Abstract
The mammalian placenta is the site of nutrient and gas exchange between the mother and fetus, and is comprised of two principal cell types, trophoblasts and endothelial cells. Proper placental development requires invasion and differentiation of trophoblast cells, together with coordinated fetal vasculogenesis and maternal vascular remodeling. Disruption in these processes can result in placental pathologies such as preeclampsia (PE), a disease characterized by late gestational hypertension and proteinuria. Epidermal Growth Factor Like Domain 7 (EGFL7) is a largely endothelial-restricted secreted factor that is critical for embryonic vascular development, and functions by modulating the Notch signaling pathway. However, the role of EGFL7 in placental development remains unknown. In this study, we use mouse models and human placentas to begin to understand the role of EGFL7 during normal and pathological placentation. We show that Egfl7 is expressed by the endothelium of both the maternal and fetal vasculature throughout placental development. Importantly, we uncovered a previously unknown site of EGFL7 expression in the trophoblast cell lineage, including the trophectoderm, trophoblast stem cells, and placental trophoblasts. Our results demonstrate significantly reduced Egfl7 expression in human PE placentas, concurrent with a downregulation of Notch target genes. Moreover, using the BPH/5 mouse model of PE, we show that the downregulation of Egfl7 in compromised placentas occurs prior to the onset of characteristic maternal signs of PE. Together, our results implicate Egfl7 as a possible factor in normal placental development and in the etiology of PE.
Keywords: placenta, preeclampsia, trophoblast, endothelium, EGFL7, Notch signaling
1. Introduction
The placenta serves as the site of contact for the maternal and embryonic circulatory systems to enable nutrient and gas exchange, and contains two primary functional cell types, trophoblast and endothelial cells. Proper placental development requires invasion and differentiation of trophoblast cells, as well as coordinated maternal vascular remodeling and fetal vasculogenesis (Norwitz et al., 2001). Any disruption in these processes can result in placental pathologies, including preeclampsia (PE). PE is a leading cause of maternal and fetal morbidity and mortality worldwide, and the only resolutive treatment is delivery of the baby and placenta. Although the pathophysiology of PE remains largely unknown, inadequate trophoblast cell invasion, endothelial cell dysfunction, dysregulated uteroplacental vascularization, and an imbalance of pro- and anti-angiogenic growth factors have been implicated in the disease (Young et al., 2010).
Recent studies have implicated Notch signaling, an evolutionarily conserved pathway that is important for cell fate specification, proliferation, and patterning (Phng and Gerhardt, 2009), in placental development and the pathogenesis of PE. Targeted mutations of Notch signaling components in the mouse result in placental defects, demonstrating their vital roles for proper placental development (Duarte et al., 2004; Fischer et al., 2004; Hunkapiller et al., 2011; Krebs et al., 2000). In addition, Notch pathway proteins are downregulated in trophoblast cells, endothelial cells, and stromal cells of third trimester placentas from human preeclamptic patients as compared to normal patients (Cobellis et al., 2007).
Epidermal Growth Factor Like Domain 7 (EGFL7) is a secreted factor that is present in both soluble and extracellular matrix-bound forms (Fitch et al., 2004). Egfl7 expression in the developing embryo is largely restricted to endothelial progenitors and actively proliferating endothelium (Campagnolo et al., 2005; Fitch et al., 2004; Soncin et al., 2003). However, it is also found in embryonic stem cells, pre- and peri-implantation embryos, primordial germ cells, and some CNS neurons (Campagnolo et al., 2008; Fitch et al., 2004; Parker et al., 2004; Schmidt et al., 2009; Soncin et al., 2003). EGFL7 is largely downregulated during late embryogenesis and in the quiescent endothelium of the adult mouse, and is upregulated during pathological and physiological angiogenesis, such as in the uterus (Campagnolo et al., 2005; Fitch et al., 2004; Soncin et al., 2003). Egfl7 is upregulated in response to, and has a protective effect against, hypoxia (Badiwala et al., 2010; Gustavsson et al., 2007; Xu et al., 2008). It acts as a chemoattractant for endothelial cells and plays an important role in their proliferation and migration (Campagnolo et al., 2005; Durrans and Stuhlmann, 2010; Schmidt et al., 2007). EGFL7 functions in vascular patterning, stratification, and tubulogenesis in vitro and in the mouse and zebrafish (Campagnolo et al., 2005; Durrans and Stuhlmann, 2010; Nichol et al., 2010; Parker et al., 2004). EGFL7 has been shown to modulate the Notch signaling cascade by acting either as a Notch agonist, such as in the developing embryo, or as a Notch antagonist, such as in the postnatal retina and neural stem cells (Nichol et al., 2010; Schmidt et al., 2009).
Despite its key role in early embryogenesis, vascular development, and modulation of Notch signaling, the expression pattern and function of EGFL7 in normal and PE placentas is poorly understood. In this study, we investigated the expression pattern of EGFL7 in normal murine and human placentas. Rodents and primates both undergo hemochorial placentation (Cross et al., 2003). Despite some structural differences, the trophoblast cell types and the molecular pathways driving placental development are highly conserved between mouse and human (Cross et al., 2003; Georgiades et al., 2002; Hu and Cross, 2010; Rossant and Cross, 2001). Importantly, the labyrinth in the mouse placenta is analogous to the chorionic villi in human placentas, whereas the junctional zone in mice is analogous to the cytotrophoblast cell columns (Rossant and Cross, 2001) or the basal plate in humans (Georgiades et al., 2002).
In addition to examining the expression profile of Egfl7 during normal placental development, this study investigates a potential role for EGFL7 in preeclampsia by analyzing human PE placentas and compromised placentas from the BPH/5 murine PE model. The BPH/5 mouse strain exhibits the characteristic PE signs of late-gestational hypertension, proteinuria, and endothelial dysfunction (Davisson et al., 2002; Dokras et al., 2006). BPH/5 mice also show fetoplacental defects such as impaired endothelial cell branching, maternal spiral artery remodeling, and reduced fetal labyrinth depth (Dokras et al., 2006). Here we have described the spatiotemporal expression profile of Egfl7 in placental endothelial cells in the mouse and human. We uncovered a previously unknown site of EGFL7 localization in the non-endothelial trophoblast lineage, beginning at the blastocyst stage and becoming restricted to a subset of differentiated trophoblast cells. Furthermore, we provide evidence that a downregulation of EGFL7 is associated with human PE and the BPH/5 mouse model of PE, and this downregulation is concomitant with a decrease in Notch target gene expression.
2. Results
2.1 EGFL7 is expressed by maternal and fetal endothelial cells in the mouse placenta throughout gestation
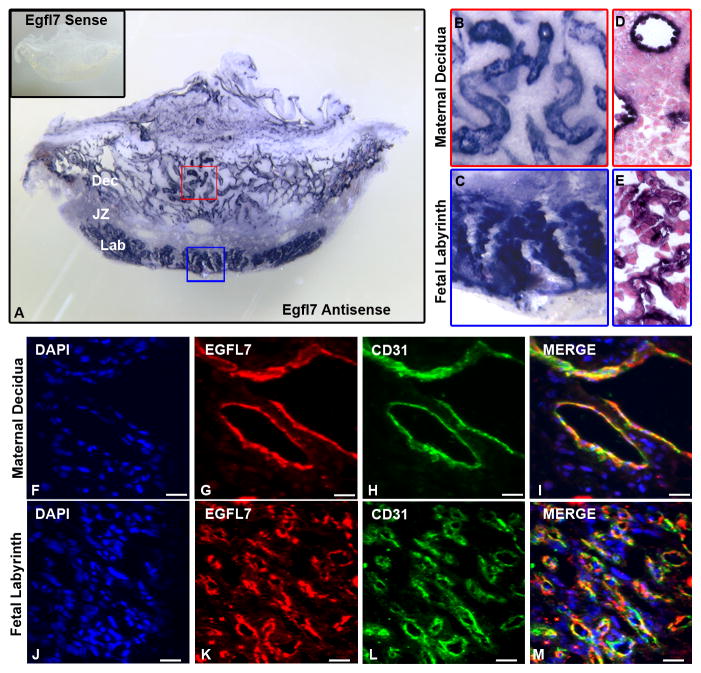
To obtain a temporal and spatial expression profile for Egfl7 throughout pregnancy, we used the mouse as a model system. To localize expression of Egfl7 transcripts in the maternal and fetal placental vasculature, RNA in situ hybridization (ISH) was performed on 100μm thick vibratome sections of C57Bl/6 mouse placentas using an Egfl7 specific riboprobe. Egfl7 transcripts were highly expressed at E10.5, and were localized to maternal vessels, including the spiral arteries of the decidua (Fig. 1A,B), and the branching vascular structures in the fetal labyrinth (Fig. 1A,C). Following ISH, specimens were embedded in paraffin, sectioned, and counterstained to analyze the cellular morphology of Egfl7 mRNA expression in the placenta. Results revealed Egfl7 transcript expression in cells lining the large lumen of maternal vessels in the decidua (Fig. 1D) and in the narrow and highly branched capillaries of the fetal labyrinth (Fig. 1E). Egfl7 transcript levels were dramatically reduced at E12.5 and nearly undetectable at E18.5 (Supplemental Fig. 1). Egfl7 sense controls (insets of Fig. 1A, Supplemental Fig. 1A,C) demonstrate specificity of the Egfl7 riboprobe.
Figure 1. EGFL7 is expressed by maternal and fetal endothelial cells in the mouse placenta.
In situ hybridization was performed to determine Egfl7 mRNA localization, using an Egfl7 riboprobe on 100μm thick vibratome sections of E10.5 C57Bl/6 placentas (A–E). Higher magnification images of boxes in (A) demonstrating Egfl7 transcript is highly expressed in the maternal decidua (B) and fetal labyrinth (C). Egfl7 sense controls (A inset) show specificity of Egfl7 riboprobe. Paraffin sections of the vibratome sections showing cellular morphology after Egfl7 riboprobe staining in the maternal decidua (D) and fetal labyrinth (E). To determine the cell types expressing EGFL7 protein in the placenta, double immunofluorescent staining was performed on E12.5 C57BL/6 placentas for EGFL7 (red), CD31 (green) and nuclear DAPI (blue) (F–M). EGFL7 colocalizes with the endothelial cell marker, CD31, in the maternal decidua (F–I) and the fetal labyrinth (J–M). Dec-Maternal Decidua, JZ-Junctional Zone, Lab-Fetal Labyrinth. Scale bar (F–M) = 20μm.
To determine the spatiotemporal expression profile of EGFL7 protein, we performed double immunofluorescent staining for EGFL7 and the pan-endothelial marker, CD31, on C57Bl/6 mouse placentas (Fig. 1-F–M, Supplemental Fig. 2). EGFL7 colocalized with CD31 in the maternal decidua and the fetal labyrinth at every stage examined, confirming that EGFL7 localized to maternal and fetal endothelial cells in the placenta.
Together, the results demonstrate that Egfl7 transcript and protein expression was dynamic throughout gestation. Egfl7 expression is high during early stages of vascular development in the placenta, with transcript expression peaking at E10.5 (Fig. 1A–E), as well as high protein expression at E10.5 and E12.5 (Fig. 1F–M, Supplemental Fig. 2A). At E18.5, a time point with fewer newly forming nascent vessels, Egfl7 transcript expression is dramatically lower (Supplemental Fig. 1D), while the protein expression persists (Supplemental Fig. 2B). Our data are consistent with previously reported Egfl7 expression data during vascular development and physiological angiogenesis, where the highest expression levels of Egfl7 are detected in actively proliferating endothelial cells, such as during formation of the initial vascular plexus during early embryogenesis (Campagnolo et al., 2005; Campagnolo et al., 2008; Durrans and Stuhlmann, 2010; Fitch et al., 2004; Nichol et al., 2010; Parker et al., 2004; Schmidt et al., 2007).
2.2 Novel EGFL7 expression in placental trophoblast cells and the trophoblast cell lineage
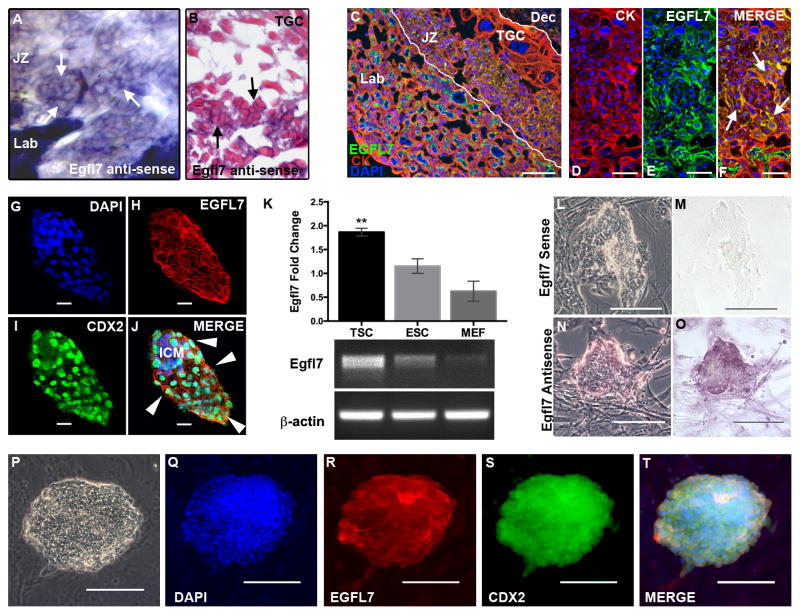
In addition to demonstrating dynamic expression of EGFL7 in the placental endothelium, our in situ hybridization images at high magnification also uncovered a novel expression domain for Egfl7 in non-endothelial cells of the junctional zone of the mouse placenta, albeit at reduced levels (Fig. 2A–B). Images of Egfl7 ISH paraffin sections revealed clusters of EGFL7 positive cells located in the junctional zone, between the fetal labyrinth and trophoblast giant cells (Fig. 2B). Double immunofluorescent staining for EGFL7 and the pan-trophoblast marker CYTOKERATIN (CK), performed on mid-gestation mouse placentas confirmed EGFL7 localization to the trophoblast lineage (Fig. 2C–F). This is the first evidence that EGFL7 localizes to trophoblast cells of the placenta.
Figure 2. EGFL7 is expressed in the trophoblast cell lineage of the mouse.
In situ hybridization was performed to determine Egfl7 mRNA localization, using an Egfl7 riboprobe on 100μm thick vibratome sections of E10.5 C57Bl/6 placentas (A). Shown is the junctional zone of the placenta (A). Paraffin sections of the vibratome section showing cellular morphology of Egfl7 riboprobe staining in the junctional zone of the placenta (B). Arrows indicate positive Egfl7 transcript signal in the junctional zone trophoblast cells. (TGC=Trophoblast Giant Cell). To determine the cell type expressing EGFL7 protein in the placenta, double immunofluorescent staining was performed on E10.5 C57BL/6 placentas (C–F) for the pan-trophoblast marker, CYTOKERATIN (CK, red), EGFL7 (green), and nuclear DAPI (blue). Cross-section of E10.5 placenta showing the fetal labyrinth (Lab), junctional zone (JZ, demarked by white lines) and maternal decidua (Dec). High magnification images (D–F) of the junctional zone demonstrating colocalization of EGFL7 with CK in the JZ trophoblast cells (arrows). Whole mount immunofluorescent staining of blastocyst-stage C57BL/6 embryos (G–J) for DAPI (blue), EGFL7 (red), and trophectoderm cell marker, CDX2 (green) reveals EGFL7 protein localization to both inner cell mass (ICM) cells and trophectoderm cells (arrowheads). Real Time RT-PCR data for Egfl7 transcript expression in trophoblast stem cells (TSC), embryonic stem cells (ESC), and mouse embryonic fibroblasts (MEF), shows a significantly higher level of Egfl7 expression in TSC,**P<0.01 (K, top). Agarose gel with products of semi-quantitative RT-PCR for TSC, ESC and MEFs are shown, using Egfl7 and β-actin specific primers (K, bottom). RNA in situ hybridization analysis of Egfl7 mRNA in TSC (L–O) defines Egfl7 transcript expression in TSC. Phase-contrast and bright-field images of TSC incubated with an Egfl7 sense control probe (L–M), and an Egfl7 antisense probe (N–O). Double immunofluorescent staining of TSC (P–T) for DAPI, EGFL7, and CDX2 demonstrating EGFL7 protein localization to TSC. Phase-contrast image of TSC colony is shown in (P). Scale bars in D–F=25μm, G–J=20μm, C and L–T=100μm.
The newly uncovered expression of EGFL7 in placental trophoblast cells prompted us to examine its temporal expression pattern during trophoblast lineage development in mice. Differentiated trophoblast cells are derived from the outer cell layer of blastocysts, the trophectoderm (Cross, 2005b). We have previously shown that Egfl7 mRNA was expressed by blastocysts and embryonic stem cells, however its spatial expression in pre-implantation embryos was not defined (Campagnolo et al., 2005; Campagnolo et al., 2008). Here, we isolated embryos from C57BL/6 mice at the blastocyst stage of development and performed whole-mount immunofluorescent staining for EGFL7 and the trophectoderm marker, CDX2. Both inner cell mass cells and trophectoderm cells expressed EGFL7 (Fig. 2G–J). Therefore, Egfl7 is expressed at the first step of trophoblast lineage development.
To further establish EGFL7 expression in the trophoblast lineage, we assayed trophoblast stem cells (TSC), a model of in vitro trophoblast cell differentiation. Multipotent TSC are derived from the trophectoderm of blastocysts, and upon removal of exogenous Fibroblast Growth Factor, they recapitulate the defining characteristics of in vivo trophoblast cell differentiation (Tanaka et al., 1998). Semi-quantitative and Real Time RT-PCR analysis showed that TSC express Egfl7 mRNA at significantly higher levels than embryonic stem cells (ESC), whereas primary mouse embryonic fibroblasts (MEF) express very low levels of Egfl7 (Fig. 2K, P<0.01). RNA in situ hybridization on TSC revealed specific localization of Egfl7 mRNA in TSC (Fig. 2L–O). EGFL7 protein was detected on cultured TSC, as shown by colocalization of EGFL7 and CDX2 using double immunofluorescent staining (Fig. 2P–T). Two additional antibodies that are directed against different regions of the EGFL7 protein gave comparable results (data not shown). Together, our results show that EGFL7 is expressed by the trophectoderm of blastocysts, in trophoblast stem cells, and a subset of differentiated trophoblast cells in the mid-gestation mouse placenta.
2.3 EGFL7 is expressed by fetal endothelial cells and trophoblast cells of human chorionic villi
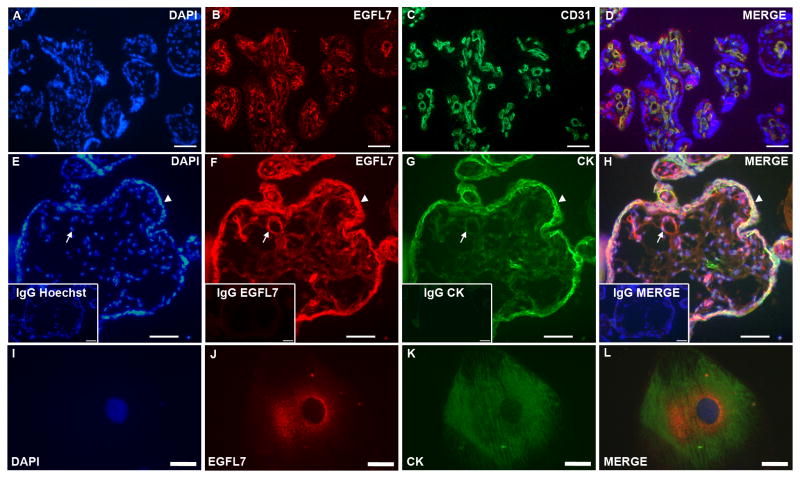
To determine if EGFL7 expression in endothelial and trophoblast cells is conserved in human placentas, chorionic villi samples were obtained from normal pregnant women with an average pregnancy duration of 39 weeks. Double immunofluorescent staining analysis revealed that EGFL7 was expressed on fetal vessels, where it colocalized with the pan-endothelial marker, CD31 (Fig. 3A–D). Importantly, EGFL7 was also expressed on the syncytiotrophoblast layer of the villi, where it colocalized with the pan-trophoblast marker CYTOKERATIN (Fig. 3E–H). To confirm this observation, EGFL7 staining was performed using two antibodies directed against different regions of the EGFL7 protein, and both gave comparable results (Supplemental Fig. 3). Of note, expression of Egfl7 was not detected in the analogous mouse syncytiotrophoblasts. To further determine if EGFL7 localized to trophoblast cells of the human placenta, we isolated cytotrophoblast cells from term placentas as previously described (Hunkapiller and Fisher, 2008). Double immunofluorescent staining showed that EGFL7 and CYTOKERATIN colocalized to the isolated cytotrophoblast cell (Fig. 3I–L). Thus, our data demonstrate that EGFL7 is expressed in endothelial cells and trophoblast cells in both mouse and human placentas.
Figure 3. EGFL7 is expressed in endothelial cells and trophoblast cells of the human placenta.
Double immunofluorescent staining of normal human placentas (A–D) for EGFL7 (red), CD31 (green), and nuclear DAPI (blue) showing localization of EGFL7 protein on endothelial cells. Double immunofluorescent staining of human chorionic villi from placentas at 40-weeks of gestation (E–H) for nuclear DAPI (blue), EGFL7 (red), and pan-trophoblast marker CYTOKERATIN (CK) (green) demonstrates EGFL7 protein localization to trophoblast cells (arrows-fetal vessels, arrowheads-trophoblasts). Control staining of a close-by section (insets, E–H) using IgG and secondary antibodies only and nuclear Hoechst (blue) shows specificity of the antibodies. Expression of EGFL7 protein is found in human cytotrophoblast cells isolated from term placentas (I–L). Cells were stained for Hoechst (blue), EGFL7 (red) and CYTOKERATIN (green) (I–L). Scale bars in E–H and insets =100μm, and I–L=30μm.
2.4 EGFL7 is downregulated in placentas of the BPH/5 mouse model of PE, prior to the onset of maternal signs of PE
To examine potential variations in the expression and spatial distribution of EGFL7 in PE, we first utilized the BPH/5 mouse model of preeclampsia. E10.5 placentas from control C57BL/6 and compromised BPH/5 fetoplacental units (see Materials and Methods) were subjected to immunofluorescent staining for EGFL7 and CD31 (Fig. 4B–I). Importantly, this time point is prior to the onset of the late-gestational signs of PE in BPH/5 mice (Davisson et al., 2002). EGFL7 protein expression was reduced in the fetal labyrinth zone of the BPH/5 placentas compared to C57BL/6 (Fig. 4C,G). Total levels of CD31 appear unchanged (Fig. 4D–E,H–I), suggesting no overall reduction in placental vascular density. The pattern of the vessels, however, appears irregular in BPH/5 placentas. In fact, a previous study reported the anatomical appearance of attenuated and irregularly branched fetal vessels in isolectin B4-stained BPH/5 placentas (Dokras et al., 2006). To provide quantitative evidence, we performed Real Time RT-PCR analysis of E10.5 placentas from C57BL/6 and affected BPH/5 fetoplacental units for Egfl7. Results demonstrated that Egfl7 mRNA levels were significantly downregulated in BPH/5 placentas compared to C57BL/6 (Fig. 4A, *P<0.05).
Figure 4. EGFL7 is downregulated in the placentas of the BPH/5 mouse model of preeclampsia.
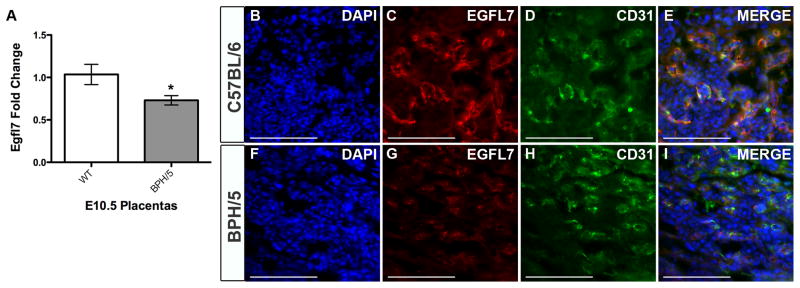
Real Time RT-PCR data for Egfl7 transcript levels in E10.5 placentas from C57BL/6 (WT) and BPH/5 mice reveal a significant decrease in Egfl7 in BPH/5 mice *P<0.05 (A). Immunofluorescent staining for EGFL7 protein (red), CD31 (green), and nuclear DAPI (blue) on the fetal labyrinth of E10.5 placentas from C57BL/6 (B–E) and BPH/5 mice (F–I) demonstrating a decrease in EGFL7 in BPH/5 placentas. Scale bar=100μm.
2.5 EGFL7 expression is significantly reduced in human preeclamptic placentas
Our results in the mouse model of PE prompted us to investigate potential variations in the expression of EGFL7 in human PE placentas. Biopsies from normal and PE human placentas were obtained and analyzed. Clinical characteristics of patients in this study are shown in Table 1 (Nelson and Burton, 2010). The study group consisted of ten early-onset preeclamptic patients with average pregnancy duration of 32 weeks. The control group consisted of ten healthy pregnant women with average pregnancy duration of 39 weeks. All patients underwent Caesarean sections. There were no differences in patient age at delivery, gravidity, or parity between the study and control groups. However, the study group exhibited lower neonatal birth weight and placental weight, higher systolic and diastolic pressure, and earlier gestational age, all of which are characteristic of PE patients.
Table 1.
Clinical characteristics of pregnancies for placentas studied. Data are presented as median ± standard deviation and analyzed using a student’s t-test.
| Parameter | Preeclampsia (n=10) | Control (n=10) | Significance |
|---|---|---|---|
| Parity | 0 ± 0.3 | 0 | |
| Gestational age (weeks) | 32 ± 2 | 39 ± 0 | <0.0001 |
| Maternal age (years) | 34 ± 4 | 35 ± 6 | 0.54 |
| Gravidity | 2 ± 1 | 2 ± 1 | 0.59 |
| Birth Weight (grams) | 1235 ± 359 | 3372 ± 354 | <0.001 |
| Birth Weight Percentile | 9 ± 3 | 63 ± 18 | <0.001 |
| Placental Weight (grams) | 236 ± 65 | 550 ± 55 | <0.001 |
| Systolic blood pressure at delivery (mm Hg) | 161 ± 11 | 130 ± 5 | <0.001 |
| Diastolic blood pressure at delivery (mm Hg) | 109 ± 8 | 82 ± 3 | <0.001 |
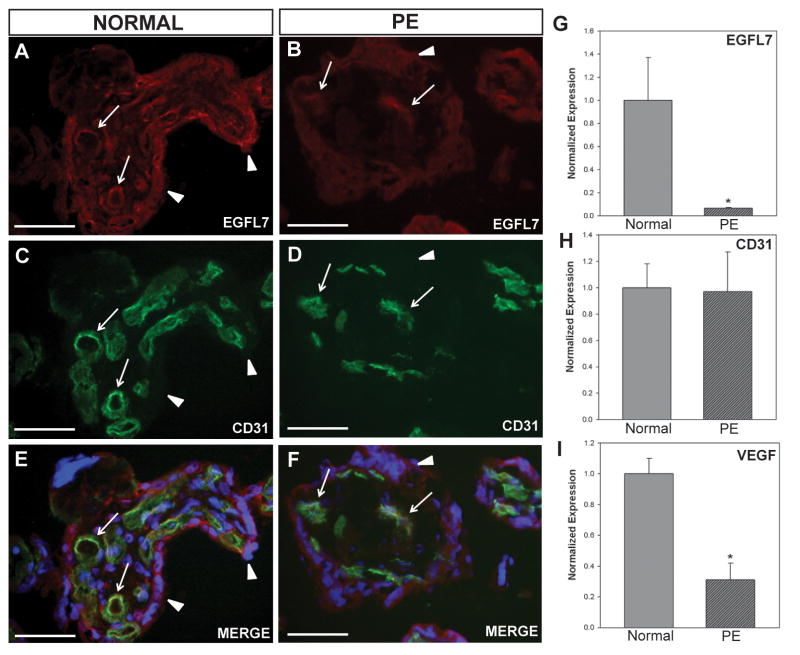
To examine the EGFL7 protein expression profile of normal and PE placentas, we performed immunofluorescent staining for EGFL7 and CD31 on human placental biopsies (Fig. 5A–F). EGFL7 was immunodetected on the endothelium and trophoblast cells of the human placenta. When comparing immunofluorescent staining of normal and PE placentas, EGFL7 appeared lower in both endothelial and trophoblast cells of PE placentas (Fig. 5A–B), whereas no major differences were observed for CD31 (Fig. 5C–D).
Figure 5. EGFL7 is significantly reduced in human preeclamptic placentas.
Double immunofluorescent staining of normal and preeclamptic (PE) human placentas (A–F) for EGFL7 protein (red), CD31 (green), and nuclear Hoechst (blue) revealing a decrease in EGFL7 protein in PE placentas (arrows-fetal vessels, arrowheads-trophoblasts). Real Time RT-PCR data for EGFL7 (G), CD31 (H), and VEGF (I) on normal and preeclamptic (PE) human placentas demonstrating a significant decrease in EGFL7 transcript levels in PE placentas, even further than the known angiogenic factor, VEGF (n=10, *P<0.05). Scale bars = 50μm.
To quantify this observation, we performed Real-Time RT-PCR analysis on normal and PE samples for EGFL7 (Fig. 5G) and CD31 (Fig. 5H). EGFL7 mRNA expression was significantly decreased by more than 10-fold in PE when compared to normal placentas (*P<0.05). In contrast, no significant difference in the level of CD31 mRNA was detected, suggesting the difference in EGFL7 transcript levels was not due to an overall reduced expression of endothelial-specific genes or a reduction in vascular density (Costa et al., 2013; Nadra et al., 2010; Nichol et al., 2010; van Tuyl et al., 2005). In our study, the reduction in EGFL7 levels in PE was more pronounced as compared to VEGF levels (Fig. 5I), a growth factor whose implication in PE has been extensively studied by others, and found to be either decreased (Cooper et al., 1996; Lyall et al., 1997; Park et al., 2010) or increased (Akercan et al., 2008; Chung et al., 2004; Geva et al., 2002) in PE placentas. Thus, EGFL7 expression is significantly downregulated in both a mouse model of PE and human PE placentas.
2.6 EGFL7 expression correlates with NOTCH signaling in normal and PE placentas
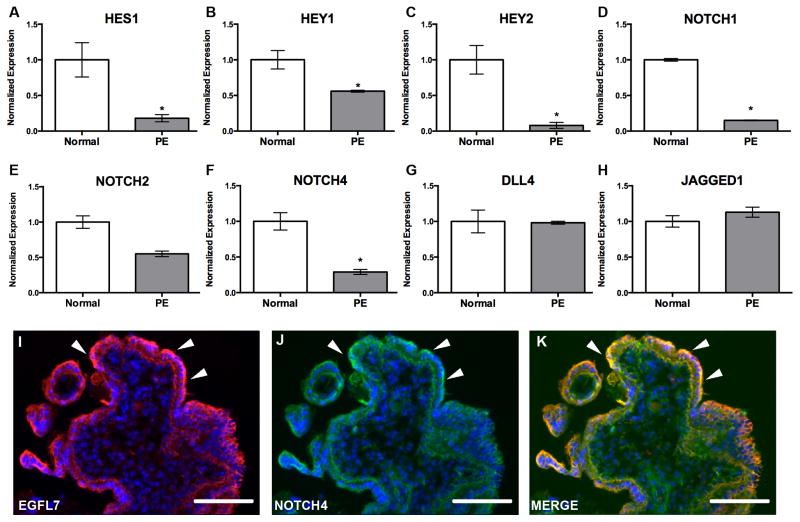
Previous studies demonstrated that EGFL7 interacts with and modulates NOTCH signaling in vitro and in vivo (Nichol et al., 2010; Schmidt et al., 2009). To examine if EGFL7 and the Notch signaling pathway are concomitantly dysregulated in PE, we performed Real-Time RT-PCR analysis of normal and human PE placentas for NOTCH pathway members and NOTCH target genes. Our results demonstrated a significant decrease in NOTCH1, NOTCH4, HEY2, HEY1, and HES1 mRNA expression (P<0.05), and a downward trend in NOTCH2 expression in PE samples when compared to normal placentas (Fig. 6A–F). In contrast, transcript levels for the Notch ligands DLL4 and JAGGED1 were unchanged (Fig. 6G–H). Additionally, double immunofluorescent staining of human placentas revealed that EGFL7 colocalized with NOTCH4 on the syncytiotrophoblast cells (Fig. 6I–K). Thus, downregulation of NOTCH signaling members and NOTCH target gene expression correlate with a downregulation of EGFL7 in PE placentas, suggesting that EGFL7 may possibly function through the Notch signaling pathway.
Figure 6. Expression of NOTCH target genes and NOTCH receptors are downregulated in PE, concomitant with EGFL7.
Real Time RT-PCR data for NOTCH pathway members on normal and preeclamptic (PE) human placentas (A–H). Gene expression analysis for HES1 (A), HEY1 (B), and HEY2 (C)demonstrating a significant decrease in all NOTCH target gene transcripts in PE. Gene expression analysis for NOTCH receptors NOTCH1 (D), NOTCH2 (E), and NOTCH4 (F) demonstrating a significant downregulation in NOTCH1 and NOTCH4, and a downward trend in NOTCH2 in PE patients. Gene expression analysis indicating no change in NOTCH ligands DLL4 (G) and JAGGED1 (H). (n=3–7, *P<0.05). Immunofluorescent staining of a cross section of human villi (I–K) for EGFL7 (red), NOTCH4 (green), and Hoechst (blue) (arrowheads-trophoblasts) shows colocalization of EGFL7 protein and NOTCH4. (*P<0.05) Scale bars = 50 μm.
3. Discussion
PE is a complex placental disease that occurs in approximately 2–7.5% of pregnancies worldwide (Dolea and AbouZahr, 2003; Wallis et al., 2008), yet the etiology of the disease remains unknown and early predictive biomarkers are lacking. Research on the human placenta has mostly been limited to studying the organ at late gestation and full term. To fully understand the early causes and progression of this placental disease, it is crucial to employ appropriate animal and in vitro models. Our results show that expression of EGFL7 is significantly reduced in compromised placentas from the BPH/5 mouse model of PE compared to control mice. These findings are corroborated by our results from human PE samples. Importantly, reduced EGFL7 expression in PE placentas did not correlate with reduced expression of the pan-endothelial marker CD31 or reduced density of CD31-positive vessels. This suggests that the change in EGFL7 was not a consequence of reduced vascular density. However, as a caveat, CD31 transcript expression is an indirect indicator for endothelial cell content and therefore may vary from the total vascular density in the placenta.
Of interest to this study, EGFL7 was identified in two recent microarray studies as one of the genes significantly reduced in human PE placentas compared to normal placentas, consistent with our data (Johansson et al., 2011; Junus et al., 2012). Importantly, PE is thought to begin much earlier in gestation than when the late-stage human placental biopsies used in most studies are obtained (Merviel et al., 2004; Waite et al., 2002). Our results describe changes in EGFL7 expression in phenotypically abnormal placentas of a PE mouse model, prior to the onset of characteristic maternal signs of PE, suggesting EGFL7 may be an early biomarker for the disease. It would be of interest to determine whether EGFL7 is altered in phenotypically abnormal placentas of other placental pathologies.
Moreover, we have shown that EGFL7 is downregulated in human placentas of early-onset PE patients. Formally, we cannot rule out the possibility that the difference in EGFL7 expression is due to a difference in gestational age (PE: week-32 versus Normal: average of 39-week gestation). However, analysis of a single placenta at week-34 of gestation from a normal pregnancy indicated that EGFL7 expression levels were similar to those in normal term placentas (data not shown). Furthermore, both early onset PE and intrauterine growth restriction (IUGR) are associated with abnormal placentation and changes in plasma levels of placental angiogenic factors, but IUGR does not present with the maternal signs of hypertension and proteinuria. A recent study demonstrated that isolated IUGR with no maternal disease is associated with the same changes in placental angiogenic factors and subclinical endothelial dysfunction as in PE (Crispi et al., 2006), suggesting the only difference between these two diseases may be maternal manifestations of the disease. Early-onset PE, which manifests by maternal hypertension and proteinuria, and progresses to a systemic hypoperfusion of multiple maternal organs, is often accompanied by abnormal fetal growth (Ness and Sibai, 2006), as in the cases of our study (9/10; 90%). Therefore, reduced EGFL7 expression correlates with abnormal placentation that may be associated with either early-onset PE or isolated IUGR. Contribution of additional facilitating factors, possibly at the level of maternal predisposition, may influence the manifestation of maternal hypertension and proteinuria.
Previously, EGFL7 expression was thought to be restricted to actively proliferating vascular endothelium and to embryonic stem cells (Campagnolo et al., 2008; Fitch et al., 2004; Schmidt et al., 2009). Our study has used multiple methods to uncover a novel and dynamic expression pattern for Egfl7 during murine placental development. Egfl7 is expressed highly in the fetal and maternal vasculature of the placenta. Its highly regulated expression pattern that correlates with expression during embryonic development suggests a potential angiogenic role in placental angiogenesis. Notably, in addition to its expression in the fetal and maternal vessels of the placenta, we showed, for the first time, that Egfl7 was also expressed in the trophoblast cell lineage, beginning at the blastocyst stage and becoming restricted to a subset of differentiated trophoblast cells in the mature placenta. Trophoblast cells are the principal non-endothelial cell populations at the feto-maternal interface. Targeted mutations in genes important for trophoblast cell differentiation result in placental defects or complete loss of placenta formation (Cross, 2005a). Interestingly, knockdown of Egfl7 in ESC decreases the proliferation rate of undifferentiated ESC and impairs endothelial cord formation in an embryoid body model of ESC differentiation (Durrans and Stuhlmann, 2010). Determining the role of Egfl7 in TSC and trophoblasts will be crucial for understanding the molecular mechanisms that control trophoblast lineage development and formation of the placenta.
EGFL7 has previously been shown to colocalize and functionally interact with NOTCH to modulate Notch signaling (Nichol et al., 2010; Schmidt et al., 2009). Notch signaling is a crucial pathway for placental development that affects both trophoblast and vascular cell function. For example, mouse strains with targeted deletions in the Notch receptors Notch1/4, their ligand Dll4, Notch target genes Hey1/2, or the Notch nuclear co-activator RBPJκ show defects in chorioallantoic branching and fetal placental angiogenesis (Duarte et al., 2004; Fischer et al., 2004; Gale et al., 2004; Gasperowicz and Otto, 2008; Krebs et al., 2004; Krebs et al., 2000; Limbourg et al., 2005; Oka et al., 1995). Mash2, a basic helix-loop-helix transcription factor that is thought to be repressed by the Notch target Hes1 (Gasperowicz and Otto, 2008), is critical for trophoblast cell fate specification in the mouse (Guillemot et al., 1994). Notch2 receptor function is crucial for formation of maternal blood sinuses and maternal spiral artery and arterial canal remodeling (Hamada et al., 2007; Hunkapiller et al., 2011). A recent study demonstrated that the Notch2 receptor is prominently expressed in the junctional zone trophoblast cells at E10.5 (Gasperowicz et al., 2013), as is EGFL7. It would be of interest to determine if EGFL7 functionally interacts with NOTCH2 in the placental junctional zone. Here we showed that NOTCH4 colocalized with EGFL7 in the placenta, and that downregulation of EGFL7 coincided with a reduction in Notch target gene expression in human PE placentas. Together, our results suggest EGFL7 may act, at least in part, as a NOTCH agonist in the placenta. Interestingly, whereas Egfl7 was found to act as an antagonist of Notch postnatally and in HUVECs (Nichol et al., 2010; Schmidt et al., 2009), embryonic overexpression of Egfl7 significantly increased Hey1 and Hey2 transcript levels, consistent with acting as a Notch agonist during embryogenesis (Nichol et al., 2010). It will be important to further explore the functional relationship between EGFL7 and the Notch signaling pathway during placental development, in addition to other pathways through which EGFL7 may be functioning.
EGFL7 gain- and loss-of-function approaches will help to fully understand the role of EGFL7 in the placenta and in placentopathies, and to dissect the underlying mechanism in both trophoblast cells and endothelial cells. It will be important to examine if endothelial and/or trophoblast-derived EGFL7 acts as an angiogenic factor in the placenta and if secreted EGFL7 acts in an autocrine or paracrine fashion, as has been suggested (Nichol and Stuhlmann, 2012). Of interest, partial embryonic lethality at mid-gestation was reported in the EGFL7 mutant mice that either overexpress EGFL7 or are EGFL7 deficient (Nichol et al., 2010; Schmidt et al., 2007). It is plausible that the partial lethality is, at least in part, due to a dysfunction in placental development.
In conclusion, our study has uncovered a novel expression domain for EGFL7 in non-endothelial trophoblast cell types. Its novel and dynamic expression pattern suggests a potential role in the molecular mechanisms that regulate trophoblast cell proliferation, differentiation, and invasion during placental development. Our study also links a significant downregulation of EGFL7 expression to PE placentas. We demonstrate that, in a PE mouse model, the change occurs in phenotypically abnormal placentas before the onset of the characteristic maternal signs of preeclampsia. It is tempting to speculate that EGFL7 could play a functional role in the pathophysiology of PE, and that EGFL7 could be an early biomarker of PE.
4. Materials and methods
4.1 Mice and placental sampling
All animal protocols were approved by the Institutional Animal Care and Use Committee at Weill Cornell Medical College and Cornell University. BPH/5 mice were from in-house colonies. C57BL/6 mice were obtained from Jackson Laboratories and served as a control for the BPH/5 strain (Davisson et al., 2002). For timed pregnancies, the day of visualization of a vaginal plug was designated embryonic day 0.5 (E0.5). Fetoplacental units of BPH/5 pregnancies are of varying status as assessed by ultrasonography in utero, ranging from normal to compromised to resorbed (Davisson et al., 2002). A subset of placentas were dissected only from E10.5 BPH/5 fetoplacental units that were compromised, i.e. with reduced fetal size, heart rate, and blood flow as determined by ultrasound as described (Davisson et al., 2002; Dokras et al., 2006). Placentas from normal or resorbed BPH/5 fetoplacental units were not sampled.
4.2 Human placenta sampling
Biopsies of placentas from normal (n=10) and early-onset PE pregnancies (n=10) were obtained from the Catholic University of Rome. The study respected the principles expressed in the Declaration of Helsinki and was approved by the Bioethical Committee of the Catholic University of Rome. For the collection of samples, all patients provided written informed consent. Clinical criteria used to define preeclampsia in our study were in accordance with the definition of the International Society for the Study of Hypertension in Pregnancy (ISSHP). In short, a patient (previously normotensive) was defined as being affected by PE if she had at least two diastolic blood pressure measurements of 90mmHg in 24 hours (≥4 hours apart), accompanied by proteinuria of at least 300 mg in a 24-hour collection of urine. Early-onset PE diagnosis was defined as the development of PE before the 34th week of gestation. All 10 PE patients displayed abnormal uterine artery Doppler velocimetry. Among PE patients, two had a normal umbilical artery Doppler velocimetry, and five patients showed altered umbilical artery Doppler data (two of which were associated with a brain sparing effect). No Doppler data were available for three PE patients. Nine out of ten (90%) of the early-onset PE pregnancies were associated with intrauterine growth restriction, defined as an estimated fetal weight below the 10th percentile for gestational age.
Chorionic villus samples were obtained from 3 healthy patients during prenatal diagnosis on the 10th week of gestation, following standard biopsy procedures. Placental villi from the third trimester of gestation were collected from 10 normotensive, healthy patients during cesarean section by multiple biopsies in the parasagittal plane with respect to the umbilical cord insertion. Similar procedures were followed for the collection of PE samples. Portions of all placental sample types were immediately frozen in liquid nitrogen or embedded in OCT. Clinical characteristics of patients in this study are shown in Table 1 (Nelson and Burton, 2010).
4.3 Cell Culture
Mouse trophoblast stem cells (TSC) were gifts from Dr. Kat Hadjantonakis (Memorial Sloan Kettering Institute, New York, NY) and Dr. Janet Rossant (Hospital for Sick Children, Toronto, Canada). TSC were cultured and maintained using published methods (Tanaka et al., 1998), on mouse embryonic fibroblasts (MEF) or with 50% MEF preconditioned medium. Primary human cytotrophoblasts were isolated from term placentas of normal patients, using a published protocol (Hunkapiller and Fisher, 2008). Cytotrophoblast cells were cultured on Matrigel-coated dishes in DMEM/H-21 medium (Gibco) supplemented with 2% Nutridoma (Roche).
4.4 RT-PCR
Placentas from C57BL/6 and BPH/5 mice were dissected and flash frozen in liquid nitrogen. TSC were grown on 60-mm plates for RNA extraction. For mouse and cell culture studies, RNA was isolated using Trizol (Invitrogen) and reverse transcribed using qScript cDNA Supermix (Quanta Biosciences). Gene expression was measured quantitatively using SYBR Green (Applied Biosciences) and specific primer sets for β-actin, Egfl7, CD31, Hes1, and Hey1/2 as described (Nichol et al., 2010). Differences among target expression were quantified using the ΔΔCT method with normalization to β-actin. For semi-quantitative RT-PCR, cDNA was amplified using the following primers:
Egfl7 (forward) 5′-CCACAAAAAGAAGAAGGCTACCC-3′
Egfl7 (reverse) 5′-TCCAAGAAGGACCCTGCTCACTC-3′
β-actin (forward) 5′-GTGGGCCGCTCTAGGCACCAA-3′
β-actin (reverse) 5′-CTCTTTGATGTCACGCACGATTTC-3′
Products were analyzed on agarose gels.
DNAse-free RNA from human placental tissue was prepared using the RNeasy Mini Kit (Qiagen). RNA quality was examined by determining the presence of ribosomal RNA bands in agarose gels. RNA was reverse transcribed using random primers and the Superscript First
Strand Synthesis System (Invitrogen) following the manufacturer’s protocol. Specific intron-spanning primers for EGFL7, CD31 and VEGF were designed using Primer Express software (Applied Biosystems, sequences are listed below). Gene expression was measured using Real Master Mix SYBR ROX (Eppendorf) and normalized to GAPDH.
EGFL7: 5′-TCGTGCAGCGTGTGTACCAG-3′, 5′-GCGGTAGGCGGTCCTATAGATG-3′
CD31: 5′-TAGCGCATGGCCTGGTTAGAG-3′, 5′-GGCGGTGCTCCCAAGTAGTCT-3′
VEGF: 5′-ATGACGAGGGCCTGGAGTGTG-3′, 5′-CCTATGTGCTGGCCTTGGTGAG-3′
GAPDH: 5′-TCGGAGTCAACGGATTTGGT-3′, 5′-GAATTTGCCATGGGTGGAAT-3′
NOTCH1: 5′-GCGGGATCCACTGTGAGAA-3′, 5′-CCGTTGAAGCAGGAGCTCTCT-3′
NOTCH2: 5′-AAAAATGGGGCCAACCGAGAC-3′, 5′-TTCATCCAGAAGGCGCACAA-3′
NOTCH4: 5′-CGGGCCTCTCTGCAACCT-3′, 5′-GACGTCTATGCCTTGGCTCAGT-3′
HES1: 5′-AAAGATAGCTCGCGGCATTC-3′, 5′-AGGTGCTTCACTGTCATTTCCA-3′
HEY1: 5′-CATCGAGGTGGAGAAGGAGAGT-3′, 5′-GACATGGAACCTAGAGCCGAACT-3′
HEY2: 5′-CGACCTCCGAGAGCGACAT-3′, 5′-CTTTGCCCCGAGTAATTGTTCT-3′
JAGGED1: 5′-GGAGGCGTGGGATTCCA-3′, 5′-CCGAGTGAGAAGCCTTTTCAATAAT-3′
DLL-4: 5′-CCAGCCAGATGGCAACTTGT-3′, 5′-CCCGAAAGACAGATAGGCTGTT-3′
4.5 RNA in situ hybridization
Egfl7 cDNA probes were generated by RT-PCR using the following primers: Egfl7 forward 5′-AGTTACTGGTGCCAGGGATG-3′ and Egfl7 reverse 5′-TCCTCCAAGAAGGACACCTG-3′. Amplicons were subcloned into pCR-2.1 or pCRII-Topo-TA vectors (Invitrogen) and linearized with SpeI or EcoRV. All probes were synthesized using DIG-labeling mix (Roche) according to manufacturer’s instructions.
In situ hybridization (ISH) was performed by modification of the protocol described by Hurtado et al (Hurtado and Mikawa, 2006). C57Bl/6 placentas were isolated, fixed in 4% paraformaldehyde, and methanol dehydrated. Placentas were rehydrated, incubated in 5% low melt agarose (BioRad) at 42°C for 2hours, and embedded in 5% low melt agarose through solidification at room temperature. Blocks were cut at 100μm thickness. Agarose was removed; sections were washed in PBT (PBS-0.01%Tween20), and treated with Proteinase K (10μg/ml, Roche) for 20min. After terminating proteinase K reaction with Glycine (2mg/ml) and postfixation with 4% paraformaldehyde-0.1% gluteraldehyde (40min), samples were washed with PBT, preincubated with hybridization buffer (50% Formamide, 200μg/ml Yeast RNA, 0.5% Chaps, 1.3X SSC, 5mM EDTA, 100μg/ml Heparin, 0.2% Tween20 in DEPC-H2O) for 1hour at 60°C, and incubated overnight with DIG-labeled RNA probes (1μg/ml) at 60°C. Samples were washed and blocked with 2% Boehringer Blocking Reagent (BBR) (Roche) in MABT for 1hour and 2%BBR-20%NGS in MABT for 1hour, then incubated with alkaline phosphatase conjugated anti-DIG (Roche) at 1:2000 overnight at 4°C. Samples were washed with MABT then NTMT (100mM NaCl, 100mM Tris-HCl pH9.5, 50mM MgCl2, 1% Tween20, 20μM Levamisole, in H2O). Signals were detected with NBT-BCIP (Roche) chromogenic substrate in NTMT at 4°C. Samples were post-fixed in 4% paraformaldehyde and imaged using a DKC-5000 (Sony) Digital Photo Camera.
Specimens were processed for paraffin sectioning as described by Hurtado, et al (Hurtado and Mikawa, 2006). Briefly, after ISH specimens were post-fixed with 4% paraformaldehyde in PBS, rocking for 48 hours at room temperature, and then overnight at 4°C. Specimens were then dehydrated stepwise in ethanol/double distilled H2O solutions as follows: 70% ethanol for 10 min, 90% ethanol for 10 min, 95% ethanol for 10 min, 3 × 100% ethanol for 10 min, 1:1 of ethanol/Citrisolv (Sigma) for 15 min. Specimens were then incubated in paraffin at 65°C as follows: 1:1 of Citrisolv/paraffin for 15 min, 3 × paraffin for 1hour, paraffin overnight, 2 × paraffin for 1hour. Specimens were then embedded in paraffin in a sagittal orientation and sectioned at 10μm thickness. Sections were rehydrated through a series of ethanol washes (100%, 90%, 85%, 70%), counterstained with Nuclear Fast Red (Sigma-Aldrich), dehydrated through a series of ethanol washes (70%, 85%, 90%, 100%), and mounted with Permount (Fisher Scientific).
For TSC, in situ hybridization was performed as above with the following modifications: TSC were cultured on 8-chamber slides (BD Biosciences) for 2 days prior to fixation and proteinase K incubation was carried out for 3min.
4.6 Antibodies
The following primary antibodies were used for all immunostaining: EGFL7 (Santa Cruz, SC-34416, 2μg/ml; R&D Systems AF3089; 2μg/ml, In-house EGFL7 (Fitch et al., 2004), 1:100), CD31 (BD Biosciences, 553370; 5μg/ml (mouse studies) or 0.78mg/ml (human studies)), CDX2 (Biogenex; CDX2-88; 25mg/ml), Cytokeratin (DakoCytomation; 53.5μg/ml (human studies)), Cytokeratin (DAKO Z0622; 12μg/ml (mouse studies)), and NOTCH4 (Santa Cruz, SC-5594; 2μg/ml). The following secondary antibodies were used: Dylight 594-donkey-α-goat (Jackson Immunoresearch, 1.5μg/ml), Alexa 488-donkey-α-goat (Jackson Immunoresearch, 1.5μg/ml) or Cy3-donkey-α-goat (Chemicon, 4μg/ml), Alexa488 donkey-α-rabbit (Jackson Immunoresearch, 1.5μg/ml) or FITC-donkey-α-rabbit (Chemicon, 4μg/ml), Alexa488 donkey-α-mouse (Jackson Immunoresearch, 1.5μg/ml (mouse studies) or Invitrogen, 4μg/ml (human studies)).
4.7 Immunofluorescence
Mouse whole fetoplacental units or placentas alone were isolated and embedded in an OCT:30% sucrose (2:1) mixture. Sections were permeablized in 0.5% Triton-X/0.1% Saponin/PBS (TSP) and blocked with 1% donkey serum in 0.1% TSP/PBS (PBS-TSP). Primary antibodies (EGFL7, CD31, CYTOKERATIN) were incubated for 3 hours at 37°C in block, then secondary antibodies in block, and mounted with Prolong Gold +DAPI (Invitrogen).
TSC were grown on 8-chamber slides (BD Biosciences) for 2–4 days and fixed with cold methanol for 5 minutes. TSC were washed in PBS-TSP and blocked with 1% donkey serum in PBS. Primary antibodies (EGFL7, CDX2) were incubated overnight at 4°C or 2hrs at RT in block, then secondary antibodies in block, and mounted as above.
For the human studies, sections were fixed in methanol for 5 minutes at −20°C and blocked in 10% donkey serum/PBS. Sections were incubated with primary antibodies (EGFL7, CD31, CYTOKERATIN, NOTCH4) in 0.1%BSA/PBS overnight at 4°C. Sections were incubated in secondary antibodies, stained with Hoechst33342 (Sigma-Aldrich), and mounted with Möwiol (Sigma-Aldrich).
Primary human cytotrophoblasts were cultured overnight, fixed with ice-cold methanol for 5 min at −20°C, and immunostained with EGFL7 and CYTOKERATIN antibodies.
All images were acquired using an Axioplan 2 imaging microscope (Carl Zeiss) or a LSM Live 5 line scanner confocal microscope (Carl Zeiss).
4.8 Whole Mount Immunostaining
Blastocyst-stage embryos were isolated by flushing uteri with M2 Medium (Sigma). Embryos were collected between E3.5 and E4.0, washed with M2 medium, fixed in 2% paraformaldehyde, and permeablized with 0.25%Triton-X/PBS. Embryos were blocked with 2.5% donkey serum/0.5%PBS-TSP/PBS, and incubated with primary antibodies (EGFL7, CDX2) overnight at 37°C in block. Blastocysts were incubated with secondary antibodies and mounted with Prolong Gold+DAPI using Fastwell spacers (Sigma-Aldrich).
4.9 Statistics
Data are represented as mean ± SEM. The data were analyzed using a student’s t-test with statistical significance defined as P < 0.05.
Supplementary Material
In situ hybridization was performed using an Egfl7 riboprobe on 100μm thick vibratome sections of E12.5 (A–C) and E18.5 (D–E) C57Bl/6 placentas. Higher magnification images of boxes in (A) demonstrating that Egfl7 transcript is expressed in the maternal decidua (B) and fetal labyrinth (C). Egfl7 transcript is largely downregulated at E18.5 (D), except in a few vascular structures resembling arterioles in the fetal labyrinth (E). Egfl7 sense controls (A, D insets) show specificity of Egfl7 riboprobe. Dec-Maternal Decidua, JZ-Junctional Zone, Lab-Fetal Labyrinth.
Double immunofluorescent staining was performed on E10.5 (A) and E18.5 (B) C57BL/6 placentas for EGFL7 (red), CD31 (green) and nuclear DAPI (blue). Images are collapsed z-stack confocal images of the maternal decidua and fetal labyrinth placental zones. EGFL7 colocalizes with the endothelial cell marker, CD31, in the maternal decidua and the fetal labyrinth. Scale bar=20μm.
(A) H&E staining of week-10 chorionic villi (left), and of week-40 chorionic villi (right) demonstrating morphology. Scale bars=50μm. (B) EGFL7 antibodies from different sources show similar staining patterns in trophoblasts. Depicted are staining of chorionic villi from placentas at week-10 of gestation for Hoechst (blue) and EGFL7 (red). Top row: EGFL7 antibody from R&D; middle row: Egfl7 antibody from Santa Cruz; bottom row: IgG control on the same chorionic villi specimen. (*-syncytiotrophoblast cell layer; arrow-inner trophoblast cell layer). Scale bar=50μm.
Highlights.
Egfl7 exhibits a dynamic spatiotemporal endothelial expression in the placenta.
A novel Egfl7 expression domain was uncovered in the trophoblast cell lineage.
Egfl7 is downregulated in compromised BPH/5 placentas prior to maternal PE signs.
Egfl7 is downregulated in human PE placentas assessed at time of delivery.
Notch target gene expression is reduced concomitantly with Egfl7 in PE placentas.
Acknowledgments
L.A.L. and H.S. wrote the manuscript. L.A.L, S.F., L.C., R.L.D. and H.S. participated in the design of the studies, and L.A.L., M.M., J.L.S., R.H., and S.S. participated in performing the studies and collecting data. L.A.L., M.M., S.S., S.F., R.L.D., L.C. and H.S. participated in the analysis of the data. The authors acknowledge Drs. Kat Hadjantonakis and Janet Rossant for providing TSC clones, Drs. Yi Zhou, Graziano Bonelli and Mallik Guruju for technical assistance, and Dr. Kathryn Bambino, Dr. Donna Nichol, and Dr. Massimo De Felici for critical reading of the manuscript. This work was supported by the National Institutes of Health (T32 HD060600, L.L.), the Association for the Study of Malformations (ASM ONLUS, L.C., S.S.), and the Qatar National Research Fund (NPRP 09-1099-3-279, R.L.D.).
Abbreviations
- EGFL7
Epidermal Growth Factor Like Domain 7
- PE
Preeclampsia
- TSC
Trophoblast Stem Cell
- ESC
Embryonic Stem Cell
- MEF
Mouse Embryonic Fibroblast
- CK
Cytokeratin
- CVS
Chorionic Villus Sample
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lauretta A. Lacko, Email: lal2018@med.cornell.edu.
Micol Massimiani, Email: micol9@libero.it.
Jenny L. Sones, Email: jll74@cornell.edu.
Romulo Hurtado, Email: roh2002@med.cornell.edu.
Silvia Salvi, Email: silvia.salvi@edu.rm.unicatt.it.
Sergio Ferrazzani, Email: s.ferrazzani@rm.unicatt.it.
Robin L. Davisson, Email: robin.davisson@cornell.edu, rld2002@med.cornell.edu.
Luisa Campagnolo, Email: campagno@med.uniroma2.it.
Heidi Stuhlmann, Email: hes2011@med.cornell.edu.
References
- Akercan F, Cirpan T, Terek MC, Ozcakir HT, Giray G, Sagol S, Karadadas N. The immunohistochemical evaluation of VEGF in placenta biopsies of pregnancies complicated by preeclampsia. Arch Gynecol Obstet. 2008;277:109–14. doi: 10.1007/s00404-007-0430-5. [DOI] [PubMed] [Google Scholar]
- Badiwala MV, Tumiati LC, Joseph JM, Sheshgiri R, Ross HJ, Delgado DH, Rao V. Epidermal growth factor-like domain 7 suppresses intercellular adhesion molecule 1 expression in response to hypoxia/reoxygenation injury in human coronary artery endothelial cells. Circulation. 2010;122:S156–61. doi: 10.1161/CIRCULATIONAHA.109.927715. [DOI] [PubMed] [Google Scholar]
- Campagnolo L, Leahy A, Chitnis S, Koschnick S, Fitch MJ, Fallon JT, Loskutoff D, Taubman MB, Stuhlmann H. EGFL7 is a chemoattractant for endothelial cells and is up-regulated in angiogenesis and arterial injury. Am J Pathol. 2005;167:275–84. doi: 10.1016/S0002-9440(10)62972-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagnolo L, Moscatelli I, Pellegrini M, Siracusa G, Stuhlmann H. Expression of EGFL7 in primordial germ cells and in adult ovaries and testes. Gene Expr Patterns. 2008;8:389–96. doi: 10.1016/j.gep.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JY, Song Y, Wang Y, Magness RR, Zheng J. Differential expression of vascular endothelial growth factor (VEGF), endocrine gland derived-VEGF, and VEGF receptors in human placentas from normal and preeclamptic pregnancies. J Clin Endocrinol Metab. 2004;89:2484–90. doi: 10.1210/jc.2003-031580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobellis L, Mastrogiacomo A, Federico E, Schettino MT, De Falco M, Manente L, Coppola G, Torella M, Colacurci N, De Luca A. Distribution of Notch protein members in normal and preeclampsia-complicated placentas. Cell Tissue Res. 2007;330:527–34. doi: 10.1007/s00441-007-0511-6. [DOI] [PubMed] [Google Scholar]
- Cooper JC, Sharkey AM, Charnock-Jones DS, Palmer CR, Smith SK. VEGF mRNA levels in placentae from pregnancies complicated by pre-eclampsia. Br J Obstet Gynaecol. 1996;103:1191–6. doi: 10.1111/j.1471-0528.1996.tb09627.x. [DOI] [PubMed] [Google Scholar]
- Costa MJ, Wu X, Cuervo H, Srinivasan R, Bechis SK, Cheang E, Marjanovic O, Gridley T, Cvetic CA, Wang RA. Notch4 is required for tumor onset and perfusion. Vasc Cell. 2013;5:7. doi: 10.1186/2045-824X-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispi F, Domínguez C, Llurba E, Martín-Gallán P, Cabero L, Gratacós E. Placental angiogenic growth factors and uterine artery Doppler findings for characterization of different subsets in preeclampsia and in isolated intrauterine growth restriction. Am J Obstet Gynecol. 2006;195:201–7. doi: 10.1016/j.ajog.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Cross JC. How to make a placenta: mechanisms of trophoblast cell differentiation in mice--a review. Placenta. 2005a;26(Suppl A):S3–9. doi: 10.1016/j.placenta.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Cross JC. How to make a placenta: mechanisms of trophoblast cell differentiation in mice--a review. Placenta. 2005b;26(Suppl A):S3–9. doi: 10.1016/j.placenta.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Cross JC, Baczyk D, Dobric N, Hemberger M, Hughes M, Simmons DG, Yamamoto H, Kingdom JC. Genes, development and evolution of the placenta. Placenta. 2003;24:123–30. doi: 10.1053/plac.2002.0887. [DOI] [PubMed] [Google Scholar]
- Davisson RL, Hoffmann DS, Butz GM, Aldape G, Schlager G, Merrill DC, Sethi S, Weiss RM, Bates JN. Discovery of a spontaneous genetic mouse model of preeclampsia. Hypertension. 2002;39:337–42. doi: 10.1161/hy02t2.102904. [DOI] [PubMed] [Google Scholar]
- Dokras A, Hoffmann DS, Eastvold JS, Kienzle MF, Gruman LM, Kirby PA, Weiss RM, Davisson RL. Severe feto-placental abnormalities precede the onset of hypertension and proteinuria in a mouse model of preeclampsia. Biol Reprod. 2006;75:899–907. doi: 10.1095/biolreprod.106.053603. [DOI] [PubMed] [Google Scholar]
- Dolea C, AbouZahr C. Global burden of hypertensive disorders of pregnancy in the year 2000, Evidence and Information for Policy (EIP) World Health Organization; Geneva: 2003. pp. 1–11. [Google Scholar]
- Duarte A, Hirashima M, Benedito R, Trindade A, Diniz P, Bekman E, Costa L, Henrique D, Rossant J. Dosage-sensitive requirement for mouse Dll4 in artery development. Genes Dev. 2004;18:2474–8. doi: 10.1101/gad.1239004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrans A, Stuhlmann H. A role for Egfl7 during endothelial organization in the embryoid body model system. J Angiogenes Res. 2010;2:4. doi: 10.1186/2040-2384-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Schumacher N, Maier M, Sendtner M, Gessler M. The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev. 2004;18:901–11. doi: 10.1101/gad.291004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch MJ, Campagnolo L, Kuhnert F, Stuhlmann H. Egfl7, a novel epidermal growth factor-domain gene expressed in endothelial cells. Dev Dyn. 2004;230:316–24. doi: 10.1002/dvdy.20063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale NW, Dominguez MG, Noguera I, Pan L, Hughes V, Valenzuela DM, Murphy AJ, Adams NC, Lin HC, Holash J, Thurston G, Yancopoulos GD. Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc Natl Acad Sci U S A. 2004;101:15949–54. doi: 10.1073/pnas.0407290101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasperowicz M, Otto F. The notch signalling pathway in the development of the mouse placenta. Placenta. 2008;29:651–9. doi: 10.1016/j.placenta.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Gasperowicz M, Rai A, Cross JC. Spatiotemporal expression of Notch receptors and ligands in developing mouse placenta. Gene Expr Patterns. 2013 doi: 10.1016/j.gep.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Georgiades P, Ferguson-Smith AC, Burton GJ. Comparative developmental anatomy of the murine and human definitive placentae. Placenta. 2002;23:3–19. doi: 10.1053/plac.2001.0738. [DOI] [PubMed] [Google Scholar]
- Geva E, Ginzinger DG, Zaloudek CJ, Moore DH, Byrne A, Jaffe RB. Human placental vascular development: vasculogenic and angiogenic (branching and nonbranching) transformation is regulated by vascular endothelial growth factor-A, angiopoietin-1, and angiopoietin-2. J Clin Endocrinol Metab. 2002;87:4213–24. doi: 10.1210/jc.2002-020195. [DOI] [PubMed] [Google Scholar]
- Guillemot F, Nagy A, Auerbach A, Rossant J, Joyner AL. Essential role of Mash-2 in extraembryonic development. Nature. 1994;371:333–6. doi: 10.1038/371333a0. [DOI] [PubMed] [Google Scholar]
- Gustavsson M, Mallard C, Vannucci SJ, Wilson MA, Johnston MV, Hagberg H. Vascular response to hypoxic preconditioning in the immature brain. J Cereb Blood Flow Metab. 2007;27:928–38. doi: 10.1038/sj.jcbfm.9600408. [DOI] [PubMed] [Google Scholar]
- Hamada Y, Hiroe T, Suzuki Y, Oda M, Tsujimoto Y, Coleman JR, Tanaka S. Notch2 is required for formation of the placental circulatory system, but not for cell-type specification in the developing mouse placenta. Differentiation. 2007;75:268–78. doi: 10.1111/j.1432-0436.2006.00137.x. [DOI] [PubMed] [Google Scholar]
- Hu D, Cross JC. Development and function of trophoblast giant cells in the rodent placenta. Int J Dev Biol. 2010;54:341–54. doi: 10.1387/ijdb.082768dh. [DOI] [PubMed] [Google Scholar]
- Hunkapiller NM, Fisher SJ. Chapter 12. Placental remodeling of the uterine vasculature. Methods Enzymol. 2008;445:281–302. doi: 10.1016/S0076-6879(08)03012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller NM, Gasperowicz M, Kapidzic M, Plaks V, Maltepe E, Kitajewski J, Cross JC, Fisher SJ. A role for Notch signaling in trophoblast endovascular invasion and in the pathogenesis of pre-eclampsia. Development. 2011;138:2987–98. doi: 10.1242/dev.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado R, Mikawa T. Enhanced sensitivity and stability in two-color in situ hybridization by means of a novel chromagenic substrate combination. Dev Dyn. 2006;235:2811–6. doi: 10.1002/dvdy.20909. [DOI] [PubMed] [Google Scholar]
- Johansson A, Løset M, Mundal SB, Johnson MP, Freed KA, Fenstad MH, Moses EK, Austgulen R, Blangero J. Partial correlation network analyses to detect altered gene interactions in human disease: using preeclampsia as a model. Hum Genet. 2011;129:25–34. doi: 10.1007/s00439-010-0893-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junus K, Centlow M, Wikström AK, Larsson I, Hansson SR, Olovsson M. Gene expression profiling of placentae from women with early- and late-onset pre-eclampsia: down-regulation of the angiogenesis-related genes ACVRL1 and EGFL7 in early-onset disease. Mol Hum Reprod. 2012;18:146–55. doi: 10.1093/molehr/gar067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs LT, Shutter JR, Tanigaki K, Honjo T, Stark KL, Gridley T. Haploinsufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes Dev. 2004;18:2469–73. doi: 10.1101/gad.1239204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, Gallahan D, Closson V, Kitajewski J, Callahan R, Smith GH, Stark KL, Gridley T. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000;14:1343–52. [PMC free article] [PubMed] [Google Scholar]
- Limbourg FP, Takeshita K, Radtke F, Bronson RT, Chin MT, Liao JK. Essential role of endothelial Notch1 in angiogenesis. Circulation. 2005;111:1826–32. doi: 10.1161/01.CIR.0000160870.93058.DD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall F, Young A, Boswell F, Kingdom JC, Greer IA. Placental expression of vascular endothelial growth factor in placentae from pregnancies complicated by pre-eclampsia and intrauterine growth restriction does not support placental hypoxia at delivery. Placenta. 1997;18:269–76. doi: 10.1016/s0143-4004(97)80061-6. [DOI] [PubMed] [Google Scholar]
- Merviel P, Carbillon L, Challier JC, Rabreau M, Beaufils M, Uzan S. Pathophysiology of preeclampsia: links with implantation disorders. Eur J Obstet Gynecol Reprod Biol. 2004;115:134–47. doi: 10.1016/j.ejogrb.2003.12.030. [DOI] [PubMed] [Google Scholar]
- Nadra K, Quignodon L, Sardella C, Joye E, Mucciolo A, Chrast R, Desvergne B. PPARgamma in placental angiogenesis. Endocrinology. 2010;151:4969–81. doi: 10.1210/en.2010-0131. [DOI] [PubMed] [Google Scholar]
- Nelson D, Burton G. A technical note to improve the reporting of studies of the human placenta. Placenta. 2010;32:195–6. doi: 10.1016/j.placenta.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Ness RB, Sibai BM. Shared and disparate components of the pathophysiologies of fetal growth restriction and preeclampsia. Am J Obstet Gynecol. 2006;195:40–9. doi: 10.1016/j.ajog.2005.07.049. [DOI] [PubMed] [Google Scholar]
- Nichol D, Shawber C, Fitch MJ, Bambino K, Sharma A, Kitajewski J, Stuhlmann H. Impaired angiogenesis and altered Notch signaling in mice overexpressing endothelial Egfl7. Blood. 2010;116:6133–43. doi: 10.1182/blood-2010-03-274860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol D, Stuhlmann H. EGFL7: a unique angiogenic signaling factor in vascular development and disease. Blood. 2012;119:1345–52. doi: 10.1182/blood-2011-10-322446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norwitz ER, Schust DJ, Fisher SJ. Implantation and the survival of early pregnancy. N Engl J Med. 2001;345:1400–8. doi: 10.1056/NEJMra000763. [DOI] [PubMed] [Google Scholar]
- Oka C, Nakano T, Wakeham A, de la Pompa JL, Mori C, Sakai T, Okazaki S, Kawaichi M, Shiota K, Mak TW, Honjo T. Disruption of the mouse RBP-J kappa gene results in early embryonic death. Development. 1995;121:3291–301. doi: 10.1242/dev.121.10.3291. [DOI] [PubMed] [Google Scholar]
- Park JS, Baik HW, Lee SK, Na WS, Song YR, Yang YS, Park MH, Hwang IT, Oh KY. Vascular endothelial growth factor, fms-like tyrosine kinase-1 (Flt-1) and soluble Flt-1 gene expressions in Korean pre-eclamptic placentas. J Obstet Gynaecol Res. 2010;36:726–32. doi: 10.1111/j.1447-0756.2010.01208.x. [DOI] [PubMed] [Google Scholar]
- Parker LH, Schmidt M, Jin SW, Gray AM, Beis D, Pham T, Frantz G, Palmieri S, Hillan K, Stainier DY, De Sauvage FJ, Ye W. The endothelial-cell-derived secreted factor Egfl7 regulates vascular tube formation. Nature. 2004;428:754–8. doi: 10.1038/nature02416. [DOI] [PubMed] [Google Scholar]
- Phng LK, Gerhardt H. Angiogenesis: a team effort coordinated by notch. Dev Cell. 2009;16:196–208. doi: 10.1016/j.devcel.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Rossant J, Cross JC. Placental development: lessons from mouse mutants. Nat Rev Genet. 2001;2:538–48. doi: 10.1038/35080570. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Paes K, De Mazière A, Smyczek T, Yang S, Gray A, French D, Kasman I, Klumperman J, Rice DS, Ye W. EGFL7 regulates the collective migration of endothelial cells by restricting their spatial distribution. Development. 2007;134:2913–23. doi: 10.1242/dev.002576. [DOI] [PubMed] [Google Scholar]
- Schmidt MH, Bicker F, Nikolic I, Meister J, Babuke T, Picuric S, Müller-Esterl W, Plate KH, Dikic I. Epidermal growth factor-like domain 7 (EGFL7) modulates Notch signalling and affects neural stem cell renewal. Nat Cell Biol. 2009;11:873–80. doi: 10.1038/ncb1896. [DOI] [PubMed] [Google Scholar]
- Soncin F, Mattot V, Lionneton F, Spruyt N, Lepretre F, Begue A, Stehelin D. VE-statin, an endothelial repressor of smooth muscle cell migration. EMBO J. 2003;22:5700–11. doi: 10.1093/emboj/cdg549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Kunath T, Hadjantonakis AK, Nagy A, Rossant J. Promotion of trophoblast stem cell proliferation by FGF4. Science. 1998;282:2072–5. doi: 10.1126/science.282.5396.2072. [DOI] [PubMed] [Google Scholar]
- van Tuyl M, Liu J, Wang J, Kuliszewski M, Tibboel D, Post M. Role of oxygen and vascular development in epithelial branching morphogenesis of the developing mouse lung. Am J Physiol Lung Cell Mol Physiol. 2005;288:L167–78. doi: 10.1152/ajplung.00185.2004. [DOI] [PubMed] [Google Scholar]
- Waite LL, Atwood AK, Taylor RN. Preeclampsia, an implantation disorder. Rev Endocr Metab Disord. 2002;3:151–8. doi: 10.1023/a:1015411113468. [DOI] [PubMed] [Google Scholar]
- Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am J Hypertens. 2008;21:521–6. doi: 10.1038/ajh.2008.20. [DOI] [PubMed] [Google Scholar]
- Xu D, Perez RE, Ekekezie II, Navarro A, Truog WE. Epidermal growth factor-like domain 7 protects endothelial cells from hyperoxia-induced cell death. Am J Physiol Lung Cell Mol Physiol. 2008;294:L17–23. doi: 10.1152/ajplung.00178.2007. [DOI] [PubMed] [Google Scholar]
- Young BC, Levine RJ, Karumanchi SA. Pathogenesis of preeclampsia. Annu Rev Pathol. 2010;5:173–92. doi: 10.1146/annurev-pathol-121808-102149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In situ hybridization was performed using an Egfl7 riboprobe on 100μm thick vibratome sections of E12.5 (A–C) and E18.5 (D–E) C57Bl/6 placentas. Higher magnification images of boxes in (A) demonstrating that Egfl7 transcript is expressed in the maternal decidua (B) and fetal labyrinth (C). Egfl7 transcript is largely downregulated at E18.5 (D), except in a few vascular structures resembling arterioles in the fetal labyrinth (E). Egfl7 sense controls (A, D insets) show specificity of Egfl7 riboprobe. Dec-Maternal Decidua, JZ-Junctional Zone, Lab-Fetal Labyrinth.
Double immunofluorescent staining was performed on E10.5 (A) and E18.5 (B) C57BL/6 placentas for EGFL7 (red), CD31 (green) and nuclear DAPI (blue). Images are collapsed z-stack confocal images of the maternal decidua and fetal labyrinth placental zones. EGFL7 colocalizes with the endothelial cell marker, CD31, in the maternal decidua and the fetal labyrinth. Scale bar=20μm.
(A) H&E staining of week-10 chorionic villi (left), and of week-40 chorionic villi (right) demonstrating morphology. Scale bars=50μm. (B) EGFL7 antibodies from different sources show similar staining patterns in trophoblasts. Depicted are staining of chorionic villi from placentas at week-10 of gestation for Hoechst (blue) and EGFL7 (red). Top row: EGFL7 antibody from R&D; middle row: Egfl7 antibody from Santa Cruz; bottom row: IgG control on the same chorionic villi specimen. (*-syncytiotrophoblast cell layer; arrow-inner trophoblast cell layer). Scale bar=50μm.