly similar film analysis (Eggan et al. 2008). In contrast, [3H]-OMAR binding is positively correlated with mRNA levels for ABHD6 (r=0.30, p=0.029) that were previously quantified by quantitative PCR (Volk et al. 2013) in the same subjects.
4. Discussion
Elucidating the nature of the deleterious relationship between cannabis use and schizophrenia requires an investigation into underlying disturbances in the endogenous cannabinoid system in the disorder. However, prior studies of CB1R in the prefrontal cortex of subjects with schizophrenia have reported apparently conflicting findings of higher CB1R receptor binding (Dean et al. 2001; Zavitsanou et al. 2004; Newell et al. 2006; Dalton et al. 2011; Jenko et al. 2012) and lower or unchanged CB1R mRNA and protein immunoreactivity levels (Koethe et al. 2007; Eggan et al. 2008; Uriguen et al. 2009; Eggan et al. 2010b). We sought to explore this apparent discrepancy by conducting CB1R receptor binding studies with a new ligand and comparing the results to measures of CB1R mRNA and protein (assessed by radioimmunocytochemistry) in nearby prefrontal cortex tissue sections from the same cohort of schizophrenia and comparison subjects. Using the novel CB1R selective ligand [3H]-OMAR, we report that schizophrenia subjects with lower CB1R mRNA and protein immunoreactivity levels also have higher levels of [3H]-OMAR binding to CB1R. The magnitude of higher [3H]-OMAR binding in the prefrontal cortex in schizophrenia that we report (+8%) is consistent with, though slightly smaller, than the reported increases in binding previously reported using other CB1R ligands in the same brain region (+19-25% using [3H]-MePPeP and [3H]-CP55940) (Dean et al. 2001; Dalton et al. 2011; Jenko et al. 2012). Furthermore, we report for the first time that [3H]-OMAR receptor binding was increased across all cortical layers in schizophrenia subjects and achieved statistical significance in layers 1, deep 3, and 5.
The combination of lower CB1R mRNA and protein immunoreactivity with higher CB1R receptor binding in the same schizophrenia subjects may reflect a redistribution of the receptor due to altered trafficking of the receptor and higher levels of membrane-bound CB1R. Thus, while the total amount of CB1R may be reduced, as suggested by lower CB1R mRNA and protein immunoreactivity levels, levels of functional CB1R that are present on the membrane and accessible to ligand binding may actually be increased. Alternatively, the presence of higher levels of [3H]-OMAR receptor binding in the face of lower CB1R protein immunoreactivity levels might reflect greater CB1R affinity which could lead to a reduced need for CB1R receptors resulting in lower CB1R transcription and translation in schizophrenia. Unfortunately, we could not directly assess CB1R affinity in this study due to the apparent receptor saturation that occurred at higher doses of OMAR ligand. Furthermore, while binding of an allosteric modulation site can induce a conformational change in CB1R which increases the affinity of an agonist ligand (i.e. [3H]-CP55940) for the orthosteric binding site on CB1R (Price et al. 2005), evidence supporting a similar affinity change for antagonist/inverse agonist ligands such as [3H]-OMAR is not currently available. As a third interpretation, lower CB1R mRNA and protein immunoreactivity levels may instead reflect a failure of CB1R-containing axon terminals to fully develop in schizophrenia. Consequently, the remaining axon terminals that contain CB1R may have a higher affinity as a compensatory response in an attempt to maintain homeostatic levels of endogenous cannabinoid signaling. Each of these interpretations is supported by the negative correlations between [3H]-OMAR receptor binding and CB1R mRNA levels in the same subjects. As a fourth interpretation, the lack of a correlation between [3H]-OMAR binding and CB1R radioimmunoreactivity levels in our study may reflect the fact that our previous radioimmunocytochemistry study employed an antibody that primarily targets a CB1R epitope present on inhibitory axon terminals and not on excitatory axon terminals (Eggan et al. 2010a) while the [3H]-OMAR ligand presumably binds to all available CB1R regardless of cell-type localization. Consequently, it may also be that CB1R levels are lower in inhibitory axons but higher in excitatory axons in schizophrenia. However, additional studies that quantify CB1R levels specifically in pyramidal neurons in schizophrenia are required to further test this interpretation. As a final interpretation, we also previously reported higher mRNA levels for ABHD6, but not diacylglyercol lipase or monoglyceride lipase, in this cohort of schizophrenia subjects (Volk et al. 2010; Volk et al. 2013). As described previously, higher levels of ABHD6, which metabolizes 2-arachidonoylglycerol, may lead to lower 2-arachidonoylglycerol levels, and, consequently, a compensatory up-regulation in the membrane localization or affinity of locally affected CB1R that does not necessitate a corresponding upstream change in CB1R transcription and translation.
Discriminating among these possible interpretations requires additional testing through animal models and additional cell-type specific measures of CB1R levels in schizophrenia. However, our findings of higher CB1R binding, even in the presence of deficits in CB1R mRNA and protein immunoreactivity levels, may have important relevance for cannabis use in schizophrenia. The consistent finding of higher CB1R binding in the present study and many others (Dean et al. 2001; Zavitsanou et al. 2004; Newell et al. 2006; Dalton et al. 2011; Jenko et al. 2012) suggests that schizophrenia subjects may be more susceptible and have a amplified response to the effects of cannabis, even in the face of lower numbers of receptors. Furthermore, if the number of CB1R-containing axon terminals is indeed reduced and higher CB1R receptor binding is a compensatory response in individuals with schizophrenia, then cannabis use may interfere with the functioning of an already disturbed signaling pathway.
Supplementary Material
Acknowledgements
The authors wish to acknowledge Elizabeth Sengupta, MA for her work in processing the tissue sections and analyzing the data.
Role of funding source
This study was supported by grants from the National Institutes of Health (MH084016 to Dr. Volk; MH043784 and DA023109 to Dr. Lewis, and MH079017 to Dr. Horti).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Dr. Volk oversaw all aspects of the design and implementation of the study and was the primary author of the manuscript. Drs. Eggan, Horti, Wong, and Lewis contributed to the design of the study and interpretation of the data. All authors contributed to and have approved the final manuscript.
Disclosures
David A. Lewis currently receives investigator-initiated research support from Bristol-Myers Squibb and Pfizer and in 2012-2014 served as a consultant in the areas of target identification and validation and new compound development to Autifony, Bristol-Myers Squibb, Concert Pharmaceuticals, and Sunovion. All other authors have nothing to disclose.
Reference List
- American Psychiatric Association . DSM-IV. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. 4. American Psychiatric Association; Washington, D.C.: 1994. [Google Scholar]
- Casadio P, Fernandes C, Murray RM, Di Forti M. Cannabis use in young people: the risk for schizophrenia. Neurosci Biobehav Rev. 2011;35:1779–1787. doi: 10.1016/j.neubiorev.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Compton MT, Kelley ME, Ramsay CE, Pringle M, Goulding SM, Esterberg ML, Stewart T, Walker EF. Association of pre-onset cannabis, alcohol, and tobacco use with age at onset of prodrome and age at onset of psychosis in first-episode patients. Am J Psychiatry. 2009;166:1251–1257. doi: 10.1176/appi.ajp.2009.09030311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza DC, Abi-Saab WM, Madonick S, Forselius-Bielen K, Doersch A, Braley G, Gueorguieva R, Cooper TB, Krystal JH. Delta-9-tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biol Psychiatry. 2005;57:594–608. doi: 10.1016/j.biopsych.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Dalton VS, Long LE, Weickert CS, Zavitsanou K. Paranoid schizophrenia is characterized by increased CB1 receptor binding in the dorsolateral prefrontal cortex. Neuropsychopharm. 2011;36:1620–1630. doi: 10.1038/npp.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean B, Sundram S, Bradbury R, Scarr E, Copolov D. Studies on [3H]CP-55940 binding in the human central nervous system: regional specific changes in density of cannabinoid-1 receptors associated with schizophrenia and cannabis use. Neuroscience. 2001;103:9–15. doi: 10.1016/s0306-4522(00)00552-2. [DOI] [PubMed] [Google Scholar]
- Eggan SM, Lewis DA. Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate neocortex: a regional and laminar analysis. Cereb Cortex. 2007;17:175–191. doi: 10.1093/cercor/bhj136. [DOI] [PubMed] [Google Scholar]
- Eggan SM, Hashimoto T, Lewis DA. Reduced cortical cannabinoid 1 receptor messenger RNA and protein expression in schizophrenia. Arch Gen Psychiatry. 2008;65:772–784. doi: 10.1001/archpsyc.65.7.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggan SM, Melchitzky DS, Sesack SR, Fish KN, Lewis DA. Relationship of cannabinoid CB1 receptor and cholecystokinin immunoreactivity in monkey dorsolateral prefrontal cortex. Neuroscience. 2010a;169:1651–1661. doi: 10.1016/j.neuroscience.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggan SM, Stoyak SR, Verrico CD, Lewis DA. Cannabinoid CB1 receptor immunoreactivity in the prefrontal cortex: Comparison of schizophrenia and major depressive disorder. Neuropsychopharm. 2010b;35:2060–2071. doi: 10.1038/npp.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H, Ravert HT, Holt DP, Dannals RF, Horti AG. Synthesis of 1-(2,4-dichlorophenyl)-4-cyano-5-(4-[11C]methoxyphenyl)-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide ([11C]JHU75528) and 1-(2-bromophenyl)-4-cyano-5-(4-[11C]methoxyphenyl)-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide ([11C]JHU75575) as potential radioligands for PET imaging of cerebral cannabinoid receptor. J Label Compd Radiopharm. 2006;49:1021–1036. [Google Scholar]
- Foti DJ, Kotov R, Guey LT, Bromet EJ. Cannabis use and the course of schizophrenia: 10-year follow-up after first hospitalization. Am J Psychiatry. 2010;167:987–993. doi: 10.1176/appi.ajp.2010.09020189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez-Buccollini JA, Proal AC, Tomaselli V, Trachtenberg M, Coconcea C, Chun J, Manschreck T, Fleming J, DeLisi LE. Association between age at onset of psychosis and age at onset of cannabis use in non-affective psychosis. Schizophr Res. 2012;139:157–160. doi: 10.1016/j.schres.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horti AG, Fan H, Kuwabara H, Hilton J, Ravert HT, Holt DP, Alexander M, Kumar A, Rahmim A, Scheffel U, Wong DF, Dannals RF. 11C-JHU75528: a radiotracer for PET imaging of CB1 cannabinoid receptors. J Nucl Med. 2006;47:1689–1696. [PubMed] [Google Scholar]
- Jenko KJ, Hirvonen J, Henter ID, Anderson KB, Zoghbi SS, Hyde TM, Deep-Soboslay A, Innis RB, Kleinman JE. Binding of a tritiated inverse agonist to cannabinoid CB1 receptors is increased in patients with schizophrenia. Schizophr Res. 2012;141:185–188. doi: 10.1016/j.schres.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koethe D, Llenos IC, Dulay JR, Hoyer C, Torrey EF, Leweke FM, Weis S. Expression of CB1 cannabinoid receptor in the anterior cingulate cortex in schizophrenia, bipolar disorder, and major depression. J Neural Transm. 2007;114:1055–1063. doi: 10.1007/s00702-007-0660-5. [DOI] [PubMed] [Google Scholar]
- Marrs WR, Blankman JL, Horne EA, Thomazeau A, Lin YH, Coy J, Bodor AL, Muccioli GG, Hu SS, Woodruff G, Fung S, Lafourcade M, Alexander JP, Long JZ, Li W, Xu C, Moller T, Mackie K, Manzoni OJ, Cravatt BF, Stella N. The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nat Neurosci. 2010;13:951–957. doi: 10.1038/nn.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell KA, Deng C, Huang XF. Increased cannabinoid receptor density in the posterior cingulate cortex in schizophrenia. Exp Brain Res. 2006;172:556–560. doi: 10.1007/s00221-006-0503-x. [DOI] [PubMed] [Google Scholar]
- Pierri JN, Chaudry AS, Woo T-U, Lewis DA. Alterations in chandelier neuron axon terminals in the prefrontal cortex of schizophrenic subjects. Am J Psychiatry. 1999;156:1709–1719. doi: 10.1176/ajp.156.11.1709. [DOI] [PubMed] [Google Scholar]
- Price MR, Baillie GL, Thomas A, Stevenson LA, Easson M, Goodwin R, McLean A, McIntosh L, Goodwin G, Walker G, Westwood P, Marrs J, Thomson F, Cowley P, Christopoulos A, Pertwee RG, Ross RA. Allosteric modulation of the cannabinoid CB1 receptor. Mol Pharmacol. 2005;68:1484–1495. doi: 10.1124/mol.105.016162. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: I. Remapping of areas 9 and 46 using quantitative criteria. Cereb Cortex. 1995;5:307–322. doi: 10.1093/cercor/5.4.307. [DOI] [PubMed] [Google Scholar]
- Uriguen L, Garcia-Fuster MJ, Callado LF, Morentin B, La Harpe R, Casado V, Lluis C, Franco R, Garcia-Sevilla JA, Meana JJ. Immunodensity and mRNA expression of A2A adenosine, D2 dopamine, and CB1 cannabinoid receptors in postmortem frontal cortex of subjects with schizophrenia: effect of antipsychotic treatment. Psychopharmacology (Berl) 2009;206:313–324. doi: 10.1007/s00213-009-1608-2. [DOI] [PubMed] [Google Scholar]
- Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- Volk DW, Eggan SM, Lewis DA. Alterations in metabotropic glutamate receptor 1alpha and regulator of G protein signaling 4 in the prefrontal cortex in schizophrenia. Am J Psychiatry. 2010;167:1489–1498. doi: 10.1176/appi.ajp.2010.10030318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk DW, Radchenkova PV, Walker EM, Sengupta EJ, Lewis DA. Cortical opioid markers in schizophrenia and across postnatal development. Cereb Cortex. 2011;22:1215–1223. doi: 10.1093/cercor/bhr202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk DW, Matsubara T, Li S, Sengupta EJ, Georgiev D, Minabe Y, Sampson A, Hashimoto T, Lewis DA. Deficits in transcriptional regulators of cortical parvalbumin neurons in schizophrenia. Am J Psychiatry. 2012;169:1082–1091. doi: 10.1176/appi.ajp.2012.12030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk DW, Siegel BI, Verrico CD, Lewis DA. Endocannabinoid metabolism in the prefrontal cortex in schizophrenia. Schizophr Res. 2013;147:53–57. doi: 10.1016/j.schres.2013.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DF, Kuwabara H, Horti AG, Brasic JR, Raymont V, Nandi A, Rahmim A, Gean E, Dannals RF, Cascella N. Cannabinoid receptor subtype 1 (CB1) distribution correlates with neuropsychiatric ratings. Society of Biological Psychiatry Abstract . 2012 [Google Scholar]
- Wong DF, Kuwabara H, Horti AG, Raymont V, Brasic J, Guevara M, Ye W, Dannals RF, Ravert HT, Nandi A, Rahmim A, Ming JE, Grachev I, Roy C, Cascella N. Quantification of cerebral cannabinoid receptors subtype 1 (CB1) in healthy subjects and schizophrenia by the novel PET radioligand [11C]OMAR. NeuroImage. 2010;52:1505–1513. doi: 10.1016/j.neuroimage.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavitsanou K, Garrick T, Huang XF. Selective antagonist [3H]SR141716A binding to cannabinoid CB1 receptors is increased in the anterior cingulate cortex in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:355–360. doi: 10.1016/j.pnpbp.2003.11.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.