Abstract
Nonadherence to immunosuppressants may play a role in late rejection in liver transplant recipients. In children, emerging data suggest that adherence can be measured by computing the standard deviation (SD) of consecutive blood levels of tacrolimus, resulting in a number that reflects the degree of variability between individual measures (the Medication Level Variability Index, MLVI). A higher MLVI value means erratic immunosuppression, likely due to less adherence. Data on this method in adults are limited. We obtained data from the medical charts of 150 randomly selected adult recipients. The MLVI was significantly higher in patients who had biopsy-confirmed rejection (mean MLVI=3.8, SD=3.2) as compared with the rest of the cohort (mean MLVI=2.3, SD=1.5; p<0.01), and it was significantly higher in patients who had a rejection as compared with patients who had a biopsy that was not read as a rejection (mean MLVI=2.6, SD=1.6; p<0.01). The MLVI was both associated with rejection and predicted its occurrence. A threshold MLVI of 2.0 resulted in 77% sensitivity and 60% specificity in predicting rejection; a threshold of 1.8 resulted in a sensitivity of 92% and specificity of 48%. The Area Under the Curve (AUC) in a Receiver Operating Characteristic (ROC) curve analysis was 0.71 (95% CI: 0.61–0.81). The MLVI is associated with and can predict rejection, possibly related to nonadherence, in adult liver transplant recipients.
Keywords: tacrolimus, medication adherence, noncompliance
Introduction
Consistent immunosuppression cannot be achieved if patients take their medications incorrectly. Nonadherence is the most common cause of late acute rejection in pediatric liver transplant recipients (1–7). Although data suggest that nonadherence is associated with poor outcomes in adult transplant recipients as well (8), the topic of nonadherence as an important factor in rejection has not been studied as extensively in adults. Developing strategies to aggressively identify and treat erratic immunosuppression due to nonadherence ranks highly on any research or clinical agenda that attempts to improve posttransplant outcomes in children (NIH liver action plan, 9; NIDDK panel, 10). One could argue that it should also be a focus of research in adults; a majority of pediatric liver transplant recipients transition into adult services and may retain at least some childhood risks, including the risk of nonadherence.
Blood levels of tacrolimus are routinely monitored as standard of care after solid organ transplantation. It is possible to evaluate the degree of fluctuation between individual blood levels for a given patient over time by computing the standard deviation (SD) of consecutive blood levels of tacrolimus. The resulting variable, the Medication Level Variability Index (MLVI), reflects the degree of fluctuation between individual blood levels (higher MLVI value = more fluctuation). When a threshold was applied to this variable in pediatric recipients, it was shown to be significantly associated with acute rejection, in the United States and elsewhere (2,6,7,11).
The MLVI is easy to use, and it requires almost no additional expense to calculate. Its use to monitor adherence can inform behavioral interventions that may, in turn, reduce rejection episodes in children (12–14), although intervention studies that used this index to monitor adherence to date have been pilot studies, not well-powered randomized controlled trials (RCTs). If this method were proven to be as predictive of nonadherence-related poor outcomes in adults as well, it could enable routine identification and management of nonadherence in this population. When evaluating the use of the MLVI, however, several reasons that would render this index less precise or less applicable to adults should be considered. For example, the indications for liver transplantation in adults are somewhat different from the indications most commonly encountered in children. Biliary atresia, the leading indication for liver transplantation in children (15), does not recur after liver transplantation and is not typically associated with significant extrahepatic disease. In contrast, common indications for liver transplantation in adults include hepatitis C, fatty liver disease and alcohol related liver disease (16). Auto-immune hepatitis (AIH) can also recur after liver transplantation; in some cases, rejection and recurrence can be difficult to tease apart (17). Therefore, as compared with pediatric recipients, an adverse outcome after liver transplantation in adults is more likely to be related to the underlying disease process which may still be present. Because the MLVI is not sensitive to intrinsic risks conferred by specific disease processes, it may be less predictive of graft dysfunction in adults, or the cutoff threshold for increased risk (the MLVI value beyond which rejection risk appears to be substantially increased, reflecting an unacceptable variation in medication blood levels) might differ.
One previous study explored the relationship between the MLVI and rejection/poor outcomes in adult liver transplant recipients (18). This large and detailed exploratory investigation found a correlation between a higher MLVI and increased risk for graft rejection. However, this study did not define a “screening threshold” for rejection, and it was not hypothesis-driven, increasing the risk of inadvertent spurious results.
We conducted a hypothesis-driven, retrospective chart review of adult liver transplant recipients. Although the chart review was retrospective, the study defined the hypotheses a-priori and used the pediatric data as preliminary findings. As such, this is not an exploratory study but rather an attempt to validate previously reported findings in an adult cohort. Our pre-defined primary hypothesis was that a higher MLVI would be associated with a higher likelihood of biopsy-proven graft rejection, whether it was used as a continuous variable or as a dichotomy with a cutoff point of 2.5 (as derived from the pediatric literature, 13). In a secondary analysis, we evaluated whether, in addition to being associated with rejection, the MLVI would also predict it (whether patients who had a rejection had a high MLVI before it occurred). Also, because nonadherence is expected to be associated with rejection but less so with other causes of graft dysfunction, we hypothesized that the MLVI would be associated with rejection but not with having had a biopsy done for other reasons. We expected that the MLVI threshold, which is reported at 2–3.5 in various pediatric populations (13), will be similar in adults, although it might carry less predictive value than in children. We also expected the measure to have an area under the receiver operating characteristic curve (AUC) of at least 0.7 in the predictive analytic model, fulfilling the general criterion of a “potentially useful laboratory test” (19).
Methods
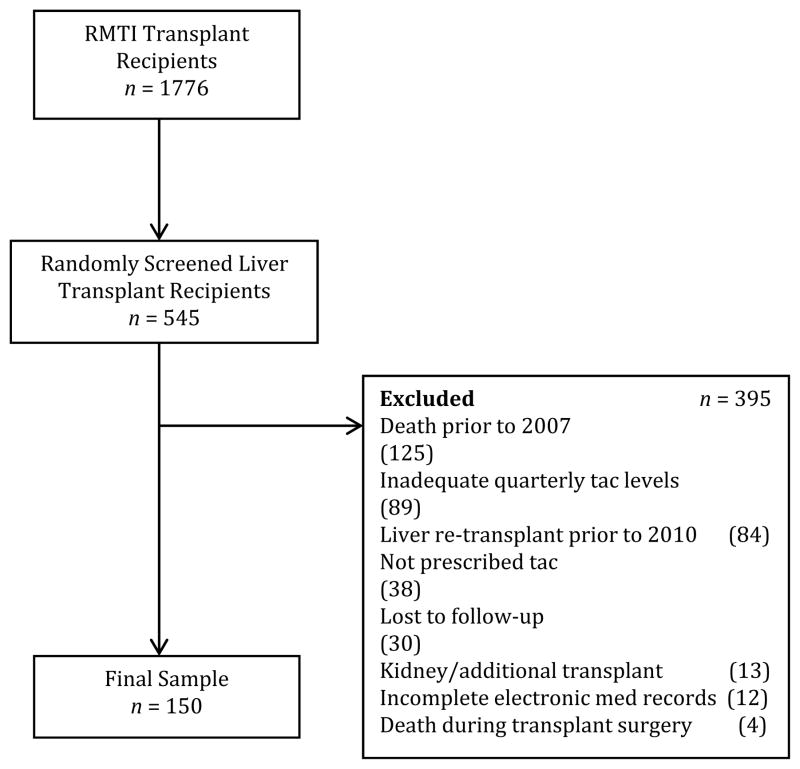
We first identified all transplant recipients whose medical records were available at the adult liver transplant program at the Recanati-Miller Transplant Institute (RMTI) at the Mount Sinai Medical Center in New York between 2007 and 2010 (n=1776). Patient charts were selected for review by randomly selecting a letter of the alphabet, identifying patients whose last name begins with this letter, and repeating this process until a sufficient number of eligible patients was identified, aiming at a final sample size of 150 participants. As it turned out, 545 patients were screened. Figure 1 summarizes the selection process. Patients were selected for the study if they met the following criteria:
FIGURE 1.
Enrollment flowchart.
Inclusion criteria:
Received a liver transplant for any reason between 1988 and 2010
Were prescribed tacrolimus between 2007 and 2010 for maintenance immunosuppression; patients taking other immunosuppressant drugs concurrently with tacrolimus were also included in the study
Had at least three serum tacrolimus levels at intervals of approximately three months recorded between 2007 and 2010
Exclusion criteria:
Death prior to 2007 even though the medical record was still available during the initial screening (n=125)
Inadequate number of serum tacrolimus levels (n=89)
Liver retransplant prior to 2010 regardless of the cause (n=84)
Not prescribed tacrolimus (n=38)
Lost to follow up (n=30)
Kidney or other additional transplant (n=13)
Incomplete electronic medical records (n=12)
Death occurring during transplant surgery (n=4)
The program did not perform any “protocol” biopsies during the study period; all biopsies were clinically warranted to investigate abnormal liver chemistry tests. For the final group of 150 recipients, we obtained the following data: gender, race, age at transplant, age at entrance into the study (i.e., age at the first time that a tacrolimus level was recorded during the period reviewed for this study), diagnosis at time of transplant, serum tacrolimus levels, and pathology reports from any liver biopsies performed six months or later post-transplant during the study follow-up time. At our institution, patients are asked to have their immunosuppressant blood level checked at least every three months post-transplant, and more if clinically indicated. Serum tacrolimus levels, which were obtained as a part of routine management, were recorded beginning at least six months post-transplant at intervals of approximately every three months for the entire duration of follow-up between 2007 and 2010. When more than one tacrolimus level was recorded in a quarter, the level associated with the interval closest to 3 months, by the day, was selected. Biopsy results were based upon the official pathology report at our center (not on chart notes), distinguishing between cellular rejection and any other reading. Chart reviewers were blinded to the patients’ biopsy status when entering the tacrolimus level data.
This study was approved by the Icahn School of Medicine at Mount Sinai’s Institutional Review Board as exempt. It involved a retrospective chart review and was analyzed without patient identifiers.
For each subject, if there was at least one biopsy-proven episode of rejection in the study period, it was entered as a positive value (positive rejection) for the purpose of the main analysis. Thus, even if a subject had more than one rejection episode, it was counted as one event for the primary analysis (yes/no rejection occurring during the follow-up period, regardless of the number of rejections). In secondary analyses, we distinguished between patients who had one biopsy-proven rejection and those who had more than one. Also, we looked to see whether the MLVI differed between those diagnosed with AIH and the rest of the cohort. We then examined whether the results would change if we removed this group from the main analysis. Particular attention was placed on those diagnosed with AIH because they are a potential high-risk group in which recurrent disease is common (17). Additionally, at our center, patients diagnosed with AIH may receive a more intense schedule of immunosuppression: maintenance steroids are added to tacrolimus per protocol.
In some cases, tacrolimus levels were reported as “undetectable”. Our patients may obtain results at local laboratories of their choosing and these different labs have different thresholds for what is considered “undetectable.” At our center, target levels are never chosen to be in the “undetectable” range. Because of the different thresholds in different labs, we were not confident that assigning any fixed number to substitute an “undetectable” reading would be reasonable. We decided to discard “undetectable” readings from the main analyses, but we do report the number (and percentage) of such readings in this cohort.
To verify whether our exclusion criteria resulted in a substantial selection bias, we compared baseline characteristics of patients with less than three blood levels to the selected cohort (course characteristics cannot be compared, because many of those patients turned out to be receiving care elsewhere, which is the reason for having few levels recorded in our charts).
To verify whether patients who were identified by the MLVI as nonadherent and had a rejection were already suspected of nonadherence by the treating team, we reviewed all of the charts of patients with an “above threshold” MLVI to try to capture instances in which nonadherence was mentioned.
Statistical methods
All statistical analyses were completed using IBM SPSS statistical package version 20.0 (IBM, Armonk, NY) and predefined the level of significance as p≤0.05. For the primary analysis, independent samples t-tests (two-tailed) and chi-square analyses were conducted to examine the relationship between MLVI values, as both a continuous and categorical variable, and graft rejection. We also performed a t-test to determine whether the presence of “undetectable” levels in a patient significantly predicted rejection (if it was predictive, this would have suggested that discarding those data could have had a significant effect on the final results). When Levene’s Test for Equality of Variance was statistically significant, the Welch-Satterthwaite correction (the default method used by the SPSS software) was applied. Given that no preliminary data exist about the validity of a certain threshold of this method in adults, there were no parameters available to include in a formal power analysis in order to determine the sample size. Since this index seemed to have identified most rejection episodes in cohorts of n = ~100 children (2,6,7), we decided that a somewhat larger sample of 150 would be expected to show the same effects in adults, even if the index’s performance may be a bit less reliable in adults. Consistent with the way this index was previously used (2) and in order to mimic the clinical reality in which those tests would be performed, we decided to include all extant data in the primary analysis: blood-level outliers were not discarded. But because outliers could be present, and if so, may have a significant effect, we evaluated in a secondary analysis the effect of outliers on the main outcome measure. To do so, we first calculated the mean of all MLVI’s for the entire cohort. Then, we compiled a list of all individual MLVI’s that were more than 2 standard deviations above that mean. We then excluded the subjects whose MLVI’s were above that threshold and re-analyzed the new sample.
For the predictor analysis, we re-calculated all MLVI’s while including only blood level values that were obtained more than a month before the index rejection occurred, and no post-rejection levels, attempting to mitigate putative effects of peri-rejection liver dysfunction on blood levels and, therefore, on the index. The area under receiver operating characteristic curve (AUC ROC) was used to evaluate the predictive ability of MLVI in the predictor analysis model.
To determine whether race, gender, or age affected the predictive value of the MLVI in this cohort, we performed a multivariate logistic regression to predict rejection. These demographic variables, culled from the medical charts, were entered into the analytic model to determine whether they can explain or mitigate the association between the MLVI and rejection.
Tacrolimus levels which were repeated one day or less apart were averaged because taking levels so close in time might mean that the physician was concerned about a lab error. To determine whether averaged values may have affected the results of the main analyses, we re-analyzed the data, replacing averaged values with the closest measure in time.
Results
Patient characteristics for the final group of 150 patients are presented in Table 1. Figure 1 presents the selection process for participation in this study. Table 2 compares baseline characteristics between patients who were included in the study and patients who were excluded due to not having enough blood tacrolimus levels recorded.
TABLE 1.
Patient characteristics
| n=150 (%) | |
|---|---|
| Sex | |
| Male | 98 (65) |
| Female | 52 (35) |
| Mean Age ± SD | |
| At Entrance into Study | 54 ± 13 years |
| At Transplant | 50 ± 13 years |
| Age at Entrance into Study | |
| 18 – 20 | 2 (1) |
| 21 – 30 | 9 (6) |
| 31 – 40 | 10 (7) |
| 41 – 50 | 20 (13) |
| 51 – 60 | 63 (42) |
| 61 – 70 | 39 (26) |
| 71 – 80 | 7 (5) |
| Age at Transplant | |
| 1 – 18 | 1 (<1) |
| 19 – 20 | 6 (4) |
| 21 – 30 | 9 (6) |
| 31 – 40 | 10 (7) |
| 41 – 50 | 30 (20) |
| 51 – 60 | 70 (47) |
| 61 – 70 | 20 (13) |
| 71 – 80 | 4 (3) |
| Diagnosis at Time of Transplant | |
| Hepatitis C | 77 (51) |
| Auto-Immune Hepatitis | 21 (14) |
| Hepatitis B | 11 (8) |
| Alcohol Related Cirrhosis | 8 (5) |
| Toxic/Drug Induced Hepatitis | 6 (4) |
| Primary Biliary Cirrhosis | 6 (4) |
| Fulminant Hepatic Failure | 3 (2) |
| Other (not specified) | 18 (12) |
| Rejection | |
| Yes | 47 (31) |
| No | 103 (69) |
| Biopsy | |
| Yes | 113 (75) |
| No | 37 (25) |
n = total sample size
TABLE 2.
Patient characteristics (Included patients versus those excluded due to insufficient levels)
| Included Patients | Excluded Patients (Not Enough Levels Recorded) | ||
|---|---|---|---|
| n=150 (%) | n=87 (%) | p | |
| Sex | |||
| Male | 98 (65) | 51 (59) | .303 |
| Female | 52 (35) | 36 (41) | |
| Mean Age ± SD | |||
| At Entrance into Study | 54 ± 13 years | 57 ± 13 years | .091 |
| Diagnosis at Time of Transplant | |||
| Hepatitis C | 77 (51) | 41 (47) | .107 |
| Auto-Immune Hepatitis | 21 (14) | 11 (13) | |
| Hepatitis B | 11 (8) | 4 (5) | |
| Alcohol Related Cirrhosis | 8 (5) | 8 (9) | |
| Toxic/Drug Induced Hepatitis | 6 (4) | 0 (0) | |
| Primary Biliary Cirrhosis | 6 (4) | 4 (4) | |
| Fulminant Hepatic Failure | 3 (2) | 5 (6) | |
| Nonalcoholic Steatohepatitis | 0 (0) | 3 (3) | |
| Other (not specified) | 18 (12) | 11 (13) | |
Note: n = total sample size; 89 patients had insufficient tacrolimus levels, and 2 of these patients were missing baseline information and so were excluded from this analysis
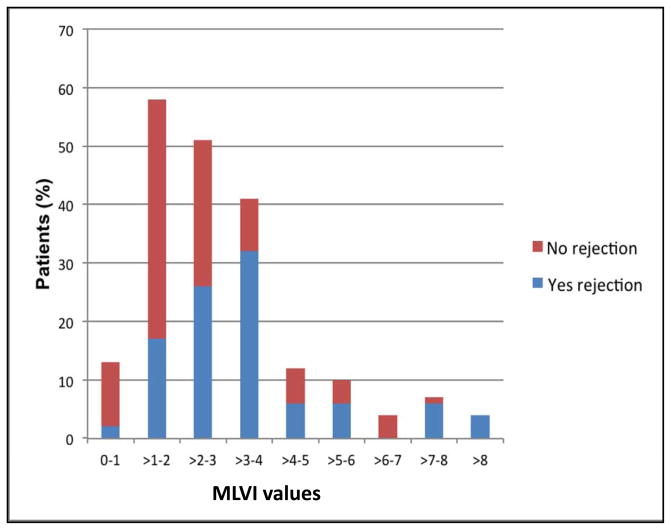
The numbers of tacrolimus troughs levels in the three groups were as follows: Patients who had a biopsy that was read as rejection: mean=9.8, range=3–22, patients who had a biopsy that was read as anything but rejection: mean=8.3, range=3–38, and patients who had no biopsy performed: mean=5.4, range=3–13; p=0.38. Table 3 presents data in which the MLVI is used as a continuous variable. The primary analysis showed that MLVI’s were significantly higher in patients with biopsy-confirmed rejection (mean=3.8, SD=3.2) as compared with the rest of the group (mean=2.3, SD=1.5); t(55.15)= –3.164; p<0.01. Figure 2 presents the distribution of MLVI’s as a function of the rejection status. As can be seen, 2% of all patients with rejection and 10% of all patients without rejection had an MLVI range of 0–1. Patients with MLVI’s of more than 8 all had a documented rejection, and those represented 4% of the “rejection” cohort.
TABLE 3.
MLVI values by groups
| Groups | n |
Group mean (SD) MLVI |
p-value |
|---|---|---|---|
| Rejection | 47 | 3.8 (3.2) | 0.003 |
| Rest of cohort | 103 | 2.3 (1.5) | |
| Rejection | 47 | 3.8 (3.2) | 0.008 |
| Biopsy, no rejection | 66 | 2.6 (1.6) | |
| Biopsy, no rejection | 66 | 2.6 (1.6) | 0.411 |
| Rest of cohort | 84 | 3.0 (2.7) |
Note: MLVI = Medication Level Variability Index
FIGURE 2.
Distribution of MLVI values, by rejection status. Patient (%) denotes the percent of patients amongst all of the patients in the rejection – blue, or no rejection – red categories in each MLVI value range (each category adds up to 100%). Note: MLVI=Medication Level Variation Index.
To investigate whether the index distinguishes between rejection and other reasons for adverse outcomes, we compared MLVI’s of patients who had a biopsy-confirmed rejection to MLVI’s of patients whose outcome was compromised enough to mandate a diagnostic biopsy, but in which the finding was anything but rejection. Table 3 shows that MLVI values were significantly higher in patients with biopsy-confirmed rejection (mean=3.8, SD=3.2) as compared with those who had a biopsy without rejection (mean=2.6, SD=1.6); t(111)= –2.709; p<0.01. Hence, a higher MLVI was associated with rejection but not poor outcomes in general.
Table 4 displays the index as a threshold variable (less than vs. equal or greater than 2.5 units). When a threshold of 2.5 is used, the likelihood of an MLVI = 2.5 is significantly higher amongst those with rejection, p<0.001. Additionally, there was a significant difference between patients who had a biopsy-proven rejection vs. patients who had a biopsy but no rejection (p<0.001). Lastly, patients who had a biopsy but no rejection were not more likely to have an above-threshold MLVI as compared with the general cohort (p=0.41).
TABLE 4.
Distribution of number of rejections per MLVI threshold
| Groups | MLVI ≥ 2.5 (%) | MLVI < 2.5 (%) | p-value |
|---|---|---|---|
| Rejection | 35 (74.5) | 12 (25.5) | <0.001 |
| Rest of cohort | 32 (31.1) | 71 (68.9) | |
| Rejection | 35 (74.5) | 12 (25.5) | <0.001 |
| Biopsy performed, no rejection | 27 (40.9) | 39 (59.1) | |
| Biopsy performed, no rejection | 27 (40.9) | 39 (59.1) | 0.412 |
| Rest of cohort | 40 (47.6) | 44 (52.4) |
Note: MLVI = Medication Level Variability Index
Of 35 patients who had an above-threshold MLVI and turned out to also have a rejection, we found a note identifying not taking the medications as an issue in only 2 cases.
Secondary analyses
There was no significant difference in MLVI values between those who have had one episode of rejection (mean=4.04, SD=3.77) and those who have had multiple episodes of rejection (mean=3.5, SD=1.46); t(45)=.503, p=0.62. There were no significant differences in MLVI values between those with AIH (mean=2.6, SD=1.88) and the rest of the cohort (mean=2.8, SD=2.31); t(148)=.487, p=0.63. When those diagnosed with AIH were removed from the main analyses, there were still significant differences in MLVI values between rejection (mean=3.7, SD=3.24) and non-rejection groups (mean=2.4, SD=1.49); t(127)= –3.271; p=0.001. When removing those with AIH in the biopsy-only group, there were still significant differences in MLVI values between patients who had a biopsy that showed rejection (mean=3.7, SD=3.24) and those who had a biopsy that did not show rejection (mean=2.8, SD=1.63); t(96)= –1.968; p=0.05.
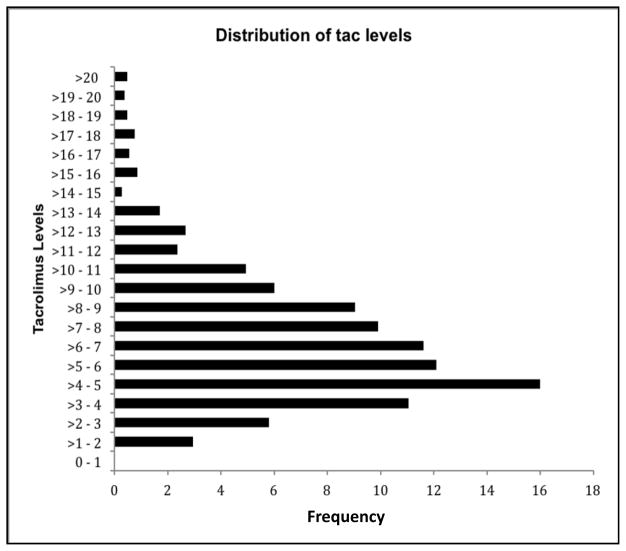
“Undetectable” levels appeared in 17 out of the 1050 readings that were used for this study (1.6%), in 12 patients of 150 (8% of patients). The presence of an “undetectable value” in a patient was not associated with rejection (p=0.48), or with the performance of a biopsy for any reason in this cohort (p=0.38). The distribution of tacrolimus blood levels in the entire sample is presented in Figure 3. Note that this figure presents actual levels, not MLVI’s. The mean and standard deviation for the whole sample’s MLVI distribution were: mean=2.8; SD=2.25. Two standard deviations above the mean is 7.30. There were 5 MLVI’s above the “mean plus two standard deviation” threshold: 7.34, 7.83, 7.88, 11.58, 20.80. Four out of those 5 subjects had a biopsy-confirmed rejection (all but the subject with the MLVI of 7.34). When those cases were discarded, the difference between the “rejection” vs. “no rejection” groups remained significant, whether or not the MLVI’s were treated as a dichotomy (putative outliers did not significantly impact the conclusion). Results using the MLVI’s as a continuous variable while discarding outliers were as follows: Rejection (n=43, mean=3.1, SD=1.18) versus no rejection (n=102, mean=2.3, SD=1.37); p=0.001; Biopsy-confirmed rejection (mean=3.1, SD=1.2) versus biopsy with no rejection (mean=2.5, SD=1.45); p=0.04; Biopsy with no rejection (mean=2.5, SD=1.45) versus rest of cohort (mean=2.5, SD=1.31); p=0.79.
FIGURE 3.
Distribution of tacrolimus blood level values in the entire cohort.
Results when using the MLVI threshold while discarding outliers were as follows, with percentage of those with an MLVI = 2.5 provided: Rejection (69.8%) versus rest of cohort (30.4%); p<.001; Biopsy-confirmed rejection (69.8%) versus biopsy with no rejection (38.5%); p=.001; Biopsy with no rejection (38.5%) versus rest of cohort (45.0%); p=0.43.
When the 38 averaged values were replaced with the closest measure in time, results did not significantly change (all previously significant results remained significant).
We then ran a multivariate logistic regression that included age at enrollment, race (classified as Caucasian, Black, Latino, Asian, Native American or Other), and gender as well as the MLVI. The demographic variables were not independent predictors of rejection in this cohort (in the primary analysis, p=0.07 for age, p=0.93 for race, p=0.29 for gender; in the predictor analysis, presented below, p=0.11 for age, p=0.93 for race, p=0.23 for gender). In both of these models, the MLVI remained the only significant predictor (p<0.05).
Predictor analyses: for this analysis, we excluded all tacrolimus levels that were obtained less than a month before the index rejection has occurred, and all post-rejection levels. This has resulted in an exclusion of 21 patients who had a rejection but did not have enough tacrolimus levels recorded before it happened, potentially biasing the analysis away from an important result due to removal of potentially nonadherent patients. This also represents a substantial reduction in our sample size and power. The results, however, remained essentially the same. For the main comparison, when used as a continuous variable, the mean MLVI value in the “confirmed Rejection” (n=26) group was 3.9, (SD=3.8) as compared with a “no rejection” (n=103) mean MLVI of 2.3, (SD=1.5); p=0.05. When used as a dichotomy (cutoff at SD>2.5), 58% of patients who had a confirmed rejection had an above-threshold MLVI, as compared with only 31% of those with no rejection; p=0.01. Differences between patients who had a biopsy that did not reveal a rejection and the rest of the cohort remained insignificant as in the primary analysis (p=0.96 when the MLVI is a continuous variable, p=0.48 when the MLVI is used as threshold).
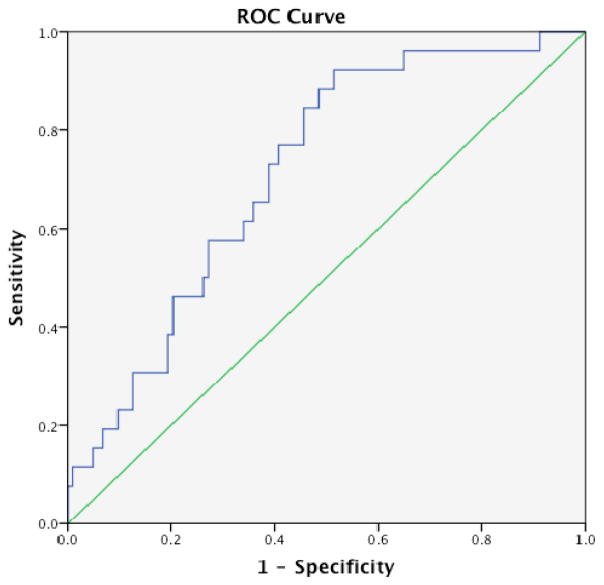
Figure 4 is an ROC curve calculated from the predictor analytic model. The AUC is 0.71 (95% CI: 0.61–0.81). Table 5 represents the specificity-sensitivity values for potential cutoff points. A cutoff value of 2.0 resulted in 77% sensitivity and 60% specificity in predicting rejection; a threshold of 1.8 resulted in a sensitivity of 92% and specificity of 48%.
FIGURE 4.
Receiver operating characteristic (ROC) curve for the relationship between MLVI values and rejection. Diagonal segments are produced by ties. The area under the curve is 0.71. Note: MLVI=Medication Level Variation Index.
TABLE 5.
MLVI cutoff values and associated sensitivity and specificity (based on Receiver Operating Curve analysis results)
| MLVI | Sensitivity (%) | Specificity (%) |
|---|---|---|
| 1.0 | 96 | 13 |
| 1.8 | 92 | 48 |
| 2.0 | 77 | 60 |
| 2.6 | 58 | 72 |
| 3.0 | 46 | 79 |
| 3.5 | 31 | 82 |
| 4.0 | 31 | 87 |
Note: MLVI = Medication Level Variability Index
Discussion
As hypothesized, the MLVI, an index representing the degree of fluctuation between individual medication blood levels in adult liver transplant recipients, calculated by computing the standard deviation of a series of tacrolimus levels, differentiated patients who had or did not have rejection. A higher degree of fluctuation (higher MLVI) is both associated with and is predictive of rejection. As also hypothesized, a threshold MLVI of 2.5–2.6 is associated with rejection. Values of 1.8–2.0 can be used to predict rejection risk. The AUC that we found for the ability of the marker to predict rejection, 0.71, is considered an acceptable value for a biomarker as compared with other biomarkers of disease (for example, see reference 20).
This study showed a correlation between the MLVI and a specific type of adverse posttransplant outcome (rejection); we did not examine less frequent outcomes such as death or graft loss. Those outcomes are also important and can be included, perhaps, in future studies. Our study did not address the reason for the adverse outcome. We believe that a likely reason for the association is patient nonadherence, because the index was shown to correlate with electronic monitoring of adherence (21), and because MLVI values have been shown in preliminary studies to be responsive to behavioral interventions (12,14), establishing that the index likely measures a behavioral construct.
The fact that the index was both associated with and also predictive of rejection suggests that the MLVI identifies a construct that is not in itself influenced much by the rejection itself (otherwise, it would not have been predictive but merely associated with rejection). This further suggests that this index accurately identified nonadherent patients, not patients with metabolic or absorption issues. Our study was not designed to firmly establish whether the clinical teams already suspected that the at-risk patients identified by the MLVI were nonadherent. However, only 2 of the 35 patients with an MLVI of 2.5 or higher who had a rejection had a notation in the chart related to a suspicion of nonadherence. Our previous results in children (2) showed a lack of correlation of the MLVI with clinician perception of patient adherence. Those results strongly suggest, but do not yet firmly establish, that this index is capable of identifying at-risk patients who would have otherwise been missed.
Calculating MLVI’s is not the same as looking at one blood level. Factors that influence blood levels of tacrolimus (such as absorption issues) may or may not influence the index because absorption issues may lead to low but not necessarily variable levels. Even if levels do vary because of variable absorption, the degree of the effect on blood levels might not be the same as the effect of not taking the medication at all (if a threshold value is applied to the MLVI, as we had done, absorption issues may fall below that threshold). The same is true for prescription practices: while the amount of prescribed medication is clearly related to the level of that medication, variable prescription practices may not lead to the same degree of fluctuation or poor outcomes as patient nonadherence. Medical prescription practices in pediatric liver transplant recipients were shown to be related to some degree of fluctuation in blood levels (22), but a later analysis showed that tightening prescription practices did not reduce rejection rates (6), establishing that only “above threshold” fluctuation, which is not responsive to an intervention related to prescribing practices, matters inasmuch as transplant outcomes are considered.
More complex statistical modelling (e.g., mixed models), which can extract point estimates and variation for each patient and also take into account correlation of values closer in time, may have been better at capturing the nuances of blood level variation. But our study did not try to evaluate the best way to capture variation. Rather, we attempted to validate the least complex method, or index, that can be easily used in practice to predict rejections.
Relatively new methods to monitor and improve adherence in a broad spectrum of medical illnesses, including electronic monitoring devices and text messaging, have been described as somewhat effective (23–29). Those are indirect measures of adherence that require patients to be motivated to engage in additional activities related to their medical care. This motivation may be lacking in nonadherent patients who, by definition, are not following treatment recommendations. Therefore, the use of external adherence measurement devices may select for more adherent patients (exactly the opposite of the aim of using such devices, which is to detect nonadherence). Multi-pronged approaches have been proposed as ways to improve accuracy of detection (30, 31), but those approaches have not been conclusively validated, and they may put unsustainable burden on patients or clinicians (32). Perhaps because direct, objective measurement of adherence is difficult, almost all published adherence interventions are administered to entire clinic populations rather than to patients who are known to be nonadherent (13). There is, therefore, particular interest in developing a simple, objective method that would allow efficient targeting of patients without additional burden and little or no additional expense. Although preliminary data suggest that the use of the MLVI to monitor adherence and inform interventions is feasible and promising (12,14), only well-powered RCTs can conclusively determine whether an approach is in fact clinically useful. We are not aware of any published well-powered RCTs to date that show that the use of any adherence detection method can improve posttransplant rejection outcomes. One recent rigorous intervention study (using medication refill rates as the adherence monitoring method) did not show an improvement in rejection rates or medical outcomes, although adherence improved amongst enrolled patients (33). It is possible that the most nonadherent patients failed to consent or did not adhere to study procedures. This study and others demonstrate that engaging the most nonadherent patients in an intervention is quite challenging. It is, therefore, hardly a forgone conclusion that interventions would help. It is important to evaluate the costs and medical effects of a monitoring program coupled with an intervention in a rigorous study before any recommendations can be made about monitoring adherence in practice.
The relatively low frequency of blood tests might seem to hinder real-time use of this index, but the fact that it was already used for monitoring in preliminary intervention studies (12,14) suggests that this theoretical consideration might be overcome. We tried to examine how to treat outliers (very high levels of tacrolimus) both in calculating the index, and in practice. Very high levels of tacrolimus (for example, a blood level of 20 or 30 and above) may be due to peak (non-trough) blood draws. They could also be real trough, and thus toxic, levels. If those outliers are not trough levels, they represent nonadherence to the recommendation to obtain a trough level. Hence, high levels of tacrolimus which may result in a higher MLVI may not necessarily be “outliers”, in that high levels may be related to nonadherence (to the recommendation to obtain a trough level) rather than being a statistical oddity. This may explain why either discarding or including “outliers” did not have any effect on the primary analytic results in this study. Our results should caution clinicians against discarding unusually high tacrolimus levels and treating them as a laboratory mistake. Rather, we found that those “outliers” are associated with a higher risk for rejection (they do not occur at random). We believe that our results suggest that in clinical practice, “outliers” should be treated with caution, as a possible indication that “something is amiss with the patient”.
Our study was not designed to thoroughly evaluate the effect of variables such as race or socioeconomic status (SES) on the index. We found that neither race, as recorded in patients’ medical charts, nor age or gender was associated with rejection in this cohort. But our dataset is limited and it is possible that larger studies will find different results, especially if race is prospectively recorded using detailed and standardized definitions. Nonadherence may explain the association – if one exists – between poor outcomes and race or SES; if so, interventions to monitor and improve adherence would be indicated as a way to mitigate this relationship (34).
Ours is a single-center, retrospective study. The shortcomings of a retrospective chart review include the lack of ability to standardize the outcome measure. But a retrospective design does offer a substantial benefit, specifically in adherence research. Prospective studies almost invariably require a consent process, and some form of commitment to enhanced follow-up. Therefore, prospective studies of adherence frequently result in the exclusion of the most nonadherent patients, who are by definition the least likely to consent to research and adhere to the study’s follow-up requirements. This inadvertently leads to a biased sample – an oversampling of patients with good outcomes, while excluding the patients who are most likely to suffer from adverse outcomes (35, 36). Although we have taken care to ensure random selection of participants, our inclusion/exclusion criteria, as in any study, may have resulted in a sample that is not fully representative. In particular, the inclusion criterion which mandated that the chart has a record of a certain number of tacrolimus levels may have led to an over-representation of those who are most likely to be closely monitored. In our cohort, 31.3% of patients had a rejection. The rate of rejection in a published study of liver transplant recipients was 11.5% to 23.9% (37). It, therefore, appears that indeed we may have somewhat oversampled those with rejection. This potential oversampling, while noted as a threat to generalizability, is not necessarily a limitation. By somewhat oversampling those with adverse outcomes, we likely increased the power of this study to show differences between groups, and it also appears that we avoided the “classic” adherence study limitation of oversampling patients with good outcomes (35).
In summary, we found that the MLVI, an index that is easy to calculate and inexpensive to use, is associated with and predictive of a specific adverse outcome (rejection), likely related to nonadherence to tacrolimus prescription, in adult liver transplant recipients. A threshold value of 1.8–2.6 can be used, depending on the aim of the investigator (screening versus intervention research). Our results, while encouraging, should not be taken as final proof of the concept that the Index can be used clinically in adults to assess and address nonadherence, because of the limitations that were discussed above. Prospective intervention studies are essential before firm conclusions can be drawn about the utility of this, or any other, adherence measure.
Acknowledgments
Grants and Financial Support: This study was funded by grant # 3R01DK080740-04S1 (NIH/NIDDK) to Christina Supelana and Eyal Shemesh.
Abbreviations
- AIH
autoimmune hepatitis
- AUC
area under the curve
- ROC
receiver operating characteristic
- TAC
tacrolimus
- MLVI
Medication Level Variability Index
Footnotes
Conflicts of Interest: The authors of this manuscript have no conflicts of interest to disclose.
References
- 1.Shemesh E, Shneider BL, Emre S. Adherence to medical recommendations in pediatric transplant recipients: time for action. Pediatr Transplant. 2008;12(3):281–3. doi: 10.1111/j.1399-3046.2008.00920.x. [DOI] [PubMed] [Google Scholar]
- 2.Shemesh E, Shneider BL, Savitzky JK, Arnott L, Gondolesi GE, Krieger NR, et al. Medication adherence in pediatric and adolescent liver transplant recipients. Pediatrics. 2004;113(4):825–32. doi: 10.1542/peds.113.4.825. [DOI] [PubMed] [Google Scholar]
- 3.Shemesh E. Nonadherence to medications following pediatric liver transplantation. Pediatr Transplant. 2004;8(6):600–5. doi: 10.1111/j.1399-3046.2004.00238.x. [DOI] [PubMed] [Google Scholar]
- 4.Molmenti E, Mazariegos G, Bueno J, Cacciarelli T, Alasio T, Khanna A, et al. Noncompliance after pediatric liver transplantation. Transplant Proc. 1999;31(1–2):408. doi: 10.1016/s0041-1345(98)01682-0. [DOI] [PubMed] [Google Scholar]
- 5.Shemesh E, Lurie S, Stuber ML, Emre S, Patel Y, Vohra P, et al. A pilot study of posttraumatic stress and nonadherence in pediatric liver transplant recipients. Pediatrics. 2000;105(2):E29. doi: 10.1542/peds.105.2.e29. [DOI] [PubMed] [Google Scholar]
- 6.Venkat VL, Nick TG, Wang Y, Bucuvalas JC. An objective measure to identify pediatric liver transplant recipients at risk for late allograft rejection related to non-adherence. Pediatr Transplant. 2008;12(1):67–72. doi: 10.1111/j.1399-3046.2007.00794.x. [DOI] [PubMed] [Google Scholar]
- 7.Stuber ML, Shemesh E, Seacord D, Washington J, 3rd, Hellemann G, McDiarmid S. Evaluating non-adherence to immunosuppressant medications in pediatric liver transplant recipients. Pediatr Transplant. 2008;12(3):284–8. doi: 10.1111/j.1399-3046.2008.00923.x. [DOI] [PubMed] [Google Scholar]
- 8.Mor E, Gonwa TA, Husberg BS, Goldstein RM, Klintmalm GB. Late-onset acute rejection in orthotopic liver transplantation--associated risk factors and outcome. Transplantation. 1992;54(5):821–4. doi: 10.1097/00007890-199211000-00010. [DOI] [PubMed] [Google Scholar]
- 9.National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases. Action Plan for Liver Disease Research. [Accessed July 6, 2013];Executive Summary. :6. http://liverplan.niddk.nih.gov. Updated June 9, 2009.
- 10.Bucuvalas JC, Alonso E, Magee JC, Talwalkar J, Hanto D, Doo E. Improving long-term outcomes after liver transplantation in children. Am J Transplant. 2008;8(12):2506–13. doi: 10.1111/j.1600-6143.2008.02432.x. [DOI] [PubMed] [Google Scholar]
- 11.Pollock-Barziv SM, Finkelstein Y, Manlhiot C, Dipchand AI, Hebert D, Ng VL, et al. Variability in tacrolimus blood levels increases the risk of late rejection and graft loss after solid organ transplantation in older children. Pediatr Transplant. 2010;14(8):968–75. doi: 10.1111/j.1399-3046.2010.01409.x. [DOI] [PubMed] [Google Scholar]
- 12.Shemesh E, Annunziato RA, Shneider BL, Dugan CA, Warshaw J, Kerkar N, Emre S. Improving adherence to medications in pediatric liver transplant recipients. Pediatr Transplant. 2008;12(3):316–23. doi: 10.1111/j.1399-3046.2007.00791.x. [DOI] [PubMed] [Google Scholar]
- 13.Shemesh E, Fine RN. Is calculating the standard deviation of tacrolimus blood levels the new gold standard for evaluating non-adherence to medications in transplant recipients? Pediatr Transplant. 2010;14(8):940–3. doi: 10.1111/j.1399-3046.2010.01396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Annunziato RA, Baisley MC, Arrato N, Barton C, Henderling F, Arnon R, Kerkar N. Strangers headed to a strange land? A pilot study of using a transition coordinator to improve transfer from pediatric to adult services. J Pediatr. 2013 Dec;163(6):1628–33. doi: 10.1016/j.jpeds.2013.07.031. [DOI] [PubMed] [Google Scholar]
- 15.McDiarmid SV, Anand R, Lindblad AS. Studies of pediatric liver transplantation: 2002 update. An overview of demographics, indications, timing, and immunosuppressive practices in pediatric liver transplantation in the United States and Canada. Pediatr Transplant. 2004;8(3):284–94. doi: 10.1111/j.1399-3046.2004.00153.x. [DOI] [PubMed] [Google Scholar]
- 16.Fink SA, Brown RS. Liver transplantation: Indications, preoperative evaluation and posttransplantation management. In: Dancygier H, editor. Clinical Hepatology: Principles and practice of hepatobiliary diseases. 1. New York, NY: Springer; 2009. pp. 1353–81. [Google Scholar]
- 17.Hübscher SG. Recurrent autoimmune hepatitis after liver transplantation: diagnostic criteria, risk factors, and outcome. Liver Transpl. 2001;7(4):285–91. doi: 10.1053/jlts.2001.23085. [DOI] [PubMed] [Google Scholar]
- 18.Lieber SR, Volk ML. Non-adherence and graft failure in adult liver transplant recipients. Dig Dis Sci. 2013;58(3):824–34. doi: 10.1007/s10620-012-2412-0. [DOI] [PubMed] [Google Scholar]
- 19.Wians FH. Clinical laboratory tests: which, why, and what do the results mean? LabMedicine. 2009;40:105–113. [Google Scholar]
- 20.Karabudak AA, Hafner J, Shetty V, Chen S, Secord AA, Morse MA, Philip R. Autoantibody biomarkers identified by proteomics methods distinguish ovarian cancer from non-ovarian cancer with various CA-125 levels. J Cancer Res Clin Oncol. 2013;139(10):1757–70. doi: 10.1007/s00432-013-1501-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerkar N, Annunziato RA, Foley L, Shmeidler J, Rumbo C, Emre S, et al. Prospective analysis of nonadherence in autoimmune hepatitis: a common problem. J Pediatr Gastroenterol Nutr. 2006;43(5):629–34. doi: 10.1097/01.mpg.0000239735.87111.ba. [DOI] [PubMed] [Google Scholar]
- 22.Bucuvalas JC, Ryckman FC, Arya G, Andrew B, Lesko A, Cole CR, et al. A novel approach to managing variation: outpatient therapeutic monitoring of calcineurin inhibitor blood levels in liver transplant recipients. J Pediatr. 2005;146(6):744–50. doi: 10.1016/j.jpeds.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 23.De Geest S, Abraham I, Dunbar-Jacob J. Measuring transplant patients’ compliance with immunosuppressive therapy. West J Nurs Res. 1996;18(5):595–605. doi: 10.1177/019394599601800509. [DOI] [PubMed] [Google Scholar]
- 24.Christensen A, Christrup LL, Fabricius PE, Chrostowska M, Wronka M, Narkiewicz K, Hansen EH. The impact of an electronic monitoring and reminder device on patient compliance with antihypertensive therapy: a randomized controlled trial. J Hypertens. 2010;28(1):194–200. doi: 10.1097/HJH.0b013e328331b718. [DOI] [PubMed] [Google Scholar]
- 25.Drent G, Haagsma EB, Geest SD, van den Berg AP, Ten Vergert EM, van den Bosch HJ, et al. Prevalence of prednisolone (non)compliance in adult liver transplant recipients. Transpl Int. 2005;18:960–966. doi: 10.1111/j.1432-2277.2005.00170.x. [DOI] [PubMed] [Google Scholar]
- 26.Fennie KP, Bova CA, Williams AB. Adjusting and censoring electronic monitoring device data. Implications for study outcomes. J Acquir Immune Defic Syndr. 2006;43(suppl):S88–95. doi: 10.1097/01.qai.0000248336.97814.2f. [DOI] [PubMed] [Google Scholar]
- 27.Rand CS, Wise RA. Measuring adherence to asthma medication regimens. Am J Crit Care Med. 1994;149 (suppl):S69–76. doi: 10.1164/ajrccm/149.2_Pt_2.S69. [DOI] [PubMed] [Google Scholar]
- 28.Cramer J. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27(5):1218–24. doi: 10.2337/diacare.27.5.1218. [DOI] [PubMed] [Google Scholar]
- 29.Riekert KA, Rand CS. Electronic monitoring of medication adherence. When is high-tech best? J Clin Psychol Med Settings. 2002;9(1):25–34. [Google Scholar]
- 30.Martin L, Williams SL, Haskard KB, DiMatteo MR. The challenge of patient adherence. Therapeut Clin Risk Manag. 2005;1(3):189–199. [PMC free article] [PubMed] [Google Scholar]
- 31.Pai AL, Rausch J, Tackett A, Marsolo K, Drotar D, Goebel J. System for integrated adherence monitoring: real-time non-adherence risk assessment in pediatric kidney transplantation. Pediatr Transplant. 2012 Jun;16(4):329–34. doi: 10.1111/j.1399-3046.2012.01657.x. [DOI] [PubMed] [Google Scholar]
- 32.Shemesh E. Measuring adherence to medications: are complex methods superior to simple ones? Pediatr Transplant. 2012 Jun;16(4):315–7. doi: 10.1111/j.1399-3046.2012.01676.x. [DOI] [PubMed] [Google Scholar]
- 33.Chisholm-Burns MA, Spivey CA, Graff Zivin J, Lee JK, Sredzinski E, Tolley EA. Improving outcomes of renal transplant recipients with behavioral adherence contracts: a randomized controlled trial. Am J Transplant. 2013 Sep;13(9):2364–73. doi: 10.1111/ajt.12341. [DOI] [PubMed] [Google Scholar]
- 34.Shemesh E, Howell EA, Annunziato R, Kleinman LC. Racial and economic disparities in transplant outcomes: the not-so-hidden morbidities. Liver Transpl. 2014 Jan;20(1):4–6. doi: 10.1002/lt.23801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Powell LH, Calvin JE, Jr, Richardson D, Janssen I, Mendes de Leon CF, Flynn KJ, et al. Self-management counseling in patients with heart failure: the heart failure adherence and retention randomized behavioral trial. JAMA. 2010;304(12):1331–8. doi: 10.1001/jama.2010.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molloy GJ, O’Carroll RE, Witham MD, McMurdo ME. Interventions to enhance adherence to medications in patients with heart failure: a systematic review. Circ Heart Fail. 2012;5(1):126–133. doi: 10.1161/CIRCHEARTFAILURE.111.964569. [DOI] [PubMed] [Google Scholar]
- 37.Maluf DG, Stravitz RT, Cotterell AH, Posner MP, Nakatsuka M, Sterling RK, et al. Adult living donor versus deceased donor liver transplantation: a 6-year single center experience. Am J Transplant. 2005;5(1):149–156. doi: 10.1111/j.1600-6143.2004.00654.x. [DOI] [PubMed] [Google Scholar]