Abstract
PC12 cells are used to study the signaling mechanisms underlying the neurotrophic and neuroprotective activities of pituitary adenylate cyclase-activating polypeptide (PACAP) and nerve growth factor (NGF). Previous microarray experiments indicated that serpinb1a was the most induced gene after 6 h of treatment with PACAP or NGF. The present study confirmed that serpinb1a is strongly activated by PACAP and NGF in a time-dependent manner with a maximum induction (~50-fold over control) observed after 6 h of treatment. Co-incubation with PACAP and NGF resulted in a synergistic up-regulation of serpinb1a expression (200-fold over control), suggesting that PACAP and NGF act through complementary mechanisms. Consistently, PACAP-induced serpinb1a expression was not blocked by TrkA receptor inhibition. Nevertheless, the stimulation of serpinb1a expression by PACAP and NGF was significantly reduced in the presence of ERK, calcineurin, PKA, p38 and PI3K inhibitors, indicating that the two trophic factors share some common pathways in the regulation of serpinb1a. Finally, functional investigations conducted with siRNA revealed that serpinb1a is not involved in the effects of PACAP and NGF on PC12 cell neuritogenesis, proliferation or body volume but mediates their ability to block caspase-3/7 activity and to promote PC12 cell survival.
Keywords: caspase, cell death, cell survival, nerve growth factor, neuroprotection, pituitary adenylate cyclase-activating polypeptide, siRNA
Introduction
Pituitary adenylate cyclase-activating polypeptide (PACAP) is a 38-amino acid peptide that was first isolated from ovine hypothalamic extracts for its ability to stimulate adenylyl cyclase in rat anterior pituitary cells (Miyata et al. 1989). PACAP belongs to the secretin-glucagon-vasoactive intestinal polypeptide (VIP) superfamily and its sequence has been remarkably well conserved during evolution (Vaudry et al. 2009), suggesting that it must regulate important biological functions. Three PACAP receptors have been cloned: the PACAP-selective receptor PAC1 and the VIP/PACAP mutual receptors VPAC1 and VPAC2 (Harmar et al. 2012). All PACAP receptors belong to the seven-transmembrane domain G protein-coupled receptor superfamily and activate several signaling pathways including the cAMP / PKA (Spengler et al. 1993), PLC / PKC (Spengler et al. 1993), MAPK cascades (Moroo et al. 1998) and calcium fluxes (Chatterjee et al. 1996). Among its numerous biological actions, PACAP induces neurite outgrowth in cerebellar granule neurons (Gonzalez et al. 1997), inhibits cell proliferation in the developing cerebral cortex (Suh et al. 2001), and reduces apoptosis in chick neuroblasts (Erhardt and Sherwood 2004).
Nerve growth factor (NGF), a member of the neurotrophin family (Levi-Montalcini 1987), binds and activates both the tyrosine kinase member A receptor (TrkA) (Klein et al. 1991) and the p75 neurotrophin receptor (p75-NTR) (Chao 1994). TrkA is a transmembrane protein with an extracellular immunoglobulin G portion for ligand binding and a cytoplasmic tyrosine kinase domain (Chao 1994, Patapoutian and Reichardt 2001). Binding of NGF to TrkA leads, by autophosphorylation on the Tyr490 residue, to the formation of a long-lived protein complex that activates the small monomeric GTP-binding proteins Rap1 (Wu et al. 2001) and Raf-1 (Soderholm et al. 2001). NGF induces for instance neurite outgrowth in hippocampal neurons from newborn rats (Shao et al. 1993) and rescues sympathetic neurons from programmed cell death (Edwards et al. 1991).
The neurotrophic effects of PACAP and NGF have been intensively investigated by using the well characterized rat adrenal pheochromocytoma PC12 cell line in which they promote neurite outgrowth (Greene and Tischler 1976, Deutsch and Sun 1992), inhibit cell proliferation (Greene and Tischler 1976, Vaudry et al. 2002b), and reduce apoptosis (Batistatou and Greene 1993, Tanaka et al. 1997). Some of the transduction pathways involved in these effects are now well characterized (Vaudry et al. 2002a). In particular, neurite outgrowth is induced through phosphorylation of the extracellular signal-regulated kinase (ERK) MAP kinase but, while NGF acts through both a Ras- and Rap1-dependent B-Raf activation to stimulate neurite outgrowth (York et al. 1998, Wu et al. 2001), PACAP signaling is independent of Ras (Lazarovici et al. 1998), indicating that the transduction pathways activated upstream of ERK are different. The inhibition of PC12 cells apoptosis by PACAP seems to involve, at least in part, the PKA pathway (Reglodi et al. 2004) while NGF would prevent apoptosis through the phosphoinositide-3 kinase (PI3K) (Shimoke and Chiba 2001, Koh et al. 2003, Salinas et al. 2003) and Akt (Wu and Wong 2005) cascades. Up to now, very few genes involved in PC12 cells differentiation have been identified. These include fasciculation and elongation protein zeta-1 (FEZ-1) (Kuroda et al. 1999), disrupted-in-schizophrenia 1 (DISC-1) (Miyoshi et al. 2003), deleted in colorectal cancer (DCC) (Lawlor and Narayanan 1992), and early growth response 1 (Egr1) (Ravni et al. 2008). In order to get a more comprehensive view of the molecular events occurring after PACAP or NGF treatment, transcriptional investigations have been conducted (Angelastro et al. 2000, Vaudry et al. 2002b, Grumolato et al. 2003a, Ishido and Masuo 2004, Lee et al. 2005, Ravni et al. 2008) and serine (or cysteine) proteinase inhibitor, clade B, member 1a (serpinb1a) was found to be the gene that exhibits the highest level of induction after 6 h of treatment with either PACAP or NGF (Ravni et al. 2008). So far, more than 500 serpins have been identified in the three major phyla (Bacteria, Archæa and Eukarya) as well as in several eukaryotic viruses (van Gent et al. 2003). Malfunction of serpins results in a number of diseases including emphysema, thrombosis, cirrhosis, dementia, tissue self-destruction and hypersensitivity of the immune system (Irving et al. 2000, van Gent et al. 2003). Serpins are classified into 16 clades (A-P) based on their phylogenetic relationships (Silverman and Lomas 2004). The ov-serpins or clade B serpins, identified according to their amino acid sequence similarities with the chicken ovalbumin, constitute the largest group of serpins. In contrast to most other serpins that are secreted in the blood circulation to control proteolytic cascades, the ov-serpins lack a classical secretory signal peptide and reside primarily within cells with a cytoplasmic or nucleocytoplasmic distribution (Silverman et al. 2004). The majority of ov-serpins inhibits serine and/or papain-like cysteine proteinases and protects cells from exogenous and endogenous proteinase-mediated injury (Silverman et al. 2004). In mouse, four homologs of human serpinb1 have been identified and named serpinb1a, serpinb1b, serpinb1c, and serpinb1-ps1 (Benarafa et al. 2002). Both homologous human serpinb1 and murine serpinb1a are expressed in a wide range of tissues with the highest levels of expression in bone marrow, brain, spleen, and pancreas (Benarafa et al. 2002). Serpins participate to many biological activities (e.g. phagocytosis, blood coagulation, complement activation, fibrinolysis, programmed cell death, inflammation) and some of them have been reported to act as neurotrophic factors. For instance, serpinf1, also known as pigment epithelium-derived factor (PEDF), promotes survival and differentiation of retinal photoreceptors (Cayouette et al. 1999, Jablonski et al. 2000), cerebellar granule neurons (Yabe et al. 2005) and spinal motor neurons (Houenou et al. 1999). SerpinI1, or neuroserpin, is involved in neurite outgrowth (Hill et al. 2000, Parmar et al. 2002) and provides neuronal protection in pathologies such as cerebral ischemia (Yepes et al. 2000) or epilepsy (Miranda and Lomas 2006). Serpin2, alternatively named protease nexin-1 (PN-1), plays a role in regulation of neurite outgrowth (Cunningham and Gurwitz 1989). On the basis of these data, we hypothesized that serpinb1a could be implicated in some of the neurotrophic effects induced by PACAP and NGF. The present study investigated the signaling pathways involved in PACAP and NGF regulation of serpinb1a expression in PC12 cells and revealed that this protein acts as an antiapoptotic factor through inhibition of caspases-3/7.
Materials and methods
Reagents
The 38-amino acid isoform of PACAP and VIP were synthesized by solid phase methodology as previously described (Bourgault et al. 2008). U0126 was purchased from Promega (Charbonnieres, France). Ascomycin, cycloheximide, D600, dibutyryl-cyclic AMP (dbcAMP), forskolin, Gö6983, H7, H89, JNK inhibitor 1, K-252a, LY294002, PD98059, phorbol-12-myristate-13-acetate (PMA) and SB203580 were obtained from Calbiochem (San Diego, CA, USA). Actinomycin D, cyclosporin A, NGF (7S from murine submaxillary gland) and rapamycin were from Sigma (St. Louis, MO, USA).
Cell culture and treatment
PC12-G rat pheochromocytoma cells (Rausch et al. 1988) were grown in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Carlsbad, CA, USA) supplemented with 7% horse serum (Cambrex, Emerainville, France), 7% heat-inactivated fetal bovine serum (Sigma), 2.5% HEPES buffer (Invitrogen), 1% glutamine (Invitrogen), 100 units/mL penicillin and 100 µg/mL streptomycin antibiotic (Invitrogen). Two days before the experiments, PC12 cells were plated on 10 cm2 petri dishes or 12-well plates pre-coated with poly-L-lysine (Sigma). Cells were then cultured at 37°C in a 10% CO2 atmosphere. Inhibitors were added 30 min prior treatment with control medium, PACAP (10−7 M) or NGF (100 ng/ml).
Measurement of mRNA expression
Total RNA was extracted by the guanidium thiocyanate-phenol-chloroform method with Tri® Reagent (Sigma) and further purified using the RNeasy® Mini Kit (Qiagen, Courtaboeuf, France). Contaminating genomic DNA was removed by treatment with DNAse I (Qiagen) and cDNA were synthesized from 5 µg of RNA using the ImProm II™ Reverse Transcription System (Promega). Quantitative PCR was performed on cDNA in the presence of a Mastermix (Applied Biosystems, Courtaboeuf, France) containing dNTPs, MgCl2 and the SYBR® Green reporter dye along with specific primers, using the ABI Prism® 7000 sequence Detection System (Applied Biosystems). The cDNA-generated signals in samples were internally corrected for variations in amounts of input mRNA with the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA signal. The following primers, designed with the Primer Express® software (Applied Biosystems), were used: GAPDH-forward 5’-CAGCCTCGTCTCATAGACAAGATG-3’, GAPDH-reverse 5’-CAATGTCCACTTTGTCACAAGAGAA-3' (NM_017008; Rn.54911), serpinb1a-forward 5’-TCACACTCTGAAACTTGCCAACA-3’, serpinb1a-reverse 5’-CCAAGTCAGCACCATACATCTTCT-3’ (NM_001031642; Rn.103402), serpin b5-forward 5’-AACAGCTCCATTAAGGAACTCACA-3’, serpin b5-reverse 5’-GTAGGCAGCATTAACCACAAGGAT-3’ (NM_057108; Rn.25752). GAPDH and serpin b5 primers were used at a concentration of 300 nM while serpinb1a primers were used at a concentration of 500 nM.
Inhibition of serpinb1a protein expression
Transfection of small interfering RNA (siRNA) into PC12 cells was performed with the Amaxa Nucleofector™ Kit V (Amaxa, Koeln, Germany) according to the instructions of the manufacturer as previously described (Ravni et al. 2008, Dejda et al. 2010). Briefly, 2×106 cells were resuspended into 120 µL of Nucleofector solution containing 20 µg of siRNA. Immediately after electroporation in a Nucleofector™ II apparatus, DMEM was added and cells were cultured at 37°C. Each siRNA was designed to target serpinb1a mRNA sequence (Hp flexible siRNA, Qiagen) and the level of this inhibition was assessed by quantitative PCR. Sequences of the siRNA used in this study were GAG GAG AAA TTC ATG AAA CAA (Control siRNA, which did not affect serpinb1a expression), TGG CTA CAT TTC GGA TCT GAA, and ATC TGT GAA GAT GAT GTA TCA (serpinb1a siRNA and supplemental serpinb1a siRNA, respectively, which both significantly reduced serpinb1a expression by approximately 90%).
Assessment of cell proliferation and cell size
Two days after treatment, PC12 cells were washed with phosphate buffered saline (PBS) and detached by incubation with Esgro Complete™ Accutase® (Chemicon International, Temecula, CA, USA) at 37°C for 15 min. Cell size and number were measured with a Z2 Beckman Coulter counter (Beckman, Miami, FL, USA) with lower and upper limits set to 10 and 17 µm, respectively, as previously described (Ravni et al. 2008).
Assessment of neuronal differentiation
Two days after treatment, images of PC12 cells were randomly acquired on a Leica DM IRB/E inverted microscope (Leica, Heidelberg, Germany) and analyzed with the Metamorph® software, version 6.3 (Molecular Devices, Sunnyvale, CA, USA). Neuronal differentiation was evaluated by counting the percentage of cells bearing neurites, the number of neurites per cell, and total neurite outgrowth for each cell as previously described (Ravni et al. 2008).
Assessment of apoptosis
After two days of culture in the presence of serum, PC12 cells were washed twice and cultured in serum free medium for 12 h before treatment with PACAP or NGF. Six hours after treatment, cells were washed and re-suspended in serum-free medium at 4°C and treated with the fluorometric Apo-ONE® Homogeneous Caspases-3/7 Assay kit (Promega). Fluorescence was then measured over a 6-h period on a microplate reader FlexStation II (Molecular Devices) as previously reported (Dejda et al. 2010).
Assessment of cell survival
After two days of culture in the presence of serum, PC12 cells were washed twice and cultured in serum free medium for 12 h before treatment with PACAP or NGF. Two days after treatment, cells were washed with PBS and detached by incubation with Esgro Complete™ Accutase® (Chemicon International) at 37°C for 15 min. The number of surviving cells was counted with a Z2 Beckman Coulter counter. Qualitative visualization of cell survival 48 h after PACAP or NGF treatment was conducted by using LIVE/DEAD® Viability/Cytotoxicity Kit for mammalian cells (Invitrogen) according to the manufacturer protocol, as previously reported (Vaudry et al. 2002a). Briefly, cells were incubated for 20 min with a solution of calcein (producing green fluorescence in living cells) and ethidium homodimer-1 (producing red fluorescence in dead cells). Images of PC12 cells were randomly acquired on a Leica DM IRB/E inverted microscope (Leica).
Statistical analysis
Data are presented as the mean ± S.E.M. from at least three independent experiments in which each experimental condition were performed in triplicate. Statistical analyses were conducted using a Kruskal-Wallis test, followed by Dunn’s post-hoc test or by Mann-Whitney test using the PRISM software (GraphPad Software, San Diego, CA, USA).
Results
Effect of PACAP and NGF on serpinb1a mRNA expression in PC12 cells
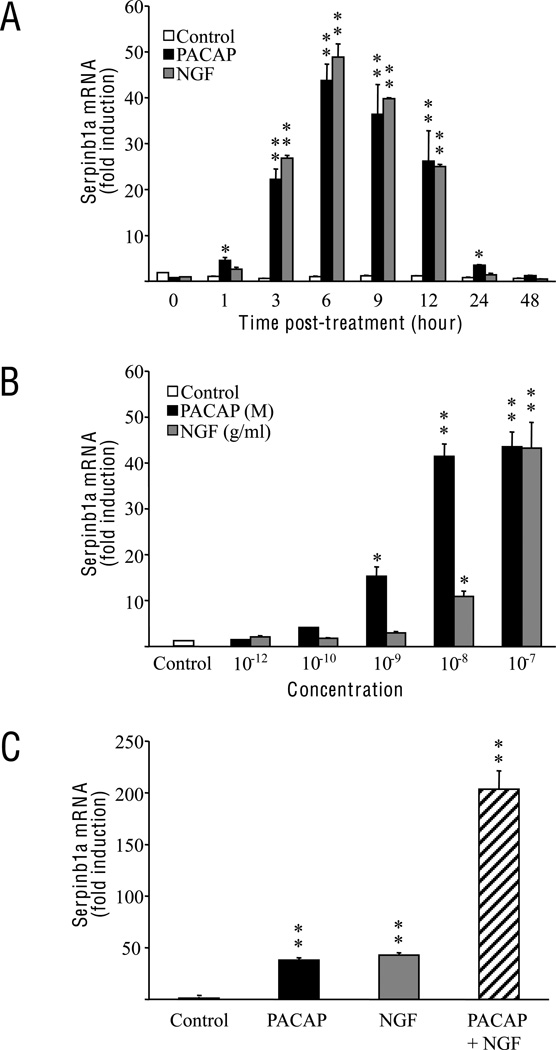
Incubation of PC12 cells with 10−7 M PACAP caused a significant increase in serpinb1a mRNA expression after only 1 h of treatment (Fig. 1A). The maximum effect (~40-fold over control) was observed after 6 h of treatment; then serpinb1a mRNA gradually declined and returned to basal level within 48 h. The time-course effect of NGF on serpinb1a mRNA expression was very similar to that of PACAP (Fig. 1A). Since a 6-h exposure to PACAP or NGF provoked the strongest increase in serpinb1a mRNA expression, gene induction was measured after 6 h of treatment in all subsequent experiments. In order to look at the specificity of this PACAP-induced transcriptional effect on serpinb1a, we also measured mRNA expression of serpinb5, another member of serpin family also known as maspin. Contrary to its stimulatory effect on serpinb1a expression, PACAP (10−7 M) did not affect the mRNA expression level of serpinb5 (data not shown). Incubation of PC12 cells with graded concentrations of PACAP (from 10−12 to 10−7 M) and NGF (from 10−12 to 10−7 g/ml) for 6 h resulted in a dose-dependent stimulation of serpinb1a gene expression (Fig. 1B). A significant increase in serpinb1a mRNA level was observed in the presence of 10−9 M PACAP and 10−8 g/ml NGF. The strongest increase in serpinb1a expression was obtained with 10−7 M PACAP and 100 ng/ml NGF and these two concentrations were used for subsequent experiments. Treatment of PC12 cells with both PACAP and NGF led to a synergistic increase of serpinb1a expression that exceeded 200-fold over control (Fig. 1C).
Fig. 1.
Effects of PACAP and NGF treatment on serpinb1a mRNA expression in PC12 cells. (A) Time-course effect: Cells were cultured in the presence of PACAP (10−7 M) or NGF (100 ng/ml) for durations ranging from 0 to 48 hours. (B) Dose effect: Cells were cultured 6 hours in the presence of PACAP (10−12 to 10−7 M) or NGF (10−12 to 10−7 g/ml). (C) Combined effect: Cells were cultured 6 hours in the presence of PACAP (10−7 M) and/or NGF (100 ng/ml). Quantitative PCR results were corrected using the GAPDH signal as internal control and expressed as the mean fold induction (± S.E.M.) of serpinb1a mRNA level compared to the control. * p<0.05 and ** p<0.01 versus control.
Transduction pathways involved in the regulation of serpinb1a expression by PACAP and NGF
The ability of PACAP and NGF to stimulate serpinb1a transcription was abolished when PC12 cells were pretreated with the RNA polymerase blocker actinomycin D, but was not modified when they were exposed to the protein synthesis inhibitor cycloheximide (Table 1).
Table 1.
Effect of transcription or translation inhibitors on PACAP- and NGF-induced serpinb1a expression in PC12 cells.
| PACAP | NGF | |
|---|---|---|
| No inhibitor | 39.8 ± 2.3** | 43.3 ± 2.6** |
| Actinomycin D | 1.4 ± 0.1## | 1.6 ± 0.2## |
| Cycloheximide | 38.4 ± 3.4NS | 37.7 ± 2.5NS |
Cells were pre-incubated for 30 min with the RNA polymerase blocker actinomycin D (10 µM) or the protein synthesis inhibitor cycloheximide (0.5 µg/ml) and then cultured 6 hours in the presence of PACAP (10−7 M) or NGF (100 ng/ml). Quantitative PCR results were corrected using the GAPDH signal as internal control and expressed as the mean fold induction (± S.E.M.) of serpinb1a mRNA expression compared to the control without any treatment (neither PACAP or NGF nor inhibitors).
p<0.01 vs. control;
p<0.01 vs. corresponding treatment without inhibitor;
NS, not significantly different from corresponding treatment without inhibitor.
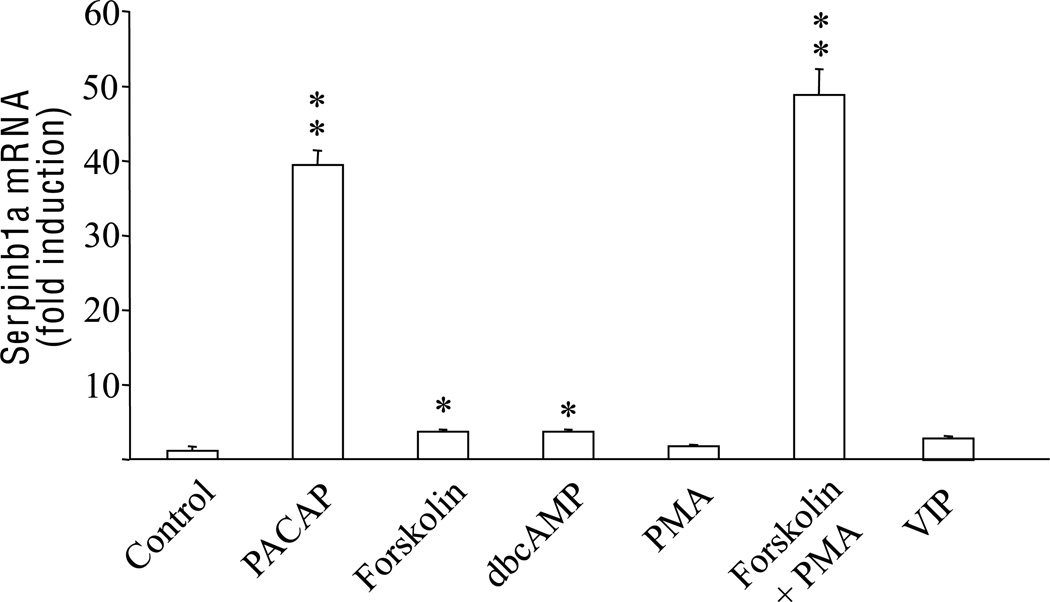
Exposure of PC12 cells to the adenylyl cyclase activator forskolin or to the cell permeant cAMP analog dbcAMP only induced a ~ 3.5-fold increase in serpinb1a mRNA expression, i.e. 10–12 times weaker than PACAP or NGF (Fig. 2). Activation of PKC with PMA did not affect serpinb1a mRNA expression level but concomitant incubation with both forskolin and PMA potentialized the response to forskolin, leading to an increase in serpinb1a gene transcription that was similar to the one observed after PACAP or NGF treatment (Fig. 2). On the other hand, the serpinb1a mRNA expression was not modulated in PC12 cells in response to a treatment with VIP, which is consistent with previous observations showing that PC12 cells express the PACAP-selective receptor PAC1 but not the VIP/PACAP mutual receptors VPAC1 and VPAC2 (Ravni et al. 2006b).
Fig. 2.
Effects of cAMP and PKC activation as well as VIP treatment on serpinb1a mRNA expression in PC12 cells. Cells were cultured 6 h in the presence of PACAP (10−7 M), the cAMP activator forskolin (25 µM), the cAMP analog dbcAMP (10−3 M), the PKC activator PMA (10−7 M), a combination of PMA plus forskolin, or VIP (10−7 M). Quantitative PCR results were corrected using the GAPDH signal as internal control and expressed as the mean fold induction (± S.E.M.) of serpinb1a mRNA level compared to the control without activator. * p<0.05 and ** p<0.01 versus control.
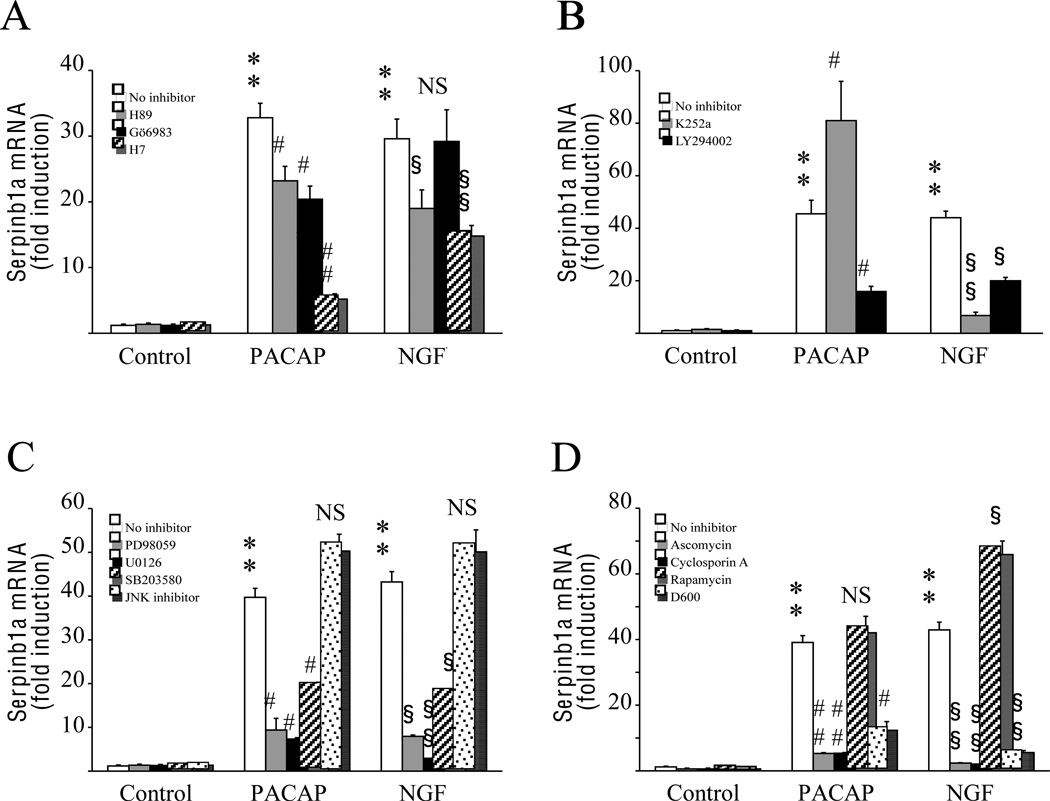
Preincubation of PC12 cells with the selective PKA inhibitor H89 significantly reduced the effect of both PACAP and NGF on serpinb1a gene expression while the PKC inhibitor Gö6983 only attenuated the response to PACAP (Fig. 3A). In the presence of the PKA/PKC inhibitor H7, the effect of PACAP and NGF on serpinb1a gene expression was reduced by 6- and 2-fold, respectively (Fig. 3A). The reduction of the effect of NGF on serpinb1a expression by H7 was similar to that observed with H89 while H7 almost completely blocked the ability of PACAP to stimulate serpinb1a expression. The trkA inhibitor K252a increased by l.7-fold the PACAP-evoked stimulation of serpinb1a mRNA level but diminished by more than 6 times the ability of NGF to increase serpinb1a expression (Fig. 3B). The PI3K inhibitor LY294003 induced a reduction of the effect of both PACAP (2.9-fold) and NGF (2.2-fold) on serpinb1a mRNA expression (Fig. 3B). Inhibition of ERK1/2 with PD98059 or U0126 and blockage of the p38 MAPK with SB203580 provoked a 2- to 15-fold decrease of the induction of serpinb1a mRNA expression by PACAP or NGF (Fig. 3C). In contrast, the JNK inhibitor had no effect on PACAP-or NGF-induced serpinb1a gene transcription (Fig. 3C). The calcineurin inhibitors ascomycin and cyclosporin A induced a 7- and 20-fold reduction, respectively, of the effects of PACAP and NGF on serpinb1a mRNA expression (Fig. 3D). In contrast, the specific FKBP12 inhibitor rapamycin had no effect on PACAP-induced serpinb1a expression but significantly increased NGF induction by 1.5-fold (Fig. 3D). However, blockade of voltage-sensitive calcium channels with D600 reduced by 3.5- and 8-fold the effects of PACAP and NGF, respectively, on serpinb1a mRNA levels (Fig. 3D).
Fig. 3.
Investigation of the transduction pathways involved in the regulation of serpinb1a expression by PACAP and NGF in PC12 cells. Cells were pre-incubated for 30 min with inhibitors and then cultured for 6 h in the presence of PACAP (10−7 M) or NGF (100 ng/ml). (A) Effect of protein kinase inhibitors on PACAP- and NGF-induced serpinb1a expression. Cells were pre-incubated with the PKA inhibitor H89 (10 µM), the PKC inhibitor Gö6983 (1 mM), or the PKA/PKC inhibitor H7 (50 µM). (B) Effect of TrkA and PI3K inhibitors on PACAP- and NGF-induced serpinb1a expression. Cells were pre-incubated with the TrkA inhibitor K252a (1 µM) or the PI3K inhibitor LY294002 (20 µM). (C) Effect of MAP kinase inhibitors on PACAP- and NGF-induced serpinb1a expression. Cells were pre-incubated with the MEK inhibitors PD98059 (50 µM) or U0126 (25 µM), the p38 inhibitor SB203580 (10 µM), or the JNK inhibitor I (2 µM). (D) Effect of calcineurin inhibitors and calcium channel blockers on PACAP- and NGF-induced serpinb1a expression. Cells were pre-incubated with the calcineurin inhibitors ascomycin (100 nM) or cyclosporin A (100 nM), the FKBP12 inhibitor rapamycin (100 nM), or the calcium channel blocker D600 (30 µM). Quantitative PCR results were corrected using the GAPDH signal as internal control and expressed as the mean fold induction (± S.E.M.) of serpinb1a mRNA level compared to the control without inhibitor. ** p<0.01 versus control; # p<0.05 and ## p<0.01 versus PACAP without inhibitor; § p<0.05 and §§ p<0.01 versus NGF without inhibitor; NS, not significantly different from corresponding treatment without inhibitor.
Involvement of serpinb1a in the neurotrophic effects of PACAP and NGF
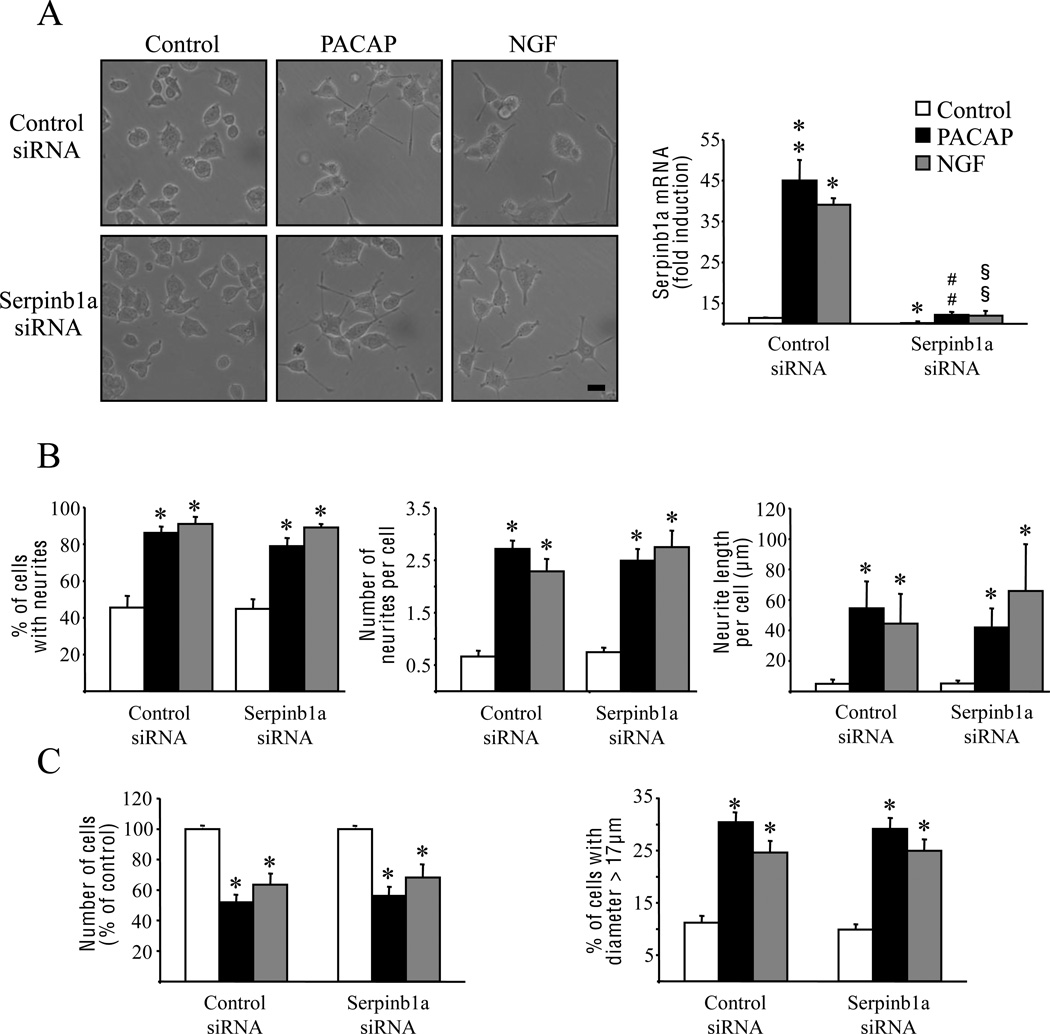
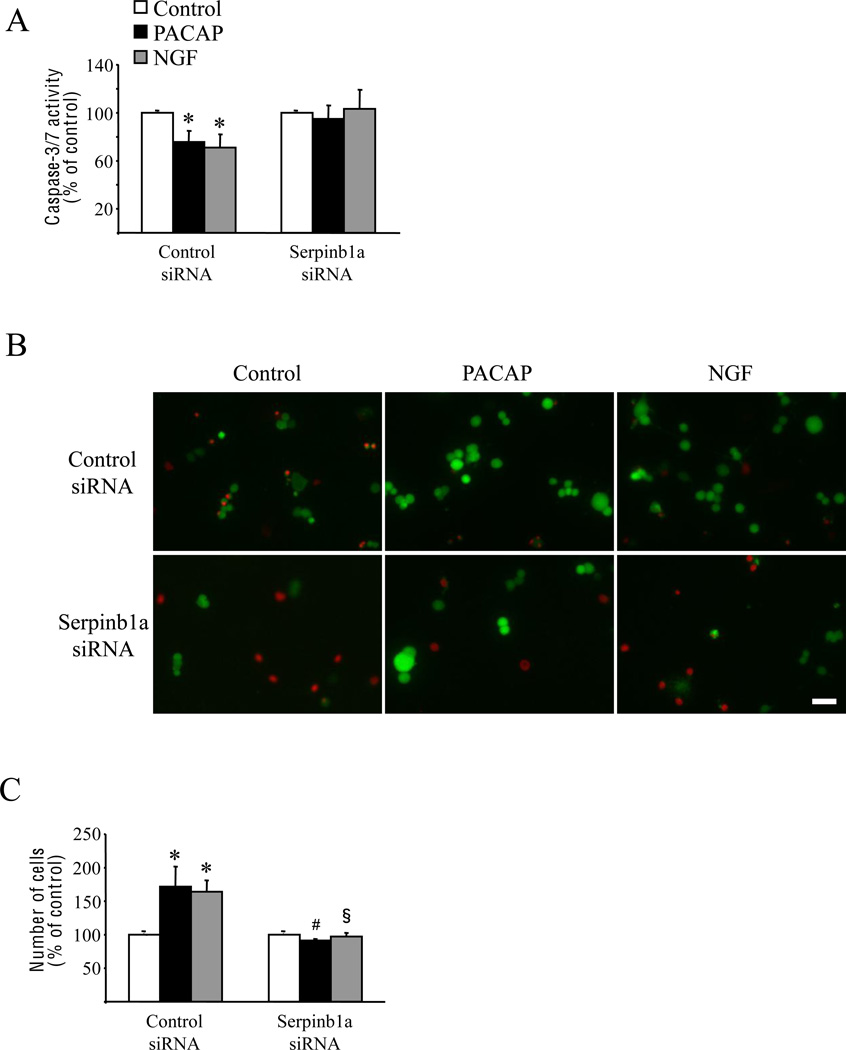
Transfection of PC12 cells with siRNA showed that control siRNA did not alter serpinb1a mRNA increase by PACAP or NGF while specific-serpinb1a siRNA reduced by approximately 90% PACAP- and NGF-induced serpinb1a mRNA expression (Fig. 4A). Blockage of serpinb1a mRNA induction by specific siRNA did not modify the effect of PACAP or NGF on neurite outgrowth (Fig. 4B), growth arrest or cell size (Fig. 4C). In contrast, serpinb1a siRNA blocked the inhibitory effect of PACAP and NGF on caspases-3/7 activity (Fig. 5A) and totally abolished the antiapoptotic effects of PACAP and NGF on PC12 cells cultured in serum-free conditions (Fig. 5B and C). These effects of serpinb1a blockade on caspases-3/7 activity and cell survival were confirmed by transfecting a distinct inhibitory serpinb1a siRNA, the sequence of which is specified in the materials and methods section (data not shown).
Fig. 4.
Involvement of serpinb1a in the neurotrophic effects of PACAP and NGF on PC12 cells. Cells were transfected with control siRNA or siRNA directed against serpinb1a mRNA, and were treated with PACAP (10−7 M) or NGF (100 ng/ml) 2 days later. (A) Effect of anti-serpinb1a siRNA on cell differentiation (photomicrographs) and serpinb1a mRNA expression after treatment with PACAP or NGF for 24 h and 6 h, respectively. Quantitative PCR results were corrected using the GAPDH signal as internal control and expressed as the mean fold induction (± S.E.M.) of serpinb1a mRNA level compared to untreated cells transfected with control siRNA. (B) Neuritogenesis assessment by measuring the percentage of cells with neurites, the number of neurites per cell, and the overall neurite outgrowth after 48 h of treatment with PACAP or NGF in the presence of control siRNA or anti-serpinb1a siRNA. (C) Quantification of cell proliferation and cell size by counting the number of cells and the percentage of cells with a diameter greater than 17 µm after 48 hours of treatment with PACAP or NGF in the presence of control siRNA or anti-serpinb1a siRNA. * p<0.05 and ** p<0.01 versus control in the presence of control siRNA; ## p<0.01 versus PACAP in the presence of control siRNA; §§ p<0.01 versus NGF in the presence of control siRNA. Scale bar = 15 µm.
Fig. 5.
Involvement of serpinb1a in PACAP- and NGF-induced survival of PC12 cells. Cells were transfected with control siRNA or siRNA directed against serpinb1a mRNA and were serum-deprived 2 days later. After 12 h in serum-free conditions, cells were treated with PACAP (10−7 M) or NGF (100 ng/ml). (A) Quantification of caspases-3/7 activity after 6 h of treatment with PACAP or NGF. (B) Typical microphotographs illustrating the effect of anti-serpinb1a siRNA on cell survival. Two days after PACAP or NGF treatment, living cells were labeled with calcein (green fluorescence) and dead cells were labeled with ethidium homodimer-1 (red fluorescence). (C) Quantification of cell survival by counting the number of cells after 48 h of treatment with PACAP or NGF in serum-deprived conditions. * p<0.05 versus control in the presence of control siRNA; # p<0.05 versus PACAP in the presence of control siRNA; § p<0.05 versus NGF in the presence of control siRNA. Scale bar = 100 µm.
Discussion
In previous studies, the PC12 cell line has been used to investigate the various effects of PACAP and NGF on neuronal differentiation including neurite outgrowth, inhibition of cell division and neuroprotection (Vaudry et al. 2002a, Grumolato et al. 2003b, Sakai et al. 2004, Ravni et al. 2008). Like NGF, which is the classical inducer of neuronal differentiation (Greene and Tischler 1976), PACAP promotes growth arrest and causes neuritogenesis in PC12 cells (Deutsch and Sun 1992). In order to identify genes involved in PC12 cell differentiation, we previously investigated the trancriptome of PC12 cells exposed to PACAP or NGF during 6 h (Ravni et al. 2008). Among the 99 transcripts induced by PACAP and the 114 induced by NGF, 19 were shared by the two neurotrophic factors and serpinb1a represented one of the most induced genes by both PACAP and NGF. The aim of the present study was thus to elucidate the signal transduction pathway(s) leading to PACAP- and NGF-evoked serpinb1a transcription and to determine the functional role of this gene.
The obtained results confirmed that both PACAP and NGF markedly increase serpinb1a expression. In PC12 cells, PACAP acts through the seven-transmembrane G protein-coupled receptor PAC1 (Ravni et al. 2006a) while NGF activates the TrkA receptor tyrosine kinase and p75-NTR (Berg et al. 1991, Cordon-Cardo et al. 1991). The fact that the two trophic factors exerted similar effects on serpinb1a kinetics and on some signaling pathway components suggested an indirect regulation by PACAP through activation of the neurotrophin receptor, as previously reported (Lazarovici and Fink 1999, Rajagopal et al. 2004). However, this hypothesis was invalidated by the fact that PACAP-induced serpinb1a mRNA expression was not blocked by K252a, a TrkA tyrosine kinase inhibitor (Lee et al. 2002). On the other hand, K252a actually blocked the NGF-induced increase in serpinb1a expression, confirming the involvement of TrkA in the antiapoptotic effects of NGF. Thus, although some regulatory elements are different, PACAP and NGF transduction pathways proceed through several common actors including ERK and PKA. Accordingly, several studies have already pointed out the ability of PACAP and NGF to activate transduction pathways that converge to have an effect on the same target genes (Hashimoto et al. 2000, Vaudry et al. 2002c). Nevertheless, the synergistic effect of PACAP and NGF on PACAP (Hashimoto et al. 2000) and serpinb1a gene expression suggests that besides the common pathways activated by both trophic factors, complementary mechanisms are also presumably induced. Actually, the synergistic stimulation of serpinb1a co-treatment by PACAP and NGF, illustrates the possible cooperation between a G protein-coupled receptor and tyrosine kinase receptor as already reported for cell differentiation (Sakai et al. 2004). As previously shown by microarray, PACAP and NGF activate common and distinct mechanisms (Ravni et al. 2008), suggesting that they both exert neurotrophic activities even if their functions are not exactly the same. Actually, PACAP is acting rapidly on the cells but its action may be transient while NGF initiates slower response but induces a greater final effect. In the presence of both factors, the cell generates faster response (Sakai et al. 2004) which could be a way to adapt the cellular response to the physiological situation.
PACAP simultaneously activates several signaling pathways that mediate its various effects (Vaudry et al. 2000b, Ravni et al. 2008). For instance, neuritogenesis is activated through a cAMP- and ERK-dependent, PKA/PKC-independent pathway while cell size increase involves ERK and PKA, but not PKC (Ravni et al. 2008, Emery and Eiden 2012). The fact that multiple transduction pathways promote serpinb1a expression suggests that this gene could be involved in several cellular functions. Nevertheless, investigations achieved by using siRNA revealed that in contrast to what has recently been reported by Watanabe and collaborators (Watanabe et al. 2012), in our hands serpinb1a inhibition with two different siRNA does not affect the ability of PACAP or NGF to promote neuritogenesis, induce growth arrest, or increase cell size. In contrast, it appeared that blockade of serpinb1a expression suppressed the ability of PACAP and NGF to inhibit caspases-3/7 activity. Moreover, siRNA targeting serpinb1a abrogated the protective effect of PACAP and NGF on cell death induced by serum deprivation. Serpinb1a might exert its antiapoptotic effect by directly inhibiting the activity of the cysteine protease caspases-3/7, as already reported for other serpins (Antoku et al. 1997). For instance, the viral CrmA serpin, a caspase inhibitor whose physiological target is likely to be the apical caspase in the cascade (Zhou et al. 1997), uses such mechanisms to block programmed cell death induced by Fas (Tewari and Dixit 1995). Serpin1 of Arabidopsis thaliana was also reported to act as a suicide inhibitor for metacaspase-9 (Vercammen et al. 2006). Nevertheless, serpinb1a might also exert its antiapoptotic effect indirectly, by changing the stability of bcl2 and Bax proteins as previously reported for serpinb5 in tumor cells (Zhang et al. 2005). Interestingly among the different pathways involved in the regulation of serpinb1a, the cAMP/PKA has been reported in several cellular models to be involved in neuroprotection (Vaudry et al. 2003, Racz et al. 2007, Baxter et al. 2011). Furthermore, inhibition of both PKA and PKC pathways, which induce a very strong inhibition of serpin b1a have also been previously shown to be required for the inhibition of caspase-3/7 by PACAP (Vaudry et al. 2000a). The PKA and PKC pathways seem to play a key role in the regulation of serpin b1a by PACAP if we consider that incubation with the PKA stimulator forskolin, together with the PKA stimulator PMA, mimics the effect of PACAP on serpin b1a expression. But, as shown with the Gö6983 compound, the PKC pathway does not seem to mediate the activation by NGF, which is consistent with the idea that PKC is not involved in the effects of NGF (Sigmund et al. 1990). This observation suggests that besides PKA, NGF must activate another pathway that remains to be identified and which would explain the synergistic action of both PACAP and NGF.
The present study established that the antiapoptotic effect of PACAP and NGF in PC12 cells involves serpinb1a expression. Thus, serpinb1a should be considered as another serpin family member acting as a neurotrophic factor. Taking into account that PACAP and NGF block programmed cell death in many neuronal cell types (Seaborn et al. 2011), it will now be of great interest to investigate the possible antiapoptotic action of serpinb1a overexpression in some neurodegenerative pathologies such as Alzheimer’s disease.
Acknowledgments
The authors wish to thank Mr. Colas Calbrix for skillful technical assistance. A.R. was the recipient of a doctoral fellowship from the Ministry of Education. T.S. was the recipient of an INSERM postdoctoral fellowship. D.V. and H.V. are Affiliated Professors at the INRS — Institut Armand-Frappier. This study was supported by INSERM (U982), the National Institute of Mental Health (NIMH) Intramural Research Program, an INSERM-FRSQ program (to A.F. and D.V.), the Interreg TC2N project and the Région Haute-Normandie.
Abbreviations used
- Akt
v-akt murine thymoma viral oncogene homolog
- AP-1
activator protein 1
- cAMP
cyclic adenosine monophosphate
- caspases
cysteine-dependent aspartate-directed proteases
- dbcAMP
N6, 2'-O-dibutyryladenosine 3'–5'-cyclic monophosphate
- DCC
deleted in colorectal cancer
- DISC-1
disrupted-in-schizophrenia 1
- DMEM
Dulbecco’s modified Eagle’s medium
- Egr1
early growth response 1
- ERK
extracellular signal-regulated kinase
- FEZ-1
fasciculation and elongation protein zeta-1
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- JNK
c-Jun NH2-terminal kinase
- MAPK
mitogen-activated protein kinase
- NF-κB
nuclear factor-kappa B
- NFATs
nuclear factor of activated T cells
- NGF
nerve growth factor
- p75-NTR
p75 neurotrophin receptor
- PACAP
pituitary adenylate cyclase-activating polypeptide
- PAC1
specific PACAP receptor
- PBS
phosphate buffered saline
- PC12
rat adrenal pheochromocytoma cell
- PEDF
pigment epithelium-derived factor
- PI3K
phosphatidylinositol 3-kinase
- PKA
protein kinase A
- PKC
protein kinase C
- PLC
phospholipase C
- PMA
phorbol-12-myristate-13-acetate
- PN-1
protease nexin-1
- serpin
serine (or cysteine) peptidase inhibitor
- siRNA
small interfering RNA
- TrkA
tyrosine kinase member A receptor
- VIP
vasoactice intestinal polypeptide
- VPAC1
VIP and PACAP receptor 1
- VPAC2
VIP and PACAP receptor 2
Footnotes
Authors declare that they have no conflict of interest.
References
- Angelastro JM, Klimaschewski L, Tang S, Vitolo OV, Weissman TA, Donlin LT, Shelanski ML, Greene LA. Identification of diverse nerve growth factor-regulated genes by serial analysis of gene expression (SAGE) profiling. Proc Natl Acad Sci USA. 2000;97:10424–10429. doi: 10.1073/pnas.97.19.10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoku K, Liu Z, Johnson DE. Inhibition of caspase proteases by CrmA enhances the resistance of human leukemic cells to multiple chemotherapeutic agents. Leukemia. 1997;11:1665–1672. doi: 10.1038/sj.leu.2400805. [DOI] [PubMed] [Google Scholar]
- Batistatou A, Greene LA. Internucleosomal DNA cleavage and neuronal cell survival/death. J Cell Biol. 1993;122:523–532. doi: 10.1083/jcb.122.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter PS, Martel MA, McMahon A, Kind PC, Hardingham GE. Pituitary adenylate cyclase-activating peptide induces long-lasting neuroprotection through the induction of activity-dependent signaling via the cyclic AMP response element-binding protein-regulated transcription co-activator 1. J Neurochem. 2011;118:365–378. doi: 10.1111/j.1471-4159.2011.07330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarafa C, Cooley J, Zeng W, Bird PI, Remold-O'Donnell E. Characterization of four murine homologs of the human ov-serpin monocyte neutrophil elastase inhibitor MNEI (SERPINB1) J Biol Chem. 2002;277:42028–42033. doi: 10.1074/jbc.M207080200. [DOI] [PubMed] [Google Scholar]
- Berg MM, Sternberg DW, Hempstead BL, Chao MV. The low-affinity p75 nerve growth factor (NGF) receptor mediates NGF-induced tyrosine phosphorylation. Proc Natl Acad Sci U S A. 1991;88:7106–7110. doi: 10.1073/pnas.88.16.7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgault S, Vaudry D, Botia B, Couvineau A, Laburthe M, Vaudry H, Fournier A. Novel stable PACAP analogs with potent activity towards the PAC1 receptor. Peptides. 2008;29:919–932. doi: 10.1016/j.peptides.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Cayouette M, Smith SB, Becerra SP, Gravel C. Pigment epithelium-derived factor delays the death of photoreceptors in mouse models of inherited retinal degenerations. Neurobiol Dis. 1999;6:523–532. doi: 10.1006/nbdi.1999.0263. [DOI] [PubMed] [Google Scholar]
- Chao MV. The p75 neurotrophin receptor. J Neurobiol. 1994;25:1373–1385. doi: 10.1002/neu.480251106. [DOI] [PubMed] [Google Scholar]
- Chatterjee TK, Sharma RV, Fisher RA. Molecular cloning of a novel variant of the pituitary adenylate cyclase-activating polypeptide (PACAP) receptor that stimulates calcium influx by activation of L-type calcium channels. J Biol Chem. 1996;271:32226–32232. doi: 10.1074/jbc.271.50.32226. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C, Tapley P, Jing SQ, Nanduri V, O'Rourke E, Lamballe F, Kovary K, Klein R, Jones KR, Reichardt LF, et al. The trk tyrosine protein kinase mediates the mitogenic properties of nerve growth factor and neurotrophin-3. Cell. 1991;66:173–183. doi: 10.1016/0092-8674(91)90149-s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham DD, Gurwitz D. Proteolytic regulation of neurite outgrowth from neuroblastoma cells by thrombin and protease nexin-1. J Cell Biochem. 1989;39:55–64. doi: 10.1002/jcb.240390107. [DOI] [PubMed] [Google Scholar]
- Dejda A, Chan P, Seaborn T, Coquet L, Jouenne T, Fournier A, Vaudry H, Vaudry D. Involvement of stathmin 1 in the neurotrophic effects of PACAP in PC12 cells. J Neurochem. 2010;114:1498–1510. doi: 10.1111/j.1471-4159.2010.06873.x. [DOI] [PubMed] [Google Scholar]
- Deutsch PJ, Sun Y. The 38-amino acid form of pituitary adenylate cyclase-activating polypeptide stimulates dual signaling cascades in PC12 cells and promotes neurite outgrowth. J Biol Chem. 1992;267:5108–5113. [PubMed] [Google Scholar]
- Edwards SN, Buckmaster AE, Tolkovsky AM. The death programme in cultured sympathetic neurones can be suppressed at the posttranslational level by nerve growth factor, cyclic AMP, and depolarization. J Neurochem. 1991;57:2140–2143. doi: 10.1111/j.1471-4159.1991.tb06434.x. [DOI] [PubMed] [Google Scholar]
- Emery AC, Eiden LE. Signaling through the neuropeptide GPCR PAC1 induces neuritogenesis via a single linear cAMP- and ERK-dependent pathway using a novel cAMP sensor. Faseb J. 2012 doi: 10.1096/fj.11-203042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt NM, Sherwood NM. PACAP maintains cell cycling and inhibits apoptosis in chick neuroblasts. Mol Cell Endocrinol. 2004;221:121–134. doi: 10.1016/j.mce.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Gonzalez BJ, Basille M, Vaudry D, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide promotes cell survival and neurite outgrowth in rat cerebellar neuroblasts. Neuroscience. 1997;78:419–430. doi: 10.1016/s0306-4522(96)00617-3. [DOI] [PubMed] [Google Scholar]
- Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Nad Acad Sci USA. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumolato L, Elkahloun AG, Ghzili H, Alexandre D, Coulouarn C, Yon L, Salier JP, Eiden LE, Fournier A, Vaudry H, Anouar Y. Microarray and suppression subtractive hybridization analyses of gene expression in pheochromocytoma cells reveal pleiotropic effects of pituitary adenylate cyclase-activating polypeptide on cell proliferation, survival, and adhesion. Endocrinology. 2003a;144:2368–2379. doi: 10.1210/en.2002-0106. [DOI] [PubMed] [Google Scholar]
- Grumolato L, Louiset E, Alexandre D, Ait-Ali D, Turquier V, Fournier A, Fasolo A, Vaudry H, Anouar Y. PACAP and NGF regulate common and distinct traits of the sympathoadrenal lineage: effects on electrical properties, gene markers and transcription factors in differentiating PC12 cells. Eur J Neurosci. 2003b;17:71–82. doi: 10.1046/j.1460-9568.2003.02426.x. [DOI] [PubMed] [Google Scholar]
- Harmar AJ, Fahrenkrug J, Gozes I, Laburthe M, May V, Pisegna JR, Vaudry D, Vaudry H, Waschek JA, Said SI. Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR Review 1. Br J Pharmacol. 2012;166:4–17. doi: 10.1111/j.1476-5381.2012.01871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto H, Hagihara N, Koga K, Yamamoto K, Shintani N, Tomimoto S, Mori W, Koyama Y, Matsuda T, Baba A. Synergistic induction of pituitary adenylate cyclase-activating polypeptide (PACAP) gene expression by nerve growth factor and PACAP in PC12 cells. J Neurochem. 2000;74:501–507. doi: 10.1046/j.1471-4159.2000.740501.x. [DOI] [PubMed] [Google Scholar]
- Hill RM, Parmar PK, Coates LC, Mezey E, Pearson JF, Birch NP. Neuroserpin is expressed in the pituitary and adrenal glands and induces the extension of neurite-like processes in AtT-20 cells. Biochem J. 2000;345(Pt 3):595–601. [PMC free article] [PubMed] [Google Scholar]
- Houenou LJ, D'Costa AP, Li L, Turgeon VL, Enyadike C, Alberdi E, Becerra SP. Pigment epithelium-derived factor promotes the survival and differentiation of developing spinal motor neurons. J Comp Neurol. 1999;412:506–514. [PubMed] [Google Scholar]
- Irving JA, Pike RN, Lesk AM, Whisstock JC. Phylogeny of the serpin superfamily: implications of patterns of amino acid conservation for structure and function. Genome Res. 2000;10:1845–1864. doi: 10.1101/gr.gr-1478r. [DOI] [PubMed] [Google Scholar]
- Ishido M, Masuo Y. Transcriptome of pituitary adenylate cyclase-activating polypeptide-differentiated PC12 cells. Regul Pept. 2004;123:15–21. doi: 10.1016/j.regpep.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Jablonski MM, Tombran-Tink J, Mrazek DA, Iannaccone A. Pigment epithelium-derived factor supports normal development of photoreceptor neurons and opsin expression after retinal pigment epithelium removal. J Neurosci. 2000;20:7149–7157. doi: 10.1523/JNEUROSCI.20-19-07149.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R, Jing SQ, Nanduri V, O'Rourke E, Barbacid M. The trk proto-oncogene encodes a receptor for nerve growth factor. Cell. 1991;65:189–197. doi: 10.1016/0092-8674(91)90419-y. [DOI] [PubMed] [Google Scholar]
- Koh SH, Kim SH, Kwon H, Park Y, Kim KS, Song CW, Kim J, Kim MH, Yu HJ, Henkel JS, Jung HK. Epigallocatechin gallate protects nerve growth factor differentiated PC12 cells from oxidative-radical-stress-induced apoptosis through its effect on phosphoinositide 3-kinase/Akt and glycogen synthase kinase-3. Brain Res Mol Brain Res. 2003;118:72–81. doi: 10.1016/j.molbrainres.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Kuroda S, Nakagawa N, Tokunaga C, Tatematsu K, Tanizawa K. Mammalian homologue of the Caenorhabditis elegans UNC-76 protein involved in axonal outgrowth is a protein kinase C zeta-interacting protein. J Cell Biol. 1999;144:403–411. doi: 10.1083/jcb.144.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor KG, Narayanan R. Persistent expression of the tumor suppressor gene DCC is essential for neuronal differentiation. Cell Growth Differ. 1992;3:609–616. [PubMed] [Google Scholar]
- Lazarovici P, Fink D., Jr Heterologous upregulation of nerve growth factor-TrkA receptors in PC12 cells by pituitary adenylate cyclase-activating polypeptide (PACAP) Mol Cell Biol Res Commun. 1999;2:97–102. doi: 10.1006/mcbr.1999.0158. [DOI] [PubMed] [Google Scholar]
- Lazarovici P, Jiang H, Fink D., Jr The 38-amino-acid form of pituitary adenylate cyclase-activating polypeptide induces neurite outgrowth in PC12 cells that is dependent on protein kinase C and extracellular signal-regulated kinase but not on protein kinase A, nerve growth factor receptor tyrosine kinase, p21(ras) G protein, and pp60(c-src) cytoplasmic tyrosine kinase. Mol Pharmacol. 1998;54:547–558. doi: 10.1124/mol.54.3.547. [DOI] [PubMed] [Google Scholar]
- Lee FS, Rajagopal R, Kim AH, Chang PC, Chao MV. Activation of Trk neurotrophin receptor signaling by pituitary adenylate cyclase-activating polypeptides. J Biol Chem. 2002;277:9096–9102. doi: 10.1074/jbc.M107421200. [DOI] [PubMed] [Google Scholar]
- Lee KH, Ryu CJ, Hong HJ, Kim J, Lee EH. CDNA microarray analysis of nerve growth factor-regulated gene expression profile in rat PC12 cells. Neurochem Res. 2005;30:533–540. doi: 10.1007/s11064-005-2688-y. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- Miranda E, Lomas DA. Neuroserpin: a serpin to think about. Cell Mol Life Sci. 2006;63:709–722. doi: 10.1007/s00018-005-5077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, Culler MD, Coy DH. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun. 1989;164:567–574. doi: 10.1016/0006-291x(89)91757-9. [DOI] [PubMed] [Google Scholar]
- Miyoshi K, Honda A, Baba K, Taniguchi M, Oono K, Fujita T, Kuroda S, Katayama T, Tohyama M. Disrupted-In-Schizophrenia 1, a candidate gene for schizophrenia, participates in neurite outgrowth. Mol Psychiatry. 2003;8:685–694. doi: 10.1038/sj.mp.4001352. [DOI] [PubMed] [Google Scholar]
- Moroo I, Tatsuno I, Uchida D, Tanaka T, Saito J, Saito Y, Hirai A. Pituitary adenylate cyclase activating polypeptide (PACAP) stimulates mitogen-activated protein kinase (MAPK) in cultured rat astrocytes. Brain Res. 1998;795:191–196. doi: 10.1016/s0006-8993(98)00291-1. [DOI] [PubMed] [Google Scholar]
- Parmar PK, Coates LC, Pearson JF, Hill RM, Birch NP. Neuroserpin regulates neurite outgrowth in nerve growth factor-treated PC12 cells. J Neurochem. 2002;82:1406–1415. doi: 10.1046/j.1471-4159.2002.01100.x. [DOI] [PubMed] [Google Scholar]
- Patapoutian A, Reichardt LF. Trk receptors: mediators of neurotrophin action. Curr Opin Neurobiol. 2001;11:272–280. doi: 10.1016/s0959-4388(00)00208-7. [DOI] [PubMed] [Google Scholar]
- Racz B, Gallyas F, Jr, Kiss P, Tamas A, Lubics A, Lengvari I, Roth E, Toth G, Hegyi O, Verzal Z, Fabricsek C, Reglodi D. Effects of pituitary adenylate cyclase activating polypeptide (PACAP) on the PKA-Bad-14-3-3 signaling pathway in glutamate-induced retinal injury in neonatal rats. Neurotoxicity research. 2007;12:95–104. doi: 10.1007/BF03033918. [DOI] [PubMed] [Google Scholar]
- Rajagopal R, Chen ZY, Lee FS, Chao MV. Transactivation of Trk neurotrophin receptors by G-protein-coupled receptor ligands occurs on intracellular membranes. J Neurosci. 2004;24:6650–6658. doi: 10.1523/JNEUROSCI.0010-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch DM, Iacangelo AL, Eiden LE. Glucocorticoid- and nerve growth factor-induced changes in chromogranin A expression define two different neuronal phenotypes in PC12 cells. Mol Endocrinol. 1988;2:921–927. doi: 10.1210/mend-2-10-921. [DOI] [PubMed] [Google Scholar]
- Ravni A, Bourgault S, Lebon A, Chan P, Galas L, Fournier A, Vaudry H, Gonzalez B, Eiden LE, Vaudry D. The neurotrophic effects of PACAP in PC12 cells: control by multiple transduction pathways. J Neurochem. 2006a;98:321–329. doi: 10.1111/j.1471-4159.2006.03884.x. [DOI] [PubMed] [Google Scholar]
- Ravni A, Eiden LE, Vaudry H, Gonzalez BJ, Vaudry D. Cycloheximide treatment to identify components of the transitional transcriptome in PACAP-induced PC12 cell differentiation. J Neurochem. 2006b;98:1229–1241. doi: 10.1111/j.1471-4159.2006.03962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravni A, Vaudry D, Gerdin MJ, Eiden MV, Falluel-Morel A, Gonzalez BJ, Vaudry H, Eiden LE. A cAMP-dependent, protein kinase A-independent signaling pathway mediating neuritogenesis through Egr1 in PC12 cells. Mol Pharmacol. 2008;73:1688–1708. doi: 10.1124/mol.107.044792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reglodi D, Fabian Z, Tamas A, Lubics A, Szeberenyi J, Alexy T, Toth K, Marton Z, Borsiczky B, Roth E, Szalontay L, Lengvari I. Effects of PACAP on in vitro and in vivo neuronal cell death, platelet aggregation, and production of reactive oxygen radicals. Regul Pept. 2004;123:51–59. doi: 10.1016/j.regpep.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Sakai Y, Hashimoto H, Shintani N, Katoh H, Negishi M, Kawaguchi C, Kasai A, Baba A. PACAP activates Rac1 and synergizes with NGF to activate ERK1/2, thereby inducing neurite outgrowth in PC12 cells. Brain Res Mol Brain Res. 2004;123:18–26. doi: 10.1016/j.molbrainres.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Salinas M, Diaz R, Abraham NG, Ruiz de Galarreta CM, Cuadrado A. Nerve growth factor protects against 6-hydroxydopamine-induced oxidative stress by increasing expression of heme oxygenase-1 in a phosphatidylinositol 3-kinase-dependent manner. J Biol Chem. 2003;278:13898–13904. doi: 10.1074/jbc.M209164200. [DOI] [PubMed] [Google Scholar]
- Seaborn T, Masmoudi-Kouli O, Fournier A, Vaudry H, Vaudry D. Protective effects of pituitary adenylate cyclase-activating polypeptide (PACAP) against apoptosis. Current pharmaceutical design. 2011;17:204–214. doi: 10.2174/138161211795049679. [DOI] [PubMed] [Google Scholar]
- Shao N, Wang H, Zhou T, Liu C. 7S nerve growth factor has different biological activity from 2.5S nerve growth factor in vitro. Brain Res. 1993;609:338–340. doi: 10.1016/0006-8993(93)90893-r. [DOI] [PubMed] [Google Scholar]
- Shimoke K, Chiba H. Nerve growth factor prevents 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced cell death via the Akt pathway by suppressing caspase-3-like activity using PC12 cells: relevance to therapeutical application for Parkinson's disease. Journal of neuroscience research. 2001;63:402–409. doi: 10.1002/1097-4547(20010301)63:5<402::AID-JNR1035>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Sigmund O, Naor Z, Anderson DJ, Stein R. Effect of nerve growth factor and fibroblast growth factor on SCG10 and c-fos expression and neurite outgrowth in protein kinase C-depleted PC12 cells. J Biol Chem. 1990;265:2257–2261. [PubMed] [Google Scholar]
- Silverman GA, Lomas DA. Serpin identification, production, and characterization. Methods. 2004;32:71–72. doi: 10.1016/s1046-2023(03)00199-3. [DOI] [PubMed] [Google Scholar]
- Silverman GA, Whisstock JC, Askew DJ, Pak SC, Luke CJ, Cataltepe S, Irving JA, Bird PI. Human clade B serpins (ov-serpins) belong to a cohort of evolutionarily dispersed intracellular proteinase inhibitor clades that protect cells from promiscuous proteolysis. Cell Mol Life Sci. 2004;61:301–325. doi: 10.1007/s00018-003-3240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderholm H, Olsson A, Lavenius E, Ronnstrand L, Nanberg E. Activation of Ras, Raf-1 and protein kinase C in differentiating human neuroblastoma cells after treatment with phorbolester and NGF. Cell Signal. 2001;13:95–104. doi: 10.1016/s0898-6568(00)00141-8. [DOI] [PubMed] [Google Scholar]
- Spengler D, Waeber C, Pantaloni C, Holsboer F, Bockaert J, Seeburg PH, Journot L. Differential signal transduction by five splice variants of the PACAP receptor. Nature. 1993;365:170–175. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- Suh J, Lu N, Nicot A, Tatsuno I, DiCicco-Bloom E. PACAP is an anti-mitogenic signal in developing cerebral cortex. Nat Neurosci. 2001;4:123–124. doi: 10.1038/83936. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Koshimura K, Murakami Y, Sohmiya M, Yanaihara N, Kato Y. Neuronal protection from apoptosis by pituitary adenylate cyclase-activating polypeptide. Regul Pept. 1997;72:1–8. doi: 10.1016/s0167-0115(97)01038-0. [DOI] [PubMed] [Google Scholar]
- Tewari M, Dixit VM. Fas- and tumor necrosis factor-induced apoptosis is inhibited by the poxvirus crmA gene product. J Biol Chem. 1995;270:3255–3260. doi: 10.1074/jbc.270.7.3255. [DOI] [PubMed] [Google Scholar]
- van Gent D, Sharp P, Morgan K, Kalsheker N. Serpins: structure, function and molecular evolution. Int J Biochem Cell Biol. 2003;35:1536–1547. doi: 10.1016/s1357-2725(03)00134-1. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Chen Y, Hsu CM, Eiden LE. PC12 cells as a model to study the neurotrophic activities of PACAP. Ann N Y Acad Sci. 2002a;971:491–496. doi: 10.1111/j.1749-6632.2002.tb04513.x. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Chen Y, Ravni A, Hamelink C, Elkahloun AG, Eiden LE. Analysis of the PC12 cell transcriptome after differentiation with pituitary adenylate cyclase-activating polypeptide (PACAP) J Neurochem. 2002b;83:1272–1284. doi: 10.1046/j.1471-4159.2002.01242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudry D, Falluel-Morel A, Basille M, Pamantung TF, Fontaine M, Fournier A, Vaudry H, Gonzalez BJ. Pituitary adenylate cyclase-activating polypeptide prevents C2 ceramide-induced apoptosis of cerebellar granule cells. Journal of neuroscience research. 2003;72:303–316. doi: 10.1002/jnr.10530. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BK, Hashimoto H, Galas L, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev. 2009;61:283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Pamantung TF, Fontaine M, Fournier A, Vaudry H. The neuroprotective effect of pituitary adenylate cyclase-activating polypeptide on cerebellar granule cells is mediated through inhibition of the CED3-related cysteine protease caspase-3/CPP32. Proc Natl Acad Sci U S A. 2000a;97:13390–13395. doi: 10.1073/pnas.97.24.13390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev. 2000b;52:269–324. [PubMed] [Google Scholar]
- Vaudry D, Stork PJ, Lazarovici P, Eiden LE. Signaling pathways for PC12 cell differentiation: making the right connections. Science. 2002c;296:1648–1649. doi: 10.1126/science.1071552. [DOI] [PubMed] [Google Scholar]
- Vercammen D, Belenghi B, van de Cotte B, Beunens T, Gavigan JA, De Rycke R, Brackenier A, Inze D, Harris JL, Van Breusegem F. Serpin1 of Arabidopsis thaliana is a suicide inhibitor for metacaspase 9. J Mol Biol. 2006;364:625–636. doi: 10.1016/j.jmb.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Akimoto Y, Yugi K, Uda S, Chung J, Nakamuta S, Kaibuchi K, Kuroda S. Latent process genes for cell differentiation are common decoders of neurite extension length. Journal of cell science. 2012 doi: 10.1242/jcs.097709. [DOI] [PubMed] [Google Scholar]
- Wu C, Lai CF, Mobley WC. Nerve growth factor activates persistent Rap1 signaling in endosomes. J Neurosci. 2001;21:5406–5416. doi: 10.1523/JNEUROSCI.21-15-05406.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu EH, Wong YH. Pertussis toxin-sensitive Gi/o proteins are involved in nerve growth factor-induced pro-survival Akt signaling cascade in PC12 cells. Cell Signal. 2005;17:881–890. doi: 10.1016/j.cellsig.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Yabe T, Kanemitsu K, Sanagi T, Schwartz JP, Yamada H. Pigment epithelium-derived factor induces pro-survival genes through cyclic AMP-responsive element binding protein and nuclear factor kappa 13 activation in rat cultured cerebellar granule cells: Implication for its neuroprotective effect. Neuroscience. 2005;133:691–700. doi: 10.1016/j.neuroscience.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Yepes M, Sandkvist M, Wong MK, Coleman TA, Smith E, Cohan SL, Lawrence DA. Neuroserpin reduces cerebral infarct volume and protects neurons from ischemia-induced apoptosis. Blood. 2000;96:569–576. [PubMed] [Google Scholar]
- York RD, Yao H, Dillon T, Ellig CL, Eckert SP, McCleskey EW, Stork PJ. Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature. 1998;392:622–626. doi: 10.1038/33451. [DOI] [PubMed] [Google Scholar]
- Zhang W, Shi HY, Zhang M. Maspin overexpression modulates tumor cell apoptosis through the regulation of Bcl-2 family proteins. BMC Cancer. 2005;5:50. doi: 10.1186/1471-2407-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Snipas S, Orth K, Muzio M, Dixit VM, Salvesen GS. Target protease specificity of the viral serpin CrmA. Analysis of five caspases. J Biol Chem. 1997;272:7797–7800. doi: 10.1074/jbc.272.12.7797. [DOI] [PubMed] [Google Scholar]