INTRODUCTION
CVID is the most prevalent symptomatic primary immunodeficiency,1 and is characterized by predisposition to sinopulmonary infections as well as susceptibility to non-infectious complications such as autoimmunity and malignancy.2 Chronic lung disease is among the most common complications of CVID, affecting 29 - 58% of patients, depending on the study population.3-5 Ten years ago it was established by Routes and colleagues that interstitial lung disease (ILD) significantly worsens survival in CVID.6 However, established CVID lung disease has proven difficult to treat and is not reversed by conventional immunoglobulin (Ig) replacement therapy in most patients,7 though it may improve pulmonary function testing.8,9
Radiologic evaluation of lungs in CVID demonstrates a variety of chronic pulmonary findings, including air trapping, bronchial wall thickening, bronchiectasis, emphysema, ground glass opacities, parenchymal consolidation, pulmonary nodules, and/or scarring/fibrosis.7,10-12 Lung pathology may reveal ILD with manifestations of pulmonary lymphoid hyperplasia (PLH), which includes follicular bronchiolitis, lymphocytic interstitial pneumonitis (LIP), and nodular lymphoid hyperplasia.6,13-15 Additionally, granulomatous lung disease is found in many cases16-18 and organizing pneumonia (OP) in some.19-21 Granulomatous-lymphocytic interstitial lung disease (GLILD) has been used as an encompassing term for this combination of pathologic findings in CVID.6
The pathogenesis of lung disease in CVID is not well understood. Although as many as 50% of CVID patients reportedly develop bronchiectasis, not all of whom have or progress to ILD.11 Development of lung disease in CVID patients has previously been associated with a low CD4+:CD8+ T cell ratio in bronchoalveolar lavage22 as well as reductions in peripheral CD8+ T cells6 and fewer numbers of IgM-IgD-CD27+ isotype-switched as well as IgM+CD27+ memory B cells,23,24 in some, but not all studies.25 Epstein-Barr Virus may be associated with PLH,26 including subjects with HIV,27 however EBV has not been found in lung biopsies from CVID patients with PLH.13 Similarly, human herpesvirus-8 was associated with GLILD in one study,28 though this has not yet been confirmed. Non-infectious pathogenic mechanisms for the development of CVID lung disease have also been proposed, including aberrant B cell lymphoproliferation29 and T cell-driven autoimmunity.14 Through retrospective chart review, we found bronchiectasis to be associated with history of pneumonia and reduced CD4+ T cells in CVID. In contrast, patients with CT evidence of ILD shared clinical and radiologic characteristics that differed from those with bronchiectasis alone or no CT chest findings. Additionally, the presence of numerous pulmonary nodules was linked to autoimmunity, elevation of IgM, and increased CD4+:CD8+ T cell ratio, while progression to ground glass opacity was associated with elevated peripheral monocytes and increased prevalence of liver disease.
METHODS
Study Design
This study was conducted through retrospective review of the electronic medical record from Mount Sinai Hospital in New York. Electronic medical records and supplemental material are available for patient encounters from January 2003 until present. Patients with the ICD-9 code for CVID (279.06) who had either a CT scan of the chest or tissue biopsy pathology report in the medical record were selected. One hundred and twenty-six patients were identified using these initial search parameters. These records were then screened to confirm that the diagnostic criteria of CVID were met based upon markedly low IgG and IgA and/or IgM (IgG ≤ 400 mg/dL, IgA< 45 mg/dL, IgM < 35 mg/dL), poor response to vaccines, and exclusion of other causes of hypogammaglobulinemia.30 The study required: (1) one or more radiology reports of CT chest and (2) availability of peripheral blood leukocyte counts and quantitative immunoglobulin levels. Patients with known hematological malignancy were excluded. Out of the 126 patients identified in the initial screen, 41 were excluded because the diagnostic guidelines for CVID could not be confirmed and/or the patient had a hematological malignancy. Out of the remaining 85 patients, 21 were excluded due to absence of CT chest and 3 were excluded because laboratory results were not available. The remaining 61 CVID patients were included in the analysis. This study was approved by the Institutional Review Board of the Icahn School of Medicine at Mount Sinai.
Data Collection
Radiology reports from all CT chest scans were reviewed, and the presence of bronchiectasis, emphysematous changes, ground glass opacities, hilar adenopathy, and the number of pulmonary nodules, if any, were recorded. If the radiology report used a term such as “extensive” or “numerous” to signify a number of nodules that was too high to count, the patient was included in the ≥ 5 nodule category. Nodules 1 mm or larger were counted. Patient age and sex as well as history of pneumonia, autoimmune hemolytic anemia (AIHA) or immune thrombocytopenic purpura (ITP), splenomegaly or splenectomy, liver disease (defined as the presence of both alkaline phosphatase > 110 U/L and abdominal imaging demonstrating parenchymal disease), and enteropathy (based on suggestive clinical history with presence of colitis, enteritis, intraepithelial lymphocytosis, and/or villous blunting found on endoscopic biopsy) were derived from the medical record. The numbers of subjects with obstructive (7%), restrictive (53%), and normal (40%) spirometry results are similar to studies of CVID previously published.17,24 The most consistent pulmonary function test result in our cohort of CVID lung disease patients was impaired DLCO (80%), in agreement with other reports.13,17,31 DLCO in our cohort ranged from 28 to 117%.
Means of up to 5 of the most recent laboratory values in the medical record were used to quantify neutrophils, monocytes, eosinophils, and serum IgG, IgA, and IgM. Treatment IgG level was determined as the average of IgG values after 2 or more years of immunoglobulin replacement therapy. Flow cytometry of at least 1 sample, and as many as 3 averaged together, was used to quantify CD19+ total B cells, CD3+ total T cells, CD3+CD4+ helper T cells, and CD3+CD8+ cytotoxic T cells as well as the percentage of total B cells of IgM-IgD- isotype-switched and IgM+CD27+ memory B cells.
Statistical Analysis
Associations between pathologic or radiologic findings and categorical clinical parameters were assessed using Fisher's exact tests. Differences in continuous laboratory values between groups were assessed using one-way ANOVA tests. If an ANOVA F-test yielded a p value of less than 0.05, differences between patients with a specific radiologic finding and those in other groups were assessed using Dunnett's test which adjusts for multiple comparisons. Non-normally distributed laboratory values (diagnostic IgG, IgA, IgM, neutrophils, monocytes, NK cells, CD8+ T cells, total B cells, CD27+IgM+ B cells, and isotype-switched memory B cells) were rank-transformed prior to analysis.
Negative predictive value (NPV), positive predictive value (PPV), odds ratio (OR), sensitivity, and specificity were calculated to examine the predictive utility of specific clinical and laboratory parameters within our patient cohort. For these statistical analyses, laboratory cut-offs were established at 700 CD4+ T cells/ L, midpoint of the normal distribution of values within our cohort, and 500 monocytes/ L, which was rounded from the median value in our cohort of 460.
RESULTS
Radiologic studies
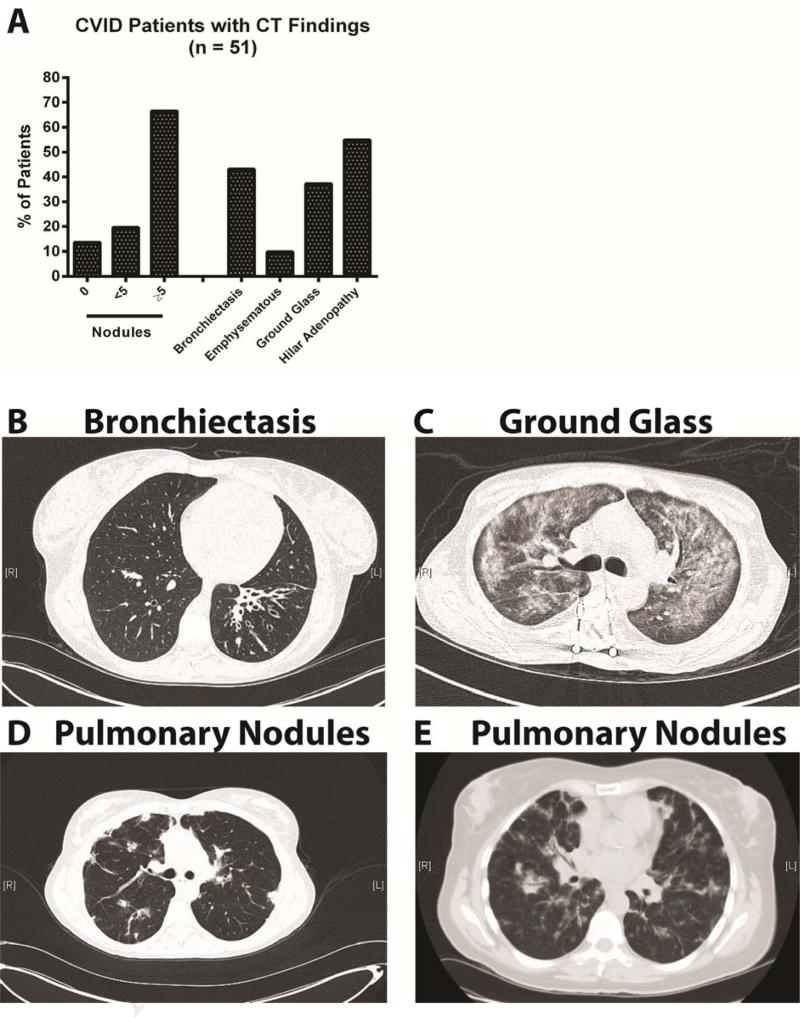
Of the 61 CVID patients in this study, 34 were female and 27 were male. The age range of subjects was 14 – 89 years, with a median age of 47. Baseline IgG, averaged IgA, IgM, and post treatment IgG values, as well as medical complications are listed in Table 1. Ten of these subjects did not have CT abnormalities. For the 51 subjects with radiologic findings, 34 (67%) had ≥ 5 pulmonary nodules, 22 (43%) had bronchiectasis, and 18 (37%) had ground glass opacity (Figure 1A). For illustration, examples of chest findings are: a 32 year-old female with a history of pneumonia with severe left lobe bronchiectasis and bronchial wall thickening (Figure 1B), a 29 year-old male with extensive ground glass appearance throughout the lungs (Figure 1C), and a 52 year-old female with splenomegaly, bilateral pulmonary nodules, bronchiectasis, and atypical lymphoid hyperplasia on lung biopsy (Figure 1D). A final subject shown here is a 63 year-old female with hepatosplenomegaly and colitis with nodular densities throughout the lungs in a somewhat peripheral pattern and ground glass changes; a biopsy demonstrated bronchiolocentric interstitial pneumonia with fibrosis, lymphoid hyperplasia, organizing pneumonia (OP), and occasional poorly formed granulomas (Figure 1E).
Table 1.
Patient Characteristics
| Patient Characteristics | n = 61 | |
|---|---|---|
| Age range (median) | 14 – 89 (47) | |
| Female (%) | 34 (56) | |
| Medical Complications | Number of subjects (%) | |
| History of Pneumonia | 33 (54) | |
| Splenomegaly/Splenectomy | 27 (44) | |
| AIHA/ITP | 23 (38) | |
| Liver Disease | 8 (13) | |
| Enteropathy | 6 (10) | |
| Laboratory Characteristics | Range (median) | Normal Reference Range |
| Diagnostic IgG (mg/dL) | 8 – 400 (169) | 600 - 1600 |
| Therapeutic IgG (mg/dL) | 307.4 – 1312 (806.3) | 600 – 1600 |
| IgA (mg/dL) | 2 – 78.3 (5) | 70 – 400 |
| IgM (mg/dL) | 0 – 243 (12.9) | 40 – 230 |
Figure 1.
Chest CT in CVID. (A) Overall findings. (B) Left lobe bronchiectasis and bronchial wall thickening. (C) Diffuse ground glass. (D) Bilateral nodules and bronchiectasis. (E) Nodules with ground glass.
Clinical and radiologic observations
Patients with ground glass opacity or ≥ 5 pulmonary nodules were younger than those with bronchiectasis but no ILD (median ages 33 and 35 versus 67 years) (Table 2). The radiologic finding of bronchiectasis was not commonly observed in patients with ILD on CT scans, as only a minority of patients with either ground glass opacity (33%) or ≥ 5 pulmonary nodules (35%) also had bronchiectasis. However, pulmonary nodules were commonly observed with ground glass opacity, as nearly 90% of CVID patients with ground glass also had one or more pulmonary nodules, suggesting that these radiologic features are common aspects of ILD.32,33 Given the significant radiologic overlap among those with ground glass opacity and pulmonary nodules, these 39 patients were grouped together as “ILD” for statistical analysis. CVID ILD patients were then compared with the 22 “non-ILD” subjects, consisting of the 10 patients with no CT findings, 9 patients with bronchiectasis only, and 3 subjects with 1 to 4 pulmonary nodules but no other chest abnormalities. CVID ILD was significantly associated with splenectomy/splenomegaly (p < 0.0001), history of AIHA/ITP (p < 0.0001), and liver disease (p < 0.05). There was no significant difference in patients with and without ILD for history of pneumonia or enteropathy. Only subjects with ILD had liver disease, and those with ground glass opacity had the highest percentage of liver abnormalities (28%).
Table 2.
Associations of Clinical and Radiological Characteristics
| Ground Glass Opacitya | ≥ 5 Pulmonary Nodulesa | Bronchiectasis Only | No Lung Diseaseb | |
|---|---|---|---|---|
| Number of Subjects | 18 | 34 | 9 | 13 |
| Female (%) | 20 (56) | 20 (59) | 5 (56) | 7 (54) |
| Median Age (% > 50 years) | 44 (33) | 45 (35) | 60 (67) | 43 (39) |
| Subjects (%) with: | ||||
| Ground Glass | 18(100) | 13 (38) | 0 (0) | 0 (0) |
| ≥ 1 Nodule | 16 (89) | 34 (100) | 4 (44) | 3 (23) |
| Bronchiectasis | 6 (33) | 12 (35) | 9 (100) | 0 (0) |
| Hilar Adenopathy | 11 (61) | 24 (71) | 3 (33) | 0 (0) |
| History of Pneumonia | 8 (44) | 21 (62) | 7 (78) | 7 (54) |
| Splenomegaly/Splenectomy | 10 (56) | 24 (71) | 1 (11) | 1 (8) |
| AIHA/ITP | 9 (50) | 21 (62) | 0 (0) | 1 (8) |
| Liver Disease | 5 (28) | 6 (18) | 0 (0) | 0 (0) |
| Enteropathy | 2 (11) | 3 (9) | 1 (11) | 1 (8) |
| ILD | No ILD | |||
| Number of Subjects | 39 | 22 | ||
| Subjects (%) with: | p value | |||
| History of Pneumonia | 22 (56) | 14 (64) | 0.78 | |
| Splenomegaly/Splenectomy | 24 (63) | 2 (9) | <0.0001 | |
| AIHA/ITP | 22 (56) | 1 (5) | <0.0001 | |
| Liver Disease | 8 (21) | 0 (0) | 0.042 | |
| Enteropathy | 4 (10) | 2 (9) | 1.00 | |
AIHA = autoimmune hemolytic anemia ILD = interstitial lung disease ITP = immune thrombocytopenic purpura
patients included in both groups if both ground glass opacity and > 5 pulmonary nodules were present
includes patients with no CT findings and those with 1-4 pulmonary nodules but no bronchiectasis or ground glass opacity
Correlation of clinical and laboratory data with radiologic findings
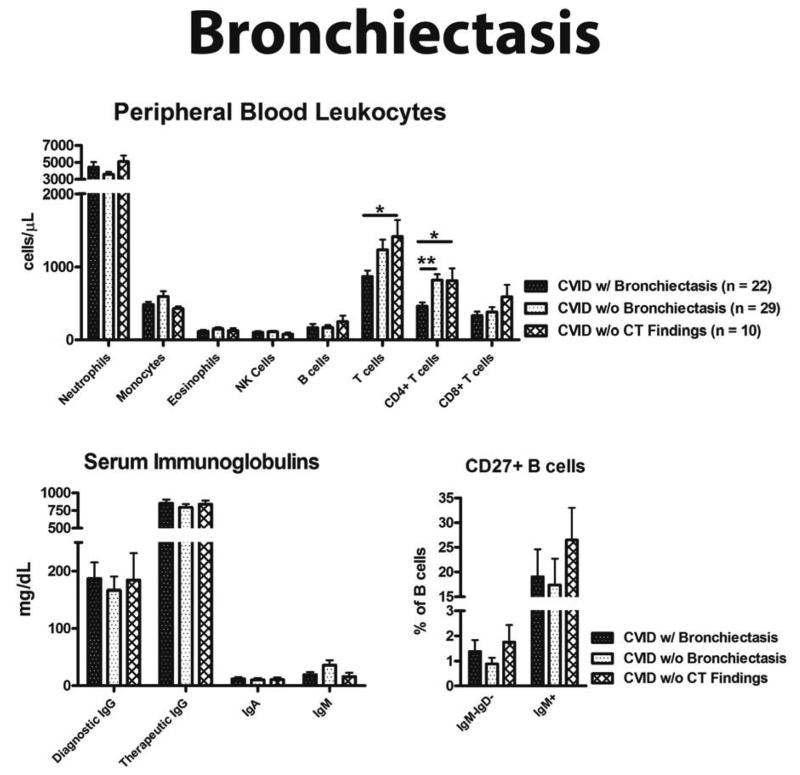
CVID patients with bronchiectasis had significantly fewer CD4+ T cells than CVID subjects with other CT findings (p < 0.01), and fewer total CD3+ T cells as well as CD4+ T cells compared to those with no CT findings (p < 0.05) (Figure 2). CD4+ T cell count < 700 cells/ L had a sensitivity and NPV of about 80% for bronchiectasis, though only a moderate specificity (55%) (Table 3). Adding an age cut off of ≥ 50 years increased specificity to 90%, with a PPV of 79%. The odds of having bronchiectasis was 9 times greater for patients ≥ 50 years of age with CD4+ T cells < 700/ L, and 4.5 times greater for those with a history of pneumonia and CD4+ T cells < 700/ L.
Figure 2.
Laboratory associations with bronchiectasis. (A) Peripheral blood leukocytes. (B) Quantitative serum immunoglobulins. (C) CD27+ B cell percentage. * = p value < 0.05, ** = p value < 0.01
Table 3.
Predictive value of clinical and laboratory parameters.
| CT Finding | Parameters | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | OR (95% CI) |
|---|---|---|---|---|---|---|
| Bronchiectasis | CD4 < 700 | 82% (60– 95) | 55% (36 – 73) | 56% (38 – 74) | 81% (58 – 94) | 5.5 (1.5 – 20) |
| CD4 < 700 AND PNA Hx | 62% (39 – 82) | 74% (55 – 88) | 62% (39 – 82) | 74% (55 – 88) | 4.7 (1.4 – 15) | |
| CD4 < 700 AND Age > 50 years | 50% (28 – 72) | 90% (74 – 98) | 79% (49 – 95) | 72% (55 – 85) | 9.3 (2.2 – 40) | |
| > 5 Nodules | (1) CD4: CD8 > 2 (2) AIHA or ITP Hx (3) IgM > 18 |
|||||
| At least 1 | 92% (78 – 98) | 50% (29 – 71) | 74% (59 – 86) | 80% (52 – 95) | 11 (2.7 – 47) | |
| At least 2 | 63% (44 – 80) | 93% (76 – 99) | 91% (70 – 99) | 69% (52 – 84) | 22 (4.3 – 109) | |
| All 3 | 18% (7 – 35) | 100% (87 – 100) | 100% (54 – 100) | 48% (34 – 62) | 12 (0.7 – 225) | |
| Ground Glass | monocytes > 500 | 67% (41 – 87) | 79% (64 – 90) | 57% (34 – 78) | 85% (70 – 94) | 7.6 (2.2 – 26) |
| monocytes > 500 AND SMB < 0.5% | 41% (19 – 67) | 91% (77 – 97) | 64% (30 – 90) | 79% (65 – 90) | 6.7 (1.6 – 27) |
NPV = negative predictive value, PNA Hx = pneumonia history, OR = odds ratio, PPV = positive predictive value, SMB = switched memory B cell, 95% CI = 95% confidence interval
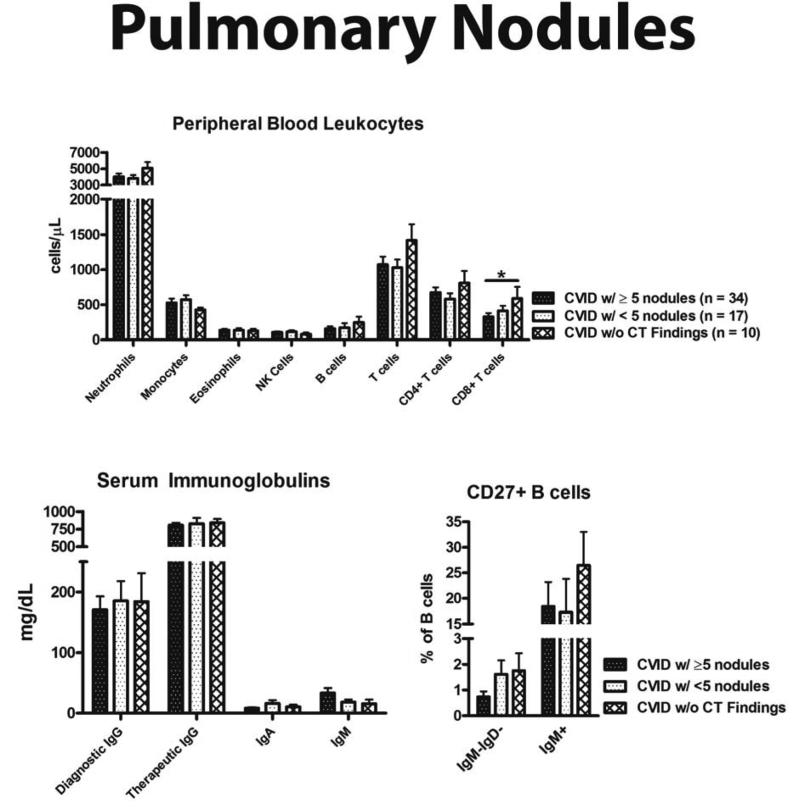
Subjects with ≥ 5 pulmonary nodules had fewer CD8+ T cells (p < 0.05), but similar numbers of CD4+ T cells and total CD3+ T cells to that of CVID patients without CT findings (Figure 3). Three parameters differentiated CVID patients with ≥ 5 pulmonary nodules quite effectively: (1) CD4+:CD8+ T cell ratio > 2, (2) history of AIHA or ITP, and (3) serum IgM > 18 mg/dL (Table 3). If none of these parameters were met, a patient was not likely to have nodular lung disease, with a NPV of 80%. Meeting two of these parameters increased specificity and PPV both over 90%, and the odds for developing nodular lung disease was increased twentyfold. Subjects meeting three of these parameters all had ≥ 5 pulmonary nodules.
Figure 3.
Laboratory associations with ≥ 5 pulmonary nodules. (A) Peripheral blood leukocytes. (B) Quantitative serum immunoglobulins. (C) CD27+ B cell percentage. * = p value < 0.05
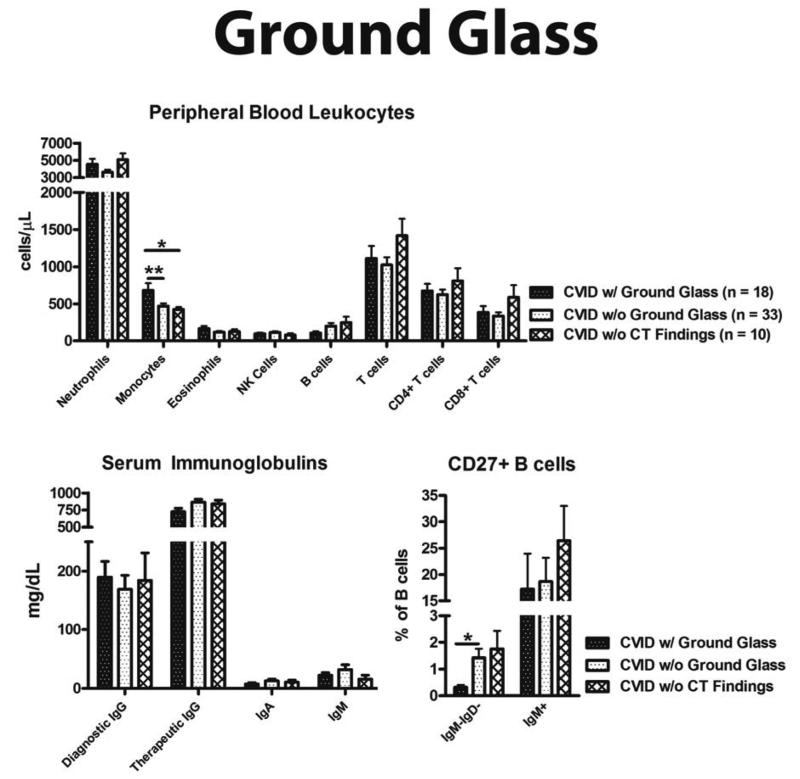
Patients with ground glass opacity were differentiated by both a statistically significant elevation in monocytes and reduction in CD19+IgM-IgD-CD27+ isotype-switched memory B cells (Figure 4). Lower monocyte count was useful for excluding the likelihood of ground glass opacity, as monocytes ≤ 500 cells/ L had a NPV of 85% (Table 3). Adding the parameter of < 0.5% CD19+IgM-IgD-CD27+ isotype-switched memory B cells to this monocyte count increased specificity for ground glass opacity to over 90% and the odds of this CT finding sevenfold.
Figure 4.
Laboratory associations with ground glass. (A) Peripheral blood leukocytes. (B) Quantitative serum immunoglobulins. (C) CD27+ B cell percentage. * = p value < 0.05, ** = p value < 0.01
There were no significant differences in serum Ig (diagnostic IgG, therapeutic IgG, IgA, or IgM) between patients with and without bronchiectasis, ≥ 5 pulmonary nodules, ground glass opacity, or no CT findings. However, we did note that the highest serum IgM values occurred in patients with ≥ 5 pulmonary nodules. There were no significant differences in total B cells, IgM+CD27+ B cells, NK cells, eosinophils, or neutrophils.
Lung pathology
Twelve patients had lung biopsies, all with ≥ 5 pulmonary nodules on CT scan (Table 4). All the patients underwent lung biopsy as a result of chest consolidation on CT in the setting of lymphadenopathy, to rule-out infection and lymphoma as well as characterize ILD, if present. Eleven of the 12 biopsies demonstrated PLH,34 and 3 had granulomas. LIP and OP may lead to ground glass opacity on CT,33,35,36 and we found this to be the case in 4 of 6 patients. Moreover, the finding of LIP and/or OP was statistically associated with a peripheral monocyte count ≥ 500 cells/μL (p = 0.015).
Table 4.
Lung pathology.
| Subject | Type | Pathology Diagnosis | PLH | Granulomas |
|---|---|---|---|---|
| 1 | thoracoscopic | reactive lymphoid hyperplasia | yes | No |
| 2 | thoracoscopic | atypical nodular lymphoid infiltrate with poorly formed granulomas | yes | Yes |
| 3 | thoracoscopic | atypical lymphoid hyperplasia | yes | No |
| 4 | thoracoscopic | follicular bronchiolitis with fibrosis and loosely formed granulomas | yes | Yes |
| 5 | transbronchial | follicular bronchiolitis | yes | No |
| 6 | thoracoscopic | atypical reactive lymphoid infiltrate with organizing pneumonia | yes | No |
| 7 | thoracoscopic | lymphocytic interstitial pneumonia with follicular bronchiolitis | yes | no |
| 8 | transbronchial | organizing pneumonia | no | no |
| 9 | thoracoscopic | bronchiolocentric interstitial pneumonia with fibrosis, lymphoid hyperplasia, organizing pneumonia, and occasional poorly formed granulomas | yes | yes |
| 10 | transbronchial | lymphocytic interstitial pneumonia | yes | no |
| 11 | thoracoscopic | lymphocytic interstitial pneumonia with follicular bronchiolitis and organizing pneumonia | yes | no |
| 12 | transbronchial | atypical lymphoid hyperplasia | yes | no |
| Parameter | FB or LH only (n = 6) | LIP or OP (n = 6) | p value |
|---|---|---|---|
| > 5 Nodules | 6 | 6 | 1.00 |
| Ground Glass | 2 | 4 | 0.567 |
| Monocytes > 500 | 0 | 5 | 0.015 |
FB = follicular bronchiolitis, LH = lymphoid hyperplasia, LIP = lymphocytic interstitial pneumonia, OP = organizing pneumonia
DISCUSSION
We analyzed a group of 61 CVID patients who had a chest CT scan to seek clinical and/or laboratory correlations with specific radiologic findings. As CT scan of the chest was not routinely obtained on all CVID patients, it is possible that there is a bias towards more severe disease in this study as many subjects had imaging done for clinical symptoms. Many patients are referred to our institution because of abnormal CT chest scans in the setting of CVID, further contributing to the high prevalence of ILD in our cohort. Indeed, the most frequent radiologic finding in this study, pulmonary nodules, is the most common radiologic presentation of ILD in CVID.6,37,38 It is the high prevalence of ILD, however, that allows this study to uniquely classify divergent radiologic manifestations of CVID lung disease.
Those with bronchiectasis only, likely to be the most common radiologic abnormality in CVID patients overall,37 had the highest prevalence of pneumonia, oldest median age, and lowest percentage of medical complications other than enteropathy. Brochiectasis has previously been associated with history of pneumonia in CVID.38 The high sensitivity of a CD4+ T cell count < 700 cells/μL in our cohort strongly suggests that lower CD4+ T cell counts heighten susceptibility to bronchiectasis in CVID. Advancing age and history of pneumonia may compound the susceptibility imparted by a low CD4+ T cell count, as evidenced by the high PPV when these additional parameters are included. Precursor findings to bronchiectasis can be identified on a CT scan of the chest, as bronchial wall thickening with dilation may progress to bronchiectasis.19 Thus, low CD4+ T cell count and/or a history of chronic bronchitis or pneumonia in a patient with CVID may suggest the need for interval CT scans of the chest even in the absence of active respiratory symptoms. The European Society for Immunodeficiencies registry recently identified an association between low IgM levels and bronchiectasis in CVID,39 supporting previous reports of a protective role of IgM in the lung.23,40 Our study may not have been adequately powered to detect a decreased IgM in bronchiectasis subjects. Further studies are needed to examine the possibility for prevention and treatment of bronchiectasis in CVID, though usage of prophylactic antibiotics41 and increased Ig replacement dosage42 may be efficacious.
We found ILD to be associated with younger age and liver disease, as well as AIHA and ITP, lymphoid hyperplasia, splenomegaly or splenectomy as previously reported.10,43,44 There was no apparent association of ILD with history of pneumonia or enteropathy. Elevated CD4+:CD8+ T cell ratio, serum IgM, and history of AIHA and ITP were strongly associated with extensive pulmonary nodules, which we found to almost exclusively represent PLH as in prior reports.6,13 Emergence of PLH can be indicative of more systemic lymphoid hyperplasia, as these CVID patients also had higher prevalence of hilar adenopathy and splenomegaly or splenectomy. Despite antibody deficiency, PLH in CVID is characterized by actively proliferating ectopic lung follicles,13 which may promote expansion of CD4+ T cells (relative to CD8+ T cells) and possibly the IgM elevation noted in this study. Indeed, CVID patients with lymphoproliferative complications have an increase in activated CD4+ T cells,45 and higher serum IgM has been shown to be a predictor of polyclonal lymphocytic infiltration in CVID.3 Additionally, the heightened prevalence of AIHA or ITP may also be correlated with lymphoid hyperplasia, as benign proliferation in the spleen46 or in gastric-associated lymphoid tissue47 may be associated with ITP. Clearly, the propensity for many CVID patients to develop benign lymphoid proliferations deserves further study.48
Subjects with ground glass opacity could be differentiated by a significantly elevated peripheral blood monocyte count and the highest percentage of concurrent liver disease. Of note, subjects in our study with biopsy-proven LIP and/or OP were significantly more likely to have monocytes ≥ 500 cells/μL, in addition to having ground glass opacity on CT scan. Thus, elevated monocytes may be indicative of an evolution of ILD that manifests as ground glass opacity on CT and LIP or OP on biopsy. Of the 61 subjects, a lung biopsy had been performed in 12, thus precluding further comments on associations of pathology with the laboratory parameters noted here. It remains to be seen whether similar inflammatory progression underlies granulomatous disease that can occur in the lungs, as well as liver, lymph nodes, skin, or spleen, of CVID patients.17,49
Our data suggest parameters that are associated with selected radiologic findings in CVID which may provide insight into the determinants of chronic pulmonary complications in this disease. While bronchiectasis appears to develop over time as a result of heightened susceptibility to infection, ILD generally occurred in younger CVID patients with concurrent autoimmunity and lymphoid hyperplasia. Furthermore, a subset of ILD patients may progress to a heightened inflammatory state characterized by increased peripheral monocytes, liver involvement, LIP or OP on lung biopsy, and ground glass opacity on CT chest scan. The results of this study suggest that divergent immunological processes underlie the distinct clinical manifestations of bronchiectasis and ILD in CVID.
Acknowledgments
Funding: Supported by the Thrasher Research Fund Early Career Award, Baxter-Clinical Immunology Society Senior Fellowship Award, Jeffrey Modell Foundation, National Institutes of Health grants AI 048693 and AI 061093, and the David S. Gottesman Immunology Chair.
ABBREVIATIONS
- AIHA
autoimmune hemolytic anemia
- CT
computed tomography
- CVID
common variable immunodeficiency
- DLCO
diffusing capacity of the lung for carbon monoxide
- GLILD
granulomatous-lymphocytic interstitial lung disease
- Ig
immunoglobulin
- ILD
interstitial lung disease
- ITP
immune thrombocytopenic purpura
- LIP
lymphocytic interstitial pneumonitis
- NPV
negative predictive value
- OP
organizing pneumonia
- OR
odds ratio
- PLH
pulmonary lymphoid hyperplasia
- PPV
positive predictive value
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors declare that they have no conflict of interest.
REFERENCES
- 1.Cunningham-Rundles C, Maglione PJ. Common variable immunodeficiency. J Allergy Clin Immunol. 2012;129(5):1425–1426. e3. doi: 10.1016/j.jaci.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 2.Chapel H, Cunningham-Rundles C. Update in understanding common variable immunodeficiency disorders (CVIDs) and the management of patients with these conditions. Br J Haematol. 2009;145(6):709–27. doi: 10.1111/j.1365-2141.2009.07669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapel H, Lucas M, Lee M, Bjorkander J, Webster D, Grimbacher B, et al. Common variable immunodeficiency disorders: division into distinct clinical phenotypes. Blood. 2008;112(2):277–86. doi: 10.1182/blood-2007-11-124545. [DOI] [PubMed] [Google Scholar]
- 4.Quinti I, Soresina A, Spadaro G, Martino S, Donnanno S, Agostini C, et al. Long-term follow-up and outcome of a large cohort of patients with common variable immunodeficiency. J Clin Immunol. 2007;27(3):308–16. doi: 10.1007/s10875-007-9075-1. [DOI] [PubMed] [Google Scholar]
- 5.Resnick ES, Moshier EL, Godbold JH, Cunningham-Rundles C. Morbidity and mortality in common variable immune deficiency over 4 decades. Blood. 2012;119(7):1650–7. doi: 10.1182/blood-2011-09-377945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bates CA, Ellison MC, Lynch DA, Cool CD, Brown KK, Routes JM. Granulomatous-lymphocytic lung disease shortens survival in common variable immunodeficiency. J Allergy Clin Immunol. 2004;114(2):415–21. doi: 10.1016/j.jaci.2004.05.057. [DOI] [PubMed] [Google Scholar]
- 7.Gregersen S, Aalokken TM, Mynarek G, Fevang B, Holm AM, Ueland T, et al. Development of pulmonary abnormalities in patients with common variable immunodeficiency: associations with clinical and immunologic factors. Ann Allergy Asthma Immunol. 2010;104(6):503–10. doi: 10.1016/j.anai.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 8.Arish N, Eldor R, Fellig Y, Bogot N, Laxer U, Izhar U, et al. Lymphocytic interstitial pneumonia associated with common variable immunodeficiency resolved with intravenous immunoglobulins. Thorax. 2006;61(12):1096–7. doi: 10.1136/thx.2004.029819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Gracia J, Vendrell M, Alvarez A, Pallisa E, Rodrigo MJ, de la Rosa D, et al. Immunoglobulin therapy to control lung damage in patients with common variable immunodeficiency. Int Immunopharmacol. 2004;4(6):745–53. doi: 10.1016/j.intimp.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Bondioni MP, Soresina A, Lougaris V, Gatta D, Plebani A, Maroldi R. Common variable immunodeficiency: computed tomography evaluation of bronchopulmonary changes including nodular lesions in 40 patients. Correlation with clinical and immunological data. J Comput Assist Tomogr. 2010;34(3):395–401. doi: 10.1097/RCT.0b013e3181cad9da. [DOI] [PubMed] [Google Scholar]
- 11.Touw CM, van de Ven AA, de Jong PA, Terheggen-Lagro S, Beek E, Sanders EA, et al. Detection of pulmonary complications in common variable immunodeficiency. Pediatr Allergy Immunol. 2010;21(5):793–805. doi: 10.1111/j.1399-3038.2009.00963.x. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez Perez ER. Granulomatous lymphocytic interstitial lung disease. Immunol Allergy Clin North Am. 2012;32(4):621–32. doi: 10.1016/j.iac.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Maglione PJ, Ko HM, Beasley MB, Strauchen JA, Cunningham-Rundles C. Tertiary lymphoid neogenesis is a component of pulmonary lymphoid hyperplasia in patients with common variable immunodeficiency. J Allergy Clin Immunol. 2014;133(2):535–542. doi: 10.1016/j.jaci.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park JH, Levinson AI. Granulomatous-lymphocytic interstitial lung disease (GLILD) in common variable immunodeficiency (CVID). Clin Immunol. 2010;134(2):97–103. doi: 10.1016/j.clim.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Kohler PF, Cook RD, Brown WR, Manguso RL. Common variable hypogammaglobulinemia with T-cell nodular lymphoid interstitial pneumonitis and B-cell nodular lymphoid hyperplasia: different lymphocyte populations with a similar response to prednisone therapy. J Allergy Clin Immunol. 1982;70(4):299–305. doi: 10.1016/0091-6749(82)90066-5. [DOI] [PubMed] [Google Scholar]
- 16.Fasano MB, Sullivan KE, Sarpong SB, Wood RA, Jones SM, Johns CJ, et al. Sarcoidosis and common variable immunodeficiency. Report of 8 cases and review of the literature. Medicine (Baltimore) 1996;75(5):251–61. doi: 10.1097/00005792-199609000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Bouvry D, Mouthon L, Brillet PY, Kambouchner M, Ducroix JP, Cottin V, et al. Granulomatosis-associated common variable immunodeficiency disorder: a case-control study versus sarcoidosis. Eur Respir J. 2013;41(1):115–22. doi: 10.1183/09031936.00189011. [DOI] [PubMed] [Google Scholar]
- 18.Sugino K, Uekusa T, Homma S. Granulomatous-lymphocytic Interstitial Lung Disease in a Patient with Common Variable Immunodeficiency. Intern Med. 2013;52(23):2683–4. doi: 10.2169/internalmedicine.52.1222. [DOI] [PubMed] [Google Scholar]
- 19.Hampson FA, Chandra A, Screaton NJ, Condliffe A, Kumararatne DS, Exley AR, et al. Respiratory disease in common variable immunodeficiency and other primary immunodeficiency disorders. Clin Radiol. 2012;67(6):587–95. doi: 10.1016/j.crad.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 20.Wislez M, Sibony M, Naccache JM, Liote H, Carette MF, Oksenhendler E, et al. Organizing pneumonia related to common variable immunodeficiency. case report and literature review. Respiration. 2000;67(4):467–70. doi: 10.1159/000029552. [DOI] [PubMed] [Google Scholar]
- 21.Boujaoude Z, Arya R, Rafferty W, Dammert P. Organising pneumonia in common variable immunodeficiency. BMJ Case Rep. 2013:2013. doi: 10.1136/bcr-2013-008905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gregersen S, Holm AM, Fevang B, Ueland T, Sikkeland LI, Aalokken TM, et al. Lung disease, T-cells and inflammation in common variable immunodeficiency disorders. Scand J Clin Lab Invest. 2013;73(6):514–22. doi: 10.3109/00365513.2013.819523. [DOI] [PubMed] [Google Scholar]
- 23.Carsetti R, Rosado MM, Donnanno S, Guazzi V, Soresina A, Meini A, et al. The loss of IgM memory B cells correlates with clinical disease in common variable immunodeficiency. J Allergy Clin Immunol. 2005;115(2):412–7. doi: 10.1016/j.jaci.2004.10.048. [DOI] [PubMed] [Google Scholar]
- 24.Detkova D, de Gracia J, Lopes-da-Silva S, Vendrell M, Alvarez A, Guarner L, et al. Common variable immunodeficiency: association between memory B cells and lung diseases. Chest. 2007;131(6):1883–9. doi: 10.1378/chest.06-2994. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez-Ramon S, Radigan L, Yu JE, Bard S, Cunningham-Rundles C. Memory B cells in common variable immunodeficiency: clinical associations and sex differences. Clin Immunol. 2008;128(3):314–21. doi: 10.1016/j.clim.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barbera JA, Hayashi S, Hegele RG, Hogg JC. Detection of Epstein-Barr virus in lymphocytic interstitial pneumonia by in situ hybridization. Am Rev Respir Dis. 1992;145(4 Pt 1):940–6. doi: 10.1164/ajrccm/145.4_Pt_1.940. [DOI] [PubMed] [Google Scholar]
- 27.Andiman WA, Eastman R, Martin K, Katz BZ, Rubinstein A, Pitt J, et al. Opportunistic lymphoproliferations associated with Epstein-Barr viral DNA in infants and children with AIDS. Lancet. 1985;2(8469-70):1390–3. doi: 10.1016/s0140-6736(85)92557-7. [DOI] [PubMed] [Google Scholar]
- 28.Wheat WH, Cool CD, Morimoto Y, Rai PR, Kirkpatrick CH, Lindenbaum BA, et al. Possible role of human herpesvirus 8 in the lymphoproliferative disorders in common variable immunodeficiency. J Exp Med. 2005;202(4):479–84. doi: 10.1084/jem.20050381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prasse A, Kayser G, Warnatz K. Common variable immunodeficiency-associated granulomatous and interstitial lung disease. Curr Opin Pulm Med. 2013;19(5):503–9. doi: 10.1097/MCP.0b013e3283642c47. [DOI] [PubMed] [Google Scholar]
- 30.Conley ME, Notarangelo LD, Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan-American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies). Clin Immunol. 1999;93(3):190–7. doi: 10.1006/clim.1999.4799. [DOI] [PubMed] [Google Scholar]
- 31.Kainulainen L, Varpula M, Liippo K, Svedstrom E, Nikoskelainen J, Ruuskanen O. Pulmonary abnormalities in patients with primary hypogammaglobulinemia. J Allergy Clin Immunol. 1999;104(5):1031–6. doi: 10.1016/s0091-6749(99)70085-0. [DOI] [PubMed] [Google Scholar]
- 32.Hare SS, Souza CA, Bain G, Seely JM, Frcpc, Gomes MM, et al. The radiological spectrum of pulmonary lymphoproliferative disease. Br J Radiol. 2012;85(1015):848–64. doi: 10.1259/bjr/16420165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee KS, Kullnig P, Hartman TE, Muller NL. Cryptogenic organizing pneumonia: CT findings in 43 patients. AJR Am J Roentgenol. 1994;162(3):543–6. doi: 10.2214/ajr.162.3.8109493. [DOI] [PubMed] [Google Scholar]
- 34.Guinee DG., Jr. Update on nonneoplastic pulmonary lymphoproliferative disorders and related entities. Arch Pathol Lab Med. 2010;134(5):691–701. doi: 10.5858/134.5.691. [DOI] [PubMed] [Google Scholar]
- 35.Dalvi V, Gonzalez EB, Lovett L. Lymphocytic interstitial pneumonitis (LIP) in Sjogren's syndrome: a case report and a review of the literature. Clin Rheumatol. 2007;26(8):1339–43. doi: 10.1007/s10067-006-0351-x. [DOI] [PubMed] [Google Scholar]
- 36.Filipek MS, Thompson ME, Wang PL, Gosselin MV, S LP. Lymphocytic interstitial pneumonitis in a patient with systemic lupus erythematosus: radiographic and high-resolution CT findings. J Thorac Imaging. 2004;19(3):200–3. doi: 10.1097/01.rti.0000099464.94973.51. [DOI] [PubMed] [Google Scholar]
- 37.Obregon RG, Lynch DA, Kaske T, Newell JD, Jr., Kirkpatrick CH. Radiologic findings of adult primary immunodeficiency disorders. Contribution of CT. Chest. 1994;106(2):490–5. doi: 10.1378/chest.106.2.490. [DOI] [PubMed] [Google Scholar]
- 38.Quinti I, Soresina A, Guerra A, Rondelli R, Spadaro G, Agostini C, et al. Effectiveness of immunoglobulin replacement therapy on clinical outcome in patients with primary antibody deficiencies: results from a multicenter prospective cohort study. J Clin Immunol. 2011;31(3):315–22. doi: 10.1007/s10875-011-9511-0. [DOI] [PubMed] [Google Scholar]
- 39.Gathmann B, Mahlaoui N, for C, Gerard L, Oksenhendler E, Warnatz K, et al. Clinical picture and treatment of 2212 patients with common variable immunodeficiency. J Allergy Clin Immunol. 2014 doi: 10.1016/j.jaci.2013.12.1077. doi: 10.1016/j.jaci.2013.12.1077. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 40.Micol R, Kayal S, Mahlaoui N, Beaute J, Brosselin P, Dudoit Y, et al. Protective effect of IgM against colonization of the respiratory tract by nontypeable Haemophilus influenzae in patients with hypogammaglobulinemia. J Allergy Clin Immunol. 2012;129(3):770–7. doi: 10.1016/j.jaci.2011.09.047. [DOI] [PubMed] [Google Scholar]
- 41.Altenburg J, de Graaff CS, Stienstra Y, Sloos JH, van Haren EH, Koppers RJ, et al. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non-cystic fibrosis bronchiectasis: the BAT randomized controlled trial. JAMA. 2013;309(12):1251–9. doi: 10.1001/jama.2013.1937. [DOI] [PubMed] [Google Scholar]
- 42.Bonilla FA, Bernstein IL, Khan DA, Ballas ZK, Chinen J, Frank MM, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. Ann Allergy Asthma Immunol. 2005;94(5 Suppl 1):S1–63. doi: 10.1016/s1081-1206(10)61142-8. [DOI] [PubMed] [Google Scholar]
- 43.Torigian DA, LaRosa DF, Levinson AI, Litzky LA, Miller WT., Jr. Granulomatouslymphocytic interstitial lung disease associated with common variable immunodeficiency: CT findings. J Thorac Imaging. 2008;23(3):162–9. doi: 10.1097/RTI.0b013e318166d32f. [DOI] [PubMed] [Google Scholar]
- 44.Wehr C, Kivioja T, Schmitt C, Ferry B, Witte T, Eren E, et al. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood. 2008;111(1):77–85. doi: 10.1182/blood-2007-06-091744. [DOI] [PubMed] [Google Scholar]
- 45.Mouillot G, Carmagnat M, Gerard L, Garnier JL, Fieschi C, Vince N, et al. B-cell and T-cell phenotypes in CVID patients correlate with the clinical phenotype of the disease. J Clin Immunol. 2010;30(5):746–55. doi: 10.1007/s10875-010-9424-3. [DOI] [PubMed] [Google Scholar]
- 46.Daridon C, Loddenkemper C, Spieckermann S, Kuhl AA, Salama A, Burmester GR, et al. Splenic proliferative lymphoid nodules distinct from germinal centers are sites of autoantigen stimulation in immune thrombocytopenia. Blood. 2012;120(25):5021–31. doi: 10.1182/blood-2012-04-424648. [DOI] [PubMed] [Google Scholar]
- 47.Franchini M, Vescovi PP, Garofano M, Veneri D. Helicobacter pylori-associated idiopathic thrombocytopenic purpura: a narrative review. Semin Thromb Hemost. 2012;38(5):463–8. doi: 10.1055/s-0032-1305781. [DOI] [PubMed] [Google Scholar]
- 48.da Silva SP, Resnick E, Lucas M, Lortan J, Patel S, Cunningham-Rundles C, et al. Lymphoid proliferations of indeterminate malignant potential arising in adults with common variable immunodeficiency disorders: unusual case studies and immunohistological review in the light of possible causative events. J Clin Immunol. 2011;31(5):784–91. doi: 10.1007/s10875-011-9565-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ardeniz O, Cunningham-Rundles C. Granulomatous disease in common variable immunodeficiency. Clin Immunol. 2009;133(2):198–207. doi: 10.1016/j.clim.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]