Abstract
Hepatocellular carcinoma (HCC) is the fourth cause of cancer related mortality, and its incidence is rapidly increasing. Viral hepatitis, alcohol abuse, and exposure to hepatotoxins are major risk factors, but nonalcoholic fatty liver disease (NAFLD) associated with obesity, insulin resistance, and type 2 diabetes, is an increasingly recognized trigger, especially in developed countries. Older age, severity of insulin resistance and diabetes, and iron overload have been reported to predispose to HCC in this context. Remarkably, HCCs have been reported in non-cirrhotic livers in a higher proportion of cases in NAFLD patients than in other etiologies. Inherited factors have also been implicated to explain the different individual susceptibility to develop HCC, and their role seems magnified in fatty liver, where only a minority of affected subjects progresses to cancer. In particular, the common I148M variant of the PNPLA3 gene influencing hepatic lipid metabolism influences HCC risk independently of its effect on the progression of liver fibrosis. Recently, rare loss-of-function mutations in Apolipoprotein B resulting in very low density lipoproteins hepatic retention and in Telomerase reverse transcriptase influencing cellular senescence have also been linked to HCC in NAFLD. Indeed, hepatic stellate cells senescence has been suggested to bridge tissue aging with alterations of the intestinal microbiota in the pathogenesis of obesity-related HCC. A deeper understanding of the mechanisms mediating hepatic carcinogenesis during insulin resistance, and the identification of its genetic determinants will hopefully provide new diagnostic and therapeutic tools.
Keywords: Nonalcoholic fatty liver disease, Steatosis, Hepatocellular carcinoma, Cirrhosis, Fibrogenesis, Liver disease, Genetics, Single nucleotide polymorphism, Patatin-like phospholipase domain-containing 3
Core tip: Nonalcoholic fatty liver disease (NAFLD) associated with obesity, insulin resistance, and type 2 diabetes, is an increasingly recognized trigger of hepatocellular carcinoma (HCC), even in non-cirrhotic livers. The role of inherited factors seems magnified in fatty liver. The common I148M variant of the PNPLA3 gene influencing hepatic lipid metabolism has been associated with HCC. Rare loss-of-function mutations in apolipoprotein B resulting in very low density lipoproteins hepatic retention and in telomerase reverse transcriptase influencing senescence have been linked to HCC in NAFLD. Hepatic stellate cells senescence has also been suggested to bridge tissue aging with microbiota alterations in the pathogenesis of obesity-related HCC.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third cause of cancer-related mortality worldwide. It represents 90% of primary liver cancers, and its incidence is increasing[1,2].
The major risk factor for HCC is represented by chronic hepatitis, mostly related to hepatitis B and hepatitis C virus (HCV) infection[3]. Excessive alcohol intake, hereditary hemochromatosis, and other inherited diseases account for additional cases, and represent an important cofactor in patients with viral hepatitis[4-7]. However, the fraction of cases arising in the context of chronic liver damage associated with nonalcoholic fatty liver (NAFLD) related to systemic insulin resistance and obesity[8] is rapidly increasing, especially in Western countries[9].
The majority of HCC cases occurs in individuals with cirrhosis, a subversion of liver histological architecture due to progressive fibrosis representing per se a pre-cancerous condition. Overall, one-third of cirrhotic individuals will develop HCC during lifetime[10], at an incidence of 1%-8% per year. Male gender, older age, cigarette smoking, obesity, the metabolic syndrome, and type 2 diabetes are major risk factors, together with viral features, the severity of liver damage, and serum alpha-fetoprotein levels[11-15].
Besides viral, environmental, behavioral, and metabolic features, inherited factors have also been implicated to explain the different susceptibility to develop HCC. Family history and ethnicity are strongly associated with HCC, particularly in areas characterized by a high prevalence of chronic infection with hepatitis viruses. In the last years, several studies have demonstrated the contribution of single nucleotide polymorphisms (SNPs) to the predisposition towards HCC[16,17].
Liver fat deposition, most frequently related to systemic insulin resistance (IR) defines NAFLD[18], which is considered the hepatic manifestation of the metabolic syndrome. In susceptible individuals NAFLD is associated with inflammation and fibrogenesis, i.e., nonalcoholic steatohepatitis (NASH)[19], which can progress to severe fibrosis and lead to HCC[20] even before cirrhosis ensues. As NAFLD is now the most frequent liver disease affecting at least 25% of the United States and European population[21,22], it is predicted that it will fuel a rise HCC cases in the future, reducing the benefit of new effective antiviral therapies and of hepatitis B virus (HBV) vaccine campaigns[23].
HEPATOCELLULAR CARCINOMA AND STEATOSIS
NAFLD is now a recognized and increasingly observed cause of HCC[7]. In the Newcastle area, United Kingdom, where the incidence of HCC was historically low due to eliminare do a low prevalence of viral hepatitis, NAFLD became the leading etiological factor associated with HCC by 2010[9].
It should be noted that besides cases ascribed to NAFLD, 5%-30% of HCCs are cryptogenic, i.e. do not have any identifiable risk factor[24], and it has been suggested that they represent the evolution of severe forms of NAFLD (“burn out nonalcoholic steatohepatitis”)[20]. Several case-control studies have described that HCC patients with cryptogenic cirrhosis tend to have typical clinical features of NASH, compared with sex- and age-matched HCC patients arising in cirrhosis of well-defined etiology[20,25,26]. In a South Korean study in 480 HCC patients, cryptogenic cirrhosis accounted for 7% of cases, and was associated with older age, metabolic syndrome and tumor characteristics, all features that have been associated with NAFLD-related HCC[27].
However, given the enormous prevalence of the metabolic syndrome, in the absence of other cofactors such as viral infections and alcohol abuse, HCC remains a distinctly rare complication of obesity, type 2 diabetes, and NAFLD[18], although the exact incidence of this complication is not known.
Most NAFLD-related HCCs are believed to develop in the background of a cirrhotic liver[20,28-30]. However, the incidence of HCC in patients with cirrhosis or advanced fibrosis related to NAFLD seems lower to that conferred by other etiologies, such as CHC, and requires the presence of other metabolic cofactors such as a long history of diabetes[31], and typically develop in older males[32,33]. Notwithstanding, due to the diffusion of the vaccination against HBV, the availability of novel antiviral treatments, and the epidemics of obesity, NASH is projected to become the leading cause of HCC in Western countries in the next future.
Steatohepatitis represents also a key step in the development of progressive liver damage and HCC in patients with chronic alcohol abuse[34,35]. Evidence is accumulating that steatosis is a risk factor for HCC also in patients with chronic HCV infection, persisting after viral eradication[36-38]. In addition, a specific subtype of HCC (steatohepatitic HCC) have been described in HCV positive patients with risk factors for NAFLD, suggesting the existence of a specific NASH-HCC pathway[39].
HCC may develop in patients with NASH and even simple steatosis even in the absence of advanced liver fibrosis[7,33,40]. In a small number of cases NAFLD may progress to HCC in the background of metabolic syndrome especially with type 2 diabetes and obesity[41]. Indeed, HCCs arising in patients with features of metabolic syndrome are larger, more differentiated and mainly occur in the absence of significant fibrosis[42] compared than those arising in chronic viral hepatitis. An overview of the literature supporting increased incidence of HCC in nonalcoholic fatty liver disease without cirrhosis is presented in Table 1.
Table 1.
Literature supporting increased incidence of hepatocellular carcinoma diagnosed in non-cirrhotic liver in nonalcoholic fatty liver disease
| Ref. | Cases (n) | Other etiologies (n) | Cryptogenic cirrhosis (n) | Design |
| Marrero et al[25] | 105 | 75 | 30 | Population study |
| Bugianesi et al[20] | 641 | 597 | 44 | Retrospective case-control study |
| Regimbeau et al[26] | 210 | 192 | 18 | Case-control study |
| Lee et al[27] | 480 | 446 | 34 | Case-control study |
| Pekow et al[36] | 32 | 32 | - | Retrospective case-control study |
| Ascha et al[30] | 89 | 64 | 25 | Case-control study |
HCC: Hepatocellular carcinoma; NAFLD: Nonalcoholic fatty liver disease.
In conclusion, the different natural history, specific histhological features of hepatocellular carcinoma in individuals with fatty liver and inflammation suggests a specific mechanism underlying the NAFLD-HCC carcinogenesis.
HEPATOCELLULAR CARCINOMA: ASSOCIATION WITH OBESITY, INSULIN RESISTANCE, AND TYPE 2 DIABETES
Accumulating epidemiological studies indicate that overweight and obesity, the major determinant of insulin resistance and NAFLD at population level, and whose prevalence is markedly increasing, are associated with higher risk of HCC. In a prospective population-based study conducted in a large cohort of United States adults stratified by severity of overweight, HCC-related death rate was 4.5-fold higher in men with a body mass index higher than 35 kg/m2 than in men with a normal body mass index (BMI)[14].
Overall evidence indicate that the risk of liver cancer is 17% and 89% higher in persons who are overweight and obese, respectively, than in those of normal weight, and that the relationship is stronger in men than in women[43]. Obesity also increases the risk of HCC in subjects with cirrhosis. Among 19271 United States patients who underwent liver transplantation with cirrhosis, obesity was an independent predictor of HCC in patients with alcoholic and cryptogenic cirrhosis[44].
Epidemiological studies have also established an independent association between type 2 diabetes and HCC. The first population study conducted in United States revealed that diabetes was associated with 2.3-fold increase in the risk of HCC, regardless of the presence of other major HCC risk factors[45]. Consistently, El-Serag et al[31] carried out a large prospective cohort study, using the national database of the Department of Veterans Affairs, to examine HCC risk according to the presence of diabetes, and found that among men with diabetes the risk for HCC was doubled. Interestingly, it has been hypothesized that a high concentration of insulin and insulin growth factor 1 (IGF1) in type 2 diabetics may have a hepatocarcinogenic effect[46]. This would suggest that glucose lowering medications counteracting insulin resistance, such as metformin, could reduce the risk of HCC in diabetics[47-49], but this hypothesis is still based on indirect evidence and remains to be proven.
Results of a meta-analysis which included four studies (three prospective cohorts and one case-control study) considering 829651 participants supported an increased risk of HCC among individuals with metabolic syndrome[50].
The population attributable fractions for established HCC risk factors in the United States have been estimated in a population-based case-control study. Among individual risk factors, diabetes/obesity had the greatest population attributable fraction. Overall, these data imply that in Western countries decreasing diabetes and obesity would be able to reduce the HCC incidence more than the eradication of any other risk factors, including viral hepatitis[49].
Furthermore, the co-presence of both diabetes and obesity may have a synergistic effect on hepatic carcinogenesis in other etiologies. In 23820 Taiwan residents infected with HBV or HCV followed up for 14 years, HCC risk was more than 100-fold higher in those with both obesity and diabetes[51].
Summarizing, obesity is a strong risk factor for HCC development especially in men. The presence of metabolic syndrome and diabetes increases the risk exponentially in obese individuals (Table 2).
Table 2.
Ascertained risk factors for hepatocellular carcinoma in fatty liver
| Risk factors |
| Age |
| Male gender |
| HCV/HBV[3,30,31,36-38] |
| Alcohol abuse[34,35] |
| Severity of NAFLD/NASH[25,28-30,33,39] |
| Diabetes/obesity[26,31,43-46] |
| Iron overload[69-71] |
| Genetic variants (PNPLA3, APOB, TERT)[92-94,100,104,111,113,114] |
| Hepatic stellate cells (HSCs) activation and senescence[118,119] |
HCV: Hepatitis C virus; HBV: Hepatitis B virus; NAFLD: Nonalcoholic fatty liver disease; NASH: Nonalcoholic steatohepatitis; PNPLA3: Patatin-like phospholipase domain-containing protein 3; APOB: Apolipoprotein B; TERT: Telomerase reverse transcriptase.
PATHOGENESIS
The pathways linking steatosis to HCC are still under definition. Potential carcinogenic mechanisms specifically related to steatosis and steatohepatitis[7,52] are represented by (1) the low-grade inflammatory status associated with obesity and the metabolic syndrome, with increased release of tumor necrosis factors (TNF)-alpha and IL-6, which has proliferative and anti-apoptotic effects through the activation of the transcription factor STAT3[53]; (2) altered release of adipokines influencing insulin resistance and inflammation[54]; (3) increased lipogenesis and cellular availability of fatty acids supporting energy for rapidly growing cells[55,56]; (4) lipotoxicity influencing intracellular signaling pathways[57]; (5) oxidative stress and DNA damage related to increased lipid peroxidation and mitochondrial damage; and (6) hyperinsulinemia determining activation of proliferative pathways in hepatocytes and increased release of insulin-like growth factor-1 (IGF-1)[58].
Summarizing, intranuclear damage and changes predisposing to HCC may be due to activation of insulin mediated proliferative pathways, specific cytokine release, intracellular lipid species, oxidative stress, and mitochondrial damage.
OTHER ENVIRONMENTAL RISK FACTORS: IRON OVERLOAD?
Liver iron overload, which is frequently observed in patients with chronic HCV hepatitis, alcoholic liver disease, NAFLD, NASH and end-stage liver disease, may also exert a carcinogenic effect thus facilitating HCC development. Oxidative stress resulting in the production of oxygen free radicals is considered the main mechanism underlying the progression of liver damage toward HCC in genetic hemochromatosis[59,60]. Secondary iron overload worsens liver damage progression in other chronic liver disease as well[61,62]. In particular, hepatic iron accumulation seems to increase the risk of NASH progression[63], and to be involved in the pathogenesis of insulin resistance[64-67], while iron depletion by phlebotomy can improve insulin sensitivity and liver function in patients with NAFLD[68]. In a large case series of histological NAFLD from Italy, liver iron depots were more frequent and larger in patients who developed HCC than in those who did not, and excess iron was mainly located within non-parenchymal cells. Experimental studies indicate that the cellular site of iron deposition in the liver is important for its carcinogenic activity[69,70]. Summarizing, iron may be a risk factor for HCC, thus implying that iron chelation/depletion may represent a strategy to prevent liver disease progression and HCC patients with NAFLD and increased iron stores, identified by the presence of increased ferritin levels[71,72] (Table 2).
GENETICS
Familial, epidemiologic and twin studies suggest that heritability plays a major role in the susceptibility towards NAFLD and progressive forms of liver diseases associated with steatosis with possible evolution to HCC[73], and both steatosis and genetics contribute to HCC risk[15,17]. Following the completion of the Human Genome Project and the trail of technological advancements, in the last years genomewide association studies (GWAS) that screen the whole DNA for the association with a specific phenotype have unrevealed the main common genetic risk factor for complex traits and diseases, such as hepatic fat content. Besides viral, environmental factors, behavioral habits and metabolic features also inherited factors have been implicated in the pathogenesis of HCC. Family history and ethnicity are strong risk factor for HCC and both candidate genes studies and GWAS are beginning to discover the genetic determinants that may contribute to HCC susceptibility[16]. Recently, the first GWAS have discovered new candidates, such as the 1p36.22 locus in Chinese patients with chronic HBV infection[74], and the MICA rs2596542 and rs1012068 DEPDC5 single nucleotide polymorphisms (SNPs) in Japanese patients with CHC[75,76], which however still require functional clarification and replication in other ethnic groups.
Rapidly evolving knowledge has been generated suggesting that NAFLD, fibrosis progression, and HCC share common genetic determinants[77]. There is also evidence that rare variants associated with a severe phenotype strongly contribute to steatosis and HCC risk in specific individuals and their families[78] (Table 2).
Common variants: Patatin-like phospholipase domain-containing 3
In 2008, two independent GWAS linked the common rs738409 C>G SNP encoding for the I148M sequence variant of Patatin-like phospholipase domain-containing protein 3 (PNPLA3 or adiponutrin) with hepatic fat content, steatosis and ALT levels[79,80]. The 148M allele causes a critical aminoacidic substitution next to the catalytic domain, reducing the access of substrates and reducing the PNPLA3 enzymatic activity towards glycerolipids, thereby favoring to the development of macrovesicular steatosis[81-83], partly mediated by very low density lipoprotein retention[83]. Furthermore, the 148M risk allele favors disease progression in NAFLD[84-86], ALD[85,87], chronic HCV hepatitis[88,89], and also in liver diseases unrelated to steatosis[90,91].
Most importantly, the I148M variant has been associated with the risk of HCC independently of its effect on the progression of liver fibrosis[92,93]. We first described an association between 148M PNPLA3 variant and HCC in a retrospective cohort of 325 patients affected by chronic hepatitis C where homozygosity for the 148M risk allele conferred a 2.2-fold higher risk of HCC, independently of cirrhosis, age, diabetes, male gender, and eradication of viral hepatitis[88]. These data have been replicated in patients with HCV cirrhosis and alcoholic cirrhosis[92,94-96]. After a large European effort, a very recent meta-analysis of the literature based on individual patient data of 2503 cirrhotic patients concluded that the I148M is an independent risk factor for HCC in cirrhosis, and that the size effect is larger in alcoholic than in HCV related liver disease[97].
The predisposing effect of the I148M seems also very strong in the context of severe obesity. Indeed, in 3473 obese subjects included in the Swedish Obese Study (SOS) cohort followed up for 15 years, the 148M risk allele conferred a very large increase in the risk of HCC in subjects on conventional treatment, whereas the effect was not detected in those who improved metabolic disease following weight loss due to bariatric surgery[98]. Importantly, the I148M variant is also a strong risk factor for HCC in patients with histological NAFLD[99].
Finally, the I148M variant may also influence the biological features, natural history, and response to treatment of HCC. In 460 HCC Italian patients, PNPLA3 148M was over-represented in NAFLD and ALD-related HCCs, and liver cancers arising in these subjects were associated with earlier age at presentation, lower differentiation grade and larger diffusion at the diagnosis, and with a worse prognosis[100]. These data are in line with results obtained in a United States cohort[101].
In conclusion, the common PNPLA3 I148M variant is a strong determinant of hepatic fat content and in the presence of triggering factors (especially metabolic rather than viral) strongly predisposes to HCC[77].
Rare variants
Apolipoprotein B: Hypobetalipoproteinemia (HBL) represents a rare disorder of lipoprotein metabolism characterized by reduced plasma levels of total cholesterol, low-density lipoprotein-cholesterol (LDL-C) and apolipoprotein B (APOB). Familial HBL (FHBL) is the most frequent monogenic form of HBL and it is associated with loss-of-function mutations in APOB gene. Fatty liver is a characteristic feature of FHBL as a consequence of impaired hepatic export of very low density lipoproteins. Development of HCC in the absence of cirrhosis was anedoctically reported in a subject with FHBL due to a truncated form of ApoB[102], as well as cryptogenic cirrhosis due to a truncated form of ApoB[103].
Recently, evaluating by whole exome sequencing a large family in which it was observed cosegregation of FHBL with fatty liver and HCC, Cefalù et al[104] identified a novel nonsense mutation in ApoB gene (c.6718A>T, K2240X) associated with the phenotype. Therefore, APOB mutations represent a paradigm of rare variant influencing liver fat content and HCC risk. In the general perspective, the liver fat retention observed in individuals with the common PNPLA3 variant due to the defective very low density lipoprotein secretion[83] may indicate a common pathway for these genetic conditions.
Telomerase: The human telomerase reverse transcriptase (TERT) gene encodes the catalytic reverse transcriptase subunit of telomerase that maintain telomere length[105]. Increased telomerase activity has been indicated as one of the hallmarks of human cancer, and transcriptional up-regulation of TERT gene is the major cause of its cancer-specific activation[106-108]. On the other hand, telomere diseases, exemplified by dyskeratosis congenita, are characterized by premature senescence of the stem cell compartment, tissue fibrosis and increased cancer risk due to chromosome instability[108]. TERT mutations have been associated with a spectrum of familial hepatic liver disease often characterized by histological steatosis and iron accumulation, possibly as a consequence of hepatocellular senescence[109]. TERT mutations are enriched in cirrhosis, being observed in 3%-8% of unselected patients with different liver diseases, in line with increased telomere attrition in cirrhosis, and suggesting a role of cellular aging in liver disease progression[110,111]. In addition, studies on TERT-deficient mice have demonstrated that telomere shortening reduces the response to acute and chronic liver damage inducing the formation of steatosis and fibrosis[111]. TERT overexpression is frequently observed in HCC related to chronic hepatitis[112], favoring the replication of stem cell compartment. Point mutations in the promoter of TERT gene increase telomerase expression and are somatically acquired during hepatic carcinogenesis, especially in males with chronic HCV hepatitis[113]. On the other hand, HCC cases were reported only in a few patients with loss-of-function TERT mutations. Recently, we reported the occurrence of HCC in a subject with cirrhosis associated with NAFLD and diabetes, who carried the novel c.2062 C>G TERT mutation encoding for the protein Glu668Asp substitution in exon 5 of TERT[114]. This mutation is located in the reverse transcriptase domain of the protein, leading to reduced telomere lenght by interfering with enzymatic activity[115]. Additional studies are ongoing to evaluate whether telomerase complex mutations are highly enriched in patients who develop HCC in NAFLD. In conclusion, the occurrence of HCC in the context of NAFLD, which at the population level can be considered a rare disease, seems to be influenced by common genetic variants as PNPLA3 and by rare genetic variants such as those described in APOB and TERT genes[79].
STELLATE CELLS: A NEW PLAYER IN OBESITY-RELATED HEPATIC CARCINOGENESIS
Cellular senescence is a stress response occurring in normal cells, which blocks proliferation through checkpoint activation and cell-cycle arrest[116]. It occurs not only in hepatocytes, but also in nonparenchymal liver cells, and in particular hepatic stellate cells (HSCs), which are key players in the progression of liver fibrosis and hepatic carcinogenesis[117]. Senescent HSCs are biologically active and characterized by a secretory profile called senescence-associated secretory phenotype (SASP), composed of inflammatory cytokines, chemokines and proteases, which may promote hepatic carcinogenesis[118]. In experimental models, dietary or genetic obesity induces alterations of the gut microbiota increasing the levels of microbial metabolites such as deoxycholic acid (DCA), which are DNA damaging. The enterohepatic circulation can bring these metabolites to the liver inducing a SASP phenotype in activated HSCs, which in turn secrete tumor-promoting factors and favor HCC development even in absence of fibrosis. In healthy male volunteers, high-fat diet results in higher fecal DCA levels, suggesting that DCA-induced senescent HSCs may partly contribute to obesity-associated HCC by SASP also in humans. Moreover, signs of cellular senescence and SASP were directly observed in HSCs not related to fibrotic areas, but located in the proximity of HCC lesions occurring in patients with NASH. These findings suggest that senescent HSCs are characterized by an inflammatory but not fibrogenic phenotype and may contribute to explain NASH-associated hepatic carcinogenesis in non-cirrhotic livers[32,119]. In conclusion, improved understanding of the role of genetic predisposition, the type of diet, and gut microbiota may lead to new therapeutic approaches to prevent and control liver cancer related to NAFLD[120].
CONCLUSION
NAFLD is increasingly being recognized as a frequent cause of HCC, especially in Western countries, and is a contributing factor in a large fraction of cases related to past or active viral hepatitis and alcohol abuse. Besides viral, environmental factors, behavioral habits and metabolic features, inherited factors have been implicated in the pathogenesis of HCC. In particular, the common I148M variant of the PNPLA3 gene has been associated with HCC risk independently of the effect on the progression of liver fibrosis. Recently, rare functional mutations in genes influencing hepatic lipid metabolism and cellular senescence have also been linked to HCC in NAFLD. Finally, it has been recently suggested that HSCs senescence may represent a common effector in hepatic carcinogenesis of the alterations in gut microbiota, genetic factors and steatohepatitis. Better understanding the driving factors and genetics HCC in patients with obesity and diabetes will provide new therapeutic and diagnostic targets and facilitate the prevention and early diagnosis of the disease. The currently ascertained risk factors for HCC in NAFLD are presented in Figure 1.
Figure 1.
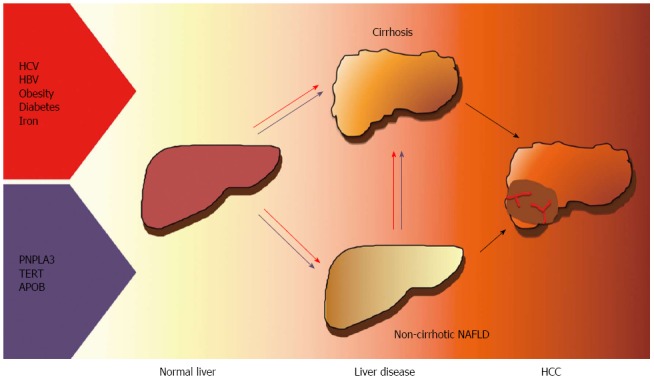
Graphical display of the ascertained risk factors for hepatocellular carcinoma in nonalcoholic fatty liver disease. HCC: Hepatocellular carcinoma; NAFLD: Nonalcoholic fatty liver disease; Red lines: Metabolic risk factors; Violet lines: Genetic risk factors; HCV: Hepatitis C virus; HBV: Hepatitis B virus; TERT: Telomerase reverse transcriptase; APOB: Apolipoprotein B; PNPLA3: Patatinlike phospholipase domain-containing protein 3.
Footnotes
P- Reviewer: van Erpecum K, Yamagiwa S S- Editor: Nan J L- Editor: A E- Editor: Zhang DN
References
- 1.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 2.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 4.Fracanzani AL, Fargion S, Stazi MA, Valenti L, Amoroso P, Cariani E, Sangiovanni A, Tommasini M, Rossini A, Bertelli C, et al. Association between heterozygosity for HFE gene mutations and hepatitis viruses in hepatocellular carcinoma. Blood Cells Mol Dis. 2005;35:27–32. doi: 10.1016/j.bcmd.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Rudnick DA, Perlmutter DH. Alpha-1-antitrypsin deficiency: a new paradigm for hepatocellular carcinoma in genetic liver disease. Hepatology. 2005;42:514–521. doi: 10.1002/hep.20815. [DOI] [PubMed] [Google Scholar]
- 6.Fracanzani AL, Piperno A, Valenti L, Fraquelli M, Coletti S, Maraschi A, Consonni D, Coviello E, Conte D, Fargion S. Hemochromatosis in Italy in the last 30 years: role of genetic and acquired factors. Hepatology. 2010;51:501–510. doi: 10.1002/hep.23333. [DOI] [PubMed] [Google Scholar]
- 7.Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J Hepatol. 2012;56:1384–1391. doi: 10.1016/j.jhep.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 8.Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, Forlani G, Melchionda N. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450–455. doi: 10.1016/s0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- 9.Dyson J, Jaques B, Chattopadyhay D, Lochan R, Graham J, Das D, Aslam T, Patanwala I, Gaggar S, Cole M, et al. Hepatocellular cancer: the impact of obesity, type 2 diabetes and a multidisciplinary team. J Hepatol. 2014;60:110–117. doi: 10.1016/j.jhep.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Sangiovanni A, Prati GM, Fasani P, Ronchi G, Romeo R, Manini M, Del Ninno E, Morabito A, Colombo M. The natural history of compensated cirrhosis due to hepatitis C virus: A 17-year cohort study of 214 patients. Hepatology. 2006;43:1303–1310. doi: 10.1002/hep.21176. [DOI] [PubMed] [Google Scholar]
- 11.El-Serag HB, Richardson PA, Everhart JE. The role of diabetes in hepatocellular carcinoma: a case-control study among United States Veterans. Am J Gastroenterol. 2001;96:2462–2467. doi: 10.1111/j.1572-0241.2001.04054.x. [DOI] [PubMed] [Google Scholar]
- 12.Marrero JA, Fontana RJ, Fu S, Conjeevaram HS, Su GL, Lok AS. Alcohol, tobacco and obesity are synergistic risk factors for hepatocellular carcinoma. J Hepatol. 2005;42:218–224. doi: 10.1016/j.jhep.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Trichopoulos D, Bamia C, Lagiou P, Fedirko V, Trepo E, Jenab M, Pischon T, Nöthlings U, Overved K, Tjønneland A, et al. Hepatocellular carcinoma risk factors and disease burden in a European cohort: a nested case-control study. J Natl Cancer Inst. 2011;103:1686–1695. doi: 10.1093/jnci/djr395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 15.Turati F, Talamini R, Pelucchi C, Polesel J, Franceschi S, Crispo A, Izzo F, La Vecchia C, Boffetta P, Montella M. Metabolic syndrome and hepatocellular carcinoma risk. Br J Cancer. 2013;108:222–228. doi: 10.1038/bjc.2012.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nahon P, Zucman-Rossi J. Single nucleotide polymorphisms and risk of hepatocellular carcinoma in cirrhosis. J Hepatol. 2012;57:663–674. doi: 10.1016/j.jhep.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 17.Turati F, Edefonti V, Talamini R, Ferraroni M, Malvezzi M, Bravi F, Franceschi S, Montella M, Polesel J, Zucchetto A, et al. Family history of liver cancer and hepatocellular carcinoma. Hepatology. 2012;55:1416–1425. doi: 10.1002/hep.24794. [DOI] [PubMed] [Google Scholar]
- 18.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 19.Day CP. From fat to inflammation. Gastroenterology. 2006;130:207–210. doi: 10.1053/j.gastro.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 20.Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, Musso A, De Paolis P, Capussotti L, Salizzoni M, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134–140. doi: 10.1053/gast.2002.34168. [DOI] [PubMed] [Google Scholar]
- 21.Lazo M, Hernaez R, Eberhardt MS, Bonekamp S, Kamel I, Guallar E, Koteish A, Brancati FL, Clark JM. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2013;178:38–45. doi: 10.1093/aje/kws448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thoraval F. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol. 2013;58:593–608. doi: 10.1016/j.jhep.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol. 2013;10:656–665. doi: 10.1038/nrgastro.2013.183. [DOI] [PubMed] [Google Scholar]
- 24.Siegel AB, Zhu AX. Metabolic syndrome and hepatocellular carcinoma: two growing epidemics with a potential link. Cancer. 2009;115:5651–5661. doi: 10.1002/cncr.24687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002;36:1349–1354. doi: 10.1053/jhep.2002.36939. [DOI] [PubMed] [Google Scholar]
- 26.Regimbeau JM, Colombat M, Mognol P, Durand F, Abdalla E, Degott C, Degos F, Farges O, Belghiti J. Obesity and diabetes as a risk factor for hepatocellular carcinoma. Liver Transpl. 2004;10:S69–S73. doi: 10.1002/lt.20033. [DOI] [PubMed] [Google Scholar]
- 27.Lee SS, Jeong SH, Byoun YS, Chung SM, Seong MH, Sohn HR, Min BY, Jang ES, Kim JW, Park GJ, et al. Clinical features and outcome of cryptogenic hepatocellular carcinoma compared to those of viral and alcoholic hepatocellular carcinoma. BMC Cancer. 2013;13:335. doi: 10.1186/1471-2407-13-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimada M, Hashimoto E, Taniai M, Hasegawa K, Okuda H, Hayashi N, Takasaki K, Ludwig J. Hepatocellular carcinoma in patients with non-alcoholic steatohepatitis. J Hepatol. 2002;37:154–160. doi: 10.1016/s0168-8278(02)00099-5. [DOI] [PubMed] [Google Scholar]
- 29.Mori S, Yamasaki T, Sakaida I, Takami T, Sakaguchi E, Kimura T, Kurokawa F, Maeyama S, Okita K. Hepatocellular carcinoma with nonalcoholic steatohepatitis. J Gastroenterol. 2004;39:391–396. doi: 10.1007/s00535-003-1308-3. [DOI] [PubMed] [Google Scholar]
- 30.Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 31.El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460–468. doi: 10.1053/j.gastro.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 32.Takuma Y, Nouso K. Nonalcoholic steatohepatitis-associated hepatocellular carcinoma: our case series and literature review. World J Gastroenterol. 2010;16:1436–1441. doi: 10.3748/wjg.v16.i12.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yasui K, Hashimoto E, Komorizono Y, Koike K, Arii S, Imai Y, Shima T, Kanbara Y, Saibara T, Mori T, et al. Characteristics of patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2011;9:428–33; quiz e50. doi: 10.1016/j.cgh.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 34.Teli MR, Day CP, Burt AD, Bennett MK, James OF. Determinants of progression to cirrhosis or fibrosis in pure alcoholic fatty liver. Lancet. 1995;346:987–990. doi: 10.1016/s0140-6736(95)91685-7. [DOI] [PubMed] [Google Scholar]
- 35.Valenti L, Fracanzani AL, Fargion S. The immunopathogenesis of alcoholic and nonalcoholic steatohepatitis: two triggers for one disease? Semin Immunopathol. 2009;31:359–369. doi: 10.1007/s00281-009-0152-9. [DOI] [PubMed] [Google Scholar]
- 36.Pekow JR, Bhan AK, Zheng H, Chung RT. Hepatic steatosis is associated with increased frequency of hepatocellular carcinoma in patients with hepatitis C-related cirrhosis. Cancer. 2007;109:2490–2496. doi: 10.1002/cncr.22701. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka A, Uegaki S, Kurihara H, Aida K, Mikami M, Nagashima I, Shiga J, Takikawa H. Hepatic steatosis as a possible risk factor for the development of hepatocellular carcinoma after eradication of hepatitis C virus with antiviral therapy in patients with chronic hepatitis C. World J Gastroenterol. 2007;13:5180–5187. doi: 10.3748/wjg.v13.i39.5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nkontchou G, Ziol M, Aout M, Lhabadie M, Baazia Y, Mahmoudi A, Roulot D, Ganne-Carrie N, Grando-Lemaire V, Trinchet JC, et al. HCV genotype 3 is associated with a higher hepatocellular carcinoma incidence in patients with ongoing viral C cirrhosis. J Viral Hepat. 2011;18:e516–e522. doi: 10.1111/j.1365-2893.2011.01441.x. [DOI] [PubMed] [Google Scholar]
- 39.Salomao M, Yu WM, Brown RS, Emond JC, Lefkowitch JH. Steatohepatitic hepatocellular carcinoma (SH-HCC): a distinctive histological variant of HCC in hepatitis C virus-related cirrhosis with associated NAFLD/NASH. Am J Surg Pathol. 2010;34:1630–1636. doi: 10.1097/PAS.0b013e3181f31caa. [DOI] [PubMed] [Google Scholar]
- 40.Alexander J, Torbenson M, Wu TT, Yeh MM. Non-alcoholic fatty liver disease contributes to hepatocarcinogenesis in non-cirrhotic liver: a clinical and pathological study. J Gastroenterol Hepatol. 2013;28:848–854. doi: 10.1111/jgh.12116. [DOI] [PubMed] [Google Scholar]
- 41.Rahman R, Hammoud GM, Almashhrawi AA, Ahmed KT, Ibdah JA. Primary hepatocellular carcinoma and metabolic syndrome: An update. World J Gastrointest Oncol. 2013;5:186–194. doi: 10.4251/wjgo.v5.i9.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paradis V, Zalinski S, Chelbi E, Guedj N, Degos F, Vilgrain V, Bedossa P, Belghiti J. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology. 2009;49:851–859. doi: 10.1002/hep.22734. [DOI] [PubMed] [Google Scholar]
- 43.Larsson SC, Wolk A. Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br J Cancer. 2007;97:1005–1008. doi: 10.1038/sj.bjc.6603932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nair S, Verma S, Thuluvath PJ. Obesity and its effect on survival in patients undergoing orthotopic liver transplantation in the United States. Hepatology. 2002;35:105–109. doi: 10.1053/jhep.2002.30318. [DOI] [PubMed] [Google Scholar]
- 45.Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut. 2005;54:533–539. doi: 10.1136/gut.2004.052167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 47.Hassan MM, Curley SA, Li D, Kaseb A, Davila M, Abdalla EK, Javle M, Moghazy DM, Lozano RD, Abbruzzese JL, et al. Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma. Cancer. 2010;116:1938–1946. doi: 10.1002/cncr.24982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lai SW, Chen PC, Liao KF, Muo CH, Lin CC, Sung FC. Risk of hepatocellular carcinoma in diabetic patients and risk reduction associated with anti-diabetic therapy: a population-based cohort study. Am J Gastroenterol. 2012;107:46–52. doi: 10.1038/ajg.2011.384. [DOI] [PubMed] [Google Scholar]
- 49.Welzel TM, Graubard BI, Quraishi S, Zeuzem S, Davila JA, El-Serag HB, McGlynn KA. Population-attributable fractions of risk factors for hepatocellular carcinoma in the United States. Am J Gastroenterol. 2013;108:1314–1321. doi: 10.1038/ajg.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jinjuvadia R, Patel S, Liangpunsakul S. The association between metabolic syndrome and hepatocellular carcinoma: systemic review and meta-analysis. J Clin Gastroenterol. 2014;48:172–177. doi: 10.1097/MCG.0b013e3182a030c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen CL, Yang HI, Yang WS, Liu CJ, Chen PJ, You SL, Wang LY, Sun CA, Lu SN, Chen DS, et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135:111–121. doi: 10.1053/j.gastro.2008.03.073. [DOI] [PubMed] [Google Scholar]
- 52.Stickel F, Hellerbrand C. Non-alcoholic fatty liver disease as a risk factor for hepatocellular carcinoma: mechanisms and implications. Gut. 2010;59:1303–1307. doi: 10.1136/gut.2009.199661. [DOI] [PubMed] [Google Scholar]
- 53.Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marra F, Bertolani C. Adipokines in liver diseases. Hepatology. 2009;50:957–969. doi: 10.1002/hep.23046. [DOI] [PubMed] [Google Scholar]
- 55.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 56.Yamashita T, Honda M, Takatori H, Nishino R, Minato H, Takamura H, Ohta T, Kaneko S. Activation of lipogenic pathway correlates with cell proliferation and poor prognosis in hepatocellular carcinoma. J Hepatol. 2009;50:100–110. doi: 10.1016/j.jhep.2008.07.036. [DOI] [PubMed] [Google Scholar]
- 57.Vinciguerra M, Carrozzino F, Peyrou M, Carlone S, Montesano R, Benelli R, Foti M. Unsaturated fatty acids promote hepatoma proliferation and progression through downregulation of the tumor suppressor PTEN. J Hepatol. 2009;50:1132–1141. doi: 10.1016/j.jhep.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 58.Gallagher EJ, LeRoith D. Minireview: IGF, Insulin, and Cancer. Endocrinology. 2011;152:2546–2551. doi: 10.1210/en.2011-0231. [DOI] [PubMed] [Google Scholar]
- 59.Houglum K, Ramm GA, Crawford DH, Witztum JL, Powell LW, Chojkier M. Excess iron induces hepatic oxidative stress and transforming growth factor beta1 in genetic hemochromatosis. Hepatology. 1997;26:605–610. doi: 10.1002/hep.510260311. [DOI] [PubMed] [Google Scholar]
- 60.Valenti L, Fracanzani AL, Rametta R, Fraquelli M, Soverini G, Pelusi S, Dongiovanni P, Conte D, Fargion S. Effect of the A736V TMPRSS6 polymorphism on the penetrance and clinical expression of hereditary hemochromatosis. J Hepatol. 2012;57:1319–1325. doi: 10.1016/j.jhep.2012.07.041. [DOI] [PubMed] [Google Scholar]
- 61.Alla V, Bonkovsky HL. Iron in nonhemochromatotic liver disorders. Semin Liver Dis. 2005;25:461–472. doi: 10.1055/s-2005-923317. [DOI] [PubMed] [Google Scholar]
- 62.Dongiovanni P, Fracanzani AL, Cairo G, Megazzini CP, Gatti S, Rametta R, Fargion S, Valenti L. Iron-dependent regulation of MDM2 influences p53 activity and hepatic carcinogenesis. Am J Pathol. 2010;176:1006–1017. doi: 10.2353/ajpath.2010.090249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fargion S, Mattioli M, Fracanzani AL, Sampietro M, Tavazzi D, Fociani P, Taioli E, Valenti L, Fiorelli G. Hyperferritinemia, iron overload, and multiple metabolic alterations identify patients at risk for nonalcoholic steatohepatitis. Am J Gastroenterol. 2001;96:2448–2455. doi: 10.1111/j.1572-0241.2001.04052.x. [DOI] [PubMed] [Google Scholar]
- 64.Bugianesi E, Manzini P, D’Antico S, Vanni E, Longo F, Leone N, Massarenti P, Piga A, Marchesini G, Rizzetto M. Relative contribution of iron burden, HFE mutations, and insulin resistance to fibrosis in nonalcoholic fatty liver. Hepatology. 2004;39:179–187. doi: 10.1002/hep.20023. [DOI] [PubMed] [Google Scholar]
- 65.Mendler MH, Turlin B, Moirand R, Jouanolle AM, Sapey T, Guyader D, Le Gall JY, Brissot P, David V, Deugnier Y. Insulin resistance-associated hepatic iron overload. Gastroenterology. 1999;117:1155–1163. doi: 10.1016/s0016-5085(99)70401-4. [DOI] [PubMed] [Google Scholar]
- 66.Valenti L, Fracanzani AL, Bugianesi E, Dongiovanni P, Galmozzi E, Vanni E, Canavesi E, Lattuada E, Roviaro G, Marchesini G, et al. HFE genotype, parenchymal iron accumulation, and liver fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2010;138:905–912. doi: 10.1053/j.gastro.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 67.Dongiovanni P, Ruscica M, Rametta R, Recalcati S, Steffani L, Gatti S, Girelli D, Cairo G, Magni P, Fargion S, et al. Dietary iron overload induces visceral adipose tissue insulin resistance. Am J Pathol. 2013;182:2254–2263. doi: 10.1016/j.ajpath.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 68.Valenti L, Fracanzani AL, Dongiovanni P, Bugianesi E, Marchesini G, Manzini P, Vanni E, Fargion S. Iron depletion by phlebotomy improves insulin resistance in patients with nonalcoholic fatty liver disease and hyperferritinemia: evidence from a case-control study. Am J Gastroenterol. 2007;102:1251–1258. doi: 10.1111/j.1572-0241.2007.01192.x. [DOI] [PubMed] [Google Scholar]
- 69.Stál P, Hultcrantz R, Möller L, Eriksson LC. The effects of dietary iron on initiation and promotion in chemical hepatocarcinogenesis. Hepatology. 1995;21:521–528. doi: 10.1002/hep.1840210237. [DOI] [PubMed] [Google Scholar]
- 70.Carthew P, Nolan BM, Smith AG, Edwards RE. Iron promotes DEN initiated GST-P foci in rat liver. Carcinogenesis. 1997;18:599–603. doi: 10.1093/carcin/18.3.599. [DOI] [PubMed] [Google Scholar]
- 71.Sorrentino P, D’Angelo S, Ferbo U, Micheli P, Bracigliano A, Vecchione R. Liver iron excess in patients with hepatocellular carcinoma developed on non-alcoholic steato-hepatitis. J Hepatol. 2009;50:351–357. doi: 10.1016/j.jhep.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 72.Dongiovanni P, Fracanzani AL, Fargion S, Valenti L. Iron in fatty liver and in the metabolic syndrome: a promising therapeutic target. J Hepatol. 2011;55:920–932. doi: 10.1016/j.jhep.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 73.Dongiovanni P, Anstee QM, Valenti L. Genetic predisposition in NAFLD and NASH: impact on severity of liver disease and response to treatment. Curr Pharm Des. 2013;19:5219–5238. doi: 10.2174/13816128113199990381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang H, Zhai Y, Hu Z, Wu C, Qian J, Jia W, Ma F, Huang W, Yu L, Yue W, et al. Genome-wide association study identifies 1p36.22 as a new susceptibility locus for hepatocellular carcinoma in chronic hepatitis B virus carriers. Nat Genet. 2010;42:755–758. doi: 10.1038/ng.638. [DOI] [PubMed] [Google Scholar]
- 75.Kumar V, Kato N, Urabe Y, Takahashi A, Muroyama R, Hosono N, Otsuka M, Tateishi R, Omata M, Nakagawa H, et al. Genome-wide association study identifies a susceptibility locus for HCV-induced hepatocellular carcinoma. Nat Genet. 2011;43:455–458. doi: 10.1038/ng.809. [DOI] [PubMed] [Google Scholar]
- 76.Miki D, Ochi H, Hayes CN, Abe H, Yoshima T, Aikata H, Ikeda K, Kumada H, Toyota J, Morizono T, et al. Variation in the DEPDC5 locus is associated with progression to hepatocellular carcinoma in chronic hepatitis C virus carriers. Nat Genet. 2011;43:797–800. doi: 10.1038/ng.876. [DOI] [PubMed] [Google Scholar]
- 77.Valenti L, Dongiovanni P, Ginanni Corradini S, Burza MA, Romeo S. PNPLA3 I148M variant and hepatocellular carcinoma: a common genetic variant for a rare disease. Dig Liver Dis. 2013;45:619–624. doi: 10.1016/j.dld.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 78.Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, McCarthy MI, Ramos EM, Cardon LR, Chakravarti A, et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yuan X, Waterworth D, Perry JR, Lim N, Song K, Chambers JC, Zhang W, Vollenweider P, Stirnadel H, Johnson T, et al. Population-based genome-wide association studies reveal six loci influencing plasma levels of liver enzymes. Am J Hum Genet. 2008;83:520–528. doi: 10.1016/j.ajhg.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.He S, McPhaul C, Li JZ, Garuti R, Kinch L, Grishin NV, Cohen JC, Hobbs HH. A sequence variation (I148M) in PNPLA3 associated with nonalcoholic fatty liver disease disrupts triglyceride hydrolysis. J Biol Chem. 2010;285:6706–6715. doi: 10.1074/jbc.M109.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang Y, He S, Li JZ, Seo YK, Osborne TF, Cohen JC, Hobbs HH. A feed-forward loop amplifies nutritional regulation of PNPLA3. Proc Natl Acad Sci USA. 2010;107:7892–7897. doi: 10.1073/pnas.1003585107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pirazzi C, Adiels M, Burza MA, Mancina RM, Levin M, Ståhlman M, Taskinen MR, Orho-Melander M, Perman J, Pujia A, et al. Patatin-like phospholipase domain-containing 3 (PNPLA3) I148M (rs738409) affects hepatic VLDL secretion in humans and in vitro. J Hepatol. 2012;57:1276–1282. doi: 10.1016/j.jhep.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 84.Valenti L, Al-Serri A, Daly AK, Galmozzi E, Rametta R, Dongiovanni P, Nobili V, Mozzi E, Roviaro G, Vanni E, et al. Homozygosity for the patatin-like phospholipase-3/adiponutrin I148M polymorphism influences liver fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51:1209–1217. doi: 10.1002/hep.23622. [DOI] [PubMed] [Google Scholar]
- 85.Stickel F, Buch S, Lau K, Meyer zu Schwabedissen H, Berg T, Ridinger M, Rietschel M, Schafmayer C, Braun F, Hinrichsen H, et al. Genetic variation in the PNPLA3 gene is associated with alcoholic liver injury in caucasians. Hepatology. 2011;53:86–95. doi: 10.1002/hep.24017. [DOI] [PubMed] [Google Scholar]
- 86.Sookoian S, Pirola CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. 2011;53:1883–1894. doi: 10.1002/hep.24283. [DOI] [PubMed] [Google Scholar]
- 87.Tian C, Stokowski RP, Kershenobich D, Ballinger DG, Hinds DA. Variant in PNPLA3 is associated with alcoholic liver disease. Nat Genet. 2010;42:21–23. doi: 10.1038/ng.488. [DOI] [PubMed] [Google Scholar]
- 88.Valenti L, Rumi M, Galmozzi E, Aghemo A, Del Menico B, De Nicola S, Dongiovanni P, Maggioni M, Fracanzani AL, Rametta R, et al. Patatin-like phospholipase domain-containing 3 I148M polymorphism, steatosis, and liver damage in chronic hepatitis C. Hepatology. 2011;53:791–799. doi: 10.1002/hep.24123. [DOI] [PubMed] [Google Scholar]
- 89.Trépo E, Pradat P, Potthoff A, Momozawa Y, Quertinmont E, Gustot T, Lemmers A, Berthillon P, Amininejad L, Chevallier M, et al. Impact of patatin-like phospholipase-3 (rs738409 C& gt; G) polymorphism on fibrosis progression and steatosis in chronic hepatitis C. Hepatology. 2011;54:60–69. doi: 10.1002/hep.24350. [DOI] [PubMed] [Google Scholar]
- 90.Valenti L, Maggioni P, Piperno A, Rametta R, Pelucchi S, Mariani R, Dongiovanni P, Fracanzani AL, Fargion S. Patatin-like phospholipase domain containing-3 gene I148M polymorphism, steatosis, and liver damage in hereditary hemochromatosis. World J Gastroenterol. 2012;18:2813–2820. doi: 10.3748/wjg.v18.i22.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Viganò M, Valenti L, Lampertico P, Facchetti F, Motta BM, D’Ambrosio R, Romagnoli S, Dongiovanni P, Donati B, Fargion S, et al. Patatin-like phospholipase domain-containing 3 I148M affects liver steatosis in patients with chronic hepatitis B. Hepatology. 2013;58:1245–1252. doi: 10.1002/hep.26445. [DOI] [PubMed] [Google Scholar]
- 92.Trepo E, Guyot E, Ganne-Carrie N, Degre D, Gustot T, Franchimont D, Sutton A, Nahon P, Moreno C. PNPLA3 (rs738409 C& gt; G) is a common risk variant associated with hepatocellular carcinoma in alcoholic cirrhosis. Hepatology. 2012;55:1307–1308. doi: 10.1002/hep.25518. [DOI] [PubMed] [Google Scholar]
- 93.Corradini SG, Burza MA, Molinaro A, Romeo S. Patatin-like phospholipase domain containing 3 sequence variant and hepatocellular carcinoma. Hepatology. 2011;53:1776; author reply 1777. doi: 10.1002/hep.24244. [DOI] [PubMed] [Google Scholar]
- 94.Falleti E, Fabris C, Cmet S, Cussigh A, Bitetto D, Fontanini E, Fornasiere E, Bignulin S, Fumolo E, Bignulin E, et al. PNPLA3 rs738409C/G polymorphism in cirrhosis: relationship with the aetiology of liver disease and hepatocellular carcinoma occurrence. Liver Int. 2011;31:1137–1143. doi: 10.1111/j.1478-3231.2011.02534.x. [DOI] [PubMed] [Google Scholar]
- 95.Nischalke HD, Berger C, Luda C, Berg T, Müller T, Grünhage F, Lammert F, Coenen M, Krämer B, Körner C, et al. The PNPLA3 rs738409 148M/M genotype is a risk factor for liver cancer in alcoholic cirrhosis but shows no or weak association in hepatitis C cirrhosis. PLoS One. 2011;6:e27087. doi: 10.1371/journal.pone.0027087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guyot E, Sutton A, Rufat P, Laguillier C, Mansouri A, Moreau R, Ganne-Carrié N, Beaugrand M, Charnaux N, Trinchet JC, et al. PNPLA3 rs738409, hepatocellular carcinoma occurrence and risk model prediction in patients with cirrhosis. J Hepatol. 2013;58:312–318. doi: 10.1016/j.jhep.2012.09.036. [DOI] [PubMed] [Google Scholar]
- 97.Trépo E, Nahon P, Bontempi G, Valenti L, Falleti E, Nischalke HD, Hamza S, Corradini SG, Burza MA, Guyot E, et al. Association between the PNPLA3 (rs738409 C& gt; G) variant and hepatocellular carcinoma: Evidence from a meta-analysis of individual participant data. Hepatology. 2014;59:2170–2177. doi: 10.1002/hep.26767. [DOI] [PubMed] [Google Scholar]
- 98.Burza MA, Pirazzi C, Maglio C, Sjöholm K, Mancina RM, Svensson PA, Jacobson P, Adiels M, Baroni MG, Borén J, et al. PNPLA3 I148M (rs738409) genetic variant is associated with hepatocellular carcinoma in obese individuals. Dig Liver Dis. 2012;44:1037–1041. doi: 10.1016/j.dld.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 99.Liu YL, Patman GL, Leathart JB, Piguet AC, Burt AD, Dufour JF, Day CP, Daly AK, Reeves HL, Anstee QM. Carriage of the PNPLA3 rs738409 C & gt; G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J Hepatol. 2014;61:75–81. doi: 10.1016/j.jhep.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 100.Valenti L, Motta BM, Soardo G, Iavarone M, Donati B, Sangiovanni A, Carnelutti A, Dongiovanni P, Rametta R, Bertelli C, et al. PNPLA3 I148M polymorphism, clinical presentation, and survival in patients with hepatocellular carcinoma. PLoS One. 2013;8:e75982. doi: 10.1371/journal.pone.0075982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hassan MM, Kaseb A, Etzel CJ, El-Serag H, Spitz MR, Chang P, Hale KS, Liu M, Rashid A, Shama M, et al. Genetic variation in the PNPLA3 gene and hepatocellular carcinoma in USA: risk and prognosis prediction. Mol Carcinog. 2013;52 Suppl 1:E139–E147. doi: 10.1002/mc.22057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lonardo A, Tarugi P, Ballarini G, Bagni A. Familial heterozygous hypobetalipoproteinemia, extrahepatic primary malignancy, and hepatocellular carcinoma. Dig Dis Sci. 1998;43:2489–2492. doi: 10.1023/a:1026646618643. [DOI] [PubMed] [Google Scholar]
- 103.Bonnefont-Rousselot D, Condat B, Sassolas A, Chebel S, Bittar R, Federspiel MC, Cazals-Hatem D, Bruckert E. Cryptogenic cirrhosis in a patient with familial hypocholesterolemia due to a new truncated form of apolipoprotein B. Eur J Gastroenterol Hepatol. 2009;21:104–108. doi: 10.1097/MEG.0b013e3282ffd9f8. [DOI] [PubMed] [Google Scholar]
- 104.Cefalù AB, Pirruccello JP, Noto D, Gabriel S, Valenti V, Gupta N, Spina R, Tarugi P, Kathiresan S, Averna MR. A novel APOB mutation identified by exome sequencing cosegregates with steatosis, liver cancer, and hypocholesterolemia. Arterioscler Thromb Vasc Biol. 2013;33:2021–2025. doi: 10.1161/ATVBAHA.112.301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dwyer J, Li H, Xu D, Liu JP. Transcriptional regulation of telomerase activity: roles of the the Ets transcription factor family. Ann N Y Acad Sci. 2007;1114:36–47. doi: 10.1196/annals.1396.022. [DOI] [PubMed] [Google Scholar]
- 106.Daniel M, Peek GW, Tollefsbol TO. Regulation of the human catalytic subunit of telomerase (hTERT) Gene. 2012;498:135–146. doi: 10.1016/j.gene.2012.01.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 108.Chaiteerakij R, Roberts LR. Telomerase mutation: a genetic risk factor for cirrhosis. Hepatology. 2011;53:1430–1432. doi: 10.1002/hep.24304. [DOI] [PubMed] [Google Scholar]
- 109.Calado RT, Regal JA, Kleiner DE, Schrump DS, Peterson NR, Pons V, Chanock SJ, Lansdorp PM, Young NS. A spectrum of severe familial liver disorders associate with telomerase mutations. PLoS One. 2009;4:e7926. doi: 10.1371/journal.pone.0007926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Calado RT, Brudno J, Mehta P, Kovacs JJ, Wu C, Zago MA, Chanock SJ, Boyer TD, Young NS. Constitutional telomerase mutations are genetic risk factors for cirrhosis. Hepatology. 2011;53:1600–1607. doi: 10.1002/hep.24173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rudolph KL, Chang S, Millard M, Schreiber-Agus N, DePinho RA. Inhibition of experimental liver cirrhosis in mice by telomerase gene delivery. Science. 2000;287:1253–1258. doi: 10.1126/science.287.5456.1253. [DOI] [PubMed] [Google Scholar]
- 112.Llovet JM, Chen Y, Wurmbach E, Roayaie S, Fiel MI, Schwartz M, Thung SN, Khitrov G, Zhang W, Villanueva A, et al. A molecular signature to discriminate dysplastic nodules from early hepatocellular carcinoma in HCV cirrhosis. Gastroenterology. 2006;131:1758–1767. doi: 10.1053/j.gastro.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 113.Killela PJ, Reitman ZJ, Jiao Y, Bettegowda C, Agrawal N, Diaz LA, Friedman AH, Friedman H, Gallia GL, Giovanella BC, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci USA. 2013;110:6021–6026. doi: 10.1073/pnas.1303607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Valenti L, Dongiovanni P, Maggioni M, Motta BM, Rametta R, Milano M, Fargion S, Reggiani P, Fracanzani AL. Liver transplantation for hepatocellular carcinoma in a patient with a novel telomerase mutation and steatosis. J Hepatol. 2013;58:399–401. doi: 10.1016/j.jhep.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 115.Liang J, Yagasaki H, Kamachi Y, Hama A, Matsumoto K, Kato K, Kudo K, Kojima S. Mutations in telomerase catalytic protein in Japanese children with aplastic anemia. Haematologica. 2006;91:656–658. [PubMed] [Google Scholar]
- 116.Collado M, Serrano M. Senescence in tumours: evidence from mice and humans. Nat Rev Cancer. 2010;10:51–57. doi: 10.1038/nrc2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee UE, Friedman SL. Mechanisms of hepatic fibrogenesis. Best Pract Res Clin Gastroenterol. 2011;25:195–206. doi: 10.1016/j.bpg.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 119.Schnabl B, Purbeck CA, Choi YH, Hagedorn CH, Brenner D. Replicative senescence of activated human hepatic stellate cells is accompanied by a pronounced inflammatory but less fibrogenic phenotype. Hepatology. 2003;37:653–664. doi: 10.1053/jhep.2003.50097. [DOI] [PubMed] [Google Scholar]
- 120.Devkota S, Turnbaugh PJ. Cancer: An acidic link. Nature. 2013;499:37–38. doi: 10.1038/nature12404. [DOI] [PubMed] [Google Scholar]


