Abstract
Work on vocal communication, influenced by a drive to understand the evolution of language, has focused on auditory processing and forebrain control of learned vocalizations. The actual hindbrain neural mechanisms used to create communication signals are understudied, in part because of the difficulty of experimental studies in species that rely on respiration for vocalization. In these experimental systems – including those that embody vocal learning – vocal behaviors have rhythmic qualities. Recent studies using molecular markers and “fictive” patterns produced by isolated brains are beginning to reveal how hindbrain circuits generate vocal patterns. Insights from central pattern generators for respiration and locomotion are illuminating common neural and developmental mechanisms. Choice of vocal patterns is responsive to socially salient input. Studies of the vertebrate social brain network suggest mechanisms used to integrate socially salient information and produce an appropriate vocal response.
Introduction
Vocal behaviors are rhythmic. Animal songs consist of repeated patters of recurring elements with characteristic temporal structures. Human vocalizations are also rhythmic. Some rhythms – laughing and crying – are blatant while others – emotional prosody – are more subtle [1]. Comparative studies of rhythmic signaling provide support for a key role of the most posterior portion of the hindbrain in vocal pattern generation [2]. Among the bony vertebrates (fish through humans) an expansion of rhombomere 8 (R8) – an embryonic segment that is the most caudal component of the hindbrain – is associated with rhythmic pattern generation (see article by Bass, this issue). Included in R8 are motor and premotor circuitry not only for vocal behavior, but also for movements of anterior appendages such as the forelimbs and pectoral fins [3]. In fish and in many birds, pectoral fins/forewings are used to produce sounds [4]. In humans and non-human primates, arms and hands co-produce the gestures that accompany vocalizations [3,5,6]. A common set of neural elements might thus govern both vocal and gestural communication with implications for the origins of human speech [7].
Here we review our current understanding of how vocal patterns are generated within the vertebrate nervous system. Recent studies using molecular markers and “fictive” patterns produced by isolated brains are beginning to illuminate hindbrain participation in vocal pattern generation. Results from the more extensively examined respiratory and locomotory circuits suggest circuit elements and ontogenetic regulatory features that could contribute to the evolution of vocal patterning. The vocal patterns used in communication coordinate social interactions: cooperative, aggressive, sexual and parental. We thus also explore recent insights into the function and evolution of the brain network underlying social judgments that must drive the expression of an appropriate, rhythmic, vocal response.
Hindbrain cell groups: respiration and vocalization
While the motor neuron pools that participate in respiration and vocalization are readily identified, the premotor pools of interneurons (i.e. neurons whose cell bodies and processes are entirely within the CNS) lie within the hindbrain reticular formation and until recently were not easy to specify. Recent insights have used an approach that parallels studies of patterns generated by the spinal cord. In developing spinal cord, an anterior to posterior Hox code determines motor neuron pool identity (reviewed in [8]). The identity of interneurons is determined by dorsal and ventral signaling centers and can be read out from gene expression patterns, particularly transcription factors. The isolated spinal cord can produce “fictive” locomotion: patterned activity on the ventral roots that matches patterns recorded during movements [9]. Studies of fictive locomotion in isolated mouse spinal cord reveal functional roles for specific interneuron pools that control motor neuron activities [10,11]. Do these observations apply as well to circuit elements within the hindbrain that control respiration and vocalization?
Hindbrain rhombomeres contain motor neuron pools for the cranial nerves; these are arrayed from anterior to posterior to match the branchial arch-derived muscle groups that they innervate (Fig. 1A). Motor neuron identity specifically – and rhombomere identity more generally – are determined by an anterior to posterior Hox code (reviewed in [12]). During development, retinoic acid signaling is responsible for posteriorizing an initial ground state ultimately resulting in an expansion of rhombomere 8 [13,14], the largest rhombomere [2]. As for the spinal cord, interneuron identities are organized dorsoventrally in stripes (Fig. 1B) whose neurons express characteristic transcription factors together with specific neurotransmitter receptors and transporters [15,16]. Analysis of morphologies and electrophysiological properties of interneurons within a hindbrain stripe reveal a common interneuron “toolkit” [15].
Fig. 1.
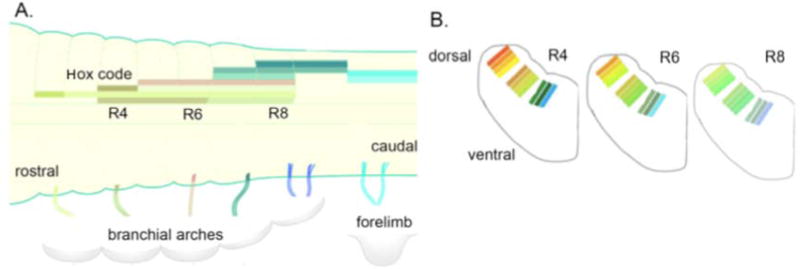
A. Hindbrain rhombomeres are specified from anterior to posterior by a Hox code; here each Hox gene is indicated by a different pastel-colored bar (note that the code can be combinatorial). Hox genes are a sub-set of homeotic genes implicated in segmental identities. The branchial arches and forelimbs are innervated by neurons whose axons form the caudal cranial nerves. Based on [17]. B. Transverse sections through the hindbrain at levels from R4 to R8. Groups of interneurons are organized in stripes (indicated by brightly colored bars; these do not match part A) and can be identified by expression of transcription factors and genes associated with neurotransmitter identity (modified from[16]). Abbreviation: R, rhombomere.
This toolkit supports distinctive and specific hindbrain components such as the preBötzinger complex, essential for respiration [18]. A genome-scale analysis of transcription factor expression has provided markers for making sense of cell groups in the hindbrain reticular formation [19]. Transcription factors are highly conserved in vertebrates and, together with information on neurotransmitter-related genes, will allow us to ask whether they can be used to outline commonalities in hindbrain neural circuits that participate in generating and modulating the rhythmic patterns of breathing and singing and perhaps locomotion as well.
Generating respiratory and vocal rhythms
Rhythmic activity that parallels respiration can be recorded from caudal cranial nerves of many vertebrates. In rodents, where the underlying circuitry has been examined in detail, this activity is generated by the ventral respiratory group (VRG), a column of interneurons in the hindbrain surrounding the nucleus ambiguus complex that includes laryngeal motor neurons [20]. Within the VRG, one set of interneurons, the preBötzinger complex (PBC), is essential for breathing. A transverse slice though the caudal medulla that includes the PBC can generate fictive breathing: rhythmic activity recorded from the hypoglossal nerve [21]. Neurons of the PBC express specific TFs (e.g. MafB) and neurotransmitter/modulator receptors (e.g. NK1) whose deletion lead to inability to breathe [18,22]. A second intrinsically rhythmically active region implicated in respiration (RTN/pFRG) surrounds the more anterior facial nucleus. This parafacial oscillator is derived from Krox20 expressing progenitors and expresses the TF Phox2b [23].
In birds, song requires precise coordination of vocal muscles with respiration, both for overall temporal structure and shaping acoustic features of notes [24]. The forebrain nucleus RA includes a ventral population of neurons that projects to elements of the hindbrain circuitry for song and a dorsal population that projects to hindbrain interneurons and motor neuron that are active during respiration [25]. Hindbrain respiratory interneurons are found in rostroventrolateral medulla and in nuclei parambigualis and retroambigualis, whose location and functional attributes are reminiscent of the VRG in mammals [26]. Respiratory-related neurons in ventrolateral medulla project via a thalamic nucleus Uva to nucleus HVC, an essential forebrain component of the song system [27,28]. In songbirds, hindbrain populations with respiratory activity have not yet been characterized by their patterns of gene expression and a more rostral respiratory group, that might be homologous to the parafacial oscillator of mammals, has not been described. A reduced, fictively breathing and/or singing preparation would be very helpful for cellular characterization of respiratory/vocal hindbrain circuitry in song birds.
Singing without breathing: frogs and fish
A vocal pattern generating circuit has been described for the South African clawed frog, Xenopus laevis (Figure 2). Frogs in this genus are air breathing but entirely aquatic, vocalizing and hearing underwater. Sound production is uncoupled from respiration; for example, glottal opening is inhibited during call production [29,30]. Calls are generated by the opening of arytenoid disks within a modified larynx in response to activity of laryngeal motor neurons [31]. The advertisement call consists of alternating fast and slow trills (trains of clicks). Fictive calling – a pattern of nerve compound action potentials (CAPs) that matches temporal features recorded during actual calling [32; Fig. 2B] – can be reliably evoked in an isolated brain by raising endogenous serotonin using reuptake inhibitors, by serotonin application or by stimulating the amygdala [33–35]. Vocal pattern generating circuitry is contained within the hindbrain and includes a more rostral, rhythmically active group of neurons located in the dorsal medulla at the level of cranial nerve V (DTAM, used as a proper noun) as well as interneurons and laryngeal motor neurons in the caudal hindbrain. Nucleus DTAM exhibits a rhythmic local field potential (Fig. 2D) that coincides with the fast trill portion of the male advertisement call [36]. If the isolated brain is transected rostral to DTAM (triangle, Fig. 2C) serotonin can still evoke fictive advertisement calling [34]. However transections just caudal to DTAM abolish this effect.
Fig. 2.
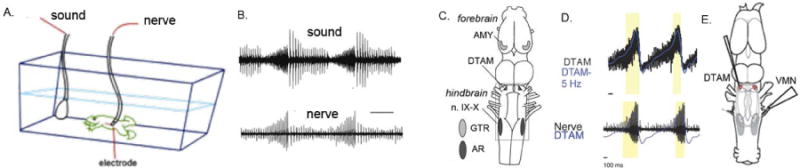
A. and B. An underwater microphone records advertisement calling in a male X. laevis and an en passant electrode records vocal nerve activity (compound action potentials) [32]. C. Components of the neural pathway for call production in X. laevis. The hindbrain VPG includes nucleus DTAM rostrally and interneurons at the anterior pole of nucleus (n.) IX–X caudally. DTAM and vocal motor neurons express androgen receptor (AR). In the forebrain, the amygdala (AMY) expresses receptor for gonadotropin (GTR) and projects to DTAM. Modified from [54]. D. Recordings of a local field potential (highlighted in yellow) from DTAM in the isolated brain (in blue) reveal activity that mirrors the fictive advertisement call pattern recorded from the vocal nerve (lower panel; figure by C. Barkan). Abbreviations: AMY, amygdala; DTAM, used as a proper noun; n. IX–X, nucleus ambiguus, VMN, vocal motor nerve.
Neurons in DTAM make excitatory, non-NMDA glutaminergic synapses on laryngeal motor neurons and inhibit glottal motor neurons via GABAergic interneurons [30]. In addition to the LFP, DTAM neurons display phasic activity (spikes that ride on the LFP) that is abolished when the connection between DTAM and laryngeal motor neurons (n. IX–X Fig. 2C) is cut. The phasic activity corresponds to trains of CAPs recorded from the vocal motor nerve (VMN, Fig. 2E) during fast trill. Local cooling of DTAM decreases fast trill click rates and thus lengthens the call [36]. Nucleus DTAM also partners with neurons in the more caudal hindbrain to set click rate. While no rhythmically active neuronal populations in the caudal hindbrain (i.e. populations resembling the VRG) have yet been identified, serotonin acts to evoke fictive calling via recruitment of rostral interneurons in the IX–X motor column (homologous to nucleus ambiguus; Fig. 2C), a location of the VRG group of mammals. A rostral position, intrinsic rhythmicity and regulation of breathing (glottal) motor neurons suggest that DTAM could be homologous to RTN/pFRG of rodents, a possibility that would be supported by common patterns of gene expression.
Fictive vocalizations can also be recorded from isolated fish brains (reviewed in [38]; see also article by Bass, this issue). In plainfin midshipmen, rhombomere 8 contains the occipital motor neurons that produce sounds as well as pacemaker and prepacemaker neurons that control sound patterning. Fish communicate acoustically using very rapid contractions of swimbladder muscles driven by network properties of input to synchronously-active sonic motor neurons [39]. Call duration is encoded by a sustained depolarization of prepacemaker neurons. Call frequency is driven by rapid membrane potential oscillations in pacemaker neurons [40]. Prepacemaker and pacemaker activity patterns are independent. Sonic motor neurons are gap-junctioned as may also be inputs from pacemaker and prepacemaker neurons, accounting for the ability of neurobiotin application to the occipital root to label the entire sonic production system (see [38]). Vocal motor neurons, pacemaker and prepacemaker neurons are located in R8. DTAM in contrast is located much more rostrally in R2/R3 [41]. The use of TFs and neurotransmitter associated genes that are informative for the hindbrain might be useful in determining whether the fish hindbrain circuit also includes a DTAM/RTN/pFRG homologue.
The ontogeny of CPGs
Locomotion
Perhaps the most well-understood vertebrate central pattern generator (CPG) is an ensemble of interneurons in the spinal cord that produces locomotion. An analysis of how this spinal CPG generates rhythms could be instructive for understanding the rhythms of vocalization. As is the case for hindbrain respiratory and vocal CPGs (see below), the spinal CPG is regulated developmentally and modulated by hormones.
In mammals, networks of spinal interneurons (sINs) are thought to serve as central pattern generators for locomotion. While the role of sINs in the successive activation of motor pools is quite well understood (e.g. inhibitory commisural neurons in left/right limb movement alternation [42]), the interneurons that set the timing (rhythm) of movements have been elusive. The expression of TFs reliably identifies classes of sINs and one (V2a interneurons) is rhythmically active during locomotion in mice. However, genetically-mediated ablation of these neurons does not abolish the rhythmic output of the SC in a fictive walking preparation [43]. In zebrafish, ablation of an analagous population does abolish the swimming rhythm, while optogenetic activation induces rhythmic activity [44]. A recent paper [45] identified a sub-class of V2a sINs that express the TF Shox2. Blocking the activity of Shox2 interneurons affects locomotor frequency leaving left-right alternation intact; these sINs may contribute to rhythmic features of limb movements. Further supporting their importance in locomotor rhythm, or in this case locomotor gait, deletion of V2a sINs produces mice that gallop at slower speeds than their wildtype littermates [46].
Swimming
The CPG for swimming in adults has been extensively analyzed in the lamprey [9]. Swimming across development also has been studied in developing Xenopus laevis tadpoles, a useful model for studying switches in spinal cord CPGs as its motor behavior changes over time: a simple escape swim response to touch in larvae, more complex swimming used for free-feeding in tadpoles, and finally, limbed movement after metamorphosis. Swimming results from the alternate firing of motor neurons from anterior to posterior on each side of the tadpole to produce axial undulation (see Figure 3). Each component of this motor response is controlled by a specific class of interneurons. The larval escape response is defined by repetitive flexion of the body to produce axial swimming and varies in frequency and duration—gentle touch results in a high frequency response which decreases over time, whereas grasping the tadpole causes the spread of a slow flexion wave from head to tail and the appearance of struggling [47]. Sensory stimulation acts through interneurons to control motor neuron firing; and it is these premotor interneurons that are essential for regulation of swimming frequency. Dorsal interneurons (dINs) in the tadpole, excitatory and ipsilateral, fire once at start of each swim cycle to initiate and maintain the swim rhythm during the escape response [48,49]. A different interneuron is recruited to control struggling behavior: the repetitively firing dINrs [50]. At high swim frequencies, a third class of ascending interneurons (aINs), ipsilateral neurons that inhibit sensory neurons and the CPG itself, is recruited to shorten the swim cycle and thus increase its frequency. Finally, commissural inhibitory interneurons (cINs) provide reciprocal inhibition of the contralateral side, resulting in the alternate contraction of the tadpole body (reviewed in [9]) These four examples of the modification of larval swimming provide evidence for the differential recruitment of interneurons in controlling locomotor rhythm, but motor neurons themselves can also play a role.
Fig. 3.
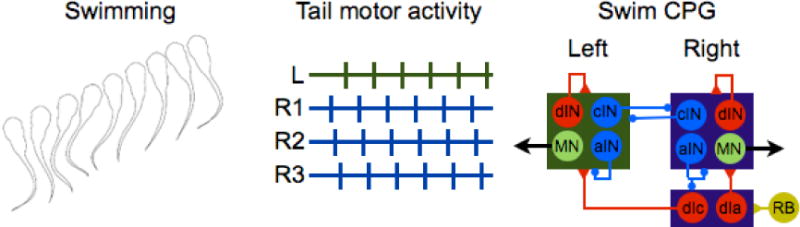
Swimming in X. laevis tapoles and the swim CPG. Both interneurons and motor neurons participate in this motor pattern. Rohon-beard sensory neurons, stimulated by touch, activate excitatory dla and dlc neurons, which in turn, stimulate dINs to control the rhythmic output of the motor neurons (MNs). Commisural cIN interneurons coordinate unilateral flexion through contralateral inhibition. Inhibitory aINs regulate high frequency swimming and inhibit sensory activation (modified from [47, 53]).
From swimming to limb movements
As the tadpole transitions from an escape response to free feeding, its swim behavior evolves from episodes of high frequency but short duration to episodes with increased frequency variability and longer duration—as the tadpoles hover and dart to find food. The cell types involved in this behavioral transition are not well understood but a role for motor neurons themselves is clear. Motor neuron axonal arbors become more restricted and as a result, their firing is less reliable. Recruitment of increasing numbers of motor neurons can result in locomotion of higher frequency or duration [51] providing yet another mechanism for varying locomotor rhythm.
The most dramatic change in motor behavior occurs during metamorphosis as tadpoles transition from swim to limb-based movement and correspondingly add a limb CPG. This transition, driven by a pulse in thyroid hormone (TH), occurs over the course of about 30 days. The mechanism through which TH secretion reconfigures the spinal CPG(s) that underlie swimming and limb movement is not known, though TH does control the innervation of the limb by regulating neurogenesis that produces motor neurons [52]. Is the swim CPG reused or is a new CPG added to control limb movement? From fictive locomotor preparations, in which isolated spinal cords are bathed in NMDA and ventral root recordings are used as a proxy for limb motion, several phases of locomotor behavior were defined during metamorphosis. At the start of metamorphosis, motor neurons fire from anterior to posterior in an alternating wave to generate the axial undulation of swimming, and as limbs develop, motor neurons innervating flexor/extensor muscles appear and gradually transition from coordinated to alternating firing [51]. The CPG that controls limb movement must be added during metamorphosis, but remains a mystery and an active area of study. The dINs of the swimming CPG in the tadpole are excitatory as are V2 and V3a interneurons that participate in limb movements. A class of interneurons, aINs in the tadpole that is similar to V1 inhibitory neurons in the mouse spinal cord, has also been shown to play a role in high frequency swimming through ablation experiments [53] but the mechanism for this effect is unclear.
Development of vocal pattern generators; modulators and hormones
Sexual differentiation of vocal behavior patterns
Vocal characteristics in the experimental model systems reviewed here differ in males and females and reflect differences in the endocrine environment during development and in adults. For example, in X. laevis, males produce 6 vocal patterns and females two, one of which- ticking- is shared with males (reviewed in [54]). During early juvenile stages both sexes produce ticking; as development proceeds the inter-click interval in male calls shortens and a burst-like pattern emerges. Gonadal androgens are required for the development of male-specific calls and androgen-treated adult females recapitulate the developmental stages seen in males on their way to producing advertisement calls [55]. Castrated adult males produced a very few, degraded advertisement calls consisting of the slow trills seen at early developmental stages [55,56]. Androgen-replaced castrated males resume some calling but full restoration requires treatment with gonadotropin [57]. Thyroid hormone secretion at specific developmental stages regulates developmental transitions, as for the switch described above from swimming to limb movement patterns. Thyroid hormone also regulates the ability to respond to gonadal steroids (competence) during development and in adulthood [58]. Because pituitary and gonadal hormone actions are global – affecting sensory input, CNS vocal circuits and sonic muscles – it has been difficult to isolate their site-specific role in the development of sexually differentiated vocal pattern generators. The issue can, however, be studied by analyzing VPGs across development and in response to hormones using fictive vocal production in isolated brains and vocal organs.
In X. laevis, following the application of serotonin, isolated male brains produce the fictive advertisement call pattern and female brains produce the fictive release call pattern [34]. In response to nerve stimulation, the isolated vocal organ of males can produce the rapid advertisement call as well as ticking; the female vocal organ cannot produce advertisement calls. The isolated vocal organ of castrated adult males is somewhat de-masculinized but maintains the ability to produce advertisement calls. This call type, though very rare, can be recorded from long-term castrated frogs (see [54]). The isolated brain of castrated males can still produce biphasic calls, albeit with slower click rates, when a serotonin agonist is applied to its target cells in the anterior portion of the IX–X motor column [56]. Taken together, these results indicate that hormone-sensitive vocal initiation must arise upstream of the hindbrain VPG. A strong candidate for initiation is a forebrain nucleus, the amygdala, that receives auditory input and innervates elements of the VPG [35] as described below.
Initiating and switching vocal responses
Vocal behaviors used in courtship vary according to social context. In X. laevis, the six calls given by males are context dependent. For example, males switch from advertisement to answer calling in response to the female fertility advertisement call rapping, are transiently vocally suppressed by the female release call ticking, and display long-lasting suppression to the calls of a vocally dominant male [54]. Broadcasts of these vocalizations produce the same effects as a vocalizing frog suggesting that acoustic cues dominate in early stages of courtship. The amygdala receives input from auditory thalamus. When the amygdala is lesioned, males do not produce socially-appropriate vocal responses to call broadcasts [35]. They respond to female rapping and ticking as though to the call of another male (i.e. prolonged vocal suppression). Even when paired with a receptive female, males do not produce the answer call. In the isolated brain, electrical stimulation of the amygdala can evoke advertisement call. Thus the X. laevis amygdala plays a key role in switching vocal patterns generated by the hindbrain VPG and does so in an acoustically-specific fashion. The amygdala is hormone-sensitive and expresses, for example, a luteinizing hormone receptor that may account for gonadotropin effects on calling in castrated males [57]. Fictive vocal production by the isolated brain will facilitate an understanding of how sensory inputs drive the VPG to produce distinctive and appropriate patterns. On a larger scale, the amygdala is part of the social decision making network [59] whose antecedents were already present in the ancestral bony vertebrates in which the rhythmic qualities of the caudal hindbrain emerged [2]. Though vocalizations leave no trace in the fossil record, the patterns of gene expression, especially hormone receptors, are remarkably conserved [59] providing an evolutionary scaffold for participation in social judgments.
Conclusions
Though the evidence is as yet fragmentary, recent studies provide support for a common “toolkit” of elements used by neurons in the hindbrain and spinal cord to generate rhythmic behaviors including vocalization, respiration and locomotion. The hindbrain and spinal cord interneurons that participate in these rhythmic behaviors express characteristic transcription factors and neurotransmitter-related genes that appear remarkably conserved from fish to mouse. Vocalizations in vertebrates are generally assumed to have arisen from neural circuits involved in respiration. Molecular markers that identify cell groups active in the control of these behaviors should prove useful in testing this idea. Sexually differentiated vocalizations accompany courtship in many species and are regulated by hormones and neuromodulators that act globally. Fictively vocalizing brain and vocal organ preparations can distinguish the sites of hormone action. A key site may be the very ancient social decision making network with its rich population of hormone responsive neurons well placed to participate in the social judgments that underlie vocal communication.
Highlights.
A hindbrain and spinal cord “toolkit” for rhythmic vocalization, respiration and locomotion.
Neurons express the same transcription factors and neurotransmitter-related genes from fish to mouse.
Sites of action for hormones and modulators can be distinguished using fictive behaviors.
The ancient social decision making neural network (SDN) participates in vocal communication.
Molecular underpinnings for the SDN include conserved hormone and neuromodulator receptors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Russell JA, Bachorowski J-A, Fernández-Dols J-M. Facial and vocal expressions of emotion. Annu Rev Psychol. 2003;54:329–349. doi: 10.1146/annurev.psych.54.101601.145102. [DOI] [PubMed] [Google Scholar]
- 2.Bass AH, Gilland EH, Baker R. Evolutionary origins for social vocalization in a vertebrate hindbrain-spinal compartment. Science. 2008;321:417–421. doi: 10.1126/science.1157632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3**.Bass AH, Chagnaud BP. Shared developmental and evolutionary origins for neural basis of vocal–acoustic and pectoral–gestural signaling. Proceedings of the National Academy of Sciences. 2012;109(Supplement 1):10677–10684. doi: 10.1073/pnas.1201886109. In this seminal review, Bass and Chagnaud point out that the motor and premotor circuitry for vocalization and “gesture” lie in the same caudal hindbrain compartment. They propose that the coupling between these two social communication systems originated with the bony fishes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bostwick KS, Elias DO, Mason A, Montealegre-Z F. Resonating feathers produce courtship song. Proceedings of the Royal Society B: Biological Sciences. 2010;277:835–841. doi: 10.1098/rspb.2009.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5*.Liebal K, Call J. The origins of non-human primates’ manual gestures. Philosophical Transactions of the Royal Society B: Biological Sciences. 2012;367:118–128. doi: 10.1098/rstb.2011.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Remedios R, Logothetis NK, Kayser C. Monkey drumming reveals common networks for perceiving vocal and nonvocal communication sounds. Proceedings of the National Academy of Sciences. 2009;106:18010–18015. doi: 10.1073/pnas.0909756106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gentilucci M, Dalla Volta R, Gianelli C. When the hands speak. Journal of Physiology-Paris. 2008;102:21–30. doi: 10.1016/j.jphysparis.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nature Reviews Genetics. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- 9.Grillner S, Jessell TM. Measured motion: searching for simplicity in spinal locomotor networks. Current Opinion in Neurobiology. 2009;19:572–586. doi: 10.1016/j.conb.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gosgnach S, Lanuza GM, Butt SJB, Saueressig H, Zhang Y, Velasquez T, Riethmacher D, Callaway EM, Kiehn O, Goulding M. V1 spinal neurons regulate the speed of vertebrate locomotor outputs. Nature. 2006;440:215–219. doi: 10.1038/nature04545. [DOI] [PubMed] [Google Scholar]
- 11.Hägglund M, Borgius L, Dougherty KJ, Kiehn O. Activation of groups of excitatory neurons in the mammalian spinal cord or hindbrain evokes locomotion. Nature Neuroscience. 2010;13:246–252. doi: 10.1038/nn.2482. [DOI] [PubMed] [Google Scholar]
- 12.Guthrie S. Patterning and axon guidance of cranial motor neurons. Nat Rev Neurosci. 2007;8:859–871. doi: 10.1038/nrn2254. [DOI] [PubMed] [Google Scholar]
- 13.Niederreithe K, Vermot J, Schuhbaur B, Chambon P, Dollé P. Retinoic acid synthesis and hindbrain patterning in the mouse embryo. Development. 2000;127:75–85. doi: 10.1242/dev.127.1.75. [DOI] [PubMed] [Google Scholar]
- 14.Dupé V, Lumsden A. Hindbrain patterning involves graded responses to retinoic acid signalling. Development. 2001;128:2199–2208. doi: 10.1242/dev.128.12.2199. [DOI] [PubMed] [Google Scholar]
- 15**.Kinkhabwala A, Riley M, Koyama M, Monen M, Satou C, Kimura Y, Higashijima S, Fetcho J. A structural and functional ground plan for neurons in the hindbrain of zebrafish. Proceedings of the National Academy of Sciences. 2011;108:1164–1169. doi: 10.1073/pnas.1012185108. In the zebrafish, stripes of hindbrain neurons with shared neurotransmitter and transcription factor expression, can be discerned throughout the hindbrain. The authors propose that each stripe contributes a set of shared neuronal components (“toolkit”) that are used to shape functional circuits and behaviors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16*.Gray PA. Transcription factors define the neuroanatomical organization of the medullary reticular formation. Frontiers in Neuroanatomy. 2013;7:1–21. doi: 10.3389/fnana.2013.00007. article 7. The author combined expression patterns of genes expressed perinatally in transgenic mice with molecular markers for excitatoty neurotransmitter associated genes and proteins to devise an organization scheme for the perenially obscure hindbrain reticular formation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiecker C, Lumsden A. Compartments and their boundaries in vertebrate brain development. Nat Rev Neurosci. 2005;6:553–564. doi: 10.1038/nrn1702. [DOI] [PubMed] [Google Scholar]
- 18.Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires preBötzinger complex neurokinin-1 receptor-expressing neurons. Nature Neuroscience. 2001;4:927–930. doi: 10.1038/nn0901-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gray PA. Mouse brain organization revealed through direct genome-scale TF expression analysis. Science. 2004;306:2255–2257. doi: 10.1126/science.1104935. [DOI] [PubMed] [Google Scholar]
- 20.Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232–241. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lieske SP, Thoby-Brisson M, Telgkamp P, Ramirez JM. Reconfiguration of the neural network controlling multiple breathing patterns: eupnea, sighs and gasps. Nature Neuroscience. 2000;3:600–607. doi: 10.1038/75776. [DOI] [PubMed] [Google Scholar]
- 22.Blanchi B, Kelly LM, Viemari J-C, Lafon I, Burnet H, Bévengut M, Tillmanns S, Daniel L, Graf T, Hilaire G, et al. MafB deficiency causes defective respiratory rhythmogenesis and fatal central apnea at birth. Nature Neuroscience. 2003;6:1091–1100. doi: 10.1038/nn1129. [DOI] [PubMed] [Google Scholar]
- 23.Thoby-Brisson M, Karlén M, Wu N, Charnay P, Champagnat J, Fortin G. Genetic i dentification of an embryonic parafacial oscillator coupling to the preBötzinger complex. Nature Neuroscience. 2009;12:1028–1035. doi: 10.1038/nn.2354. [DOI] [PubMed] [Google Scholar]
- 24.Elemans C, Mead A, Rome L, Goller F. Superfast vocal muscles control song production in songbirds. PloS one. 2008;3:e2581. doi: 10.1371/journal.pone.0002581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashmore RC, Wild JM, Schmidt MF. Brainstem and forebrain contributions to the generation of learned motor behaviors for song. Journal of Neuroscience. 2005;25:8543–8554. doi: 10.1523/JNEUROSCI.1668-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLean J, Bricault S, Schmidt MF. Characterization of respiratory neurons in the rostral ventrolateral medulla, an area critical for vocal production in songbirds. Journal of Neurophysiology. 2013;109:948–957. doi: 10.1152/jn.00595.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nottebohm F, Kelley DB, Paton J. Projections to vocal control areas of the canary telencephalon. J Comp Neurol. 1982;207:344–357. doi: 10.1002/cne.902070406. [DOI] [PubMed] [Google Scholar]
- 28.Striedter GF, Vu ET. Bilateral feedback projections to the forebrain in the premotor network for singing in zebra finches. Journal of Neurobiology. 1998;34:27–40. [PubMed] [Google Scholar]
- 29.Zornik E, Kelley DB. Breathing and calling: Neuronal networks in the Xenopus laevis hindbrain. J Comp Neurol. 2007;501:303–315. doi: 10.1002/cne.21145. [DOI] [PubMed] [Google Scholar]
- 30.Zornik E, Kelley DB. Regulation of respiratory and vocal motor pools in the isolated brain of Xenopus laevis. Journal of Neuroscience. 2008;28:612–621. doi: 10.1523/JNEUROSCI.4754-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tobias M, Kelley D. Vocalizations by a sexually dimorphic isolated larynx: Peripheral constraints on behavioral expression. Vocalizations of a sexually dimorphic isolated larynx: Peripheral constraints on behavioral expression. J Neurosci. 1987;7:3191–3197. doi: 10.1523/JNEUROSCI.07-10-03191.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamaguchi A, Kelley DB. Generating sexually differentiated vocal patterns: laryngeal nerve and EMG recordings from vocalizing male and female African clawed frogs (Xenopus laevis) J Neurosci. 2000;20:1559–1567. doi: 10.1523/JNEUROSCI.20-04-01559.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu HJ, Yamaguchi A. Endogenous serotonin scts on 5-HT2C-like receptors in key vocal areas of the brain stem to initiate vocalizations in Xenopus laevis. Journal of Neurophysiology. 2010;103:648–658. doi: 10.1152/jn.00827.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rhodes HJ, Yu HJ, Yamaguchi A. Xenopus vocalizations are controlled by a sexually differentiated hindbrain central pattern generator. Journal of Neuroscience. 2007;27:1485–1497. doi: 10.1523/JNEUROSCI.4720-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35••.Hall IC, Ballagh IH, Kelley DB. The Xenopus amygdala mediates socially appropriate vocal communication signals. Journal of Neuroscience. 2013;33:14534–14548. doi: 10.1523/JNEUROSCI.1190-13.2013. The amygdala is a nexus for social communication in Xenopus. Lesions result in socially inappropriate responses to conspecific calls and stimulation drives the hindbrain vocal pattern generator. The ability to study vocal initiation in a fictively singing preparation provides an opportunity to understand the neural basis of social judgments at the cellular level. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zornik E, Katzen AW, Rhodes HJ, Yamaguchi A. NMDAR-dependent control of call duration in Xenopus laevis. Journal of Neurophysiology. 2010;103:3501–3515. doi: 10.1152/jn.00155.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kiehn O, Eken T. Functional role of plateau potentials in vertebrate motor neurons. Current Opinion in Neurobiology. 1998;8:746–752. doi: 10.1016/s0959-4388(98)80117-7. [DOI] [PubMed] [Google Scholar]
- 38.Kelley DB, Bass AH. Neurobiology of vocal communication: mechanisms for sensorimotor integration and vocal patterning. Current Opinion in Neurobiology. 2010;20:748–753. doi: 10.1016/j.conb.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chagnaud BP, Zee MC, Baker R, Bass AH. Innovations in motoneuron synchrony drive rapid temporal modulations in vertebrate acoustic signaling. Journal of Neurophysiology. 2012;107:3528–3542. doi: 10.1152/jn.00030.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chagnaud BP, Baker R, Bass AH. Vocalization frequency and duration are coded in separate hindbrain nuclei. Nat Comms. 2011;2:346. doi: 10.1038/ncomms1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Straka H, Baker R, Gilland E. Preservation of segmental hindbrain organization in adult frogs. J Comp Neurol. 2005;494:228–245. doi: 10.1002/cne.20801. [DOI] [PubMed] [Google Scholar]
- 42.Launza G, Gosnach S, Pierani A, Jessell T, Goulding M. Genetic identification of spinal interneurons that coordinate left-right locomotor activity necessary for walking movements. Neuron. 2004;42:375–386. doi: 10.1016/s0896-6273(04)00249-1. [DOI] [PubMed] [Google Scholar]
- 43.Crone S, Quinlan K, Zagoraiou L, Droho S, Restrepo C, Lundfield L, Endo T, Setlak J, Jessell T, Kiehn O, Sharma K. Genetic ablation of V2a ipsilateral interneurons disrupts left-right locomotor coordination in mammalian spinal cord. Neuron. 2008;60:70–83. doi: 10.1016/j.neuron.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 44*.Ljunggren E, Haupt S, Ausborn J, Ampatzis K, El Manira A. Optogenetic activation of excitatory premotor interneurons Is sufficient to generate coordinated locomotor activity in larval zebrafish. The Journal of Neuroscience. 2014;34:134–139. doi: 10.1523/JNEUROSCI.4087-13.2014. Using optogenetics, the authors identify the V2a interneurons as an excitatory module in the spinal locomotor pattern generator whose activity is sufficient to initiate and maintain swimming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45•.Dougherty KJ, Zagoraiou L, Satoh D, Rozani I, Doobar S, Arber S, Jessell TM, Kiehn O. Locomotor rhythm generation linked to the output of spinal Shox2 excitatory interneurons. Neuron. 2013;80:920–933. doi: 10.1016/j.neuron.2013.08.015. The authors’ aim was to identify the rhythm-generating components of the spinal network for walking. Results indicate participation of Shox2 interneurons as participants. [DOI] [PubMed] [Google Scholar]
- 46.Crone SA, Zhong G, Harris-Warrick R, Sharma K. In mice lacking V2a interneurons, gait depends on speed of locomotion. Journal of Neuroscience. 2009;29:7098–7109. doi: 10.1523/JNEUROSCI.1206-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roberts A, Li WC, Soffe SR. How neurons generate behaviour in a hatchling amphibian tadpole: an outline. Frontiers in behavioral neuroscience. 2010;4:16. doi: 10.3389/fnbeh.2010.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li WC, Soffe SR, Wolf E, Roberts A. Persistent responses to brief stimuli: Feedback excitation among brainstem neurons. Journal of Neuroscience. 2006;26:4026–4035. doi: 10.1523/JNEUROSCI.4727-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soffe SR, Roberts A, Li WC. Defining the excitatory neurons that drive the locomotor rhythm in a simple vertebrate: insights into the origin of reticulospinal control. The Journal of Physiology. 2009;587:4829–4844. doi: 10.1113/jphysiol.2009.175208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li WC, Sautois B, Roberts A, Soffe SR. Reconfiguration of a vertebrate motor network: Specific neuron recruitment and context-dependent synaptic plasticity. Journal of Neuroscience. 2007;27:12267–12276. doi: 10.1523/JNEUROSCI.3694-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sillar KT, Combes D, Ramanathan S, Molinari M, Simmers J. Neuromodulation and developmental plasticity in the locomotor system of anuran amphibians during metamorphosis. Brain Research Reviews. 2008;57:94–102. doi: 10.1016/j.brainresrev.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 52.Marsh-Armstrong N, Cai L, Brown DD. Thyroid hormone controls the development of connections between the spinal cord and limbs during Xenopus laevis metamorphosis. Proceedings of the National Academy of Sciences. 2004;101:165–170. doi: 10.1073/pnas.2136755100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang HY, Issberner J, Sillar KT. Development of a spinal locomotor rheostat. Proceedings of the National Academy of Sciences. 2011;108:11674–11679. doi: 10.1073/pnas.1018512108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zornik E, Kelley DB. A neuroendocrine basis for the hierarchical control of frog courtship vocalizations. Frontiers in neuroendocrinology. 2011;32:353–366. doi: 10.1016/j.yfrne.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Potter KA, Bose T, Yamaguchi A. Androgen-Induced vocal transformation in adult female African clawed frogs. Journal of Neurophysiology. 2005;94:415–428. doi: 10.1152/jn.01279.2004. [DOI] [PubMed] [Google Scholar]
- 56.Zornik E, Yamaguchi A. Vocal pathway degradation in gonadectomized Xenopus laevis adults. Journal of Neurophysiology. 2011;105:601–614. doi: 10.1152/jn.00883.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang EJ, Nasipak BT, Kelley DB. Direct action of gonadotropin in brain integrates behavioral and reproductive functions. Proceedings of the National Academy of Sciences. 2007;104:2477–2482. doi: 10.1073/pnas.0608391104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robertson J, Watson J, Kelley D. Androgen directs sexual differentiation of laryngeal innervation in developing Xenopus laevis. J Neurobiol. 1994;25:1625–1636. doi: 10.1002/neu.480251213. [DOI] [PubMed] [Google Scholar]
- 59.O’Connell LA, Hofmann HA. Evolution of a vertebrate social decision-making network. Science. 336:1154–1157. doi: 10.1126/science.1218889. The evolutionary underpinnings for social decision making were investigated in more than 80 species from 5 lineages of vertebrates. Regions implicated in this crucial function are highly conserved over 450my as are receptors for neuroendocrine signals. [DOI] [PubMed] [Google Scholar]


