Abstract
Background
Cisplatin is a widely-used chemotherapeutic agent that can also cause ototoxic injury. One potential treatment for cisplatin-induced hearing loss involves the activation of endogenous inner ear stem cells, which may then produce replacement hair cells. In this series of experiments, we examined the effects of cisplatin exposure on both hair cells and resident stem cells of the mouse inner ear.
Results
Treatment for 24 hours with 10 µM cisplatin caused significant loss of hair cells in the mouse utricle, but such damage was not evident until four days after the cisplatin exposure. In addition to killing hair cells, cisplatin treatment also disrupted the actin cytoskeleton in remaining supporting cells, and lead to increased histone H2AX phosphorylation within the sensory epithelia. Finally, treatment with 10 µM cisplatin appeared to have direct toxic effects on resident stem cells in the mouse utricle. Exposure to cisplatin blocked the proliferation of isolated stem cells and prevented sphere formation when those cells were maintained in suspension culture.
Conclusion
The results suggest that inner ear stem cells may be injured during cisplatin ototoxicity, thus limiting their ability to mediate sensory repair.
Keywords: ototoxicity, hair cell, supporting cell, chemotherapy, regeneration, H2AX
INTRODUCTION
Cisplatin is an antineoplastic agent that is commonly used to treat a variety of solid tumors. The drug causes structural damage to DNA, leading to induction of apoptosis in tumor cells. Ototoxicity is a frequent side effect of cisplatin chemotherapy, usually manifesting as tinnitus and/or high-frequency hearing loss (Piel et al., 1974; Rybak et al., 2007). Approximately 15–30% of patients who receive cisplatin treatment develop irreversible sensorineural hearing loss, and most treated patients display at least some elevation of audiometric thresholds (McKeage, 1995; Nagy et al., 1999; Li et al., 2004). In addition to its effects on the cochlea, cisplatin can also damage the vestibular organs (Schaefer et al., 1981; Black et al., 1982; Myers et al., 1993; Nakayama et al., 1996; Sergi et al., 2003). Hair cells, the mechanoreceptors of the inner ear that are responsible for detection of sound and head motion, are killed by cisplatin exposure (Stadnicki et al., 1975), but the cellular basis of cisplatin ototoxicity is not fully understood. Cellular changes, such as the formation of DNA-platinum adducts, release of immune cytokines, and increased production of reactive oxygen species, have been described in the inner ear following cisplatin exposure (van Ruijven et al., 2005; So et al., 2007; Kim et al., 2010). Finally, cisplatin does not selectively target inner ear hair cells; other studies have suggested that cochlear supporting cells and spiral ganglion neurons are also damaged by cisplatin (Hinojosa et al., 1995; Zheng et al., 1995; Ramirez-Camacho et al., 2004).
One promising strategy for the treatment of sensorineural hearing loss involves the generation of replacement hair cells in the damaged ear, either through genetic targeting of surviving supporting cells or via the activation of resident stem cells (reviewed by Heller and Brigande, 2009). Conceptual support for this approach is provided by the observation that the avian inner ear can quickly regenerate after acoustic trauma or aminoglycoside toxicity (Corwin and Cotanche, 1988; Ryals and Rubel, 1988; Weisleder and Rubel, 1993). It is notable, however, that the avian ear is unable to regenerate after cisplatin ototoxicity (Slattery and Warchol, 2010), suggesting that cisplatin might impair the proliferation or transdifferentiation of inner ear supporting cells and/or stem cells. In contrast to the situation in birds, the mammalian cochlea is incapable of regeneration after hair cell injury, but the mammalian vestibular organs exhibit a limited degree of regenerative ability (e.g., Warchol 2011). In addition, the mouse utricle has been shown to contain a small population of resident stem cells that can be isolated and propagated in vitro (Li et al., 2003; Oshima et al., 2007). The present study characterized the effects of cisplatin on hair cells and resident stem cells of the mouse inner ear. We found that cisplatin treatment caused the death of hair cells in the mature mouse utricle, even when applied at relatively low doses. Although hair cell loss was not observed until several days after cisplatin exposure, immunolabeling for phosphorylated histone H2AX (p-H2AX – an indicator of DNA double-strand breaks) was detected within 24 hr of cisplatin treatment. These data indicate that cisplatin damages the genomic DNA of sensory cells, and suggests possible similarities between the toxic effects of cisplatin on tumor cells and the mechanisms of cisplatin ototoxicity. Additional experiments examined the effects cisplatin on vestibular stem cells. We found that the numbers of sphere-forming stem cells derived from the mouse utricle was nearly abolished by pretreatment of cultured utricles with low does of cisplatin. In contrast, stem cell proliferation and sphere formation were not affected by pretreatment with neomycin. These findings suggest that inner ear stem cells are targeted by cisplatin and may not be a viable means of restoring sensory function in the ear after cisplatin ototoxicity.
RESULTS
Low concentrations of cisplatin are toxic to utricular hair cells
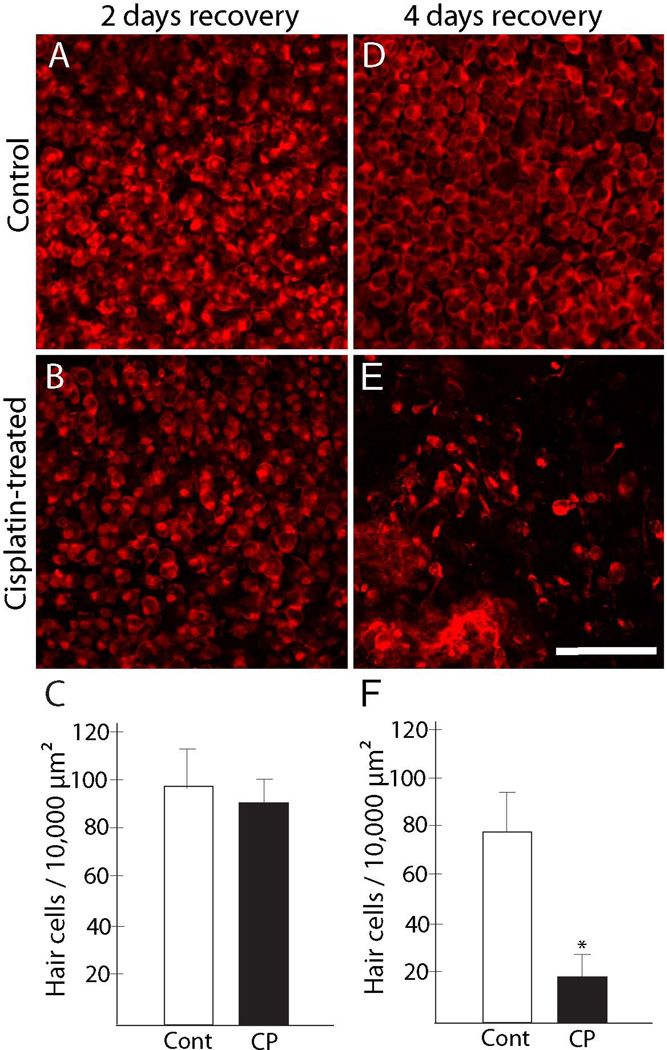
Our previous study of the avian inner ear indicated that treatment for 24 hr with 10 µM cisplatin was sufficient to cause hair death, but that the full extent of ototoxic injury was not evident until several days after the initial cisplatin exposure (Slattery and Warchol, 2010). To determine whether the mammalian ear exhibits a similar temporal response to cisplatin, utricles from adult C57BL/6 mice were treated for 24 hr with 10 µM cisplatin and then maintained for an additional 2, 4 or 7 days in cisplatin-free medium (n=10–12 utricles/condition for each timepoint, along with equal numbers of untreated controls). Following fixation, hair cells were labeled with an antibody against myosin VIIa. Specimens were imaged and surviving hair cells were quantified from two regions within the central extrastriolar portion of the sensory epithelium (see Methods). As was the case with the avian ear, we observed minimal evidence of ototoxic injury at 2 days following cisplatin treatment (Fig. 1A, B). However, specimens that were maintained for four days after cisplatin treatment displayed considerable loss of hair cells (Fig. 1D, E). Hair cell injury did not appear to be confined to a particular region, but was distributed throughout the sensory epithelium. These observations were verified by quantification of surviving hair cells (Fig. 1C, F). This injury pattern became more extensive at seven days post-cisplatin, and often culminated in the complete absence of hair cells.
Figure 1. Hair cell death is observed at 4 days after cisplatin treatment.
Mouse utricles were placed in organ culture, treated for 24 hr with 10 µM cisplatin, and then rinsed and maintained in cisplatin-free medium for an additional two or four days. Control utricles were cultured in parallel (i.e., for three or five days, total), but did not receive cisplatin. Specimens were then fixed and hair cells were immunolabeled for myosin VIIa (red). No evidence for hair cell injury was observed after three days in vitro, either in control utricles (A) or utricles treated with cisplatin (B). Quantification of hair cells in these specimens confirmed this observation (C). A small reduction in hair cell numbers was evident after five days culture in control medium (D). However, a considerable loss of hair cells was observed in utricles that were maintained for four days after cisplatin treatment (E, F, *p<0.005, t-test). CP=cisplatin-treated. Scale bar = 50 µm.
Cisplatin damages cell-cell junctions and the actin cytoskeleton
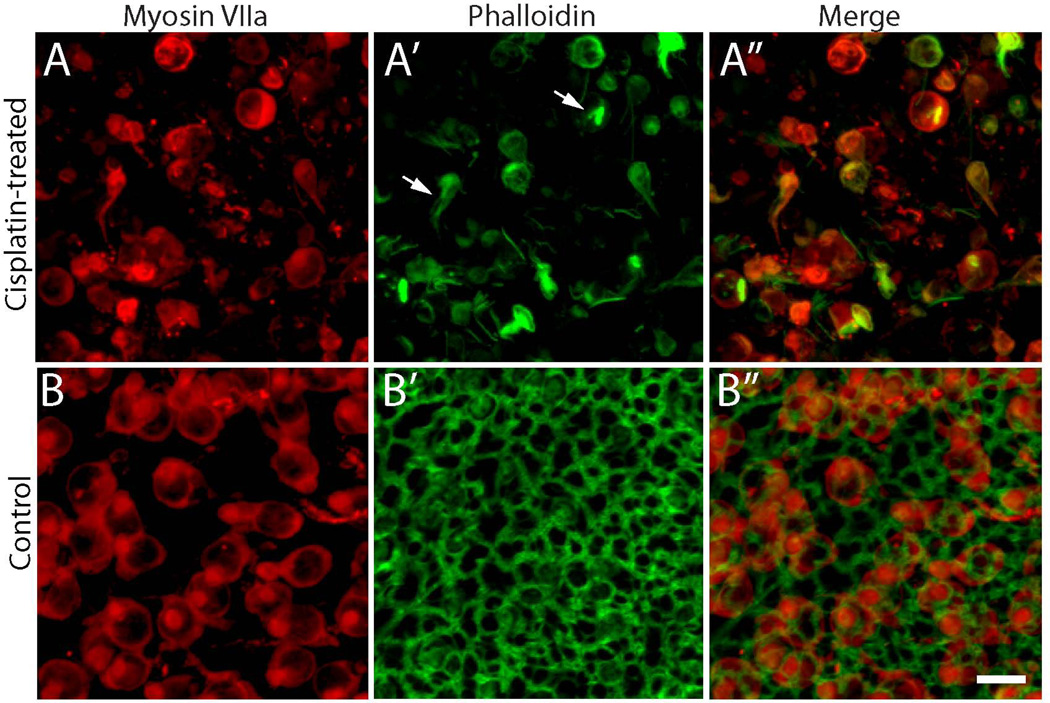
Aminoglycoside ototoxicity primarily targets sensory hair cells. In response to aminoglycoside-induced hair cell death, epithelial supporting cells quickly reform cell-cell junctions in order to preserve the barrier separating endolymph from perilymph (Raphael and Altschuler, 1991). Supporting cells in the injured ear can also undergo complex cytoskeletal rearrangements, forming actin-containing processes that phagocytose cellular debris (Bird et al., 2010). Although some previous studies have suggested that cisplatin can injure both hair cells and supporting cells (Ramirez-Camacho et al., 2004), the effects of cisplatin on the epithelial structure of the inner ear have not been thoroughly characterized. To address this issue, utricles (n=12) from adult mice were placed in organotypic culture and treated for 24 hr with 10 µM cisplatin. The specimens were then thoroughly rinsed and maintained for four days in cisplatin-free medium. Control cultures (n=12) were maintained in parallel, but did not receive cisplatin. After fixation, utricles were processed for immunohistochemical labeling of myosin VIIa (to identify hair cells) and for Alexa-488 phalloidin (to label actin filaments). Consistent with results described above, we observed a partial loss of hair cells in the cisplatin-treated specimens, compared to untreated controls (Fig 2A, B). Notably, however, phalloidin labeling revealed that nearly all cell-cell junctions were destroyed by the cisplatin treatment (Fig. 2A’), while junctional actin cables appeared normal in control utricles (Fig. 2B’). The efficacy of the phalloidin label was verified by the fact that we still observed some intact actin filaments within surviving hair cells (arrows, Fig. 2A’). This result suggests that cisplatin treatment can severely disrupt the structure of the utricle’s sensory epithelium.
Figure 2. Cisplatin treatment disrupts the actin cytoskeleton of the utricular sensory epithelium.
Utricles were cultured for 24 hr in 10 µM cisplatin and then maintained for an additional four days in cisplatin-free medium. Immunoreactivity for myosin VIIa (red) revealed fewer hair cells in cisplatin-treated specimens (A) vs. controls (B). Labeling with phalloidin (green) and imaging with confocal microscopy indicated that cell-cell junctions were severely disrupted in cisplatin-treated utricles (A’), but appeared to be intact in control utricles (B’). Notably, some filamentous actin remained within surviving hair cells after cisplatin treatment (arrows, A’). Scale bar = 10 µm.
Cisplatin treatment causes DNA damage to cells in the utricular sensory epithelium
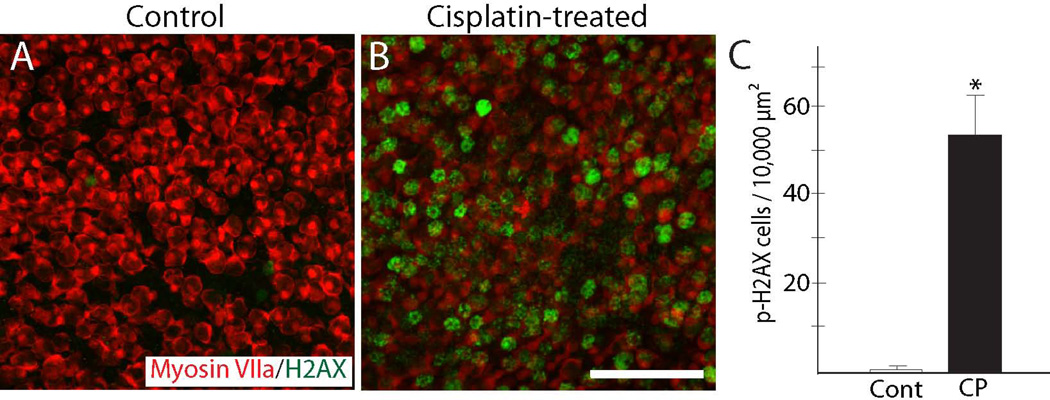
Cisplatin is thought to kill tumor cells via the formation of platinum adducts in nuclear DNA (e.g., Kelland, 2007), but it is not clear whether cisplatin ototoxicity is caused by similar DNA damage. Prior studies have shown that phosphorylation of histone H2AX is a reliable marker of cisplatin-induced DNA damage in tumor cells (Clingen et al., 2008). We examined whether cisplatin also caused induction of p-H2AX in the mouse inner ear. Utricles (n=12) were treated for 24 hr with 10 µM cisplatin and then maintained in cisplatin-free medium for an additional two days. Control utricles (n=12) were maintained in parallel, but did not receive cisplatin. Immunolabeling revealed a large increase in p-H2AX after cisplatin exposure (Fig. 3). In addition, labeling for p-H2AX was observed in cells that were myosin VIIa-labeled (hair cells), as well as in cells that did not exhibit myosin VIIa immunoreactivity (presumptive supporting cells). Notably, the increase in p-H2AX occurred prior to quantifiable hair cell loss in the cisplatin-treated utricles (e.g., Fig. 1), suggesting that DNA damage precedes hair cell apoptosis.
Figure 3. Evidence for DNA damage following cisplatin treatment.
Utricles were cultured for 24 hr in 10 µM cisplatin and then rinsed and maintained for an additional 48 hr in cisplatin-free medium. Control specimens were maintained in parallel (3 days, total), but did not receive cisplatin. After fixation, specimens were processed for immunohistochemical detection of p-H2AX (green), which identifies cell nuclei that contain double-stranded DNA lesions. Very few p-H2AX-labeled cells were observed in control utricles (A). In contrast, utricles that had received cisplatin treatment contained numerous cells that displayed evidence of DNA damage (B, C, *p<0.0005, t-test). CP=cisplatin-treated. Red: Myosin VIIa. Scale bar=50 µm.
Cisplatin targets resident stem cells in the mouse utricle
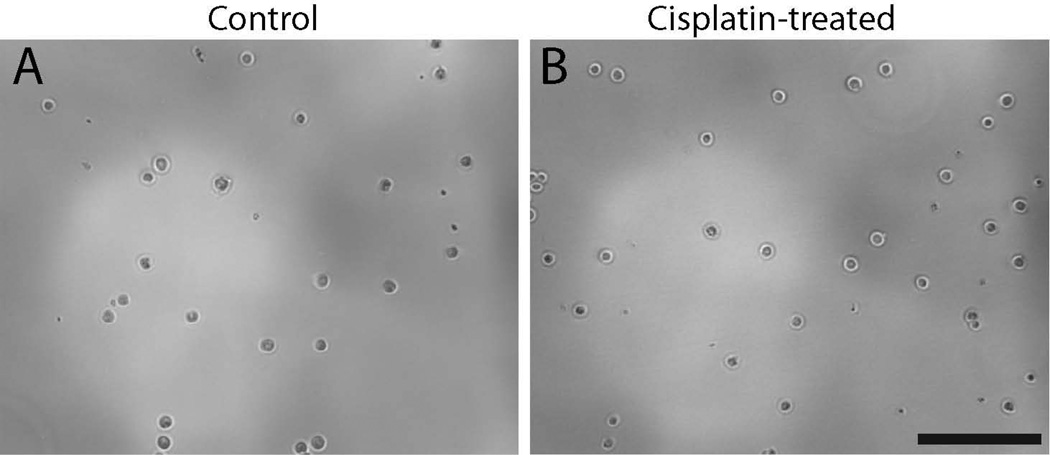
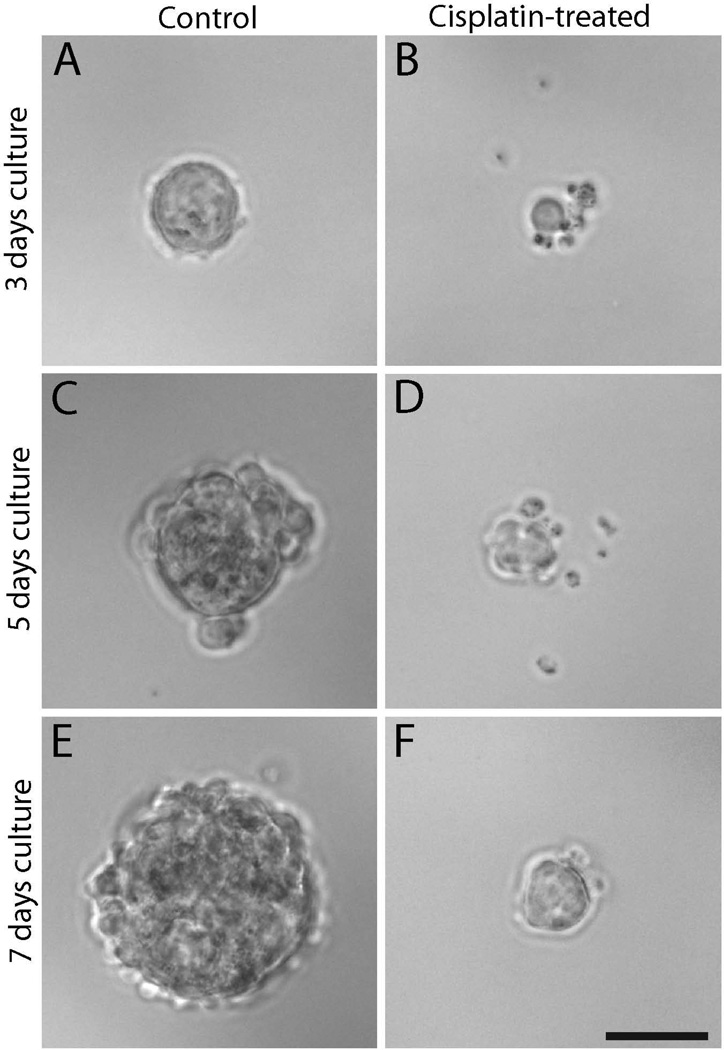
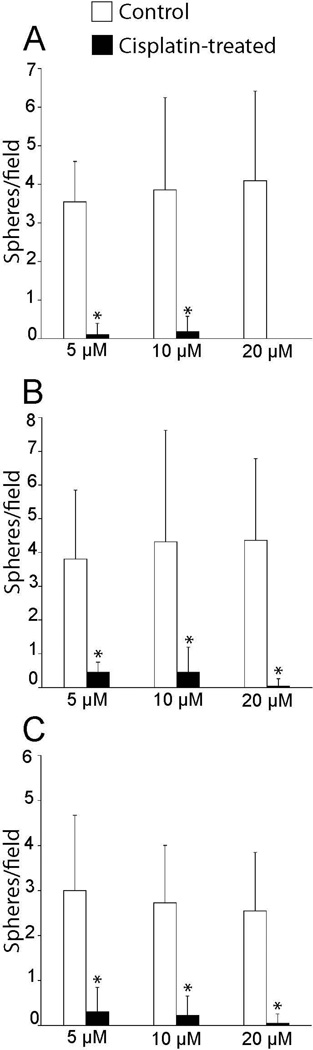
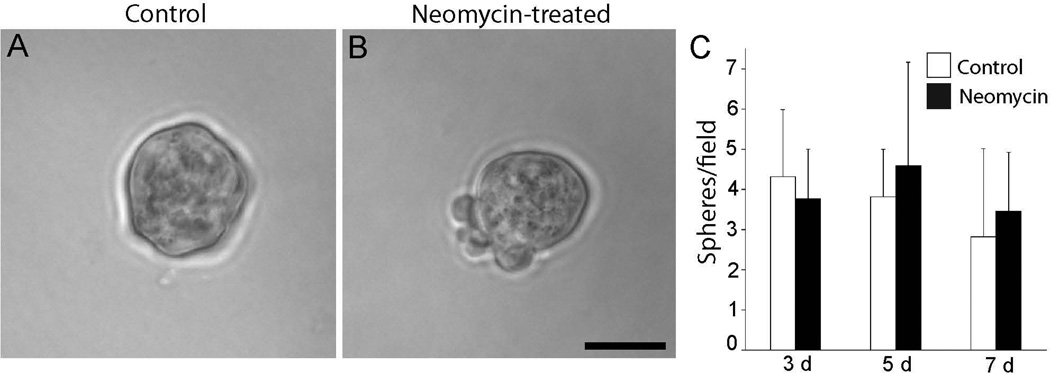
Our prior studies demonstrated that cisplatin interferes with the innate regenerative mechanism of the avian ear (Slattery and Warchol, 2010). The vestibular organs of mammals contain a small number of proliferative cells (Warchol et al., 1993), which can be isolated and propagated in vitro (Li et al., 2003). In order to determine whether those cells were affected by cisplatin exposure, we treated mouse utricles with cisplatin and then quantified the yield of derived stem cells. Utricles were explanted from mice at postnatal day 3 (when large numbers of resident stem cells are present – Oshima et al., 2007) and treated for 24 hr in 5, 10 or 20 µM cisplatin. Following thorough rinsing in fresh culture medium, we then isolated the sensory epithelia and dissociated the cells, in order to determine the number of cells with capacity for sphere formation. Immediately after dissociation of the epithelia, we found that both the cisplatin-treated and control specimens yielded approximately equal numbers of cells (~3 × 104 cells/ mL - Fig. 4). We then maintained the cells in suspension culture and quantified the numbers of spheres that had formed after 3, 5 and 7 days of incubation. At all time points, the spheres derived from cisplatin-treated utricles were smaller than those obtained from control utricles (Fig. 5). We also observed a dramatic reduction in the numbers of spheres that could be derived from cisplatin-treated epithelia, compared to untreated controls (Fig. 6). As an additional control experiment, we examined the effect of aminoglycoside ototoxicity on stem cell derivation and sphere formation. Utricles from P3 mice (n=2 groups of eight utricles) were placed in culture and treated for 24 hr with 2 mM neomycin. They were then rinsed with fresh medium and the sensory epithelia were dissociated and placed in suspension culture (as described above). The number of stem cell spheres was quantified after 3, 5 and 7 days in vitro and compared with the numbers of spheres derived from control specimens. Those data indicated that pretreatment with neomycin did not affect the stem cell population of the mouse utricle (Fig. 7).
Figure 4. Normal-appearing cells can be harvested from the utricular sensory epithelium immediately following cisplatin treatment.
Utricles were placed in organotypic culture and maintained for 24 hr in either 10 µM cisplatin or in cisplatin-free medium (controls). Specimens were then rinsed and the sensory epithelia were removed via thermolysin treatment. Isolated epithelia were pooled in groups of eight, incubated in tryspin, and dissociated via gentle trituration. We observed no differences in cell appearance in untreated specimens (A) vs. the cisplatin-treated specimens (B.) In both groups, the approximate density of viable cells (as identified by trypan blue labeling) was ~3 × 104 cells/ml. Scale bar = 100 µm.
Figure 5. Pretreatment in cisplatin inhibits the formation of stem cell-derived spheres.
Utricles from neonatal mice were treated for 24 hr in 10 µM cisplatin or in normal medium (controls). Sensory epithelia were then isolated, dissociated, and placed in suspension culture. Sphere formation (a characteristic of stem cells) was examined after three days. Control utricles yielded numerous stem cell-derived spheres (A). In contrast, many fewer spheres were observed in cultures derived from utricles that were treated with cisplatin. Spheres from cisplatin-treated utricles were typically smaller than those in control cultures (B). Similar findings were observed after five days (C and D) and seven days (E and F) of suspension culture. Scale bar = 50 µm.
Figure 6. Dose-dependent effects of cisplatin on the formation of stem cell-derived spheres.
Utricles were treated for 24 hr in various concentrations of cisplatin or in control (cisplatin-free) medium. The sensory epithelia were then isolated, dissociated and placed in suspension culture. Living cultures were visualized on an inverted microscope and the numbers of stem cell-derived spheres/visual field were quantified (field size=25.6 mm2). Such quantification occurred after three, five and seven days of suspension culture. In all cases, pretreatment in cisplatin reduced the numbers of spheres (n=22 samples/condition from four experiments, *p<0.0005, Tukey’s post-hoc test for multiple comparisons).
Figure 7. Pretreatment with neomycin does not affect the formation of stem cell spheres.
Utricles were placed in culture and treated for 24 hr with 2 mM neomycin. Sensory epithelia were then isolated, dissociated, and placed in suspension culture. The number of spheres in such cultures was quantified after three, five or seven days, and compared with sphere numbers observed in parallel cultures that had not been treated with neomycin (controls). Sphere morphology and size appeared similar in cultures derived from untreated utricles (A) vs. neomycin-treated utricles (B, C). In addition, the numbers of spheres obtained from neomycin-treated utricles was not significantly different from the numbers derived from control utricles (p=0.20–0.25, t-test). Scale bar = 50 µM.
Cisplatin pretreatment reduces stem cell proliferation
Stem cell spheres are comprised of cells that are clonally-derived from the repeated division of isolated stem cells (e.g., Martinez-Monedero et al., 2007). Since cisplatin blocks cell cycle progression in tumor cells, we next examined whether the lack of sphere formation observed after cisplatin treatment was attributable to reduced proliferation of vestibular stem cells. Mouse utricles were explanted at P3 and treated for 24 hr with 5 or 10 µM cisplatin (n=8 utricles/dose). The sensory epithelia were then isolated and dissociated into single-cell suspensions, as described above. Cells were maintained in suspension culture in medium that contained the mitotic marker BrdU. After four days, spheres were plated onto laminin/poly-l-ornithine-coated cultures wells and maintained in vitro for an additional 24 hours. At this point, specimens were fixed and processed for BrdU immunocytochemistry. Control specimens were prepared and maintained in parallel, but received either 2 mM neomycin in place of cisplatin or no ototoxin. Few spheres were detected in cultures derived from utricles that were treated with10 µM cisplatin, and none of those spheres adhered to laminin-coated dishes. However, attached spheres were successfully harvested from utricles that were treated with 5 µM cisplatin. Spheres from cisplatin-treated utricles (n=4) contained 36.7 ± 16.1 cells/sphere, while those derived from neomycin-treated (N=11) or untreated (N=12) utricles contained 47.2 ± 37.2 and 59.9 ± 39.4 cells/ sphere, respectively. We observed numerous BrdU-labeled cell nuclei in spheres derived from untreated or neomycin-treated utricles (94.0% and 94.1%, respectively; Fig. 8A, B), but very few BrdU-labeled cells were present in spheres derived from cisplatin-treated utricles (5.4%; Fig. 8C).
Figure 8. Pretreatment in cisplatin reduces proliferation of utricular stem cells.
Stem cell spheres were derived from cisplatin-treated, neomycin-treated or control utricles and maintained for four days in medium that contained BrdU. Specimens were then transferred onto laminin-coated substrates for adhesion and subsequent immunolabeling. Robust immunolabeling for BrdU (green) was observed in spheres prepared from untreated utricles (A), and from utricles that had been treated with neomycin (B). In contrast, utricles treated with 5 µM cisplatin (C) generated very few spheres, and those displayed almost no BrdU labeling. Scale bar = 50 µM.
Discussion
Cisplatin is an effective chemotherapeutic agent that is used to treat several types of solid tumors. Patients that undergo cisplatin treatment often experience permanent hearing loss, but the biological mechanisms of cisplatin ototoxicity are poorly understood. Enhanced knowledge of the direct effects of cisplatin on the sensory structures of the inner ear may suggest methods for the prevention of ototoxic injury. Also, a potential strategy for the treatment of drug-induced hearing loss is to develop biological methods for the inducing regeneration in the inner ear. Such regenerative therapies would likely involve the production of replacement hair cells from epithelial supporting cells and/or resident inner ear stem cells. However, our prior studies had shown that the avian inner ear – which has a robust regenerative ability following aminoglycoside ototoxicity – is unable to regenerate hair cells after cisplatin exposure (Slattery and Warchol, 2010), raising the question of whether the ototoxic effects of cisplatin also extend to supporting cells. The present data provide a more complete picture of cisplatin injury to the inner ear, and show that cisplatin has toxic effects on hair cells, supporting cells, and resident stem cells of the mouse utricle. Interestingly, neomycin - another ototoxic drug- did not affect sphere-forming stem cells. Based on these observations, it is likely that the development of biological therapies for remediation of cisplatin ototoxicity will present unique challenges.
Cisplatin ototoxicity in organotypic cultures
The vestibular organs of mice offer a number of experimental advantages for the study of otic development and regeneration. One drawback to the use of mice in ototoxicity research is that their vestibular organs are relatively resistant to systemic (in vivo) treatment with aminoglycosides or cisplatin. For this reason, such studies are typically conducted with organotypic cultures (Cunningham and Brandon, 2006; Schmitt et al., 2009; Slowik and Bermingham-McDonogh, 2013). Similar culture methods have been used to examine regeneration in the mouse utricle (Lambert, 1994; Meyers and Corwin, 2007; Lin et al., 2011), and to characterize the numbers and behavior of endogenous stem cells in the vestibular organs (Li et al., 2003; Oshima et al., 2007). All of the data reported here were collected from organotypic culture preparations, but the hair cell lesions were created differently from those described in earlier studies. Previous studies of cisplatin ototoxicity exposed cultured utricles to rather high concentrations of cisplatin (e.g., 50–250 µM), in order to create a significant hair cell lesion within a 24 hr exposure interval (e.g., Cunningham and Brandon, 2006; Schmitt et al., 2009). Notably, we found that treatment with lower doses of cisplatin also caused a large hair cell lesion, but that the death of hair cells was not evident until 3–4 days after the exposure. Our choice of a 10 µM dose was motivated by in vivo studies, where a single i.v. injection in guinea pigs results in a peak perilymph cisplatin concentrations of ~10 µM (Hellberg et al., 2009). Such concentrations occur within 30 min after the injection and begin to decline within an hour, and the long-term levels of cisplatin in the inner ear have not been characterized. Based on such limited in vivo data, it is not possible to specify an optimal approach for cisplatin exposure in vitro, but our observation that a relatively low concentration of cisplatin can cause hair cell injury (with a latency of several days) has permitted us to characterize some intermediate events in the ototoxic process (i.e., damage to DNA and to the cytoskeleton).
Cisplatin damages cell-cell junctions and the cytoskeleton
Previous studies of cisplatin ototoxicity have mainly focused on the loss of hair cells and diminished auditory function that occurs after cisplatin treatment. Our data suggest that, in addition to injuring hair cells, cisplatin may also damage inner ear supporting cells. Specifically, we found that incubation of mouse utricles for 24 hr in 10 µM cisplatin resulted in the disassembly of the actin cytoskeleton at the junctions of remaining supporting cells. Prior in vitro studies of both normal and transformed cells have found that comparable concentrations of cisplatin can lead to disorganization of the actin cytoskeleton (Kruidering et al., 1998; Otto et al., 2002). Moreover, systemic treatment with cisplatin has been shown to damage cochlear supporting cells in rats (Ramirez-Camacho et al., 2004). Nevertheless, our data suggest that the role of supporting cells in cisplatin ototoxicity may deserve further attention. Supporting cells are essential for maintaining ionic, chemical and mechanical homeostasis in the sensory epithelia of the inner ear. They also form a barrier between endolymph and perilymph, so as to maintain the unique ionic composition of these two fluid spaces. Injury to supporting cells would likely lead to the disruption of this barrier, permitting the flow of potassium ions into perilymph. Mixing of inner ear fluids may induce hair cell death (e.g., Bohne and Harding, 2000), so disruption of epithelial cell-cell junctions could be an important contributing factor to the loss of hair cells observed during cisplatin therapy. Additional studies – preferably conducted with in vivo models – will be necessary to evaluate this proposal.
Cisplatin causes early DNA damage in hair cells
One novel finding of our study was that exposure to cisplatin leads to DNA damage within the sensory epithelium of the utricle. Phosphorylation of histone H2AX (specifically at Ser-139) occurs in response to double-strand breaks in nuclear DNA (e.g., Mah et al., 2010), and immunohistochemical labeling of p-H2AX has been used as a marker of DNA damage in other cell types following treatment with a variety of chemotherapeutic agents (Clingen et al., 2008; Banath et al., 2010). Immunoreactivity for p-H2AX has also been demonstrated in inner ear supporting cells following ectopic over-expression of cyclinD1, suggesting that DNA damage is a consequence of abnormal cell cycle entry in hair cells (Loponen et al., 2011). To our knowledge, this is the first demonstration that cisplatin exposure can induce p-H2AX immunoreactivity in the sensory organs of the inner ear, and has several implications for the mechanisms of cisplatin ototoxicity. First, the anti-tumor properties of cisplatin are attributable to the formation of platinum-adducts, resulting in DNA damage and subsequent apoptosis (e.g., Kelland, 2007). Our data suggest that cisplatin causes a similar form of DNA damage in the inner ear, and that such damage is evident relatively early after cisplatin treatment (i.e., before hair cell loss or other epithelial pathology). Prior studies have proposed that exposure to cisplatin results in the generation of free radicals and/or inflammatory cytokines in hair cells, and these are thought to be key mediators of cisplatin ototoxicity (e.g., Schacht et al., 2012; Audo and Warchol, 2012). The present data raise the additional possibility that cisplatin may kill both tumor cells and sensory hair cells through similar biochemical mechanisms.
Cisplatin greatly reduces sphere formation by utricle-derived resident stem cells
Although the mammalian cochlea appears incapable of sensory regeneration, the vestibular organs of mammals possess a modest ability to regenerate hair cells after ototoxic injury (e.g., Forge et al., 1993; Lin et al., 2001; Golub et al., 2012). Notably, the mammalian vestibular organs contain a small number of cells that can proliferate in response to loss of hair cells (Warchol et al., 1993; Lambert, 1994; Kuntz and Oesterle, 1998), and also appear to harbor a population of cells that possess stem cell-like features (i.e., the ability to form clonally derived solid spheres when maintained in suspension culture – Li et al., 2003; Oshima et al., 2007). Studies conducted with mice have shown that both injury-evoked proliferation and the numbers of resident vestibular stem cells decrease with age (Oshima et al., 2007; Burns et al., 2012). Such observations suggest that the proliferative cells within the vestibular sensory epithelia might be identical to population of resident stem cells, but this has not been definitively established. In any case, our data show that treatment with cisplatin – but not neomycin – depletes the capacity for sphere generation among cell populations derived from utricles of neonatal mice. Given that cisplatin has been shown to target proliferative tumor cells, it is not surprising that it also affects proliferative and/or stem cells in other tissues, such as the inner ear. Together, our observations that cisplatin also causes DNA damage and disruption of the actin cytoskeleton suggest that cisplatin ototoxicity may be a complex phenomenon, which damages the ear through several and partially independent mechanisms.
Implications for sensory regeneration in humans
The inner ears of nonmammalian vertebrates are able to regenerate hair cells after acoustic trauma or aminoglycoside ototoxicity. This regenerative response is principally mediated by supporting cells, although it is possible that some nonmammalian hair cell epithelia also possess resident stem cells. It has been suggested that the activation of quiescent stem cells and/or directed transdifferentiation of supporting cells might promote repair of the human ear after ageing, acoustic trauma or ototoxic injury (e.g., Brigande and Heller, 2009). The present report that cisplatin targets supporting cells and epithelial stem cells in the mouse utricle is in general agreement with prior studies, which have shown diminished regenerative ability after cisplatin ototoxicity in birds and zebrafish (Slattery and Warchol, 2010; Mackenzie and Raible, 2012). Such findings suggest that induction of sensory regeneration after cisplatin ototoxicity may pose unique challenges. Specifically, inner ear stem cells are likely to be destroyed by cisplatin treatment and surviving supporting cells may no longer possess the potential to serve as hair cell precursors. In this regard, it is worth noting that our results are consistent with studies of the effects of anticancer therapies on neurogenesis in the mammalian CNS. Certain regions of the mammalian brain (e.g., the dentate gyrus of the hippocampus) continue to generate new neurons throughout adult life (e.g., Bonaguidi et al., 2012). Studies conducted with rodent models indicate that several chemotherapeutic drugs (including cisplatin) can suppress the proliferation of neural precursors in the hippocampus, thus blocking the production of new neurons (Dietrich et al., 2006). Treatment with cancer chemotherapy agents frequently leads to cognitive deficits, a condition known as ‘chemobrain’ (e.g., Wigmore, 2013), which may be related to this loss of ongoing neurogenesis. Such studies, together with the present data, suggest cisplatin may have complex and widely varied effects on nervous system function.
EXPERIMENTAL PROCEDURES
Animals
Mice (CD-1 and C57BL/6 strains) were obtained from Charles River (Franklin, CT) and housed within Washington University’s animal care facility. All protocols involving animals were approved by the Washington University Animal Studies Committee.
Organotypic cultures of mouse utricle
Experiments that examined epithelial effects of cisplatin were conducted on utricles taken from mature mice (C57BL/6 or CD-1 strains, age>P90), while experiments that examined the effects of cisplatin on vestibular stem cells utilized utricles taken from CD-1 mice at post natal day 3 (P3). In all cases, mice were euthanized and decapitated. Heads were placed in 70% EtOH (in order to kill surface pathogens), and the temporal bones were isolated and transferred to chilled Medium-199 with HEPES buffer and Hanks salts. Utricles were dissected away from the temporal bones and the otoconia were removed. Specimens were then placed in culture wells (MatTek, Ashland MA) that contained 100 µl of Medium-199 with Earle’s salts (2,200 mg/ l sodium bicarbonate, 0.69 mM L-glutamine, 25 mM HEPES), supplemented with 1% FBS. The cultures were maintained at 37° C in a 5% CO2/ 95% air environment for 1–7 days.
Ototoxic damage
Cisplatin (crystalline cis-platinum(II) diammine dichloride; Sigma-Aldrich) was prepared as a 2 mM stock solution (in PBS) and stored at −20°C. Organotypic cultures of utricles were treated with cisplatin (at final concentrations of 5, 10, or 20 µM) for 24 hours, and were then rinsed 3× in cisplatin-free medium. In other experiments, utricles were maintained for 24 hr in 2 mM neomycin (Taleb et al., 2008).
Sphere formation from mouse stem cells of the utricle
Utricles were isolated from P3 mice and maintained in culture for 24 hr with either 10 µM cisplatin, 2 mM neomycin, or in drug-free medium (controls). Following thorough rinsing, sphere-forming stem cells were derived from these specimens following previously published protocols (Li et al., 2003; Oshima et al., 2007; Oshima et al., 2009). Utricles (eight utricles/treatment group) were incubated for 50 min. in thermolysin (500 µg/ mL, in Medium-199) at 37°C. Using a 30-gauge needle, the sensory epithelium was removed from each utricle as a single sheet. These isolated epithelia were treated for 5 min. in 100 µl of 0.125% trypsin (in PBS). Trypsin activity was terminated by addition of 50 µl soybean trypsin inhibitor (20 mg/ml) and DNAse I inhibitor (2 mg/ml) (Worthington Biochemical; Lakewood, NJ), and were then given 50 µl of 1:1 DMEM:F12 with N2 and B27 (Invitrogen), EGF (20 ng/ ml), FGF2 (10 ng/ml), IGF-1 (50 ng/ml), heparan sulfate (50 ng/ ml) (all growth factors from Sigma-Aldrich; St. Louis, MO). Cells were dissociated by gentle trituration in pipette tips (epTIPS Filter 20–300 µl; Eppendorf; Westbury, NY). Confirmation of a near single-cell suspension was conducted by visualizing the cellular solution on an inverted microscope. The density of dissociated cells was quantified using a hemacytometer, prior to placement in sphere forming medium. The cell suspension received 1.8 ml of DMEM:F12 with N2 and B27, EGF (20 ng/ ml), FGF2 (10 ng/ml), IGF-1 (50 ng/ml), heparan sulfate (50 ng/ ml), yielding a final volume of 2 mL total volume. This mixture was filtered through a 40 µm cell strainer (BD; Franklin Lake, NJ) into a nonadherent culture dish (Greiner Bio-One; Monroe, NC). Cultures were then maintained at 37°C in a 5% CO2/ 95% air environment for either 3, 4, 5, or 7 days.
Immunohistochemistry and nucleic acid staining
Cultured utricles from adult C57BL/6 mice were fixed for 20 min. in 4% paraformaldehyde (PFA; in 0.1M phosphate buffer, pH=7.4). Specimens were then thoroughly rinsed with phosphate-buffered saline (PBS) and incubated for two hours in PBS with 5% normal horse serum (NHS) and 0.2% Triton X-100, in order to block nonspecific antibody binding. Hair cells were labeled with an antibody against myosin VIIa (rabbit polyclonal; 1:500; Proteus Biosciences). Nuclei with damaged DNA were identified using an antibody against p-H2AX (1:500; Millipore). Specimens were maintained in primary antibodies overnight at 4°C. Following treatment in primaries, the specimens were thoroughly rinsed in PBS and incubated for two hours in either Alexa-488 donkey anti-mouse, Alexa-546 donkey anti-rabbit, or Alexa-546 donkey anti-goat secondary antibodies (1:500; all Invitrogen) for two hours. Nuclei were stained with 4',6-Diamidino-2-phenyindole (DAPI; Sigma-Aldrich, 2.7 µM). Cell-cell junctions in some cultured utricles were visualized by incubation in Alexa-488 conjugated phalloidin (Invitrogen). Specimens were mounted on glass slides in 90% glycerol/ 10% PBS and coverslipped.
BrdU Processing
Cell proliferation within stem cell-derived spheres was assessed by adding the mitotic label bromodeoxyuridine (BrdU; 3 µg/ml) to the culture medium. In such experiments, spheres were maintained in 10 µg/ul for 4 days, and were then plated onto laminin/poly-l-ornithine-coated dishes (protocol in Oshima et al., 2007). Spheres were allowed to adhere for 24 hours, and were then fixed and processed for BrdU immunohistochemistry (protocol in Warchol, 2002). Nuclei were stained with DAPI, and the specimens were coverslipped prior to microscopic imaging.
Imaging and Quantification
Confocal images were obtained using either a Bio-Rad Radiance 2000 MP microscope or Zeiss LSM 700 inverted microscope. Confocal z-stacks were visualized and processed with Volocity software (PerkinElmer, Waltham, MA). Hair cells were quantified from two 40× fields (25,617 µm2) within the central extrastriolar region of each utricle, and then normalized to yield density measures of ‘Hair Cells/10,000 µm2’). The density of stem cell-derived spheres was quantified by viewing living cultures on an inverted microscope (Zeiss Axiovert 135) and counting the numbers of spheres in four randomly selected visual fields, using a 4× objective lens (field size = 25.5 mm2).
Statistics
Data were analyzed using an unpaired Student’s t-test or ANOVA with multiple comparisons (Tukey post hoc test; SPSS, Chicago, IL). All data are expressed as mean ± standard deviation.
Acknowledgements
Supported by grants R01 DC006283 and R21 DC010909 (ME Warchol), T32 DC000022 (JG Neely/JF Piccirillo), and P30 DC04665 (RA Chole) from the National Institutes of Health (NIDCD), and by a grant from the National Organization for Hearing Research (ELS)
References
- Audo I, Warchol ME. Retinal and cochlear toxicity of drugs: new insights into mechanisms and detection. Curr Opin Neurol. 2012;25:76–85. doi: 10.1097/WCO.0b013e32834ed882. [DOI] [PubMed] [Google Scholar]
- Banáth JP, Klokov D, MacPhail SH, Banuelos CA, Olive PL. Residual gamma H2AX foci as an indication of lethal DNA lesions. BMC Cancer. 2010;10:4. doi: 10.1186/1471-2407-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird JE, Daudet N, Warchol ME, Gale JE. Rapid elimination of dying sensory hair cells maintains epithelial integrity in the avian inner ear. J Neurosc. 2010;30:12545–12556. doi: 10.1523/JNEUROSCI.3042-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black FO, Myers EN, Schramm VL, Johnson J, Sigler B, Thearle PB, Burns DS. Cisplatin vestibular ototoxicity: preliminary report. Laryngoscope. 1982;92:1363–1368. [PubMed] [Google Scholar]
- Bohne BA, Harding GW. Degeneration in the cochlea after noise damage: primary versus secondary events. Am J Otol. 2000;21:505–509. [PubMed] [Google Scholar]
- Bonaguidi MA, Song J, Ming GL, Song H. A unifying hypothesis on mammalian stem cell properties in the adult hippocampus. Curr Opin Neurobiol. 2012;22:754–761. doi: 10.1016/j.conb.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigande JV, Heller S. Quo vadis, hair cell regeneration? Nat Neurosci. 2009;12:679–685. doi: 10.1038/nn.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JC, Cox BC, Thiede BR, Zuo J, Corwin JT. In vivo proliferative regeneration of balance hair cells in newborn mice. J Neurosci. 2012;32:6570–6577. doi: 10.1523/JNEUROSCI.6274-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clingen PH, Wu JY, Miller J, Mistry N, Chin Fc, Wynne P, Prise KM, Hartley JA. Histone H2AX phosphorylation as a molecular pharmacological marker for DNA interstrand crosslink cancer chemotherapy. Biochem Pharmacol. 2008;76:19–27. doi: 10.1016/j.bcp.2008.03.025. [DOI] [PubMed] [Google Scholar]
- Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acountic trauma. Science. 1988;240:1772–1774. doi: 10.1126/science.3381100. [DOI] [PubMed] [Google Scholar]
- Cunningham LL, Brandon CS. Heat shock inhibits both aminoglycoside- and cisplatin-induced sensory hair cell death. J Assoc Res Otolaryngol. 2006;7:299–307. doi: 10.1007/s10162-006-0043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich J, Han R, Yang Y, Mayer-Pröschel M, Noble M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J Biol. 2006;5:22. doi: 10.1186/jbiol50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forge A, Li L, Corwin JT, Nevill G. Ultrastructural evidence for hair cell regeneration in the mammalian inner ear. Science. 1993;259:1616–1619. doi: 10.1126/science.8456284. [DOI] [PubMed] [Google Scholar]
- Golub JS, Tong L, Ngyuen TB, Hume CR, Palmiter RD, Rubel EW, Stone JS. Hair cell replacement in adult mouse utricles after targeted ablation of hair cells with diphtheria toxin. J Neurosci. 2012;32:15093–15105. doi: 10.1523/JNEUROSCI.1709-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellberg V, Wallin I, Eriksson S, Hernlund E, Jerremalm E, Berndtsson M, Eksborg S, Arnér ES, Shoshan M, Ehrsson H, Laurell G. Cisplatin and oxaliplatin toxicity: importance of cochlear kinetics as a determinant for ototoxicity. J Natl Cancer Inst. 2009;101:37–47. doi: 10.1093/jnci/djn418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinojosa R, Riggs LC, Strauss M, Matz GJ. Temporal bone histopathology of cisplatin ototoxicity. Am J Otol. 1995;16:731–740. [PubMed] [Google Scholar]
- Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7:573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Lee JH, Kim SJ, Oh GS, Moon HD, Kwon KB, Park C, Park BH, Lee HK, Chung SY, Park R, So HS. Roles of NADPH oxidases in cisplatin-induced reactive oxygen species generation and ototoxicity. J Neurosci. 2010;30:3933–3946. doi: 10.1523/JNEUROSCI.6054-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruidering M, van de Water B, Zhan Y, Baelde JJ, Heer E, Mulder GJ, Stevens JL, Nagelkerke JF. Cisplatin effects on F-actin and matrix proteins precede renal tubular cell detachment and apoptosis in vitro. Cell Death Differ. 1998;5:601–614. doi: 10.1038/sj.cdd.4400392. [DOI] [PubMed] [Google Scholar]
- Lambert PR. Inner ear hair cell regeneration in a mammal: identification of a triggering factor. Laryngoscope. 1994;104:701–718. doi: 10.1288/00005537-199406000-00010. [DOI] [PubMed] [Google Scholar]
- Li H, Liu H, Heller S. Pluripotent stem cells from the adult mouse inner ear. Nat Med. 2003;9:1293–1299. doi: 10.1038/nm925. [DOI] [PubMed] [Google Scholar]
- Li Y, Womer RB, Silber JH. Predicting cisplatin ototoxicity in children: the influence of age and the cumulative dose. Eur J Cancer. 2004;40:2445–2451. doi: 10.1016/j.ejca.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Lin V, Golub JS, Nguyen TB, Hume CR, Oesterle EC, Stone JS. Inhibition of Notch activity promotes nonmitotic regeneration of hair cells in the adult mouse utricles. J Neurosci. 2011;31:15329–15339. doi: 10.1523/JNEUROSCI.2057-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loponen H, Ylikoski J, Albrecht JH, Pirvola U. Restrictions in cell cycle progression of adult vestibular supporting cells in response to ectopic cyclin D1 expression. PLoS One. 2011;6:e27360. doi: 10.1371/journal.pone.0027360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie SM, Raible DW. Proliferative regeneration of zebrafish lateral line hair cells after different ototoxic insults. PLoS One. 2012;12:e47257. doi: 10.1371/journal.pone.0047257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah LJ, El-Osta A, Karagiannis TC. gammaH2AX: a sensiry molecular marker of DNA damage and repair. Leukemia. 2010;24:679–686. doi: 10.1038/leu.2010.6. [DOI] [PubMed] [Google Scholar]
- Martinez-Monedero R, Oshima K, Heller S, Edge AS. The potential role of endogenous stem cells in regeneration of the inner ear. Hearing Res. 2007;227:48–52. doi: 10.1016/j.heares.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeage MJ. Comparative adverse effect profiles of platinum drugs. Drug Saf. 1995;13:228–244. doi: 10.2165/00002018-199513040-00003. [DOI] [PubMed] [Google Scholar]
- Meyers JR, Corwin JT. Shape change controls supporting cell proliferation in lesioned mammalian balance epithelium. J Neurosci. 2007;27:4313–4325. doi: 10.1523/JNEUROSCI.5023-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers SF, Blakley BW, Schwan S. Is cis-platinum vestibulotoxic? Otolaryngol Head Neck Surg. 1993;108:322–328. doi: 10.1177/019459989310800403. [DOI] [PubMed] [Google Scholar]
- Nagy JL, Adelstein DJ, Newman CW, Rybicki LA, Rice TW, Lavertu P. Cisplatin ototoxicity: the importance of baseline audiometry. Am J Clin Oncol. 1999;22:305–308. doi: 10.1097/00000421-199906000-00020. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Riggs LC, Matz GJ. Quantitative study of vestibulotoxicity induced by gentamicin or cisplatin in the guinea pig. Laryngoscope. 1996;106:162–167. doi: 10.1097/00005537-199602000-00011. [DOI] [PubMed] [Google Scholar]
- Oshima K, Senn P, Heller S. Isolation of sphere-forming stem cells from the mouse inner ear. Methods Mol Biol. 2009;493:141–162. doi: 10.1007/978-1-59745-523-7_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima K, Grimm CM, Corrales CE, Senn P, Martinez Monedero R, Geleoc GS, Edge A, Holt JR, Heller S. Differential distribution of stem cells in the auditory and vestibular organs of the inner ear. J Assoc Res Otolaryngol. 2007;8:18–31. doi: 10.1007/s10162-006-0058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto AM, Müller CS, Huff T, Hannappel E. Chemotherapeutic drugs change actin skeleton organization and the expression of beta-thymosins in human breast cancer cells. J Cancer Res Clin Oncol. 2002;128:247–256. doi: 10.1007/s00432-002-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piel IJ, Meyer D, Perlia CP, Wolfe VI. Effects of cis-diamminedichloroplatinum (NSC-119875) on hearing function in man. Cancer Chemother Rep. 1974;58:871–875. [PubMed] [Google Scholar]
- Ramirez-Camacho R, Garcia-Berrocal JR, Bujan J, Martin-Marero A, Trinidad A. Supporting cells as a target of cisplatin-induced inner ear damage: therapeutic implications. Laryngoscope. 2004;114:533–537. doi: 10.1097/00005537-200403000-00027. [DOI] [PubMed] [Google Scholar]
- Raphael Y, Altschuler RA. Scar formation after drug-induced cochlear insult. Hearing Res. 1991;57:173–183. doi: 10.1016/0378-5955(91)90034-7. [DOI] [PubMed] [Google Scholar]
- Ryals BM, Rubel EW. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science. 1988;240:1774–1776. doi: 10.1126/science.3381101. [DOI] [PubMed] [Google Scholar]
- Rybak LP, Whitworth CA, Mukherjea D, Ramkumar V. Mechanisms of cisplatin-induced ototoxicity and prevention. Hear Res. 2007;226:157–167. doi: 10.1016/j.heares.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Schacht J, Talaska AE, Rybak LP. Cisplatin and aminoglycoside antibiotics: hearing loss and its prevention. Anat Rec. 2012;295:1837–1850. doi: 10.1002/ar.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer SD, Wright CG, Post JD, Frenkel EP. Cis-platinum vestibular toxicity. Cancer. 1981;47:857–859. doi: 10.1002/1097-0142(19810301)47:5<857::aid-cncr2820470508>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Schmitt NC, Rubel EW, Nathanson NM. Cisplatin-induced hair cell death requires STAT1 and is attenuated by epigallocatechin gallate. J Neurosci. 2009;29:3843–3851. doi: 10.1523/JNEUROSCI.5842-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergi B, Ferraresi A, Troiani D, Paludetti G, Fetoni AR. Cisplatin ototoxicity in the guinea pig: vestibular and cochlear damage. Hear Res. 2003;182:56–64. doi: 10.1016/s0378-5955(03)00142-4. [DOI] [PubMed] [Google Scholar]
- Slattery EL, Warchol ME. Cisplatin ototoxicity blocks sensory regeneration in the avian inner ear. J Neurosci. 2010;30:3473–3481. doi: 10.1523/JNEUROSCI.4316-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slowik AD, Bermingham-McDonogh O. Hair cell generation by notch inhibiton in the adult mammalian critase. J Assoc Res Otolaryngol. 2013;14:813–828. doi: 10.1007/s10162-013-0414-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So H, Kim H, Lee JH, Park C, Kim Y, Kim E, Kim JK, Yun KJ, Lee KM, Lee HY, Moon SK, Lim DJ, Park R. Cisplatin cytotoxicity of auditory cells requires secretions of proinflammatory cytokines via activation of ERK and NF-kappaB. J Assoc Res Otolaryngol. 2007;8:338–355. doi: 10.1007/s10162-007-0084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadnicki SW, Fleischman RW, Schaeppi U, Merriam P. Cis-dichlorodiammineplatinum (II) (NSC-119875): hearing loss and other toxic effects in rhesus monkeys. Cancer Chemother Rep. 1975;59:467–480. [PubMed] [Google Scholar]
- Taleb M, Brandon CS, Lee FS, Lomax MI, Dillmann WH, Cunningham LL. Hsp70 inhibits aminoglycoside-induced hair cell death and is necessary for the protective effect of heat shock. J Assoc Res Otolaryngol. 2008;9:277–289. doi: 10.1007/s10162-008-0122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ruijven MW, de Groot JC, Hendriksen F, Smoorenburg GF. Immunohistochemical detection of platinated DNA in the cochlea of cisplatin-treated guinea pigs. Hear Res. 2005;203:112–121. doi: 10.1016/j.heares.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Warchol ME. Sensory regeneration in the vertebrate inner ear: differences at the levels of cells and species. Hearing Res. 2011;273:72–79. doi: 10.1016/j.heares.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Warchol ME. Cell density and N-cadherin interactions regulate cell proliferation in the sensory epithelia of the inner ear. J Neurosci. 2002;22:2607–2616. doi: 10.1523/JNEUROSCI.22-07-02607.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warchol ME, Lambert PR, Goldstein BJ, Forge A, Corwin JT. Regenerative proliferation in inner ear sensory epithelia from adult guinea pigs and humans. Science. 1993;259:1619–1622. doi: 10.1126/science.8456285. [DOI] [PubMed] [Google Scholar]
- Weisleder P, Rubel EW. Hair cell regeneration after streptomycin toxicity in the avian vestibular epithelium. J Comp Neurol. 1993;331:97–110. doi: 10.1002/cne.903310106. [DOI] [PubMed] [Google Scholar]
- Wigmore P. The effect of systemic chemotherapy on neurogenesis, plasticity and memory. Curr Topics Behav Neurosci. 2013;15:211–240. doi: 10.1007/7854_2012_235. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Stewart RR, Gao WQ. Neurotrophin-4/5 enhances survival of cultured spiral ganglion neurons and protects them from cisplatin neurotoxicity. J Neurosci. 1995;15:5079–5087. doi: 10.1523/JNEUROSCI.15-07-05079.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]