Abstract
Objective
To evaluate the effectiveness of a telephonic medication therapy management (MTM) service on reducing hospitalizations among home health patients.
Setting
Forty randomly selected, geographically diverse home health care centers in the United States.
Design
Two-stage, randomized, controlled trial with 60-day follow-up. All Medicare- insured home health care patients were eligible to participate. Twenty-eight consecutive patients within each care center were recruited and randomized to usual care or MTM intervention. The MTM intervention consisted of the following: (1) initial phone call by a pharmacy technician to verify active medications; (2) pharmacist-provided medication regimen review by telephone; and (3) follow-up pharmacist phone calls at day seven and as needed for 30 days. The primary outcome was 60-day all-cause hospitalization.
Data Collection
Data were collected from in-home nursing assessments using the OASIS-C. Multivariate logistic regression modeled the effect of the MTM intervention on the probability of hospitalization while adjusting for patients’ baseline risk of hospitalization, number of medications taken daily, and other OASIS-C data elements.
Principal Findings
A total of 895 patients (intervention n = 415, control n = 480) were block-randomized to the intervention or usual care. There was no significant difference in the 60-day probability of hospitalization between the MTM intervention and control groups (Adjusted OR: 1.26, 95 percent CI: 0.89–1.77, p = .19). For patients within the lowest baseline risk quartile (n = 232), the intervention group was three times more likely to remain out of the hospital at 60 days (Adjusted OR: 3.79, 95 percent CI: 1.35–10.57, p = .01) compared to the usual care group.
Conclusions
This MTM intervention may not be effective for all home health patients; however, for those patients with the lowest-risk profile, the MTM intervention prevented patients from being hospitalized at 60 days.
Keywords: Clinical trial, medication therapy management, home health care, hospitalization, outcomes
As health care costs continue to escalate in the United States, many stakeholders have focused on identifying effective methods for enhancing quality of care while reducing unnecessary health care costs. The cost of medication-related morbidity and mortality in the United States was estimated to be $177.4 billion in 2000, with hospital admissions accounting for approximately 70 percent of those costs (Ernst and Grizzle 2001). In a study of 400 patients discharged from hospital to home, 66 percent of adverse events, including rehospitalization, were attributed to medication-related problems (Suter 2008). Interventions to improve medication-related problems are needed, particularly during the transition from hospital to home-based care.
Medication therapy management (MTM) is one method that has been identified to reduce medication-related problems (Giberson and Yoder 2011) and potentially hospital admissions (Holland et al. 2008), where, according to the definition endorsed by 11 national pharmacy organizations in the United States, MTM is a “distinct service or group of services that optimize therapeutic outcomes for individual patients” (Bluml 2005). MTM interventions consist of five core elements: (1) comprehensive or targeted medication therapy review; (2) construction of a personal medication record; (3) development of a medication-related action plan; (4) implementation of the action plan through intervention and referral; and (5) documentation and follow-up (American Pharmacists Association 2008).
Pharmacists are the most common provider of MTM services (Center for Medicare and Medicaid Services 2010). MTM promotes cooperation among pharmacists, patients, prescribers, and other health professionals to achieve optimal patient outcomes through appropriate medication use (Center for Medicare and Medicaid Services 2005). Although numerous positive examples of pharmacist-provided services exist (Giberson and Yoder 2011), recent investigations of pharmacist-provided MTM interventions have resulted in varied conclusions (Fox et al. 2003; Pindolia, Stebelsky, and Romain 2009; Welch, Chester, and Stubbings 2009; Ward and Xu 2011), and questions remain regarding the service model needed to optimize health outcomes across various populations receiving MTM (Cranor, Buntin, and Christensen 2003; Fera, Bluml, and Ellis 2003; Isetts, Schondelmeyer, and Artz 2008; Pindolia, Stebelsky, and Romain 2009; Planas, Crosby, and Mitchell 2009). Furthermore, although previous evaluations of telephonic MTM programs have produced some promising results, the studies are limited by the use of nonrandomized, observational designs (Bunting and Cranor 2006; Bunting, Smith, and Sutherland 2008; Ramalho de Oliveira, Brummel, and Miller 2010; Zillich et al. 2012), and/or process measures or surrogate outcomes as endpoints (Hirsch, Rosenquist, and Best 2009; Pindolia, Stebelsky, and Romain 2009; Welch, Chester, and Stubbings 2009; Michaels, Jenkins, and Pruss 2010; Moczygemba et al. 2011; Zillich et al. 2012).
In recent years, Medicare has recognized the value of MTM to reduce unnecessary hospital expenditures, as all Part D sponsors are required to offer this service to optimize medication use among targeted beneficiaries, including those with multiple chronic conditions, multiple prescription medications, and significant medication expenditures (Center for Medicare and Medicaid Services 2010).
This study targeted patients admitted to the Medicare home health care episode offered by home health care agencies (HHA). Medicare home health episode patients are provided a defined 60-day covered benefit for in-home skilled care intended to restore patients’ function. As the providers of in-home care, HHAs are graded for care quality based on hospital admission rates. With health care funding moving toward value-based payment models, HHAs expect to receive differentiated payments based on quality in the near future (Suter 2008). In addition, as postacute care providers, HHAs are indirectly affected by Medicare’s 30-day readmission rate penalties being absorbed by acute care hospitals.
At the intersection of the chronically ill and the Medicare home health care benefit, we completed a prospective, pragmatic, cluster-randomized, controlled trial to examine the effectiveness of a telephonic MTM service to decrease hospital admissions among patients receiving 60-day home health care services from a national HHA. This study fills a critical gap in understanding the potential for MTM to reduce unnecessary hospitalization. Currently, there are no randomized controlled studies of MTM’s impact to reduce the incidence of hospitalizations among home health care patients and there is a need for additional evaluations of MTM programs, particularly those delivered telephonically.
Methods
Design, Setting, and Participants
This was a two-stage, randomized, controlled, pragmatic trial. A two-step process occurred to select sites and participants. First, through a partnership with a national HHA (Amedisys, Inc., Baton Rouge, LA, USA), a simple random sample of 40 coordinating home health care centers with a monthly census of 20 or more newly admitted patients were selected as sites for the study among a nationwide population of 419 care centers. The sample of sites constituted a nationally representative sample of care centers from Amedisys based on their market and geographic locations. Second, within each care center, a block randomization (using blocks of seven, constrained for equal allocation of patients to usual care or the MTM intervention) allocated 28 consecutive newly admitted home health patients to either usual care or the MTM intervention. Enrollment began on February 6, 2012, and concluded on April 13, 2012.
For this pragmatic trial, all new patients within each care center who were admitted into Medicare’s defined 60-day home health care episode were eligible, including skilled nursing care and “therapy only” patients (i.e., those receiving physical/occupational therapy services only). Medicare eligibility for home health benefits requires ordering services by a physician who reviews the need for a patient’s care and certifies that the patient is homebound (e.g., leaving the home is not generally recommended because of illness or requires assistance from devices or other parties). Patients in the study were admitted into the home health care episode from acute care hospitals or the community. Patients with a reoccurring episode of care within the past 12 months were excluded. Home health care consists of in-home episodic skilled nursing care and related physical/occupational/speech therapy as needed for homebound patients due to illness or injury. Nursing care begins with an in-home admission medical assessment and progresses based on the individual needs of the patient. The nurses complete a medication history; prepare and administer medications; monitor the effectiveness of treatment; report adverse reactions; and teach patients about their medication.
Intervention
The telephonic MTM intervention program was offered in addition to usual home health care. Upon completion of the home health nurse’s (who was blinded to the assignment of the patient) admission assessment on day one of the home health care episode, the patient’s current medication information was faxed to the MTM intervention provider (HealthStat Rx). Following a pre-MTM call by a pharmacy technician to verify medication information, the intervention aligned with the core elements of MTM (American Pharmacists Association 2008) and began with an initial telephone call to the patient and/or caregiver from a trained pharmacist. During this telephone call, the pharmacist completed a comprehensive medication therapy review to identify any medication-related problems, constructed a written personal medication record for the patient and providers, and developed a medication-related action plan. The action plan served as a patient-centered document to assist the patient and pharmacist in the resolution of identified medication-related problems. The duration of the initial pharmacist telephone call with the patient was approximately 30 minutes. The pharmacist also spent 15 minutes reviewing patient information prior to the call and 15 minutes after the call to complete documentation pertaining to the encounter. For all patients, pharmacists provided a follow-up telephone call on day seven to continue resolving medication-related problems according to the medication action plan and to identify any new medication-related problems. Additional telephone follow-up was provided as needed during the first 30 days of the 60-day home health care episode. The duration of each follow-up encounter was approximately 20 minutes.
Data Collection and Outcome Measures
All patients’ data were collected from two sources: (1) the HHA in-home nursing medical assessments via the Outcome and Assessment Information Set (OASIS-C); and (2) the MTM provider documentation system. The OASIS-C is a required standard set of nursing assessment items used by all Medicare-certified home health agencies and utilized for most home health patients, including all Medicare patients. For this study, OASIS-C data were used for determining the primary outcome, for demographic information, and for risk adjustment. The risk adjustment used in this study is the risk of acute hospitalization as applied by CMS for risk adjustment of home health patients (Nuccio, Richard, and Hittle 2011). The risk-adjusted models present outcomes for home health agency quality reports, including those publicly reported on Home Health Compare (Center for Medicare and Medicaid Services 2012a,b,c). For acute care hospitalization (ACH), there are 99 risk factors from OASIS-C data, which predict hospitalization in this population (R2 = 0.14, C-stat = 0.74). Process-related data on patients in the intervention group, including the number, type, and status of pharmacist-identified drug-related problems, were collected from the MTM documentation system.
The primary outcome was patient-level, 60-day, all-cause hospitalization. This outcome was chosen based on the requirement to report ACH rates for home health agencies to CMS for quality ratings (Center for Medicare and Medicaid Services 2012a,b,c). Secondary outcomes included 30-day all-cause hospitalization and time to first all-cause hospitalization. Planned post-hoc analyses included quartiles of patients based on their CMS risk score (Nuccio, Richard, and Hittle 2011) and subgroups of patients admitted to home health from a hospital setting as well as those with unplanned hospitalization at 60 days. Additional process-related data were collected about the provision of the intervention, such as the timing of delivery of the MTM program and the number and types of medication-related problems identified and resolved by pharmacists.
Recruitment, Randomization, and Analyses
Patients were informed of the study by their home health nurse during the initial (day one) OASIS-C in-home assessment. Patients who opted in to the study were randomized into usual care or MTM intervention at each care center by a business manager using a randomization list generated by study investigators. Nurses were blinded to the patients’ potential assignment to prevent bias during the nurses’ initial in-home assessment. All patients were followed up for 60 days from the start of their home health care episode, defined as the date of the OASIS-C initial assessment. The study was approved by the Purdue University Institutional Review Board.
Sample size estimates were based on detecting a moderate effect size (d = 0.2). Given that the historical data in this home health population showed that the care centers demonstrated significant intracluster variation, the sample size was computed using a low intracluster correlation coefficient. Using an estimated 60-day hospitalization rate of 35 percent, the likelihood of detecting a statistically significant difference in events attributable to the intervention, within 5 percent of the true population effect, is 90 percent, with a one-tailed 5 percent level of significance when there are 14 patients in the intervention cohort (and 14 in the usual care cohort) at each of 40 randomly selected patient care centers (n = 28 patients per site), for a total of 1,120 patients in the study.
A multivariate logistic regression model was fit using generalized estimating equations (GEEs) to examine the probability of the 60-day all-cause hospitalization, controlling for patient variables and clustering effects of care center. Adjustment variables included the CMS risk score for hospitalization (Nuccio, Richard, and Hittle 2011), patient age, total number of medications, ability to use a telephone, and detection of medication-related problems during the nurse’s initial in-home assessment. A similar GEE model was used to examine the secondary outcome of 30-day all-cause hospitalization. Additionally, a multivariate Cox proportional hazard model was fit to estimate the effect of the intervention on time to first hospitalization.
Results
A total of 961 patients from a targeted goal of 1,120 patients were randomized (Figure 1). Enrollment was stopped prior to attainment of the sample size goal due to operational and budgetary constraints. Twenty-three of the 40 care centers reached their enrollment goals. There were 59 patients in the intervention group who could not be reached by telephone for the MTM call, resulting in 895 patients who were included in the analyses (n = 415 intervention and n = 480 control, Figure 1). Of the cohort, 568 (64 percent) were admitted to home health directly from an acute care hospital, while 327 (36 percent) were admitted to home health from the community. There were no significant differences in the characteristics of patients at baseline between the intervention and control groups (Table 1). In general, patients in this study were 73 (±13) years old and taking 14 (±9) total medications, with 1.4 comorbid conditions.
Figure 1.
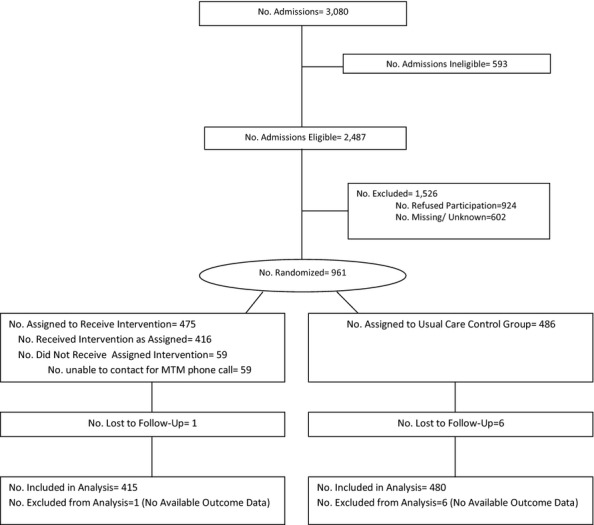
Flow Diagram of Study Participants
Table 1.
Demographic Characteristics of the Intervention and Usual Care Control Groups*
| Intervention (n = 415) | Usual Care (n = 480) | |
|---|---|---|
| Age (years), mean ± SD | 73 ± 13 | 73 ± 13 |
| Gender (female), no. (%) | 241 (58) | 296 (62) |
| Race, no. (%) | ||
| White | 315 (76) | 357 (74) |
| Black | 89 (21) | 108 (23) |
| Hispanic | 9 (2) | 10 (2) |
| Other | 2 (1) | 5 (1) |
| Admission source, no. (%) | ||
| Inpatient acute care | 184 (44) | 165 (34) |
| Rehabilitation/long-term care | 97 (23) | 125 (26) |
| Community | 131 (32) | 176 (37) |
| Other/unknown | 3 (1) | 14 (3) |
| Admission diagnosis: joint replacement aftercare, no. (%) | 183 (44) | 211 (44) |
| Admission diagnosis: Chronic disease management, no. (%) | 95 (23) | 115 (24) |
| Total medications, mean ± SD | 14 ± 11 | 13 ± 8 |
| Comorbid conditions | ||
| No. comorbidities,† mean ± SD | 1.4 ± 1.1 | 1.4 ± 1.0 |
| Heart failure, no. (%) | 54 (13) | 61 (13) |
| Chronic obstructive pulmonary disease, no. (%) | 75 (18) | 77 (16) |
| Hypertension, no. (%) | 91 (22) | 102 (21) |
| Diabetes mellitus, no. (%) | 72 (17) | 84 (18) |
There are no significant differences in the demographic data.
Calculated from the Elixhauser comorbidity index (Exilhauser, Steiner, and Harris 1998).
Overall, the MTM intervention did not significantly reduce 60-day, all-cause hospitalization compared to the usual care group (Adjusted OR: 1.26, 95 percent CI: 0.89–1.77, p = .19; Table 2). For patients in the quartile with lowest risk (based on the CMS risk score of hospitalization), the MTM intervention resulted in three times less hospitalizations at 60 days (Adjusted OR: 3.78, 95 percent CI: 1.35–10.57, p = .01).
Table 2.
All-Cause Hospitalization Outcomes for the Medication Therapy Management Intervention Compared to the Usual Care Control
| Study Population, No. | Patients Hospitalized, No. (%) | ||||||
|---|---|---|---|---|---|---|---|
| Overall | Intervention | Usual Care | Intervention | Usual Care | Unadjusted p-value | Adjusted Odds Ratio (95%) CI | Adjusted p-value |
| 60-day hospitalization | |||||||
| All participants, n = 895 | n = 415 | n = 480 | 83 (20) | 112 (23) | .26 | 1.26 (0.89, 1.77)* | .19 |
| Risk quartile 1,† n = 232 | n = 98 | n = 134 | 5 (5) | 22 (16) | .006 | 3.78 (1.35, 10.57)‡ | .01 |
| Risk quartile 2,† n = 221 | n = 103 | n = 118 | 14 (14) | 27 (23) | .06 | 2.01 (0.78, 5.15)‡ | .15 |
| Risk quartile 3,† n = 220 | n = 118 | n = 102 | 28 (24) | 18 (18) | .34 | 0.66 (0.34, 1.27)‡ | .21 |
| Risk quartile 4,† n = 222 | n = 96 | n = 126 | 36 (38) | 45 (36) | .81 | 0.85 (0.53, 1.39)‡ | .52 |
| 30-day hospitalization | |||||||
| All participants, n = 895 | n = 415 | n = 480 | 58 (14) | 83 (17) | .20 | 1.22 (0.84, 1.78)* | .30 |
| Risk quartile 1,† n = 232 | n = 98 | n = 134 | 2 (2) | 17 (13) | <.0001 | 6.82 (1.53, 30.42)‡ | .01 |
| Risk quartile 2,† n = 221 | n = 103 | n = 118 | 12 (12) | 19 (16) | .07 | 1.22 (0.47, 3.22)‡ | .68 |
| Risk quartile 3,† n = 220 | n = 118 | n = 102 | 15 (13) | 15 (15) | .16 | 0.94 (0.46, 1.95)‡ | .87 |
| Risk quartile 4,† n = 222 | n = 96 | n = 126 | 29 (30) | 32 (25) | .16 | 0.69 (0.41, 1.16)‡ | .16 |
Adjusted for risk of hospitalization based on the CMS risk score (Nuccio, Richard, and Hittle 2011) total number of medications, age, ability to use phone, and detection of medication-related problems during the nurse’s initial in-home assessment.
Risk quartiles reflect each group of participants based on their baseline risk of hospitalization from the CMS risk score (Nuccio, Richard, and Hittle 2011).
Adjusted for total number of medications, age, ability to use phone, and detection of medication problems during the nurse’s initial in-home assessment.
Similarly, among all study patients, 30-day all-cause hospitalization was not significantly different in the intervention group compared to the usual care group (Adjusted OR: 1.22, 95 percent CI: 0.84–1.78, p = .30; Table 2); while the MTM group was six times less likely to be hospitalized among patients in the quartile with the lowest risk (Adjusted OR: 6.82, 95 percent CI: 1.53–30.42, p = .01).
For all patients, time to first hospitalization was not significantly different in the MTM group than the usual care group (Adjusted HR: 0.80, 95 percent CI: 0.60–1.06, p = .12, Figure 2), but for patients in the quartile with the lowest risk, time to hospitalization was longer (Adjusted HR: 0.30, 95 percent CI: 0.11–0.81, p = .02, Figure 2).
Figure 2.
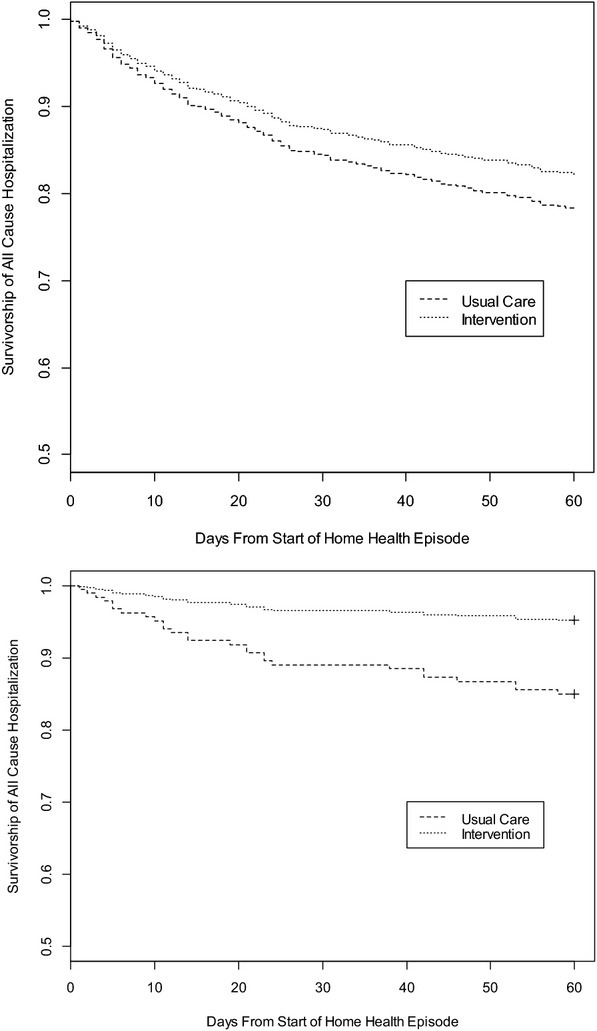
All-Cause Sixty-Day Survivorship to Time of First HospitalizationAll Participants: Usual Care and Intervention (n = 895).a Participants in Risk Quartile 1: Usual Care and Intervention (n = 232)b Note. aAdjusted HR is 0.80, 95 percent CI: 0.60–1.06, p-value = .12. Adjusted for risk of hospitalization based on the CMS risk score (Nuccio, Richard, and Hittle 2011), total number of medications, age, ability to use phone, and detection of medication-related problems during the nurses’ initial in-home assessment. bAdjusted HR: 0.30, 95 percent CI: 0.11–0.81, p-value = 0.02. Adjusted for total number of medications, age, ability to use phone, and detection of medication-related problems during the nurses’ initial in-home assessment.
The 60-day hospitalization rates for patients admitted to home health from the hospital and from the community were not significantly different in the MTM group compared to the usual care group (Hospital; Adjusted OR: 1.21, 95 percent CI: 0.81–1.81, p = .34: Community; Adjusted OR: 1.45, 95 percent CI: 0.79–2.68, p = .23).
For the subgroup of all patients with unplanned hospitalization at 60 days, the MTM intervention was not effective compared to the control group (Adjusted OR: 1.25, 95 percent CI: 0.88–1.76, p = .21).
In the intervention group, there were 892 total MTM phone calls with all 415 patients receiving the initial call and 359 patients receiving the follow-up call at day seven. The median time for the patient to receive the first MTM phone call was three days (IQR 1–4 days; range 0–21 days) after their home health admission. However, there were 59 patients who could not be reached by telephone for the initial MTM encounter (excluded from analyses) and another 15 patients who were not reached for the initial MTM encounter before being admitted to hospital (included in the analyses). During the MTM calls, 460 medication-related problems were identified by the pharmacists, with 24 percent (n = 109) requiring involvement of a physician to be resolved. The medication-related problems included issues related to untreated conditions, inappropriate drugs and/or dosages for a given condition, drug–drug interactions, and medication adherence (Table 3). At the end of the study, 414 (90 percent of all) problems were resolved, while 22 (5 percent) of the problems were not resolved because the prescriber did not accept the pharmacists’ recommendations and the remaining 5 percent of unresolved problems were related to patient factors. There was no difference in the number or type of medication-related problems based on the patients’ risk quartile.
Table 3.
Medication-Related Problems Identified by Pharmacists in the Intervention Group (n = 415)
| Problem Classification Grouped by Drug-Related Needs | Number Identified (%) | |
|---|---|---|
| Total medication-related problems | 460 | |
| Indication | Needs additional therapy* | 58 (13) |
| Unnecessary drug therapy† | 32 (7) | |
| Effectiveness | Dosage too low‡ | 15 (3) |
| Safety | Adverse drug reaction§ | 208 (45) |
| Dosage too high¶ | 16 (3) | |
| Dosage too low/high** | 59 (13) | |
| Adherence | Noncompliance†† | 72 (16) |
Untreated condition, synergistic therapy, preventative therapy.
No medical indication, recreational drug, nondrug therapy, duplicate, treating avoidable adverse reaction.
Ineffective, inappropriate frequency, duration, storage, administration.
Adverse drug reaction, patient complaint/symptom, drug allergy alert, drug-drug interaction, drug-food interaction, drug-age precaution, side effect.
Dose too high, frequency too short, duration too long.
Excessive quantity, missing information clarification.
Not available, cannot afford, cannot administer, forgets, does not understand, prefers not to take.
Discussion
To our knowledge, this is the first published study examining the relationship between an MTM intervention for Medicare patients newly admitted to home health care. As Medicare’s spending continues to grow at rates above inflation, a wide net has been cast to identify strategies to reduce the likelihood of patients being admitted and readmitted to the hospital (Center for Medicare and Medicaid Services 2012a,b,c). The introduction of risk-based funding models, such as Accountable Care Organizations (ACOs) and bundled payment fee structures, has focused health care enterprises on finding cost-saving interventions (Center for Medicare and Medicaid Services 2012a,b,c). Furthermore, Medicare Part D has promulgated MTM as a covered benefit for elderly patients at high risk for drug therapy problems, adverse drug events, and hospitalization, despite limited evidence to demonstrate its effectiveness in the entire Medicare-insured population (Smith and Clancy 2006).
This study provides evidence that this MTM intervention did not reduce the likelihood of hospitalization in the overall sample of home health patients. However, the study identified that the MTM intervention was effective at reducing the probability of hospitalization in the lowest-risk home health patients. That is, for home health patients who have the highest function and least disability, the MTM intervention is effective at reducing the risk of hospitalization. Possibly, the effectiveness of the MTM intervention is limited to those patients with the health care capacity to take and manage medications relatively independently. This telephonic MTM program may have helped facilitate those patients’ ability to appropriately manage medication. In contrast, for those patients who require more intensive assistance to take medication, this telephonic MTM program may not be sufficient to overcome the needs of these patients. Potentially a more robust, in-person or telehealth solution may be needed to accommodate the advanced needs of these patients.
These findings can be compared with several recently published studies evaluating MTM programs in somewhat similar patient populations. Among ambulatory, Medicaid-eligible patients, who were generally younger but who had more chronic disease than the patients in this study, a similar MTM intervention did not significantly reduce hospitalizations (Zillich et al. 2012). Likewise, in an ambulatory Medicare population with chronic disease, a face-to-face MTM intervention that was similar to the telephonic MTM intervention in the current study found a trend toward fewer hospitalizations, although the study was not powered to detect this outcome (Touchette et al. 2012). Our findings in home health care demonstrate that widespread implementation of MTM for all new home health patients may not be effective; but the intervention was effective at lowering hospitalizations for the lowest-risk patients.
For MTM interventions to be successful, policy and clinical decision makers should consider several implementation issues. First, the home health care service and the MTM service must be coordinated. In this study, sharing of home health care medical records, MTM records, pharmacist recommendations from the medication-related action plan, and other clinical data from the patients’ physicians were achieved through handwritten documents, mailed materials, and facsimile. A better implementation approach would integrate medical and MTM information using a shared electronic medical record. However, despite the challenges of sharing medical records in this study, a majority of the medication-related problems identified by the pharmacists were resolved, including most of those that required participation of the patient’s physician. Nevertheless, the timeliness to resolve these medication-related problems could likely be expedited through a shared electronic record.
Second, given that there is required reporting of 60-day ACH rates, identification of eligible low-risk patients and subsequent provision of the initial MTM encounter must occur as soon as possible upon initiation of the home health episode. In addition, the transitions of care process from the community and inpatient setting to home health creates the potential for medication-related problems to occur (Hume 2012). As a result, a system or process to coordinate care by rapidly assessing patients’ risk, identifying eligible patients for MTM, and providing timely MTM services would ensure successful implementation. In the current study, the median time for patients to receive the first MTM encounter was 3 days after their home health admission which may have not been rapid enough for patients at higher risk levels. Operational challenges of the study related to site training and policies and procedures slowed the time for recruitment and enrollment, increasing the time to the initial MTM encounter. In a few cases (n = 15), patients were already admitted to a hospital by the time the pharmacist reached the patient via telephone for the initial MTM encounter.
Finally, the costs associated with providing the MTM service in this study were paid by the home health agency. Yet any decrease in hospitalization and the associated cost-savings would be realized by third-party insurers. CMS and other policy makers should consider cost-sharing and risk-based payment models for MTM services in this setting, potentially through the newly created ACOs and the bundled payment demonstration projects (Center for Medicare and Medicaid Services 2012a,b,c). Future studies must be conducted to determine the overall cost-effectiveness of this MTM service and develop business models to ensure successful implementation. Additionally, studies to determine the case mix of patients who would benefit most from MTM services are needed.
The findings from this study should be considered with some limitations. First, the study did not achieve its recruitment goals due to slower than expected enrollment, combined with operational challenges at some sites. Given the power calculation’s assumed effect size and intraclass correlation were similar to levels observed in the study data, it is possible that the study did not attain sufficient statistical power to detect effects for the overall population. Home health nurses at each site delivered usual medical care, but they also were asked to recruit and enroll patients who could opt out of the study. We believe our enrollment procedures balanced the ethical need to inform patients about the study with the pragmatic need to deliver home health care services and evaluate a new program. This process represents the “real-world” challenges that would be encountered during implementation of this program, but the findings are limited to those patients who could be contacted for the MTM intervention.
The results demonstrated that low-risk, newly admitted, Medicare home health care patients who received MTM were three times less likely to be hospitalized within 60 days. In our study, we applied the CMS risk score for each patient based on patients’ OASIS-C data to determine their baseline risk of hospitalization. While applied by CMS, the CMS risk score is not based on our study population and our study outcome; therefore, it may not be the most accurate risk adjustment. Nevertheless, the CMS risk stratification algorithm is used to risk adjust ACH rates presented in home health agency quality reports, including those publicly reported using OASIS-C data for all Medicare patients.
Conclusion
This MTM intervention may not be effective for all home health patients; however, for those patients with the lowest-risk profile, the MTM intervention prevented patients from being hospitalized at 60 days.
Acknowledgments
Joint Acknowledgement/Disclosure Statement: Funding for this study was provided by Amedisys, Inc. A portion of AJZ’s time was supported by a Career Development award from the Department of Veterans Affairs, Health Services Research and Development (RCD 06-304-1). JMS is a Scholar of the Michael Smith Foundation for Health Research. MES was supported in part by a Young Investigator award (KL2RR025760) from the Indiana Clinical and Translational Sciences Institute. JLL is a CMS Innovation Advisor and is partially supported by that program.
Disclosures: Authors AJZ, MES, CKF, HJ, and JMS received funding for this study from Amedisys, Inc. Authors JLL and DD are employees of Amedisys, Inc., who provided funding for this study. JLL is Vice President of Research and Development and provided input on the study design, support for the operational logistics of the study, and contributed to the drafting of the manuscript. DD is Director of Analytics and provided the data extraction of OASIS variables for all study participants and contributed to drafting of the manuscript. Author PD is President and CEO of HealthStat Rx. His company’s pharmacists provided the MTM intervention and he contributed to the extraction of the MTM data and assisted in drafting the manuscript.
Disclaimers: None.
Previous Publications: None of the material in this manuscript has been previously published, and none of this material is under consideration or has been accepted for publication elsewhere. However, this work was presented as a podium in abstract form for the 2013 AcademyHealth Annual Meeting, June 23, in Baltimore, Maryland. The work was also presented in abstract form for an encore poster presentation at the 2013 American College of Clinical Pharmacy Annual Meeting on October 14, in Albuquerque, New Mexico.
Supporting Information
Additional supporting information may be found in the online version of this article:
Appendix SA1: Author Matrix.
References
- American Pharmacists Association. Medication Therapy Management in Pharmacy Practice: Core Elements of an MTM Service Model (Version 2.0) Journal of the American Pharmacists Association. 2008;48(3):341–53. doi: 10.1331/JAPhA.2008.08514. [DOI] [PubMed] [Google Scholar]
- Bluml BM. Definition of Medication Therapy Management: Development of Professionwide Consensus. Journal of the American Pharmacists Association. 2005;45(5):566–72. doi: 10.1331/1544345055001274. [DOI] [PubMed] [Google Scholar]
- Bunting BA. Cranor CW. The Asheville Project: Long-Term Clinical, Humanistic, and Economic Outcomes of a Community-Based Medication Therapy Management Program for Asthma. Journal of the American Pharmacists Association. 2006;46(2):133–47. doi: 10.1331/154434506776180658. [DOI] [PubMed] [Google Scholar]
- Bunting BA, Smith BH. Sutherland SE. The Asheville Project: Clinical and Economic Outcomes of a Community-Based Long-Term Medication Therapy Management Program for Hypertension and Dyslipidemia. Journal of the American Pharmacists Association. 2008;48(1):23–31. doi: 10.1331/JAPhA.2008.07140. [DOI] [PubMed] [Google Scholar]
- Center for Medicare and Medicaid Services. Department of Health and Human Services. Medicare Prescription Drug Benefit Final Rule. Federal Register. 2005;70(18):4194–585. [PubMed] [Google Scholar]
- Center for Medicare and Medicaid Services. 2010. “Medication Therapy Management (MTM) Fact Sheet” [accessed on June 5, 2012]. Available at https://www.cms.gov/PrescriptionDrugCovContra/Downloads/MTMFactSheet_2010_06-2010_final.pdf.
- Center for Medicare and Medicaid Services. 2012a. “Bundled Payments for Care Improvement” [accessed on December 5, 2012]. Available at http://innovations.cms.gov/initiatives/Bundled-Payments/index.html.
- Center for Medicare and Medicaid Services. 2012b. “Home Health Compare” [accessed on December 5, 2012]. Available at http://medicare.gov/homehealthcompare/search.aspx?AspxAutoDetectCookieSupport=1.
- Center for Medicare and Medicaid Services. 2012c. “Key CMMI Initiatives and Demonstrations” [accessed on December 5, 2012]. Available at http://www.nationalpartnership.org/site/DocServer/Key_CCMI_Initiatives_and_Demonstrations.pdf?docID=8082.
- Cranor CW, Buntin BA. Christensen DB. The Asheville Project: Long-Term Clinical and Economic Outcomes of a Community Pharmacy Diabetes Care Program. Journal of the American Pharmacists Association. 2003;43:173–84. doi: 10.1331/108658003321480713. [DOI] [PubMed] [Google Scholar]
- Ernst FR. Grizzle AJ. Drug-Related Morbidity and Mortality: Updating the Cost-of-Illness Model. Journal of the American Pharmacists Association. 2001;41(2):192–9. doi: 10.1016/s1086-5802(16)31229-3. [DOI] [PubMed] [Google Scholar]
- Exilhauser A, Steiner C. Harris DR. Comorbidity Measures for Use with Administrative Data. Medical Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- Fera T, Bluml BM. Ellis WM. Diabetes Ten City Challenge: Final Economic and Clinical Results. Journal of the American Pharmacists Association. 2003;49(3):383–91. doi: 10.1331/JAPhA.2009.09015. [DOI] [PubMed] [Google Scholar]
- Fox D, Ried LD, Klein GE, Myers W. Foli K. A Medication Therapy Management Program’s Impact on Low-Density Lipoprotein Cholesterol Goal Attainment in Medicare Part D Patients with Diabetes. Journal of the American Pharmacists Association. 2003;49(2):192–9. doi: 10.1331/JAPhA.2009.09016. [DOI] [PubMed] [Google Scholar]
- Giberson S. Yoder SL. Improving Patient and Health System Outcomes through Advanced Pharmacy Practice: A Report to the U.S. Surgeon General. Washington, DC: Office of the Chief Pharmacist, U.S. Public Health Service; 2011. [Google Scholar]
- Hirsch JD, Rosenquist A. Best BM. Evaluation of the First Year of a Pilot Program in Community Pharmacy: HIV/AIDS Medication Therapy Management for Medi-Cal Beneficiaries. Journal of Managed Care Pharmacy. 2009;15:32–41. doi: 10.18553/jmcp.2009.15.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland R, Desborough J, Goodyer L, Hall S, Wright D. Loke YK. Does Pharmacist-Led Medication Review Help to Reduce Hospital Admissions and Deaths in Older People? A Systematic Review and Meta-Analysis. British Journal of Clinical Pharmacology. 2008;65(3):303–16. doi: 10.1111/j.1365-2125.2007.03071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume AL. Improving Care Transitions: Current Practice and Future Opportunities for Pharmacists. Pharmacotherapy. 2012;32(11):e326–37. doi: 10.1002/phar.1215. [DOI] [PubMed] [Google Scholar]
- Isetts BJ, Schondelmeyer SW. Artz MB. Clinical and Economic Outcomes of Medication Therapy Management Services: The Minnesota Experience. Journal of the American Pharmacists Association. 2008;48:203–11. doi: 10.1331/JAPhA.2008.07108. [DOI] [PubMed] [Google Scholar]
- Michaels NM, Jenkins GF. Pruss DL. Retrospective Analysis of Community Pharmacists’ Recommendations in the North Carolina Medicaid Medication Therapy Management Program. Journal of the American Pharmacists Association. 2010;50:347–53. doi: 10.1331/JAPhA.2010.09021. [DOI] [PubMed] [Google Scholar]
- Moczygemba LR, Barner JC, Lawson KA, Brown CM, Gabrillo ER, Godley P. Johnsrud M. Impact of Telephone Medication Therapy Management on Medication and Health-Related Problems, Medication Adherence, and Medicare Part D Drug Costs: A 6-Month Follow Up. American Journal of Geriatric Pharmacotherapy. 2011;9(5):328–38. doi: 10.1016/j.amjopharm.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Nuccio EJ, Richard AA. Hittle DF. 2011. “Home Health Agency Quality Measures: Logistic Regression Models for Risk Adjustment” [accessed on December 5, 2012]. Available at http://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HomeHealthQualityInits/Downloads/HHQILogisticRegressionModelsforRiskAdjustment.pdf.
- Pindolia VK, Stebelsky L. Romain TM. Mitigation of Medication Mishaps Via Medication Therapy Management. Annals of Pharmacotherapy. 2009;43:611–20. doi: 10.1345/aph.1L591. [DOI] [PubMed] [Google Scholar]
- Planas LG, Crosby KM. Mitchell KD. Evaluation of a Hypertension Medication Therapy Management Program in Patients with Diabetes. Journal of the American Pharmacists Association. 2009;49:164–70. doi: 10.1331/JAPhA.2009.08164. [DOI] [PubMed] [Google Scholar]
- Ramalho de Oliveira D, Brummel AR. Miller DB. Medication Therapy Management: 10 Years of Experience in a Large Integrated Health Care System. Journal of Managed Care Pharmacy. 2010;16(3):185–95. doi: 10.18553/jmcp.2010.16.3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SR. Clancy CM. Medication Therapy Management Programs: Forming a New Cornerstone for Quality and Safety in Medicare. American Journal of Medical Quality. 2006;21:276–9. doi: 10.1177/1062860606290031. [DOI] [PubMed] [Google Scholar]
- Suter P. Home Care Agencies Take Note: The Herald of CMS ‘Never Events.’. Home Healthcare Nurse. 2008;26(10):647–8. doi: 10.1097/01.NHH.0000341231.07467.c5. [DOI] [PubMed] [Google Scholar]
- Touchette DR, Masica AL, Dolor RJ, Schumock GT, Choi YK, Kim Y. Smith SR. Safety-Focused Medication Therapy Management: A Randomized Controlled Trial. Journal of the American Pharmacists Association. 2012;52(5):603–12. doi: 10.1331/JAPhA.2012.12036. [DOI] [PubMed] [Google Scholar]
- Ward MA. Xu Y. Pharmacist-Provided Telephonic Medication Therapy Management in an MAPD Plan. The American Journal of Managed Care. 2011;17(10):e399–409. [PubMed] [Google Scholar]
- Welch EKD, Chester EA. Stubbings T. Assessment of the Impact of Medication Therapy Management Delivered to Home-Based Medicare Beneficiaries. Annals of Pharmacotherapy. 2009;43(4):603–10. doi: 10.1345/aph.1L524. [DOI] [PubMed] [Google Scholar]
- Zillich AJ, Jaynes HA, Snyder ME, Harrison J, Hudmon KS, de Moor C. French DD. Evaluation of Specialized Medication Packaging Combined with Medication Therapy Management: Adherence, Outcomes, and Costs among Medicaid Patients. Medical Care. 2012;50(6):485–93. doi: 10.1097/MLR.0b013e3182549d48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix SA1: Author Matrix.


