Abstract
Gastric cancer surgical management differs between Eastern Asia and Western countries. Extended lymphadenectomy (D2) is the standard of care in Japan and South Korea since decades, while the majority of United States patients receive at most a limited lymphadenectomy (D1). United States and Northern Europe are considered the scientific leaders in medicine and evidence-based procedures are the cornerstone of their clinical practice. However, surgeons in Eastern Asia are more experienced, as there are more new cases of gastric cancer in Japan (107898 in 2012) than in the entire European Union (81592), or in South Korea (31269) than in the entire United States (21155). For quite a long time evidence-based medicine (EBM) did not solve the question whether D2 improves long-term prognosis with respect to D1. Indeed, eastern surgeons were reluctant to perform D1 even in the frame of a clinical trial, as their patients had a very good prognosis after D2. Evidence-based surgical indications provided by Western trials were questioned, as surgical procedures could not be properly standardized. In the present study we analyzed indications about the optimal extension of lymphadenectomy in gastric cancer according to current scientific literature (2008-2012) and surgical guidelines. We searched PubMed for papers using the key words “lymphadenectomy or D1 or D2” AND “gastric cancer” from 2008 to 2012. Moreover, we reviewed national guidelines for gastric cancer management. The support to D2 lymphadenectomy increased progressively from 2008 to 2012: since 2010 papers supporting D2 have achieved a higher overall impact factor than the other papers. Till 2011, D2 was the procedure of choice according to experts’ opinion, while three meta-analyses found no survival advantage after D2 with respect to D1. In 2012-2013, however, two meta-analyses reported that D2 improves prognosis with respect to D1. D2 lymphadenectomy was proposed as the standard of care for advanced gastric cancer by Japanese National Guidelines since 1981 and was adopted as the standard procedure by the Italian Research Group for Gastric Cancer since the Nineties. D2 is now indicated as the standard of surgical treatment with curative intent by the German, British and ESMO-ESSO-ESTRO guidelines. At variance American NCCN guidelines recommend a D1+ or a modified D2 lymph node dissection. In conclusion, D2 lymphadenectomy, originally developed by Eastern surgeons, is now becoming the procedure of choice also in the West. In gastric cancer surgery EBM is lagging behind national guidelines, rather than preceding and orienting them. To eliminate this lag, EBM should value to a larger extent Eastern Asian literature and should evaluate not only the quality of the study design but also the quality of surgical procedures.
Keywords: Gastric cancer, Surgical quality, Lymphadenectomy, Evidence-based medicine, National guidelines, Eastern Asia, United States
Core tip: The extension of lymphadenectomy in advanced gastric cancer has been debated for several decades. Till recently Western surgeons supported limited lymphadenectomy in agreement with a Cochrane review and several meta-analyses, while Japanese surgeons preferred the extended procedure. Nowadays extended lymphadenectomy is considered the procedure of choice by most national guidelines. In gastric cancer surgery evidence-based medicine (EBM) is lagging behind national guidelines, rather than preceding and orienting them. To eliminate this lag, EBM should value to a larger extent Eastern Asian literature and should evaluate not only the quality of the study design but also the quality of surgical procedures.
HISTORICAL PERSPECTIVE
Eastern-Western discrepancies in gastric cancer surgery
Gastric cancer surgery differs between Eastern Asia and the United States. Extended lymphadenectomy (D2) has been the standard of care for advanced gastric cancer in Japan and South Korea since decades[1-3], while the majority of patients in the United States receive at most a limited lymphadenectomy (D1) followed by post-operative chemoradiation[4]. This difference is even more striking, if one considers that Japanese surgeons had extended lymphadenectomy to para-aortic nodes (D3 procedure), until a randomized controlled trial showed no survival advantage with respect to D2 procedure[5]. On the contrary, in the United States less than a D1 lymphadenectomy has been performed in a substantial proportion, or even in the majority of patients such as in the Intergroup 0116 trial[6].
Western countries are the leaders in medical research
United States and Northern European countries are considered the scientific leaders in medicine: indeed the most important medical journals are published in the United States [New Engl J Med, impact factor (IF) in 2012 = 51.658; Science, IF = 31.027] or in England (Lancet, IF = 39.060; Nature, IF = 38.597).
Evidence-based procedures are the cornerstone of clinical practice in Western countries. Anyway, due to the difficulty in standardizing surgical procedures, evidence-based surgical indications may not be unquestionable. The extent of lymphadenectomy in surgical management of gastric cancer proves this point.
Indeed the Western surgical approach to advanced gastric cancer was supported by a Cochrane review published in 2003[7] and 2005[8], reporting that ‘‘randomised studies show no evidence of overall survival benefit’’ after D2 dissection, ‘‘but possible benefit in T3 tumors.” These conclusions were mainly based on the results of a Dutch[9] and a British[10] trials, showing that D2 provided no 5-year survival advantage with respect to D1. Of note, the Cochrane review acknowledged that “these results may be confounded by surgical learning curves and poor surgeon compliance”[8].
However it was acknowledged that D2 lymphadenectomy was necessary to harvest at least 15 lymph nodes, i.e., to adequately stage tumours[11,12]. To circumvent this problem, nodal invasion was evaluated not only by N status but also by N ratio[13,14].
While eastern countries have the largest surgical experience
On the other hand, surgeons in Eastern Asia are more experienced, as there are more new cases of gastric cancer in Japan (107898 in 2012) than in the 28 countries of the European Union (81592), or in South Korea (31269) than in the entire United States (21155)[15] (Figure 1).
Figure 1.
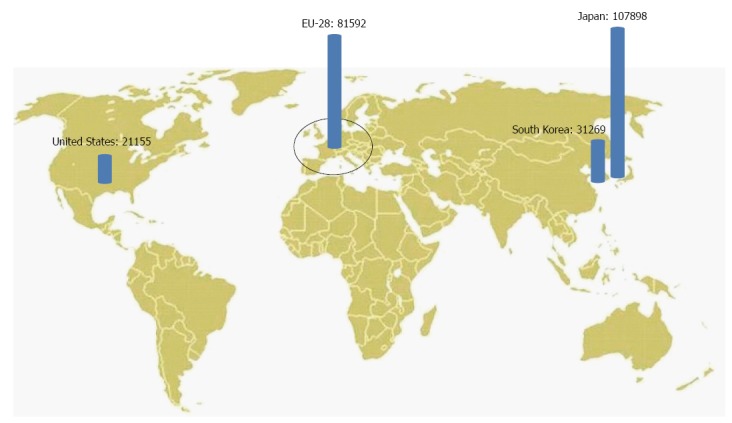
New cases of gastric cancer in 2012 in the West and in the East, according to GLOBOCAN 2012[15].
Indeed Eastern surgeons achieve better short-term results than their Western counterparts (Table 1)[16]. Of note, the two trials on which the Cochrane review was based[17,18] had been carried out by surgeons without previous training in extended lymphadenectomy, doing less than 5 five interventions per year. The limited surgical experience yielded a very high post-operative mortality after extended lymphadenectomy (9.7% in the Dutch trial and 13.5% in the British trial), a high percentage of splenectomies (37% and 65%, respectively) and pancreatectomies (30% and 56%) and a low number of retrieved nodes (median of 17 nodes in the British trial)[19]. By comparison, at the same time, mortality after D2 dissection was less than 2% in the nationwide Japanese registry[20]; likewise in a Japanese trial mortality after D2 was less than 1% and the median number of retrieved nodes was 54[21]. Of note, in the Dutch trial D2 was associated with an increased long-term survival with respect to D1 when excluding post-operative mortality (P = 0.02)[22].
Table 1.
Short-term results of gastric cancer surgery with D2 lymphadenectomy in Eastern Asia vs Europe, in clinical trials vs observational studies[16]
| Post-operative mortality | Post-operative morbidity | Median nodes retrieved | Adequate staging (≥ 15 nodes) | |
| Eastern Asia | ||||
| Trials | 0%-0.8% | 17%-21% | 54 | 100% |
| Observational | < 2% | - | - | - |
| Europe | ||||
| Trials | 5%-14% | 43%-46% | 17 | - |
| Observational | 2%-5% | 21%-35% | 25-26 | 86%-95% |
| IRGGC (VR, SI, PD) | 3.6% | 33.6% | 29 (IQ 21-38) | 93.8% |
IRGGC: Italian Research Group for Gastric Cancer.
In the meantime another randomized trial, performed in Taiwan[23], showed a mild but significant survival advantage after D2 with respect to D1; 5-year survival was 59.5% and 53.6%, respectively (P = 0.041); interestingly, none of the patients recruited died in the post-operative period[24]. Of note, a clinical trial comparing D1 and D2 could not be devised in Japan at that time, as the two procedures were not in equipoise according to Japanese surgeons. The Japan Clinical Oncology Group instead performed a trial to compare D2 and D3 lymphadenectomies[5].
In the Eastern-Western debate on gastric cancer, the Eastern position is considerably strengthened by the impressive long-term survival of Eastern patients: overall 5-year survival achieved values of 68%-74% in Japanese gastric cancer patients[2,25] whereas in Europe during the 1990s survival was three-fold lower (24%)[26].
Italian Research Group for Gastric Cancer between the East and the West
Of note, the GIRCG (Gruppo Italiano per la Ricerca sul Cancro Gastrico-Italian Research Group for Gastric Cancer) gave an important contribution to the debate on the extension of lymphadenectomy for gastric cancer.
First of all the GIRCG showed that D2 dissection is feasible on western patients with acceptable mortality and morbidity (2% and 17% respectively) rates and provides 32% probability of 5-year survival, even for patients with involvement of regional lymph nodes[27].
In 2005 De Manzoni and Verlato[28] criticized the Cochrane review on gastric cancer surgery[8] (later withdrawn[29]) for not taking into account the Japanese literature. Indeed in the past the British and American physicians gave a fundamental contribution to understanding even diseases which were rare in their countries: for instance, beta thalassemia was named Cooley’s anemia after the American physician who first described the disease in immigrant Italian children with characteristic anemia and bone deformities[30]. However, nowadays scientific methodology has spread to several countries[31], so that the leading countries in a particular medical field are often those with the highest incidence, such as Japan and South Korea for gastric cancer. Indeed “it is extremely difficult to ask Japanese surgeons, in whose series postoperative mortality is only 1%-2%, to believe in randomized clinical trials where postoperative mortality peaks to 10%-14%, irrespectively of methodological quality of those studies[16].
In 2009 Verlato et al[16] pointed out that “in this third millennium, papers dealing with surgery for gastric cancer cannot be evaluated only according to the quality of the study design, such as the Jadad score, but also the quality of surgical procedures must be taken into account”. To overcome this problem Verlato et al[16] proposed indexes of surgical quality (number of retrieved nodes, percentage of splenectomy and splenopancreasectomy, postoperative morbidity, and in-hospital mortality). Indeed, the learning curve, standardization of the procedures, poor surgical performances are among the main difficulties related to RCTs in the surgical field. Also randomization can be hampered by ethical issues, emergency setting, or need of palliative care, while patients’ and surgeons’ equipoise can be more difficult to achieve than in the medical field[32].
COMPARISON OF NATIONAL GUIDELINES AND CURRENT SCIENTIFIC RESEARCH
Extension of lymphadenectomy recommended by national guidelines
D2 was adopted as the standard of surgical treatment with curative intent by the Japanese[1,2,25,33], German[34,35] and British[36] national guidelines, by the European Society for Medical Oncology (ESMO) guidelines[37], by the joint ESMO - European Society of Surgical Oncology (ESSO) - European Society of Radiotherapy and Oncology (ESTRO) guidelines[38]. The ESMO-ESSO-ESTRO guidelines ranked the level of evidence as the highest (I) and the grade of recommendation as B (strong or moderate evidence for efficacy but with a limited clinical benefit)[38]. Of note, D2 is recommended by the Japanese guidelines since 1981[1] and by German guidelines since at least 2005[34], and D2 was adopted as the preferred lymphadenectomy within the Italian Research Group for Gastric Cancer (GIRCG) since 1992[16,27]. At variance American NCCN guidelines recommend a D1+ or a modified D2 lymph node dissection, the latter performed by experienced surgeons in high-volume centers[39].
Optimal extension of lymphadenectomy according to current scientific literature (2008-2012)
We searched PubMed for papers using the key words “lymphadenectomy or D1 or D2” AND “gastric cancer”, published in English language between 2008 and 2012. The year 2013 was not included in the systematic review, as the articles published in this year were still being introduced in medical databases which are updated with some delay. The search was limited to full-length articles in English language. In addition, bibliographies were manually inspected, to identify the relevant publications for possible inclusion.
Potentially relevant studies (n = 1174) were identified and screened for retrieval. Letters and commentaries were excluded; when duplicate articles on the same series were found, only the most recent was considered. A total of 45 full-length articles, comparing short and/or long-term effectiveness of either lymphadenectomies, were considered, 5 supporting D1, 25 supporting D2, and 15 underlying the need for further studies. Of these, 35 articles had been published on journals indexes by the Journal Citation Reports, 13 pointing out the need for further studies[40-52], 5 favouring D1[53-57], 17 supporting D2[58-74] (Table 2). Interestingly no article published on journals without impact factor was in favour of D1 lymphadenectomy, while 8 supported D2[75-82] and 2 underlined the need for more studies[83-84].
Table 2.
Summary of the systematic review of studies, comparing limited (D1) and extended (D2) lymphadenectomy and published in 2008-2012 on journals indexed by Journal Citation Reports. Articles were weighted according to 5-year impact factor (2008-2012)
| Authors | Country | Journal | Year | Study design | 5-yr IF |
| Further studies | Further studies needed | ||||
| Catalano et al[40] | Italy | Crit Rev Oncol Hematol | 2009 | Expert opinion | 4.562 |
| Coburn[41] | Canada | J Surg Oncol | 2009 | Review | 2.710 |
| D’souza et al[42] | India | J Cancer Res Ther | 2009 | Review | 0.7611 |
| Songun et al[43] | The Netherlands | Expert Rev Anticanc | 2009 | Expert opinion | 2.055 |
| Yoon et al[44] | United States | Oncologist | 2009 | Expert opinion | 5.245 |
| Coburn[45] | Canada | J Surg Oncol | 2010 | Expert opinion | 2.710 |
| de Bree et al[46] | Greece | J Surg Oncol | 2010 | Review | 2.710 |
| Degiuli et al[47] | Italy | Brit J Surg | 2010 | Clinical trial | 4.956 |
| Tanizawa et al[48] | Japan | Gastric Cancer | 2010 | Review | 3.615 |
| Maduekwe et al[49] | United States | J Gastrointest Surg | 2011 | Review | 2.766 |
| Doglietto et al[50] | Italy | Ann Ital Chir | 2012 | Expert opinion | 0.286 |
| Hundahl[51] | United States | Surg Oncol Clin N Am | 2012 | Review | 1.162 |
| Vallbohmer et al[52] | Germany | Curr Prob Surg | 2012 | Expert opinion | 2.267 |
| Overall | 35.805 | ||||
| D2 not favoured | D2 not favoured over D1 | ||||
| Lustosa et al[53] | Brazil | Acta Cir Bras | 2008 | Meta-analysis | 0.695 |
| Van Cutsem et al[54] | EORTC2 | Eur J Cancer | 2008 | Expert opinion | 5.257 |
| Yang et al[55] | China | Am J Surg | 2009 | Meta-analysis | 2.727 |
| Memon et al[56] | Australia | Ann Surg | 2011 | Meta-analysis | 8.264 |
| Wong et al[57] | United States | Curr Treat Option On | 2011 | Expert opinion | 2.4221 |
| Overall | 19.365 | ||||
| D2 favoured | D2 favoured over D1 | ||||
| Ozmen et al[58] | Turkey | J Surg Oncol | 2008 | Review | 2.710 |
| Díaz de Liaño et al[59] | Spain | Clin Transl Oncol | 2009 | Observational | 1.316 |
| Griniatsos et al[60] | Greece | World J Gastroenterol | 2009 | Observational | 2.594 |
| Kodera et al[61] | Japan | Acta Chir Belg | 2009 | Review | 0.499 |
| Roy et al[62] | India | Indian J Surg | 2009 | Review | 0.0921 |
| Sasako et al[63] | Japan | Jpn J Clin Oncol | 2010 | Expert opinion | 2.063 |
| Shi et al[64] | China | J Surg Oncol | 2010 | Review | 2.710 |
| Songun et al[65] | The Netherlands | Lancet Oncol | 2010 | Clinical trial | 21.856 |
| Tentes et al[66] | Greece | J BUON | 2010 | Observational | 0.653 |
| Hussain[67] | United Kingdom | Curr Opin Gastroen | 2011 | Expert opinion | 3.739 |
| Meyer et al[68] | Germany | Dtsch Arztebl Int | 2011 | Expert opinion | 2.988 |
| Ott et al[69] | Germany | Langenbecks Arch Surg | 2011 | Review | 1.970 |
| Saka et al[70] | Japan | Jpn J Clin Oncol | 2011 | Review | 2.063 |
| Lee et al[71] | Korea | Yonsei Med J | 2012 | Review | 1.214 |
| Sasako[72] | Japan | Surg Oncol Clin N Am | 2012 | Expert opinion | 1.162 |
| Seevaratnam et al[73] | Canada | Gastric Cancer | 2012 | Meta-analysis | 3.615 |
| Viudez-Berral et al[74] | Spain | Rev Esp Enferm Dig | 2012 | Expert opinion | 1.208 |
| Overall | 52.452 |
2012 impact factor was used, as the 5-year impact factor was not available;
EORTC: European Organisation for Research and Treatment of Cancer.
As shown in Figure 2 and in Table 2[40-74], the support to D2 lymphadenectomy increased progressively during the study period: since 2010 papers supporting D2 have achieved a higher overall IF than the other papers.
Figure 2.
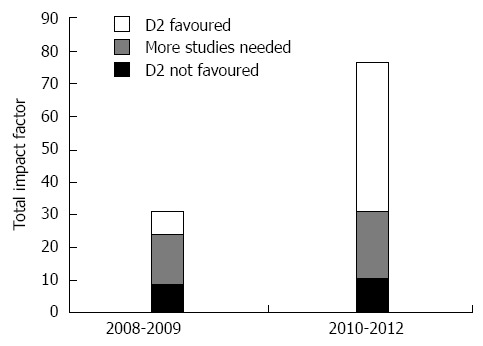
Overall Impact Factor of articles, classified as a function of D1/D2 preferences (D1 favoured, more studies needed, D2 favoured) and time (2008/09 vs 2010/12).
Initially D2 was indicated as the procedure of choice mainly by review articles and papers conveying expert opinion, while three meta-analyses[53,55,56], published between 2008 and 2011, reported that extended lymphadenectomy does not offer benefit over limited lymphadenectomy. However, in 2012 D2 was indicated as the procedure of choice in advanced gastric cancer also by a meta-analysis[73]. “Earlier trials show that D2 dissections have higher operative mortality, while recent trials have similar rates. A trend of improved survival exists among D2 patients who did not undergo resection of the spleen or pancreas, as well as for patients with T3/T4 cancers”[73].
Also the authors of the Dutch trial, after considering the 15-year follow-up results, concluded that: “Considering that a safer, spleen-preserving D2 resection is currently available in high-volume centres, and our findings of better recurrence and gastric-cancer-related survival rates, D2 resection now seems likely to be the recommended surgical approach for patients with resectable (curable) gastric cancer, despite the earlier follow-up results”[65].
The authors of another clinical trial performed in Italy[47] reported that patients undergoing D1 or D2 experienced similar post-operative morbidity (12% and 17.9% respectively) and mortality (3% and 2.2%) and concluded that “D2 dissection, in an appropriate setting, can therefore be considered a safe option for the radical management of gastric cancer in Western patients”.
In the era of tailored treatment, other Authors suggested to move “away from the D2 versus D1 debate” and to choose the extension of lymphadenectomy according to “the stage of the cancer and the age and fitness of the patient”[85]. Similarly, an expert panel, using the RAND/UCLA appropriateness methodology, concluded that “a D2 lymphadenectomy is preferred for curative-intent resection in advanced, nonmetastatic GC; and in patients with early GC or substantial comorbidities, a D1 lymphadenectomy is more appropriate”[86].
CONCLUSION
Towards an international agreement?
EBM, which till recently has not reported any advantage of extended D2 lymphadenectomy with respect to the limited D1 procedure[7,8,53,55,56], is now supporting D2. A clinical trial performed in the East highlighted a significant 5-year survival advantage after D2 with respect to D1[23]. A clinical trial performed on Western patients showed that D2 can be performed without excess post-operative morbidity or mortality[47]. The most recent meta-analyses[73,87] concluded that “D2 lymphadenectomy with spleen and pancreas preservation offers the most survival benefit”[87] for patients with advanced gastric cancer.
The latest American guidelines[39] now include not only D1+ but also “a modified D2 lymph node dissection” among recommended procedures for patients with resectable locoregional cancer, as long as the latter is “performed by experienced surgeons in high-volume centers”.
Two American authors[88] recently underlined that Eastern and Western surgeons are converging to consider D2 lymphadenectomy as the standard procedure, as the former have given up with super-extended procedures, while the latter “have increasingly accepted the importance of performing more than a D1 node dissection”.
Problems of EBM in gastric cancer surgery and possible solutions
In gastric cancer surgery EBM is lagging behind national guidelines, rather than preceding and orienting them. To eliminate this lag, EBM should value to a larger extent Eastern Asian literature[28] and should take into account not only the quality of the study design but also the quality of surgical procedures[16].
In Western countries, where the incidence of gastric cancer is getting low, centralization of gastric cancer surgery in specialized high-volume institution would also be necessary.
As pointed out by Strong and Yoon[88] one significant obstacle to implementing D2 lymphadenectomy in the West is the low volume of gastrectomies in Western centres. Indeed in the United States 80% of Medicare patients undergo gastrectomy in centers performing less than 20 procedures per year[89]. This situation reflects not only the low incidence of gastric cancer in the United States but also the surgeon’s habit to consider gastric surgery as part of general surgery[88].
However, several Northern European countries managed to achieve great improvements in gastric cancer surgery at national level. In the Netherlands survival of gastric cancer patients significantly improved after the implementation of the Dutch D1-D2 Gastric Cancer Trial, which involved substantial standardization and training[90]. In Denmark 30-d hospital mortality has decreased from 8.2% to 2.4% after centralization of gastric cancer surgery and implementation of national clinical guidelines while the proportion of patients with at least 15 lymph nodes removed has increased from 19% to 76%[91]. Centralization of gastric cancer surgery and/or audits for gastric cancer are currently implemented in the United Kingdom, Sweden, Finland, and the Netherlands[92,93].
Footnotes
P- Reviewer: Izbicki JR, Ivanov KD, Li Y, Nunobe S S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
References
- 1.Kajitani T. The general rules for the gastric cancer study in surgery and pathology. Part I. Clinical classification. Jpn J Surg. 1981;11:127–139. doi: 10.1007/BF02468883. [DOI] [PubMed] [Google Scholar]
- 2.Maruyama K, Kaminishi M, Hayashi K, Isobe Y, Honda I, Katai H, Arai K, Kodera Y, Nashimoto A. Gastric cancer treated in 1991 in Japan: data analysis of nationwide registry. Gastric Cancer. 2006;9:51–66. doi: 10.1007/s10120-006-0370-y. [DOI] [PubMed] [Google Scholar]
- 3.Sasako M, Saka M, Fukagawa T, Katai H, Sano T. Modern surgery for gastric cancer--Japanese perspective. Scand J Surg. 2006;95:232–235. doi: 10.1177/145749690609500404. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt B, Yoon SS. D1 versus D2 lymphadenectomy for gastric cancer. J Surg Oncol. 2013;107:259–264. doi: 10.1002/jso.23127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453–462. doi: 10.1056/NEJMoa0707035. [DOI] [PubMed] [Google Scholar]
- 6.Hundahl SA, Macdonald JS, Benedetti J, Fitzsimmons T; Southwest Oncology Group and the Gastric Intergroup. Surgical treatment variation in a prospective, randomized trial of chemoradiotherapy in gastric cancer: the effect of undertreatment. Ann Surg Oncol. 2002;9:278–286. doi: 10.1007/BF02573066. [DOI] [PubMed] [Google Scholar]
- 7.McCulloch P, Nita ME, Kazi H, Gama-Rodrigues J. Extended versus limited lymph nodes dissection technique for adenocarcinoma of the stomach. Cochrane Database Syst Rev. 2003;(4):CD001964. doi: 10.1002/14651858.CD001964. [DOI] [PubMed] [Google Scholar]
- 8.McCulloch P, Niita ME, Kazi H, Gama-Rodrigues JJ. Gastrectomy with extended lymphadenectomy for primary treatment of gastric cancer. Br J Surg. 2005;92:5–13. doi: 10.1002/bjs.4839. [DOI] [PubMed] [Google Scholar]
- 9.Bonenkamp JJ, Hermans J, Sasako M, van de Velde CJ, Welvaart K, Songun I, Meyer S, Plukker JT, Van Elk P, Obertop H, et al. Extended lymph-node dissection for gastric cancer. N Engl J Med. 1999;340:908–914. doi: 10.1056/NEJM199903253401202. [DOI] [PubMed] [Google Scholar]
- 10.Cuschieri A, Weeden S, Fielding J, Bancewicz J, Craven J, Joypaul V, Sydes M, Fayers P. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer. 1999;79:1522–1530. doi: 10.1038/sj.bjc.6690243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Manzoni G, Verlato G, Roviello F, Morgagni P, Di Leo A, Saragoni L, Marrelli D, Kurihara H, Pasini F. The new TNM classification of lymph node metastasis minimises stage migration problems in gastric cancer patients. Br J Cancer. 2002;87:171–174. doi: 10.1038/sj.bjc.6600432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith BR, Stabile BE. Aggressive D2 lymphadenectomy is required for accurate pathologic staging of gastric adenocarcinoma. Am Surg. 2006;72:849–852. [PubMed] [Google Scholar]
- 13.Chen S, Zhao BW, Li YF, Feng XY, Sun XW, Li W, Zhou ZW, Zhan YQ, Qian CN, Chen YB. The prognostic value of harvested lymph nodes and the metastatic lymph node ratio for gastric cancer patients: results of a study of 1,101 patients. PLoS One. 2012;7:e49424. doi: 10.1371/journal.pone.0049424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marchet A, Mocellin S, Ambrosi A, Morgagni P, Garcea D, Marrelli D, Roviello F, de Manzoni G, Minicozzi A, Natalini G, et al. The ratio between metastatic and examined lymph nodes (N ratio) is an independent prognostic factor in gastric cancer regardless of the type of lymphadenectomy: results from an Italian multicentric study in 1853 patients. Ann Surg. 2007;245:543–552. doi: 10.1097/01.sla.0000250423.43436.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] Lyon, France: International Agency for Research on Cancer, 2013. Accessed on 3/1/; 2014. Available from: http://globocan.iarc.fr. [Google Scholar]
- 16.Verlato G, Roviello F, Marchet A, Giacopuzzi S, Marrelli D, Nitti D, de Manzoni G. Indexes of surgical quality in gastric cancer surgery: experience of an Italian network. Ann Surg Oncol. 2009;16:594–602. doi: 10.1245/s10434-008-0271-x. [DOI] [PubMed] [Google Scholar]
- 17.Bonenkamp JJ, Songun I, Hermans J, Sasako M, Welvaart K, Plukker JT, van Elk P, Obertop H, Gouma DJ, Taat CW. Randomised comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet. 1995;345:745–748. doi: 10.1016/s0140-6736(95)90637-1. [DOI] [PubMed] [Google Scholar]
- 18.Cuschieri A, Fayers P, Fielding J, Craven J, Bancewicz J, Joypaul V, Cook P. Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer: preliminary results of the MRC randomised controlled surgical trial. The Surgical Cooperative Group. Lancet. 1996;347:995–999. doi: 10.1016/s0140-6736(96)90144-0. [DOI] [PubMed] [Google Scholar]
- 19.Guadagni S, Catarci M, de Manzoni G, Kinoshita T. D1 versus D2 dissection for gastric cancer. Lancet. 1995;345:1517; author reply 1517–1518. [PubMed] [Google Scholar]
- 20.Fujii M, Sasaki J, Nakajima T. State of the art in the treatment of gastric cancer: from the 71st Japanese Gastric Cancer Congress. Gastric Cancer. 1999;2:151–157. doi: 10.1007/s101200050039. [DOI] [PubMed] [Google Scholar]
- 21.Sano T, Sasako M, Yamamoto S, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K, Yamamura Y, et al. Gastric cancer surgery: morbidity and mortality results from a prospective randomized controlled trial comparing D2 and extended para-aortic lymphadenectomy--Japan Clinical Oncology Group study 9501. J Clin Oncol. 2004;22:2767–2773. doi: 10.1200/JCO.2004.10.184. [DOI] [PubMed] [Google Scholar]
- 22.Hartgrink HH, van de Velde CJ, Putter H, Bonenkamp JJ, Klein Kranenbarg E, Songun I, Welvaart K, van Krieken JH, Meijer S, Plukker JT, et al. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol. 2004;22:2069–2077. doi: 10.1200/JCO.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 23.Wu CW, Hsiung CA, Lo SS, Hsieh MC, Chen JH, Li AF, Lui WY, Whang-Peng J. Nodal dissection for patients with gastric cancer: a randomised controlled trial. Lancet Oncol. 2006;7:309–315. doi: 10.1016/S1470-2045(06)70623-4. [DOI] [PubMed] [Google Scholar]
- 24.Wu CW, Hsiung CA, Lo SS, Hsieh MC, Shia LT, Whang-Peng J. Randomized clinical trial of morbidity after D1 and D3 surgery for gastric cancer. Br J Surg. 2004;91:283–287. doi: 10.1002/bjs.4433. [DOI] [PubMed] [Google Scholar]
- 25.Nakajima T. Gastric cancer treatment guidelines in Japan. Gastric Cancer. 2002;5:1–5. doi: 10.1007/s101200200000. [DOI] [PubMed] [Google Scholar]
- 26.Berrino F, De Angelis R, Sant M, Rosso S, Bielska-Lasota M, Coebergh JW, Santaquilani M. Survival for eight major cancers and all cancers combined for European adults diagnosed in 1995-99: results of the EUROCARE-4 study. Lancet Oncol. 2007;8:773–783. doi: 10.1016/S1470-2045(07)70245-0. [DOI] [PubMed] [Google Scholar]
- 27.Roviello F, Marrelli D, Morgagni P, de Manzoni G, Di Leo A, Vindigni C, Saragoni L, Tomezzoli A, Kurihara H. Survival benefit of extended D2 lymphadenectomy in gastric cancer with involvement of second level lymph nodes: a longitudinal multicenter study. Ann Surg Oncol. 2002;9:894–900. doi: 10.1007/BF02557527. [DOI] [PubMed] [Google Scholar]
- 28.de Manzoni G, Verlato GE. Gastrectomy with extended lymphadenectomy for primary treatment of gastric cancer (Br J Surg 2005; 92: 5-13) Br J Surg. 2005;92:784. doi: 10.1002/bjs.5088. [DOI] [PubMed] [Google Scholar]
- 29.McCulloch P, Nita ME, Kazi H, Gama-Rodrigues JJ. WITHDRAWN: Extended versus limited lymph nodes dissection technique for adenocarcinoma of the stomach. Cochrane Database Syst Rev. 2012;1:CD001964. doi: 10.1002/14651858.CD001964.pub3. [DOI] [PubMed] [Google Scholar]
- 30.Vichinsky EP, MacKlin EA, Waye JS, Lorey F, Olivieri NF. Changes in the epidemiology of thalassemia in North America: a new minority disease. Pediatrics. 2005;116:e818–e825. doi: 10.1542/peds.2005-0843. [DOI] [PubMed] [Google Scholar]
- 31.International Comparative Performance of the UK Research Base - 2013. A report prepared by Elsevier for the UK’s Department of Business, Innovation and Skills (BIS) Available from: http://www.gov.uk/government/uploads/system/uploads/attachment_data/file/263729/bis-13-1297-international-comparative-performance-of-the-UK-research-base-2013.pdf.
- 32.McCulloch P, Taylor I, Sasako M, Lovett B, Griffin D. Randomised trials in surgery: problems and possible solutions. BMJ. 2002;324:1448–1451. doi: 10.1136/bmj.324.7351.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14:113–123. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 34.Moenig S, Baldus S, Bollschweiler E, Hoelscher A. Surgery of gastric cancer—quality assurance. Viszeralchirurgie. 2005;42:42–48. [Google Scholar]
- 35.Meyer HJ, Hölscher AH, Lordick F, Messmann H, Mönig S, Schumacher C, Stahl M, Wilke H, Möhler M. [Current S3 guidelines on surgical treatment of gastric carcinoma] Chirurg. 2012;83:31–37. doi: 10.1007/s00104-011-2149-x. [DOI] [PubMed] [Google Scholar]
- 36.Allum WH, Blazeby JM, Griffin SM, Cunningham D, Jankowski JA, Wong R; Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland, the British Society of Gastroenterology and the British Association of Surgical Oncology. Guidelines for the management of oesophageal and gastric cancer. Gut. 2011;60:1449–1472. doi: 10.1136/gut.2010.228254. [DOI] [PubMed] [Google Scholar]
- 37.Okines A, Verheij M, Allum W, Cunningham D, Cervantes A. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21 Suppl 5:v50–v54. doi: 10.1093/annonc/mdq164. [DOI] [PubMed] [Google Scholar]
- 38.Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D. Gastric cancer: ESMO-ESSO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 Suppl 6:vi57–vi63. doi: 10.1093/annonc/mdt344. [DOI] [PubMed] [Google Scholar]
- 39.Ajani JA, Bentrem DJ, Besh S, D’Amico TA, Das P, Denlinger C, Fakih MG, Fuchs CS, Gerdes H, Glasgow RE, et al. Gastric cancer, version 2.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2013;11:531–546. doi: 10.6004/jnccn.2013.0070. [DOI] [PubMed] [Google Scholar]
- 40.Catalano V, Labianca R, Beretta GD, Gatta G, de Braud F, Van Cutsem E. Gastric cancer. Crit Rev Oncol Hematol. 2009;71:127–164. doi: 10.1016/j.critrevonc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Coburn NG. Lymph nodes and gastric cancer. J Surg Oncol. 2009;99:199–206. doi: 10.1002/jso.21224. [DOI] [PubMed] [Google Scholar]
- 42.D’souza MA, Singh K, Shrikhande SV. Surgery for gastric cancer: an evidence-based perspective. J Cancer Res Ther. 2009;5:225–231. doi: 10.4103/0973-1482.59891. [DOI] [PubMed] [Google Scholar]
- 43.Songun I, van de Velde CJ. Optimal surgery for advanced gastric cancer. Expert Rev Anticancer Ther. 2009;9:1849–1858. doi: 10.1586/era.09.132. [DOI] [PubMed] [Google Scholar]
- 44.Yoon SS, Yang HK. Lymphadenectomy for gastric adenocarcinoma: should west meet east? Oncologist. 2009;14:871–882. doi: 10.1634/theoncologist.2009-0070. [DOI] [PubMed] [Google Scholar]
- 45.Coburn NG. Improving survival for gastric cancer patients--the role of the surgeon. J Surg Oncol. 2010;101:103–104. doi: 10.1002/jso.21437. [DOI] [PubMed] [Google Scholar]
- 46.de Bree E, Charalampakis V, Melissas J, Tsiftsis DD. The extent of lymph node dissection for gastric cancer: a critical appraisal. J Surg Oncol. 2010;102:552–562. doi: 10.1002/jso.21646. [DOI] [PubMed] [Google Scholar]
- 47.Degiuli M, Sasako M, Ponti A. Morbidity and mortality in the Italian Gastric Cancer Study Group randomized clinical trial of D1 versus D2 resection for gastric cancer. Br J Surg. 2010;97:643–649. doi: 10.1002/bjs.6936. [DOI] [PubMed] [Google Scholar]
- 48.Tanizawa Y, Terashima M. Lymph node dissection in the resection of gastric cancer: review of existing evidence. Gastric Cancer. 2010;13:137–148. doi: 10.1007/s10120-010-0560-5. [DOI] [PubMed] [Google Scholar]
- 49.Maduekwe UN, Yoon SS. An evidence-based review of the surgical treatment of gastric adenocarcinoma. J Gastrointest Surg. 2011;15:730–741. doi: 10.1007/s11605-011-1477-y. [DOI] [PubMed] [Google Scholar]
- 50.Doglietto GB, Rosa F, Bossola M, Pacelli F. Lymphadenectomy for gastric cancer: still a matter of debate? Ann Ital Chir. 2012;83:199–207. [PubMed] [Google Scholar]
- 51.Hundahl SA. Surgery for gastric cancer: what the trials indicate. Surg Oncol Clin N Am. 2012;21:79–97. doi: 10.1016/j.soc.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 52.Vallbohmer D, Oh DS, Peters JH. The role of lymphadenectomy in the surgical treatment of esophageal and gastric cancer. Curr Probl Surg. 2012;49:471–515. doi: 10.1067/j.cpsurg.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 53.Lustosa SA, Saconato H, Atallah AN, Lopes Filho Gde J, Matos D. Impact of extended lymphadenectomy on morbidity, mortality, recurrence and 5-year survival after gastrectomy for cancer. Meta-analysis of randomized clinical trials. Acta Cir Bras. 2008;23:520–530. doi: 10.1590/s0102-86502008000600009. [DOI] [PubMed] [Google Scholar]
- 54.Van Cutsem E, Van de Velde C, Roth A, Lordick F, Köhne CH, Cascinu S, Aapro M. Expert opinion on management of gastric and gastro-oesophageal junction adenocarcinoma on behalf of the European Organisation for Research and Treatment of Cancer (EORTC)-gastrointestinal cancer group. Eur J Cancer. 2008;44:182–194. doi: 10.1016/j.ejca.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 55.Yang SH, Zhang YC, Yang KH, Li YP, He XD, Tian JH, Lv TH, Hui YH, Sharma N. An evidence-based medicine review of lymphadenectomy extent for gastric cancer. Am J Surg. 2009;197:246–251. doi: 10.1016/j.amjsurg.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 56.Memon MA, Subramanya MS, Khan S, Hossain MB, Osland E, Memon B. Meta-analysis of D1 versus D2 gastrectomy for gastric adenocarcinoma. Ann Surg. 2011;253:900–911. doi: 10.1097/SLA.0b013e318212bff6. [DOI] [PubMed] [Google Scholar]
- 57.Wong J, Jackson P. Gastric cancer surgery: an American perspective on the current options and standards. Curr Treat Options Oncol. 2011;12:72–84. doi: 10.1007/s11864-010-0136-y. [DOI] [PubMed] [Google Scholar]
- 58.Ozmen MM, Ozmen F, Zulfikaroglu B. Lymph nodes in gastric cancer. J Surg Oncol. 2008;98:476–481. doi: 10.1002/jso.21134. [DOI] [PubMed] [Google Scholar]
- 59.Díaz de Liaño A, Yárnoz C, Artieda C, Aguilar R, Viana S, Artajona A, Ortiz H. Results of R0 surgery with D2 lymphadenectomy for the treatment of localised gastric cancer. Clin Transl Oncol. 2009;11:178–182. doi: 10.1007/s12094-009-0335-9. [DOI] [PubMed] [Google Scholar]
- 60.Griniatsos J, Gakiopoulou H, Yiannakopoulou E, Dimitriou N, Douridas G, Nonni A, Liakakos T, Felekouras E. Routine modified D2 lymphadenectomy performance in pT1-T2N0 gastric cancer. World J Gastroenterol. 2009;15:5568–5572. doi: 10.3748/wjg.15.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kodera E, Fujiwara M, Ito Y, Ohashi N, Nakayama G, Koike M, Nakao A. Radical surgery for gastric carcinoma: it is not an issue of whether to perform D1 or D2. Dissect as many lymph nodes as possible and you will be rewarded. Acta Chir Belg. 2009;109:27–35. doi: 10.1080/00015458.2009.11680367. [DOI] [PubMed] [Google Scholar]
- 62.Roy MK, Sadhu S, Dubey SK. Advances in the management of gastric cancer. Indian J Surg. 2009;71:342–349. doi: 10.1007/s12262-009-0092-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sasako M, Inoue M, Lin JT, Khor C, Yang HK, Ohtsu A. Gastric Cancer Working Group report. Jpn J Clin Oncol. 2010;40 Suppl 1:i28–i37. doi: 10.1093/jjco/hyq124. [DOI] [PubMed] [Google Scholar]
- 64.Shi Y, Zhou Y. The role of surgery in the treatment of gastric cancer. J Surg Oncol. 2010;101:687–692. doi: 10.1002/jso.21455. [DOI] [PubMed] [Google Scholar]
- 65.Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439–449. doi: 10.1016/S1470-2045(10)70070-X. [DOI] [PubMed] [Google Scholar]
- 66.Tentes AA, Korakianitis O, Kyziridis D, Veliovits D. Long-term results following potentially curative gastrectomy for gastric cancer. J BUON. 2010;15:504–508. [PubMed] [Google Scholar]
- 67.Hussain A. Gastric malignancy: surgical management. Curr Opin Gastroenterol. 2011;27:583–587. doi: 10.1097/MOG.0b013e32834a6d8d. [DOI] [PubMed] [Google Scholar]
- 68.Meyer HJ, Wilke H. Treatment strategies in gastric cancer. Dtsch Arztebl Int. 2011;108:698–705; quiz 706. doi: 10.3238/arztebl.2011.0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ott K, Lordick F, Blank S, Büchler M. Gastric cancer: surgery in 2011. Langenbecks Arch Surg. 2011;396:743–758. doi: 10.1007/s00423-010-0738-7. [DOI] [PubMed] [Google Scholar]
- 70.Saka M, Morita S, Fukagawa T, Katai H. Present and future status of gastric cancer surgery. Jpn J Clin Oncol. 2011;41:307–313. doi: 10.1093/jjco/hyq240. [DOI] [PubMed] [Google Scholar]
- 71.Lee JH, Kim KM, Cheong JH, Noh SH. Current management and future strategies of gastric cancer. Yonsei Med J. 2012;53:248–257. doi: 10.3349/ymj.2012.53.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sasako M. Gastric cancer eastern experience. Surg Oncol Clin N Am. 2012;21:71–77. doi: 10.1016/j.soc.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 73.Seevaratnam R, Bocicariu A, Cardoso R, Mahar A, Kiss A, Helyer L, Law C, Coburn N. A meta-analysis of D1 versus D2 lymph node dissection. Gastric Cancer. 2012;15 Suppl 1:S60–S69. doi: 10.1007/s10120-011-0110-9. [DOI] [PubMed] [Google Scholar]
- 74.Viudez-Berral A, Miranda-Murua C, Arias-de-la-Vega F, Hernández-García I, Artajona-Rosino A, Díaz-de-Liaño Á, Vera-García R. Current management of gastric cancer. Rev Esp Enferm Dig. 2012;104:134–141. doi: 10.4321/s1130-01082012000300006. [DOI] [PubMed] [Google Scholar]
- 75.Yalcin S. Gastric cancer in Turkey-a bridge between west and East. Gastrointest Cancer Res. 2009;3:29–32. [PMC free article] [PubMed] [Google Scholar]
- 76.Methasate A, Trakarnsanga A, Akaraviputh T, Chinsawangwathanakol V, Lohsiriwat D. Lymph node metastasis in gastric cancer: result of D2 dissection. J Med Assoc Thai. 2010;93:310–317. [PubMed] [Google Scholar]
- 77.Wang XN, Liang H. Some problems in the surgical treatment of gastric cancer. Chin J Cancer. 2010;29:369–373. doi: 10.5732/cjc.009.10629. [DOI] [PubMed] [Google Scholar]
- 78.An JY, Cheong JH, Hyung WJ, Noh SH. Recent evolution of surgical treatment for gastric cancer in Korea. J Gastric Cancer. 2011;11:1–6. doi: 10.5230/jgc.2011.11.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tamura S, Takeno A, Miki H. Lymph node dissection in curative gastrectomy for advanced gastric cancer. Int J Surg Oncol. 2011;2011:748745. doi: 10.1155/2011/748745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Allum WH. Optimal surgery for gastric cancer: is more always better? Recent Results Cancer Res. 2012;196:215–227. doi: 10.1007/978-3-642-31629-6_15. [DOI] [PubMed] [Google Scholar]
- 81.Bittoni A, Faloppi L, Giampieri R, Cascinu S. Selecting the best treatment for an individual patient. Recent Results Cancer Res. 2012;196:307–318. doi: 10.1007/978-3-642-31629-6_20. [DOI] [PubMed] [Google Scholar]
- 82.Zilberstein B, Mucerino DR, Yagi OK, Ribeiro-Junior U, Lopasso FP, Bresciani C, Jacob CE, Coimbra BG, Cecconello I. Results of D2 gastrectomy for gastric cancer: lymph node chain dissection or multiple node resection? Arq Bras Cir Dig. 2012;25:161–164. doi: 10.1590/s0102-67202012000300005. [DOI] [PubMed] [Google Scholar]
- 83.Akagi T, Shiraishi N, Kitano S. Lymph node metastasis of gastric cancer. Cancers (Basel) 2011;3:2141–2159. doi: 10.3390/cancers3022141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rausei S, Dionigi G, Rovera F, Boni L, Valerii C, Giavarini L, Frattini F, Dionigi R. A decade in gastric cancer curative surgery: Evidence of progress (1999-2009) World J Gastrointest Surg. 2012;4:45–54. doi: 10.4240/wjgs.v4.i3.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lamb P, Sivashanmugam T, White M, Irving M, Wayman J, Raimes S. Gastric cancer surgery--a balance of risk and radicality. Ann R Coll Surg Engl. 2008;90:235–242. doi: 10.1308/003588408X261546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brar S, Law C, McLeod R, Helyer L, Swallow C, Paszat L, Seevaratnam R, Cardoso R, Dixon M, Mahar A, et al. Defining surgical quality in gastric cancer: a RAND/UCLA appropriateness study. J Am Coll Surg. 2013;217:347–57.e1. doi: 10.1016/j.jamcollsurg.2013.01.067. [DOI] [PubMed] [Google Scholar]
- 87.Jiang L, Yang KH, Guan QL, Zhao P, Chen Y, Tian JH. Survival and recurrence free benefits with different lymphadenectomy for resectable gastric cancer: a meta-analysis. J Surg Oncol. 2013;107:807–814. doi: 10.1002/jso.23325. [DOI] [PubMed] [Google Scholar]
- 88.Strong VE, Yoon SS. Extended lymphadenectomy in gastric cancer is debatable. World J Surg. 2013;37:1773–1777. doi: 10.1007/s00268-013-2070-1. [DOI] [PubMed] [Google Scholar]
- 89.Smith DL, Elting LS, Learn PA, Raut CP, Mansfield PF. Factors influencing the volume-outcome relationship in gastrectomies: a population-based study. Ann Surg Oncol. 2007;14:1846–1852. doi: 10.1245/s10434-007-9381-0. [DOI] [PubMed] [Google Scholar]
- 90.Krijnen P, den Dulk M, Meershoek-Klein Kranenbarg E, Jansen-Landheer ML, van de Velde CJ. Improved survival after resectable non-cardia gastric cancer in The Netherlands: the importance of surgical training and quality control. Eur J Surg Oncol. 2009;35:715–720. doi: 10.1016/j.ejso.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 91.Jensen LS, Nielsen H, Mortensen PB, Pilegaard HK, Johnsen SP. Enforcing centralization for gastric cancer in Denmark. Eur J Surg Oncol. 2010;36 Suppl 1:S50–S54. doi: 10.1016/j.ejso.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 92.de Steur WO, Dikken JL, Hartgrink HH. Lymph node dissection in resectable advanced gastric cancer. Dig Surg. 2013;30:96–103. doi: 10.1159/000350873. [DOI] [PubMed] [Google Scholar]
- 93.Dikken JL, van Sandick JW, Allum WH, Johansson J, Jensen LS, Putter H, Coupland VH, Wouters MW, Lemmens VE, van de Velde CJ, et al. Differences in outcomes of oesophageal and gastric cancer surgery across Europe. Br J Surg. 2013;100:83–94. doi: 10.1002/bjs.8966. [DOI] [PubMed] [Google Scholar]


