Abstract
Objective
To describe variation across US pediatric hospitals in the utilization of resources not recommended for routine use by the AAP guideline for infants hospitalized with bronchiolitis and to examine the association between resource utilization and disposition outcomes.
Study design
We conducted a cross-sectional study of infants ≤12 months hospitalized for bronchiolitis from 2007-2012 at 42 hospitals contributing data to the Pediatric Health Information System. Patients with asthma were excluded. The primary outcome was hospital-level variation in utilization of five resources not recommended for routine use: albuterol, racemic epinephrine, corticosteroids, chest radiography and antibiotics. We also examined the association of resource utilization with length of stay (LOS) and readmission.
Results
64,994 hospitalizations were analyzed. After adjustment for patient characteristics, albuterol (median, 52.4%; range, 3.5%-81%), racemic epinephrine (20.1%; 0.6%-78.8%), and chest radiography (54.9%; 24.1%-76.7%) had the greatest variation across hospitals. Utilization of albuterol, racemic epinephrine, and antibiotics did not change significantly over time compared with small decreases in corticosteroid (3.3%) and chest radiography (8.6%) use over the study period. Utilization of each resource was significantly associated with increased LOS without concomitant decreased odds of readmission.
Conclusions
Substantial use and variation in five resources not recommended for routine use by the AAP bronchiolitis guideline persists with increased utilization associated with increased LOS without the benefit of decreased readmission. Future work should focus on developing processes that can be widely disseminated and easily implemented to minimize unwarranted practice variation when evidence and guidelines exist.
Keywords: bronchiolitis, physician practice patterns, clinical practice variation, clinical practice guidelines, hospital medicine, diagnostic tests, radiography, hospitalization, health services research, health resources, utilization, children
Acute bronchiolitis is the most frequent lower respiratory infection in infants, and the most frequent cause of hospitalization in this age group.(1-3) At a cost of more than $500 million annually, bronchiolitis is one the most expensive diseases of hospitalized children and costs appear to be increasing.(4, 5) Despite numerous evaluations of potential management strategies, effective therapies for bronchiolitis remain elusive.
When evidence is lacking for effective management of a common condition, the result is unwarranted variability in care, the variation in medical care due to differences in health system performance.(6) Clinical practice guidelines aim to decrease this unwarranted practice variation and optimize resource utilization. Several studies performed before the availability of a national bronchiolitis guideline in the United States found considerable variation in the management of bronchiolitis in the emergency department (ED) and inpatient settings, including substantial use of diagnostic tests and therapies without strong evidence to support routine use.(7-10) In October 2006, the American Academy of Pediatrics (AAP) published clinical practice guidelines to provide an evidence-based approach to the diagnosis, management and prevention of bronchiolitis.(11) This guideline recommends against routine use of chest radiography and corticosteroids, and suggested that antibiotics be used only in children with bacterial co-infection. In addition, it recommends against routine use of bronchodilators, but allows for an option of a trial of albuterol or racemic epinephrine to be continued only if the patient demonstrates objective improvement. Since guideline publication, two studies, including one using Pediatric Health Information System (PHIS) data, have documented modest declines in overall utilization of resources not routinely recommended by the AAP guideline for inpatients with bronchiolitis.(12, 13) Another nationally representative study of ED utilization of these resources found a decline in chest radiography, but no decrease in non-recommended therapies.(14) These post-guidelines studies did not address practice variation, and to our knowledge.
Our primary objective was to describe the variation across pediatric hospitals in the use of resources not routinely recommended by the AAP guideline for infants hospitalized with bronchiolitis. We also sought to examine the association between resource utilization and disposition outcomes, including length of stay and hospital readmissions.
Methods
This multicenter cross-sectional study included inpatient visits of children diagnosed with bronchiolitis. Data were from the Pediatric Health Information System (PHIS), an administrative database of 43 not-for-profit, tertiary care pediatric hospitals in the United States affiliated with the Children’s Hospital Association (CHA, Shawnee Mission, KS). This database accounts for ∼20% of annual pediatric hospitalizations in the United States. Data quality and reliability are assured through a joint effort between CHA and participating hospitals. 42 PHIS hospitals that submitted resource utilization data (e.g., pharmaceuticals, imaging, and laboratory tests) were included in this study. Data are de-identified, but encrypted medical record numbers permit identification of patients across multiple visits to the same hospital.(15) For the current study, data were included from 5 respiratory seasons, beginning in October 2007, one year after the guidelines were published, through March 2012, which was the most recent data available at time of analysis.
Patients 12 months and younger who were hospitalized with a diagnosis of bronchiolitis were eligible for inclusion. Bronchiolitis was defined by the presence of both a primary ICD-9 code (466.11, 466.19) and an APR-DRG diagnosis code of bronchiolitis (138) to minimize misclassification.(7) Given that the majority of patients with bronchiolitis at PHIS hospitals are hospitalized for 3 days or less, the study population was limited to those with a length of stay 7 days or less in order to capture patients with typical bronchiolitis that are the target of the AAP guideline. If a patient had multiple hospitalizations over the study period, we included only the first hospitalization, as patients with recurrent hospitalizations for bronchiolitis may be treated differently than those who present with their first episode. Finally, because patients with a diagnosis of asthma would be managed differently, patients with an ICD-9 code of asthma listed in any diagnosis position were excluded.
Utilization was examined for five resources not routinely recommended by the AAP guideline for bronchiolitis care – albuterol, racemic epinephrine, systemic corticosteroids, chest radiography and antibiotics. All resource use was determined using PHIS-specific Clinical Transaction Classification codes.
Outcome Measures
The primary outcome was hospital-level variation in rates of resource utilization of albuterol, racemic epinephrine, systemic corticosteroids, chest radiography and antibiotics. As secondary outcomes, we examined the association of utilization of these five resources with inpatient length of stay and hospital readmission within 3, 7 and 14 days for bronchiolitis after the index hospitalization.
Covariates
The following patient-level covariates were included to account for differences in demographics and case-mix across hospitals: age, sex, race/ethnicity, primary source of payment, and admission season and year. As a measure of patient severity, we utilized the all-patient refined-diagnosis related group (APR-DRG) severity subscore. This score represents the illness severity of hospitalized patients taking into account their entire hospitalization, separated into 4 categories from mild to extreme. They are calculated from computer algorithms on the basis of age, sex, diagnoses, procedures, and discharge status.(16, 17) The following hospital-level covariates were included to account for the effects of individual hospital traits in the outcomes: geographic location, average daily census, percent uninsured, average APR-DRG severity score of all patients cared for at the hospital for the study period, and average annual number of bronchiolitis hospitalizations at each hospital.
Statistical Analyses
Unadjusted distributions for each resource were determined by calculating the rate of subjects at each hospital who received the resource and summarizing these rates across hospitals. Unadjusted rates of utilization over time were explored using the test for linear trend. Adjusted rates were obtained by adjusting hospital-level utilization rates for patient-level characteristics. We employed a mixed-effects logistic regression model for the subject-level binary outcome of resource use (eg, chest radiography, yes/no), adjusted for patient age, race/ethnicity, year and season of presentation, insurance status and APR-DRG severity score, with hospital-specific random intercepts. Each random intercept represented the degree to which a hospital’s resource use departed from what would be expected on average for a hospital with similar case-mix. We used this model to estimate population-averaged rates of resource use expected at each hospital based on its patients’ characteristics. These expected rates were compared with observed rates at each hospital, and an adjusted rate was obtained by standardizing the unadjusted rate by this ratio of observed to expected rates of resource use.(18) To examine resource utilization at the hospital level, we assigned utilization quartiles for each resource within each hospital based on their use. We then developed a total rank score based on the quartile and sorted hospitals by that score.
The association of resource utilization with length of stay and readmission was determined using hierarchical models of mixed-effects linear regression for length of stay and logistic regression models for hospital readmissions, with hospital-specific random intercepts, adjusting for patient-level and hospital-level characteristics as fixed effects. Beta-coefficients in linear regression analyses are interpreted as the additional days in average length of stay associated with the receipt of that resource. Analyses were performed with SAS version 9.3.
Results
64,994 inpatient hospitalizations from October 2007 through March 2012 for bronchiolitis were eligible for inclusion and analyzed across the 42 PHIS hospitals (Table I; available at www.jpeds.com). The mean age of the cohort was 3.7 months (standard deviation, 3.2 months). There was a male predominance (57.7%), and government payers insured most study subjects (51.4%). Most patients were classified as having illness of minor (56.6%) or moderate (36.6%) severity and most visits occurred in the winter (65.6%). Demographic characteristics were similar across all five resources examined, with the exception of corticosteroid use, which was used more frequently in older infants (mean age 6.2 months, SD 3.3) and in a greater proportion of spring (16.5%) and summer (5.6%) visits.
Table 1. Online Only. Characteristics of Study Population.
| N=64994 | |
|---|---|
| Age (mos), Mean (SD) | 3.7 (3.2) |
| Age (mos), Median (IQR) | 3 (5) |
| Sex, %Male | 57.7 |
| Race/Ethnicity, % | |
| Non-Hisp White | 29.4 |
| Non-Hisp Black | 13.8 |
| Hispanic | 18.4 |
| Other | 5.2 |
| Unknown | 33.2 |
| Insurance, % | |
| Private | 23.4 |
| Government | 51.4 |
| Other | 6.6 |
| Unknown | 18.7 |
| Season, % | |
| Spring | 13.5 |
| Summer | 2.5 |
| Fall | 18.3 |
| Winter | 65.6 |
| Year, % | |
| 10/2007 - 09/2008 | 20.9 |
| 10/2008 - 09/2009 | 20.3 |
| 10/2009 - 09/2010 | 22 |
| 10/2010 - 09/2011 | 21.6 |
| 10/2011 - 03/2012 | 15.2 |
| Severity, % | |
| Minor | 56.6 |
| Moderate | 36.6 |
| Major | 6.1 |
| Extreme | 0.7 |
| Length of Stay (days), Median (IQR) | 2 (2) |
| Length of Stay (days), Mean (SD) | 2.8 (1.6) |
| 3-Day Readmission, % | 1.2 |
| 7-Day Readmission, % | 1.5 |
| 14-Day Readmission, % | 1.9 |
Rates of utilization of the five resources examined across hospitals are displayed in Table II. Albuterol, racemic epinephrine, and chest radiography utilization had the greatest variation of the five resources examined. Over the almost 5 year study period, utilization of albuterol (test for trend p=0.2), racemic epinephrine (p=0.1), and antibiotics (p=0.4) remained stable over time. Corticosteroid use decreased by 3.3% (12.7% to 9.4%, p=0.03) and chest radiography decreased by 8.6% (56.7% to 48.1%, p=0.003) over the study period. (Figure 1) Adjusted resource utilization of each individual process at each hospital is represented in Figure 2.
Table 2. Resource Utilization for Bronchiolitis Across 42 PHIS Hospitals.
| Unadjusted Distribution Across Hospitals (% of patients receiving process across all hospitals) |
Adjusted Distribution Across Hospitals* (% of patients receiving process across all hospitals) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Min | 25th percentile | Median | 75th percentile | Max | Min | 25th percentile | Median | 75th percentile | Max | |
| Albuterol | 3.4 | 38.9 | 56.3 | 69.7 | 84.2 | 3.5 | 38.2 | 52.4 | 67.2 | 81.0 |
| Racemic Epinephrine | 0.3 | 7.1 | 15.2 | 27.5 | 60.9 | 0.6 | 8.8 | 20.1 | 36.0 | 78.8 |
| Corticosteroids | 4.3 | 6.8 | 10.9 | 14.3 | 44.1 | 4.1 | 7.4 | 10.9 | 15.4 | 46.6 |
| Chest radiograph | 22.6 | 43.9 | 57.4 | 64.9 | 81.0 | 24.1 | 42.4 | 54.9 | 64.1 | 76.7 |
| Antibiotics | 25.4 | 32.8 | 39.0 | 41.6 | 51.5 | 27.1 | 31.4 | 38.4 | 42.8 | 50.1 |
Adjusted for patient age, race, year and season of presentation, insurance status and APR-DRG severity score, with hospital-specific random intercepts, representing the degree to which a hospital’s resource use departed from what would be expected on average for a hospital with similar case mix.
Figure 1. Resource Utilization Over Time Period of Study.
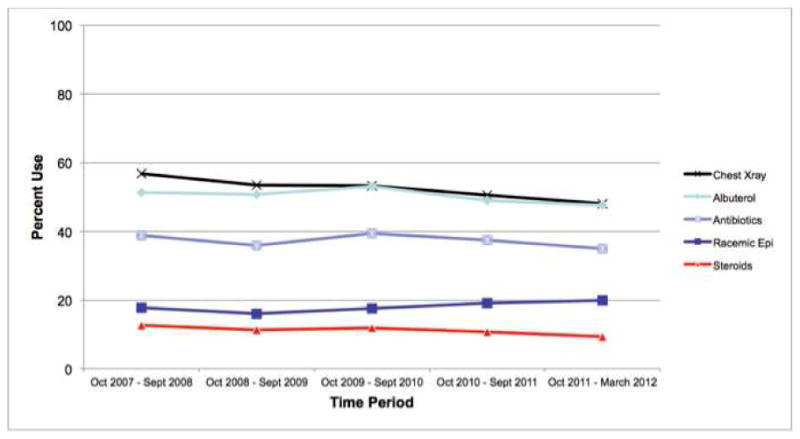
Figure 2. Adjusted Resource Use by Hospital.
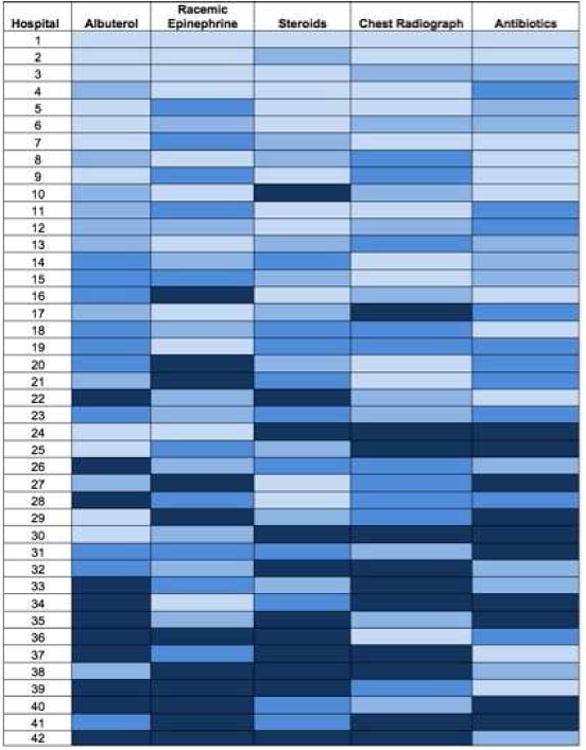
For each process listed on the horizontal axis, hospitals were ranked by their adjusted median use of the resource. Each box represents the adjusted rate of a single process at a single hospital; darker shading indicates higher adjusted rates of utilization for that process at that hospital. For each hospital, overall utilization was determined by summing that hospital’s adjusted utilization rates of the five processes. Hospitals were ordered according to their overall utilization rank, from low to high, on the vertical axis.
Mean hospital length of stay in our cohort was 2.75 days (SD, 1.56 days). In multivariable mixed-effects linear regression analyses, utilization of each of the five resources was significantly associated with increased length of stay (Table III). Receipt of albuterol (β-coefficient 0.59, 95% confidence interval [CI] 0.57, 0.62) and racemic epinephrine (β-coefficient 0.76, 95% CI 0.73, 0.79) were associated with the longest average length of stay compared with those who did not receive those medications.
Table 3. Adjusted Mixed-Effects Logistic Regression^: Association of Utilization with Length of Stay and Readmission.
| LENGTH OF STAY | READMISSION IN 3 DAYS | READMISSION IN 7 DAYS | READMISSION IN 14 DAYS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B-coefficient* | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
| Albuterol | 0.59 | 0.57, 0.62 | <.0001 | 1.04 | 0.89, 1.22 | 0.63 | 1.03 | 0.89, 1.19 | 0.68 | 1.11 | 0.98, 1.26 | 0.102 |
| Racemic Epinephrine | 0.76 | 0.73, 0.79 | <.0001 | 1.00 | 0.81, 1.23 | 0.97 | 0.99 | 0.82, 1.20 | 0.92 | 1.08 | 0.92, 1.27 | 0.360 |
| Corticosteroids | 0.43 | 0.39, 0.47 | <.0001 | 1.12 | 0.86, 1.45 | 0.41 | 1.07 | 0.85, 1.36 | 0.55 | 1.15 | 0.94, 1.40 | 0.160 |
| Chest radiograph | 0.46 | 0.43, 0.48 | <.0001 | 0.97 | 0.83, 1.14 | 0.70 | 1.04 | 0.91, 1.9 | 0.58 | 1.05 | 0.93, 1.19 | 0.427 |
| Antibiotics | 0.36 | 0.34, 0.38 | <.0001 | 0.65 | 0.55, 0.77 | <.0001 | 0.65 | 0.56, 0.76 | <.0001 | 0.70 | 0.61, 0.80 | <.0001 |
Adjusted for patient-level factors (age, sex, race, season of presentation, year of presentation, insurance status, and APR-DRG severity score) and hospital-level factors (geographic location, average daily census, average APR-DRG severity score, average annual bronchiolitis hospitalizations) as fixed effects and a hospital-specific random intercept that represents the degree to which a hospital’s process use departed from what would be expected on average for a hospital with similar case mix
The beta-coefficient in these analyses is interpreted as the additional days in average length of stay that are associated with the receipt of each process.
In our overall cohort, 1.2% of patients were readmitted within 3 days of hospital discharge, 1.5% within 7 days and 1.9% within 14 days. Use of albuterol, racemic epinephrine, corticosteroids, and chest radiograph were not associated with differences in the odds of readmission. Antibiotic use was associated with significantly decreased odds of readmission (Table III).
Discussion
This multicenter study of children hospitalized for bronchiolitis in 42 pediatric hospitals after the publication of the AAP bronchiolitis guidelines demonstrates significant and substantial variation in five resources not routinely recommended by the guideline. A key strength of our study is the examination of resource use and the outcomes of length of stay and readmission. Utilization of all five resources was associated with increased length of stay. We found that resource use was not associated with decreased odds for readmission for albuterol, racemic epinephrine, corticosteroid, or chest radiograph use, suggesting that infants who received these resources stayed in the hospital longer without the benefit of decreased readmission.
Several studies performed before the publication of the AAP guideline demonstrated significant variation in bronchiolitis management.(7, 19) Our examination of these resources after guideline publication continues to demonstrate wide variation across the cohort of 42 pediatric PHIS hospitals. Two studies have documented modest declines in overall utilization of resources not routinely recommended by the AAP guideline for inpatients with bronchiolitis.(12, 13) Our results expand upon the study by Parikh et al using the PHIS database to examine bronchiolitis management before and after the AAP guidelines. In that study, modest decreases were seen in complete blood count (5.5%), chest radiograph (9.2%), corticosteroid (8.4%) and bronchodilator (6.3%) use after guideline publication. Our study has several important differences. First, we limited our population to infants <12 months of age to minimize misclassification of bronchiolitis diagnosis. Given the variation in bronchiolitis management documented before the AAP guideline, we sought to examine the practice variation, in addition to overall utilization, across a large cohort of pediatric hospitals after publication to investigate whether such variation persisted. Although the small decreases in resource utilization in the study by Parikh et al were statistically significant, we did not consider these decreases to be clinically meaningful. There are clear examples of improvement after guideline publication and implementation that demonstrate meaningful change. One multicenter collaborative decreased total bronchodilator volume by 46% in children with bronchiolitis.(20) In community-acquired pneumonia, use of the recommended first-line therapy of amoxicillin/ampicillin increased significantly by 15% in 38 pediatric hospitals after publication of a national guideline.(21) The aggregate medians of chest radiograph use in bronchiolitis may be slowly decreasing but our results suggest that variation remains substantial. Both studies show median chest radiograph use of approximately 55%. Our study demonstrates an adjusted range of use of 24% to 77% across hospitals. The high overall rates in both studies and the substantial variation demonstrated in our results six years after guideline publication for an imaging study that has limited utility and is not recommended by the national guideline is disappointing.
We also examined albuterol and racemic epinephrine separately instead of as an aggregated measure of bronchodilator use given their distinct mechanisms of action and the varying evidence regarding their efficacy. Despite the evidence for racemic epinephrine showing small benefits over albuterol, albuterol use remains substantially higher than racemic epinephrine use, particularly after excluding patients with asthma.(22, 23) When the use of these nebulized therapies was examined individually in our study, use did not decline over the study period and the variability in the use of both agents was significant. The AAP guideline clearly recommends against routine use of these agents, but does offer an option for a carefully monitored trial of bronchodilators with objective scoring to assess improvement, which may account for the substantial variation. The fact that albuterol and racemic epinephrine utilization was associated with increased length of stay without decreased odds of readmission suggests that there is room to decrease utilization without compromising outcomes.
Both our study and the study by Parikh et al demonstrated no decline in the use of antibiotics during the study period. In addition, antibiotic use shows the narrowest range of variation of the five resources examined in this study. Antibiotic use also was the only resource of the five to be associated with decreased readmission to the hospital. The reasons for these findings are not clear and a causal relationship cannot be drawn from either study, but possible explanations include the potential that antibiotics were used appropriately in many cases or that parental perception of an antibiotic being beneficial decreased the likelihood of return to medical care.
Our study also augments previous studies by examining the effects of resource utilization on disposition outcomes after the guidelines. In 2001, Willson et al found that treatment of children with viral lower respiratory tract infection varied significantly across 10 institutions, and that institutional or provider practice patterns were not related to patient severity or recovery, but were significantly associated with higher costs and longer lengths of stay.(24) Although we did not examine costs, we found a similar association of resource utilization with length of stay after adjusting for severity, with infants who received bronchodilators, corticosteroids, or chest radiographs having a longer hospital length of stay without decreased readmission rates. Considering the quality of life and financial costs associated with the utilization of resources for which evidence is lacking, there is a clear and urgent need for interventions to minimize unwarranted use of these resources.
Our results suggest that on the national level there is a considerable opportunity to decrease unnecessary utilization. Several institutions have decreased utilization of unnecessary resources through local implementation of practice guidelines. Such declines have been more profound than the modest national declines illustrated by Parikh and Johnson.(10, 12) Enactment of a local guideline at one institution resulted in decreased admissions, decreased mean length of stay, and a decrease in nasopharyngeal washings, chest radiographs and all respiratory therapies.(25) This improvement was sustained for three years after implementation.(26) More recently, several institutions demonstrated similar results after implementation of a local guideline.(27, 28) A recent quality improvement collaborative of 17 centers shared evidence-based guidelines and protocols and implemented benchmarking in an effort to decrease utilization of unnecessary therapies in bronchiolitis. Overall, the implementation of such local guidelines amongst a collaborative resulted in a 46% decline in the volume of bronchodilators used and a 12% absolute decline in overall patients exposed to a bronchodilator.(20) These examples highlight the critical need for local implementation of evidence-based guidelines to meaningfully decrease unnecessary resource use in bronchiolitis.
There are several limitations to our analyses. First, the cross-sectional study design precludes causal inferences. Thus, even though increased resource utilization was associated with an increased length of stay, we cannot necessarily conclude that it was the cause of the increased length of stay. Although increased utilization may be a marker of increased illness severity, we attempted to adjust for illness severity in our regression models by using APR-DRG severity score. Second, there is potential for residual confounding; however, after adjusting for many factors thought to affect resource utilization such as demographics, case-mix, and severity and accounting for clustering of outcomes by hospital, there was still substantial variation in resource utilization. The magnitude of the variation is unlikely to be altered meaningfully by residual confounding. Third, there is the potential for misclassification bias due to miscoding; this is likely random and non-differential, therefore biasing our results toward the null. Fourth, we cannot assess patterns of resource use in PHIS. Therefore, we cannot determine whether a patient received a single trial of albuterol, which the AAP guidelines offer as an option, or repeated dosing on the same day. However, regardless of pattern of use, some hospitals utilize all resources, not only bronchodilators, at a high rate and others utilize all resources consistently at a low rate, making pattern of use less important in interpreting our results. Finally, our results may not be generalizable to all hospitals that care for children. Our study likely underestimates the amount of variation and utilization of these resources nationally, given prior evidence that non-pediatric focused emergency departments use non-evidence based treatments for pediatric at higher rates compared with children’s hospitals.(29)
The significant variation in use of resources not routinely recommended by the AAP bronchiolitis guidelines in this multicenter cohort of pediatric hospitals demonstrates the challenge of diffusing national guidelines into practice. The goal of the publication of practice guidelines by national societies is to influence practice. However, many guidelines are not accompanied by plans for implementation, therefore making meaningful practice change challenging. Given the success of local evidence-based guidelines, future work should focus on developing processes that can be implemented easily and then disseminated widely to improve the diffusion of evidence-based guidelines into clinical practice.
Abbreviations
- AAP
American Academy of Pediatrics
- APR-DRG
All Patient Refined-Diagnosis Related Group
- CHA
Children’s Healthcare Association
- CI
confidence interval
- ED
emergency department
- LOS
length of stay
- PHIS
Pediatric Health Information System
- SD
standard deviation
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bordley WC, Viswanathan M, King VJ, Sutton SF, Jackman AM, Sterling L, et al. Diagnosis and testing in bronchiolitis: a systematic review. Archives of pediatrics & adolescent medicine. 2004;158:119–26. doi: 10.1001/archpedi.158.2.119. [DOI] [PubMed] [Google Scholar]
- 2.King VJ, Viswanathan M, Bordley WC, Jackman AM, Sutton SF, Lohr KN, et al. Pharmacologic treatment of bronchiolitis in infants and children: a systematic review. Archives of pediatrics & adolescent medicine. 2004;158:127–37. doi: 10.1001/archpedi.158.2.127. [DOI] [PubMed] [Google Scholar]
- 3.Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980-1996. JAMA: the journal of the American Medical Association. 1999;282:1440–6. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 4.Pelletier AJ, Mansbach JM, Camargo CA., Jr Direct medical costs of bronchiolitis hospitalizations in the United States. Pediatrics. 2006;118:2418–23. doi: 10.1542/peds.2006-1193. [DOI] [PubMed] [Google Scholar]
- 5.Hasegawa K, Tsugawa Y, Brown DF, Mansbach JM, Camargo CA., Jr Trends in bronchiolitis hospitalizations in the United States, 2000-2009. Pediatrics. 2013;132:28–36. doi: 10.1542/peds.2012-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodman DC. Unwarranted variation in pediatric medical care. Pediatr Clin North Am. 2009;56:745–55. doi: 10.1016/j.pcl.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christakis DA, Cowan CA, Garrison MM, Molteni R, Marcuse E, Zerr DM. Variation in inpatient diagnostic testing and management of bronchiolitis. Pediatrics. 2005;115:878–84. doi: 10.1542/peds.2004-1299. [DOI] [PubMed] [Google Scholar]
- 8.Plint AC, Johnson DW, Wiebe N, Bulloch B, Pusic M, Joubert G, et al. Practice variation among pediatric emergency departments in the treatment of bronchiolitis. Academic emergency medicine. 2004;11:353–60. doi: 10.1197/j.aem.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Mansbach JM, Emond JA, Camargo CA., Jr Bronchiolitis in US emergency departments 1992 to 2000: epidemiology and practice variation. Pediatr Emerg Care. 2005;21:242–7. doi: 10.1097/01.pec.0000161469.19841.86. [DOI] [PubMed] [Google Scholar]
- 10.Johnson DW, Adair C, Brant R, Holmwood J, Mitchell I. Differences in admission rates of children with bronchiolitis by pediatric and general emergency departments. Pediatrics. 2002;110:e49. doi: 10.1542/peds.110.4.e49. [DOI] [PubMed] [Google Scholar]
- 11.American Academy of Pediatrics Subcommittee on Diagnosis, Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118:1774–93. doi: 10.1542/peds.2006-2223. [DOI] [PubMed] [Google Scholar]
- 12.Parikh K, Hall M, Teach SJ. Bronchiolitis management before and after the AAP guidelines. Pediatrics. 2014;133:e1–7. doi: 10.1542/peds.2013-2005. [DOI] [PubMed] [Google Scholar]
- 13.McCulloh RJ, Smitherman SE, Koehn KL, Alverson BK. Assessing the impact of national guidelines on the management of children hospitalized for acute bronchiolitis. Pediatric pulmonology. 2013 doi: 10.1002/ppul.22835. [DOI] [PubMed] [Google Scholar]
- 14.Johnson LW, Robles J, Hudgins A, Osburn S, Martin D, Thompson A. Management of bronchiolitis in the emergency department: impact of evidence-based guidelines? Pediatrics. 2013;131(Suppl 1):S103–9. doi: 10.1542/peds.2012-1427m. [DOI] [PubMed] [Google Scholar]
- 15.Fletcher DM. Achieving data quality. How data from a pediatric health information system earns the trust of its users. J AHIMA. 2004;75:22–6. [PubMed] [Google Scholar]
- 16.Hughes J. 3M Health Information Systems (HIS) APR-Drug Classification Software Overview. Utah: 3M Health Information Systems; 2009. [Google Scholar]
- 17.Sedman AB, Bahl V, Bunting E, Bandy K, Jones S, Nasr SZ, et al. Clinical redesign using all patient refined diagnosis related groups. Pediatrics. 2004;114:965–9. doi: 10.1542/peds.2004-0650. [DOI] [PubMed] [Google Scholar]
- 18.Normand SLT, Shahain DM. Statistical and Clinical Aspects of Hospital Outcomes Profiling. Statistical Science. 2007;22:206–26. [Google Scholar]
- 19.Todd J, Bertoch D, Dolan S. Use of a large national database for comparative evaluation of the effect of a bronchiolitis/viral pneumonia clinical care guideline on patient outcome and resource utilization. Arch Pediatr Adolesc Med. 2002;156:1086–90. doi: 10.1001/archpedi.156.11.1086. [DOI] [PubMed] [Google Scholar]
- 20.Ralston S, Garber M, Narang S, Shen M, Pate B, Pope J, et al. Decreasing unnecessary utilization in acute bronchiolitis care: results from the value in inpatient pediatrics network. Journal of hospital medicine : an official publication of the Society of Hospital Medicine. 2013;8:25–30. doi: 10.1002/jhm.1982. [DOI] [PubMed] [Google Scholar]
- 21.Ross RK, Hersh AL, Kronman MP, Newland JG, Metjian TA, Localio AR, et al. Impact of infectious diseases society of america/pediatric infectious diseases society guidelines on treatment of community-acquired pneumonia in hospitalized children. Clinical infectious diseases. 2014;58:834–8. doi: 10.1093/cid/ciu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gadomski AM, Brower M. Bronchodilators for bronchiolitis. Cochrane database of systematic reviews. 2010:CD001266. doi: 10.1002/14651858.CD001266.pub2. [DOI] [PubMed] [Google Scholar]
- 23.Hartling L, Bialy LM, Vandermeer B, Tjosvold L, Johnson DW, Plint AC, et al. Epinephrine for bronchiolitis. Cochrane database of systematic reviews. 2011:CD003123. doi: 10.1002/14651858.CD003123.pub3. [DOI] [PubMed] [Google Scholar]
- 24.Willson DF, Horn SD, Hendley JO, Smout R, Gassaway J. Effect of practice variation on resource utilization in infants hospitalized for viral lower respiratory illness. Pediatrics. 2001;108:851–5. doi: 10.1542/peds.108.4.851. [DOI] [PubMed] [Google Scholar]
- 25.Perlstein PH, Kotagal UR, Bolling C, Steele R, Schoettker PJ, Atherton HD, et al. Evaluation of an evidence-based guideline for bronchiolitis. Pediatrics. 1999;104:1334–41. doi: 10.1542/peds.104.6.1334. [DOI] [PubMed] [Google Scholar]
- 26.Perlstein PH, Kotagal UR, Schoettker PJ, Atherton HD, Farrell MK, Gerhardt WE, et al. Sustaining the implementation of an evidence-based guideline for bronchiolitis. Archives of pediatrics & adolescent medicine. 2000;154:1001–7. doi: 10.1001/archpedi.154.10.1001. [DOI] [PubMed] [Google Scholar]
- 27.Mittal V, Darnell C, Badawy M, Walsh B, Mehta A, Bass D, et al. Pediatric Academic Societies Annual Meeting. Washington, D.C.: May 7, 2013. Impact of Bronchiolitis Guideline Implementation on Resource Utilization. [Google Scholar]
- 28.Akenroye AT, Baskin MN, Samnaliev M, Stack AM. Impact of a Bronchiolitis Guideline on ED Resource Use and Cost: A Segmented Time-Series Analysis. Pediatrics. 2014;133:e227–e34. doi: 10.1542/peds.2013-1991. [DOI] [PubMed] [Google Scholar]
- 29.Knapp JF, Simon SD, Sharma V. Quality of care for common pediatric respiratory illnesses in United States emergency departments: analysis of 2005 National Hospital Ambulatory Medical Care Survey Data. Pediatrics. 2008;122:1165–70. doi: 10.1542/peds.2007-3237. [DOI] [PubMed] [Google Scholar]


