Abstract
Nuclear erythroid 2-related factor 2 (Nrf2) is a central regulator of antioxidative response elements-mediated gene expression. It has a significant role in adaptive responses to oxidative stress by interacting with the antioxidant response element, which induces the expression of a variety of downstream targets aimed at cytoprotection. Previous studies suggested oxidative stress and associated damage could represent a common link between different forms of diseases. Oxidative stress has been implicated in various liver diseases, including viral hepatitis, nonalcoholic fatty liver disease/steatohepatitis, alcoholic liver disease and drug-induced liver injury. Nrf2 activation is initiated by oxidative or electrophilic stress, and aids in the detoxification and elimination of potentially harmful exogenous chemicals and their metabolites. The expression of Nrf2 has been observed throughout human tissue, with high expression in detoxification organs, especially the liver. Thus, Nrf2 may serve as a major regulator of several cellular defense associated pathways by which hepatic cells combat oxidative stress. We review the relevant literature concerning the crucial role of Nrf2 and its signaling pathways against oxidative stress to protect hepatic cell from oxidative damage during development of common chronic liver diseases. We also review the use of Nrf2 as a therapeutic target to prevent and treat liver diseases.
Keywords: Nuclear erythroid 2-related factor 2, Chronic liver disease, Oxidative stress, Reactive oxygen species, Hepatic injury, Hepatic protection
Core tip: Chronic liver disease is associated with an imbalance, comprising increased reactive oxygen species and decreased net antioxidant activity. Oxidative stress plays an important role in the pathophysiological changes of liver diseases. Nuclear erythroid 2-related factor 2 (Nrf2) can activate cytoprotective genes and has a crucial role against oxidative stress to protect hepatic cells from oxidative damage. This article focuses on the activation the Nrf2-mediated antioxidant response, which prevents the progression of chronic liver disease and presents new treatment opportunities. Accordingly, integrative therapeutic strategies including Nrf2 activators have great potential as therapeutic agents against oxidative stress during chronic liver injuries.
INTRODUCTION
Nuclear erythroid 2-related factor 2 (Nrf2) is a transcription factor, first identified in 1994, that belongs to the Cap-n-collar basic leucine zipper family[1]. It has a significant role in adaptive responses to oxidative stress by interacting with antioxidant response element (ARE) sequences of antioxidant and cytoprotective genes[2]. Nrf2 is considered the main mediator of cellular adaptation to redox stress. In its inactive state, Nrf2 is located in the cytoplasm where it interacts with the actin binding protein, Kelch-like ECH associating protein 1, and is rapidly degraded by the ubiquitin-proteasome pathway. However, upon exposure to oxidative or electrophilic stress, phosphorylation of Nrf2 leads to their dissociation and subsequent translocation of Nrf2 to the nucleus[3,4]. In the nucleus, Nrf2 binds to ARE sequences and functions in partnership with other nuclear proteins as a strong transcriptional activator of ARE-responsive genes. ARE-mediated antioxidant proteins and enzymes[5], such as heme oxygenase-1 (HO-1), NAD(P)H: quinone oxidoreductase 1 (NQO1), glutathione-S-transferases (GST), group C streptococcus (GCS) are involved in the detoxification of increased electrophiles and radicals[6,7]. Therefore, the roles of the Nrf2/ARE pathway in liver diseases have been extensively investigated.
REACTIVE OXYGEN SPECIES AND THE ROLE OF Nrf2 IN CHRONIC LIVER DISEASES
Previous studies suggested that oxidative stress and associated damage could represent a common link between different forms of chronic liver injury[8-10]. The contribution of oxidative stress to lipid peroxidation is one of the critical factors involved in the genesis and the progression of nonalcoholic steatohepatitis (NASH)[11]. Viral infection or alcohol abuse greatly increases the highly variable miscoding etheno-modified DNA like epsilon A levels by triggering lipid peroxidation[12]. Oxidative stress plays an important role in the pathophysiological changes that progress to liver cirrhosis and finally to hepatocellular carcinoma. As a site of first-pass metabolism, the liver is highly susceptible to oxidative damage by reactive intermediates when it is exposed to high concentrations of xenobiotics and other chemicals. Therefore, there are several defense mechanisms to protect the liver against harmful chemicals and their potentially damaging metabolites. One of the most important protective mechanisms is the Nrf2/ARE pathway, which regulate phase II detoxifying enzyme genes and antioxidant-responsive genes, including HO-1, NQO1, GST, and GCS (Figure 1). The expression of phase II detoxifying enzyme genes in the wild-type and heterozygous Nrf2-knockout mice is clearly induced as compared to homozygous Nrf2-knockout mice in which the inducible expression of these genes is dramatically reduced[13]. NQO1 is cytoprotective against oxidative stress by scavenging superoxides, preserving various endogenous antioxidants, and catalyzing reductive metabolism of chemicals[7,14,15]. Therefore, NQO1 plays an essential role in protecting the cell against reactive oxygen species (ROS) and electrophiles. The role of Nrf2 in transcriptional activation of NQO1 was further confirmed by results from studies on Nrf2-/- mice. Mice lacking the Nrf2 gene exhibited a marked decrease in the expression and induction of NQO1[16]. In addition, the Nrf2/ARE pathway induces the expression of antioxidant and cytoprotective genes, including antioxidant proteins and enzymes[17,18]. The antioxidant proteins provide the necessary protection against oxidative and electrophilic stress[19]. Several studies have shown that Nrf2 is also a prevailing factor in the regulation of ARE-mediated activation of other defensive genes, including GST, GCS, and HO-1[20,21]. Therefore, activation of Nrf2 by glycyrrhetinic acid[22], sulforaphane[23], or caffeine[24] can induce the antioxidant enzymes system, protect the liver from oxidative stress, prevent inflammation and fibrosis, and attenuate liver injury. This indicates that Nrf2 has a crucial role against oxidative stress to protect hepatic cells from oxidative damage.
Figure 1.
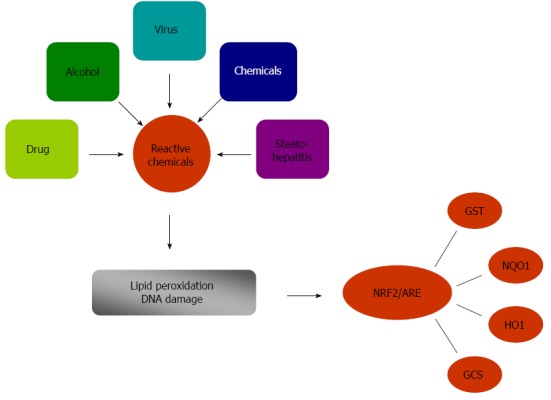
Nrf2 in chronic liver diseases. NRF2: Nuclear erythroid 2-related factor 2; ARE: Antioxidant response element; GST: Glutathione-S-transferases; NQO1: NAD(P)H: quinone oxidoreductase 1; HO1: Heme oxygenase-1; GCS: Group C streptococcus.
Nrf2 IN VIRAL HEPATITIS
Hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are major risk factors in the pathogenesis of chronic liver diseases. Permanent overproduction of viral proteins can result in increased level of radicals and other ROS[25,26]. Firstly, oxidative stress is common among HBV infected patients with chronic liver disease, and several studies have used HBV transgenic mice or HBV DNA transfection of cells in vitro to show that HBV can induce oxidative stress[27-29]. A series of studies demonstrated that HBV, via its association with mitochondria, induces oxidative stress, which in turn leads to activation of a series of transcription factors, including nuclear factor-kappaB (NF-κB), signal transducer and activator of transcription-3, and rapidly accelerated fibrosarcoma-1 (Raf-1)[30,31]. Recent research reported the capacity of HBV to stimulate the expression of a variety of cytoprotective genes that are regulated by Nrf2/ARE[32-34]. The HBV-dependent induction of these genes is primarily initiated by HBV regulatory proteins, and is mediated by methyl ethyl ketone (MEK) and c-Raf[35]. It was also demonstrated that increased augmentation of liver regeneration is regulated by Nrf2 during HBV infection, which acts as a liver regeneration and antioxidative protein and, therefore, links oxidative stress to hepatic regeneration to ensure survival of damaged cells[36,37]. Secondly, oxidative stress has been recognized as a fundamental factor in the pathological changes observed during HCV infection. Oxidative injury occurs as a direct result of HCV core protein expression both in vitro and in vivo[12]. One study demonstrated that HCV-mediated phosphorylation/activation of Nrf2 is mediated by the mitogen-activated protein (MAP) kinases (p38 MAPK) and janus kinase, and both ROS and Ca2+ signaling are necessary in the Nrf2-activation process[38,39]. Another study investigated the molecular mechanisms underlying oxidative stress and stress response induced by the individual HCV proteins and indicated that all five proteins [core, E1, E2, nonstructural protein 4B (NS4B), and nonstructural protein 5A (NS5A)] of HCV stimulated generation of ROS and Nrf2 activation by protein kinase C in response to ROS. Especially in the early stage of expression, HCV proteins induced a strong upregulation of the antioxidant defense system via Nrf2 to protect HCV infected hepatic cells from oxidative damage[40]. In addition, expression of core, E1, E2, NS4B, and NS5A proteins resulted in the activation of Nrf2 in a ROS-independent manner. The effect of core and NS5A was mediated through casein kinase 2 (CK2) and phosphoinositide-3 kinase (PI3K), whereas those of NS4B, E1, and E2, were not mediated by either protein kinase C, CK2, PI3K, p38 MAPK, or extracellular signal-regulated kinase[41,42] (Figure 2). Increasing levels of HO-1, a key cytoprotective gene, help to protect liver cells from the damaging effects of the HCV. A mechanism for this action was to increase expression of the positive transcription factor Nrf2[43]. Some studies have demonstrated that Nrf2 activation could also prevent and potentially alleviate liver diseases. These findings indicated that the anti-HCV action of drugs[44,45] was reflected the stimulation of Nrf2-mediated HO-1 expression. These results suggested that targeting the Nrf2/HO-1 signaling pathway might be a promising strategy for drug development. In conclusion, Nrf2 activation appears to be an common mechanism for potential protective effects against oxidative stress due to viral hepatitis.
Figure 2.
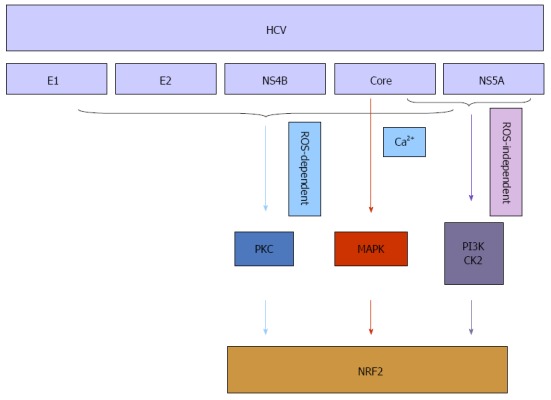
Nrf2 in chronic hepatitis C virus infection. HCV: Hepatitis C virus; NS4B: Nonstructural protein 4B; NS5A: Nonstructural protein 5A; ROS: Reactive oxygen species; PKC: Protein kinase C; MAPK: Mitogen activated protein kinase; PI3K: Phosphoinositide 3-kinase; CK2: Casein kinase 2; NRF2: Nuclear erythroid 2-related factor 2.
Nrf2 IN NAFLD/NASH
Nonalcoholic fatty liver disease (NAFLD) is a common cause of chronic liver disease worldwide, especially in developed countries[46]. The progression of NAFLD depends on multiple mechanisms operating simultaneously to produce cell injury, apoptosis, inflammation, fibrosis, and, ultimately, NASH[47,48]. Following the accumulation of triglycerides in the liver, impairment of mitochondrial respiratory chain activity results in the overproduction of ROS and the depletion of mitochondrial glutathione[49-51]. Other characteristics of NASH include reduced superoxide dismutase[52], catalase activity[53] and upregulated cytochrome P450 2A5 (CYP2A5), which is modulated through Nrf2[54] and increased lipid peroxidation within hepatocytes[55,56]. Nrf2 also modulates genes involved in metabolic regulation, which play an important role in nutrient homeostasis[57]. Nrf2 activation with 1-[2-cyano-3-,12-dioxooleana-1,9(11)-dien-28-oyl] imidazole (CDDO-Im) has been shown to effectively prevent hepatic lipid accumulation in wild-type mice, but not in Nrf2-disrupted mice[58-60]. When feeding the high-fat diet to the wild-type and the Nrf2-null mice, the wild-type mice increased hepatic fat deposition without inflammation or fibrosis (i.e., simple steatosis), while the Nrf2-null mice had significantly more hepatic steatosis and substantial inflammation[61,62]. Nrf2 expression and activation is reduced in the liver, with histological criteria of NASH[63]. Another way in which Nrf2 activation might be protective against NAFLD and NASH is through several preventive effects on inflammation[64]. Several chemotherapeutic agents have been shown in a variety of cell culture and rodent systems to induce Nrf2 and cause simultaneous repression of NF-κB[65]. Evidence shows that Punicalagin may be a useful nutrient for the treatment of NAFLD by activating Nrf2, resulting in improved mitochondrial function, elimination of oxidative stress and inflammation. Probiotics also showed remarkable induction of Nrf2 and its targeted antioxidative enzymes; they enhanced Nrf2 expression by precluding ubiquitination, which suppressed hepatic oxidative stress and prevented the progression of NAFLD[66]. Another mechanism for potential treatment of NAFLD and NASH is through activation of superoxide dismutase and catalase, which are antioxidant enzymes with decreased activity in this disease state. Finally, it is possible that activation of Nrf2 could play a role in regulating transforming growth factor-β (TGF-β). A recent study demonstrated that sulforaphane attenuates hepatic fibrosis through Nrf2-mediated inhibition of TGF-β signaling in a human hepatic stellate cell line[67]. In addition, activators of Nrf2 could abolish fibrosis in a rat model of NASH[68]. Altogether, activation of the Nrf2-mediated antioxidant response, which protects hepatic cells from oxidative damage, prevents the progression of NAFLD and presents new opportunities for treatment of NASH patients[69].
Nrf2 IN ALCOHOLIC LIVER DISEASE
Chronic alcohol consumption has long been associated with progressive liver disease[70,71]. The liver is the major site of ethanol metabolism and thus sustains the most injury from chronic alcohol consumption[72,73]. The metabolism of alcohol takes place via three main enzymatic pathways: oxidation of ethanol by alcohol dehydrogenase in hepatocytes, microsomal oxidation catalyzed by cytochrome P450 2E1 (CYP2E1), and nonoxidative metabolism catalyzed by fatty acid ethylester synthase[74-77]. First, ethanol metabolism by alcohol dehydrogenase results in acetaldehyde, which is a weak profibrogenic factor. Some important downstream effects of increased acetaldehyde production include GSH depletion, lipid peroxidation, and the generation of ROS and acetaldehyde adducts[78]. There is increasing evidence that homocysteine activates the Nrf2-mediated antioxidant response, which protects cells from oxidative damage[79], whereas Nrf2 dysregulation of GSH synthesis contributes to the pathogenesis of alcoholic liver disease (ALD)’s pathological conditions[80,81]. In alcohol-related liver disease, free radicals play a part in the pathogenesis of liver damage. Chronic ethanol treatment increases the production of ROS, lowers cellular antioxidant levels, and enhances oxidative stress in many tissues, especially the liver[82]. Second, ethanol metabolism by CYP2E1 occurs during chronic alcohol consumption, when alcohol dehydrogenase reaches saturation, and results in the generation of additional acetaldehydes, ROS, and free radicals. Activation of Nrf2 is critical in combating the oxidative stress caused by ROS generated during the normal catalytic cycle of CYP2E1. This is supported by preclinical studies showing that ethanol-induced CYP2E1 expression also results in upregulation of Nrf2 and its targets, namely HO-1[83]. It has been reported that Nrf2-null mice have increased liver-associated mortality when fed high doses of ethanol compared with wild-type mice. This detrimental effect of alcohol on Nrf2-null mice was shown to be the result of increased lipogenesis, depletion of total and mitochondrial glutathione, and a Kupffer cell-mediated aggravation of the inflammatory response. This suggested that Nrf2 plays a role in protecting against ethanol-induced damage[84]. Ethanol induced oxidative stress via induction of CYP2E1 upregulates Nrf2 activity, which in turn regulates ethanol induction of CYP2A5 and protects against ethanol-induced steatosis[85]. The prominent microvesicular steatosis and mild necrosis in hepatic histopathology were notably attenuated in accordance with the modulation of Nrf2 in clinical administration of artemisia capillaris for alcoholic-associated liver injury[86]. It remains unclear whether Nrf2 plays a major role in the pathogenesis of this disease state. Bardag-Gorce et al[87] found that the Nrf2 level was significantly decreased in the liver of a rat model of alcoholic liver disease. However, Wang et al[88] came to the opposite conclusion, that hepatic very low-density lipoprotein receptor (VLDLR) overexpression plays an important role in the pathogenesis of ALD. Oxidative stress-induced Nrf2 activation plays a critical role in alcohol-induced VLDLR upregulation in hepatocytes, and enhances VLDLR expression in primary hepatocytes[89,90]. Hence, further research is required to determine the role that Nrf2 activation might play in alleviating alcoholic liver disease.
Nrf2 IN DRUG-INDUCED LIVER INJURY (DILI)
Xenobiotic agents can initiate liver injury through reactive-intermediate formation, protein adduct accumulation, and alterations in drug-metabolizing enzymes. Acute hepatic failure secondary to acetaminophen (APAP) poisoning is associated with high mortality[91]. APAP overdose is the most frequent cause of drug-induced liver failure in the United States and most of Europe[92,93]. Therefore, APAP-induced toxicity has become an essential model for studying drug-induced liver disease[94,95]. Electrophiles, radicals, and ROS are often generated as intermediates or by-products of APAP metabolism. These reactive intermediate toxicities provoke covalent bonding with biomolecules and leads to lipid peroxidation, and ultimately oxidative stress[96-98]. Recently, studies showed that nimesulide-induced electrophile stress activates Nrf2 in human hepatocytes and mice[99]. The oxidative stress that occurs with APAP toxicity suggests a role for Nrf2 in the toxicological events of APAP. This view is supported by several studies showing that Nrf2 plays a critical role in protecting the liver against DILI. Nrf2-deficient mice are highly susceptible to APAP-induced liver injury[100]. In Nrf2-null mice, APAP exposure enhanced liver injury and mortality compared with wild-type mice[101]. In addition, the Nrf2 activator CDDO-Im is protective against APAP hepatotoxicity by inducing HO-1, NQO1, and glutamate-cysteine ligase catalytic subunit in the wild-type, but not the Nrf2-null mice[102]. However, some evidence indicates that autoprotection against APAP could contribute to this development of resistance to hepatotoxicity, and Nrf2 activation is expected to play a role in the protective adaptation. APAP treated hepatocytes showed enhanced antioxidant defense via delaying tyrosine phosphorylation of Nrf2 and its nuclear exclusion, ubiquitination and degradation[103]. Pretreatment of mice with a low hepatotoxic dose of APAP resulted in resistance to the toxicity of a subsequent higher dose of APAP. Upregulation of Lgals3, one of the genes supporting the Nrf2 hypothesis led to suppression of apoptosis and reduced mitochondrial dysfunction[104]. The mechanisms underlying the protective effects of Chinese traditional medicines against N-nitrosodimethylamine, or CCl4, or APAP-induced liver injury have been investigated. Treatment with rutin[105], safflower[106], betanin[107], or Piper puberulum[23] significantly increased Nrf2 and HO-1 expression in injured livers. These results indicated that the hepatoprotective effect of Nrf2 against DILI functions via the activation of Nrf2 and subsequent induction of the expression of genes controlled by Nrf2. Furthermore, oleanolic acid is a triterpenoid with many beneficial effects and has been demonstrated to protect against varieties of hepatotoxicants via activation of Nrf2[108]. However recently, high-doses and long-term use was reported to produce hepatotoxicity[109]. Apart from DILI, endotoxemia correlates with the degree of liver failure and may contribute to worsening of liver diseases. In most cases, lipopolysaccharide (synonymous with endotoxin) is a liver failure causing endotoxin, which lowers the hepatic GSH levels by inhibiting sumoylation of Nrf2[110]. Thus, Nrf2 may serve as a major regulator of several cellular defense associated pathways by which hepatic cells combat oxidative stress by xenobiotics.
CONCLUSION
Oxidative stress is implicated in the pathogenesis of liver disease. During oxidative stress, Nrf2 is activated to protect the liver via target gene expression. Therefore, Nrf2 activators have great potential as therapeutic agents against oxidative stress during chronic liver injury.
Footnotes
Supported by The Scientific Research Projects from the Development and Reform Commission of Hunan Province, China, No. 2011 (1318), No. 2012 (1493) and No. 2013 (1132)
P- Reviewer: Jie H, Pal A, Penkova-Radicheva M, Wateshi K S- Editor: Nan J L- Editor: Stewart G E- Editor: Zhang DN
References
- 1.Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl Acad Sci USA. 1994;91:9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaspar JW, Niture SK, Jaiswal AK. Nrf2: INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med. 2009;47:1304–1309. doi: 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Li W, Kong AN. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol Carcinog. 2009;48:91–104. doi: 10.1002/mc.20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu W, Hellerbrand C, Köhler UA, Bugnon P, Kan YW, Werner S, Beyer TA. The Nrf2 transcription factor protects from toxin-induced liver injury and fibrosis. Lab Invest. 2008;88:1068–1078. doi: 10.1038/labinvest.2008.75. [DOI] [PubMed] [Google Scholar]
- 6.Wasserman WW, Fahl WE. Functional antioxidant responsive elements. Proc Natl Acad Sci USA. 1997;94:5361–5366. doi: 10.1073/pnas.94.10.5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross D. Quinone reductases multitasking in the metabolic world. Drug Metab Rev. 2004;36:639–654. doi: 10.1081/dmr-200033465. [DOI] [PubMed] [Google Scholar]
- 8.Shin SM, Yang JH, Ki SH. Role of the Nrf2-ARE pathway in liver diseases. Oxid Med Cell Longev. 2013;2013:763257. doi: 10.1155/2013/763257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwabe RF, Brenner DA. Mechanisms of Liver Injury. I. TNF-alpha-induced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:G583–G589. doi: 10.1152/ajpgi.00422.2005. [DOI] [PubMed] [Google Scholar]
- 10.Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta. 2013;1833:3460–3470. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weltman MD, Farrell GC, Hall P, Ingelman-Sundberg M, Liddle C. Hepatic cytochrome P450 2E1 is increased in patients with nonalcoholic steatohepatitis. Hepatology. 1998;27:128–133. doi: 10.1002/hep.510270121. [DOI] [PubMed] [Google Scholar]
- 12.Okuda M, Li K, Beard MR, Showalter LA, Scholle F, Lemon SM, Weinman SA. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology. 2002;122:366–375. doi: 10.1053/gast.2002.30983. [DOI] [PubMed] [Google Scholar]
- 13.Hayes JD, Chanas SA, Henderson CJ, McMahon M, Sun C, Moffat GJ, Wolf CR, Yamamoto M. The Nrf2 transcription factor contributes both to the basal expression of glutathione S-transferases in mouse liver and to their induction by the chemopreventive synthetic antioxidants, butylated hydroxyanisole and ethoxyquin. Biochem Soc Trans. 2000;28:33–41. doi: 10.1042/bst0280033. [DOI] [PubMed] [Google Scholar]
- 14.Siegel D, Gustafson DL, Dehn DL, Han JY, Boonchoong P, Berliner LJ, Ross D. NAD(P)H: quinone oxidoreductase 1: role as a superoxide scavenger. Mol Pharmacol. 2004;65:1238–1247. doi: 10.1124/mol.65.5.1238. [DOI] [PubMed] [Google Scholar]
- 15.Siegel D, Bolton EM, Burr JA, Liebler DC, Ross D. The reduction of alpha-tocopherolquinone by human NAD(P)H: quinone oxidoreductase: the role of alpha-tocopherolhydroquinone as a cellular antioxidant. Mol Pharmacol. 1997;52:300–305. doi: 10.1124/mol.52.2.300. [DOI] [PubMed] [Google Scholar]
- 16.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 17.Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- 18.Vasiliou V, Ross D, Nebert DW. Update of the NAD(P)H: quinone oxidoreductase (NQO) gene family. Hum Genomics. 2006;2:329–335. doi: 10.1186/1479-7364-2-5-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhakshinamoorthy S, Jaiswal AK. Functional characterization and role of INrf2 in antioxidant response element-mediated expression and antioxidant induction of NAD(P)H: quinone oxidoreductase1 gene. Oncogene. 2001;20:3906–3917. doi: 10.1038/sj.onc.1204506. [DOI] [PubMed] [Google Scholar]
- 20.Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL. Nrf2, a Cap’n’Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem. 1999;274:26071–26078. doi: 10.1074/jbc.274.37.26071. [DOI] [PubMed] [Google Scholar]
- 21.Wild AC, Moinova HR, Mulcahy RT. Regulation of gamma-glutamylcysteine synthetase subunit gene expression by the transcription factor Nrf2. J Biol Chem. 1999;274:33627–33636. doi: 10.1074/jbc.274.47.33627. [DOI] [PubMed] [Google Scholar]
- 22.Chen S, Zou L, Li L, Wu T. The protective effect of glycyrrhetinic acid on carbon tetrachloride-induced chronic liver fibrosis in mice via upregulation of Nrf2. PLoS One. 2013;8:e53662. doi: 10.1371/journal.pone.0053662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Q, Zhang D, Tao N, Zhu QN, Jin T, Shi JS, Liu J. Induction of Nrf2 and metallothionein as a common mechanism of hepatoprotective medicinal herbs. Am J Chin Med. 2014;42:207–221. doi: 10.1142/S0192415X14500141. [DOI] [PubMed] [Google Scholar]
- 24.Gordillo-Bastidas D, Oceguera-Contreras E, Salazar-Montes A, González-Cuevas J, Hernández-Ortega LD, Armendáriz-Borunda J. Nrf2 and Snail-1 in the prevention of experimental liver fibrosis by caffeine. World J Gastroenterol. 2013;19:9020–9033. doi: 10.3748/wjg.v19.i47.9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hagen TM, Huang S, Curnutte J, Fowler P, Martinez V, Wehr CM, Ames BN, Chisari FV. Extensive oxidative DNA damage in hepatocytes of transgenic mice with chronic active hepatitis destined to develop hepatocellular carcinoma. Proc Natl Acad Sci USA. 1994;91:12808–12812. doi: 10.1073/pnas.91.26.12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215–229. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- 27.Severi T, Ying C, Vermeesch JR, Cassiman D, Cnops L, Verslype C, Fevery J, Arckens L, Neyts J, van Pelt JF. Hepatitis B virus replication causes oxidative stress in HepAD38 liver cells. Mol Cell Biochem. 2006;290:79–85. doi: 10.1007/s11010-006-9167-x. [DOI] [PubMed] [Google Scholar]
- 28.Bolukbas C, Bolukbas FF, Horoz M, Aslan M, Celik H, Erel O. Increased oxidative stress associated with the severity of the liver disease in various forms of hepatitis B virus infection. BMC Infect Dis. 2005;5:95. doi: 10.1186/1471-2334-5-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsieh YH, Su IJ, Wang HC, Chang WW, Lei HY, Lai MD, Chang WT, Huang W. Pre-S mutant surface antigens in chronic hepatitis B virus infection induce oxidative stress and DNA damage. Carcinogenesis. 2004;25:2023–2032. doi: 10.1093/carcin/bgh207. [DOI] [PubMed] [Google Scholar]
- 30.Waris G, Huh KW, Siddiqui A. Mitochondrially associated hepatitis B virus X protein constitutively activates transcription factors STAT-3 and NF-kappa B via oxidative stress. Mol Cell Biol. 2001;21:7721–7730. doi: 10.1128/MCB.21.22.7721-7730.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen J, Siddiqui A. Hepatitis B virus X protein stimulates the mitochondrial translocation of Raf-1 via oxidative stress. J Virol. 2007;81:6757–6760. doi: 10.1128/JVI.00172-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Severi T, Vander Borght S, Libbrecht L, VanAelst L, Nevens F, Roskams T, Cassiman D, Fevery J, Verslype C, van Pelt JF. HBx or HCV core gene expression in HepG2 human liver cells results in a survival benefit against oxidative stress with possible implications for HCC development. Chem Biol Interact. 2007;168:128–134. doi: 10.1016/j.cbi.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Chemin I, Zoulim F. Hepatitis B virus induced hepatocellular carcinoma. Cancer Lett. 2009;286:52–59. doi: 10.1016/j.canlet.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Durchdewald M, Beyer TA, Johnson DA, Johnson JA, Werner S, auf dem Keller U. Electrophilic chemicals but not UV irradiation or reactive oxygen species activate Nrf2 in keratinocytes in vitro and in vivo. J Invest Dermatol. 2007;127:646–653. doi: 10.1038/sj.jid.5700585. [DOI] [PubMed] [Google Scholar]
- 35.Schaedler S, Krause J, Himmelsbach K, Carvajal-Yepes M, Lieder F, Klingel K, Nassal M, Weiss TS, Werner S, Hildt E. Hepatitis B virus induces expression of antioxidant response element-regulated genes by activation of Nrf2. J Biol Chem. 2010;285:41074–41086. doi: 10.1074/jbc.M110.145862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dayoub R, Vogel A, Schuett J, Lupke M, Spieker SM, Kettern N, Hildt E, Melter M, Weiss TS. Nrf2 activates augmenter of liver regeneration (ALR) via antioxidant response element and links oxidative stress to liver regeneration. Mol Med. 2013;19:237–244. doi: 10.2119/molmed.2013.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dayoub R, Groitl P, Dobner T, Bosserhoff AK, Schlitt HJ, Weiss TS. Foxa2 (HNF-3beta) regulates expression of hepatotrophic factor ALR in liver cells. Biochem Biophys Res Commun. 2010;395:465–470. doi: 10.1016/j.bbrc.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 38.Burdette D, Olivarez M, Waris G. Activation of transcription factor Nrf2 by hepatitis C virus induces the cell-survival pathway. J Gen Virol. 2010;91:681–690. doi: 10.1099/vir.0.014340-0. [DOI] [PubMed] [Google Scholar]
- 39.Moriya K, Miyoshi H, Shinzawa S, Tsutsumi T, Fujie H, Goto K, Shintani Y, Yotsuyanagi H, Koike K. Hepatitis C virus core protein compromises iron-induced activation of antioxidants in mice and HepG2 cells. J Med Virol. 2010;82:776–792. doi: 10.1002/jmv.21661. [DOI] [PubMed] [Google Scholar]
- 40.Lee JC, Tseng CK, Young KC, Sun HY, Wang SW, Chen WC, Lin CK, Wu YH. Andrographolide exerts anti-hepatitis C virus activity by up-regulating haeme oxygenase-1 via the p38 MAPK/Nrf2 pathway in human hepatoma cells. Br J Pharmacol. 2014;171:237–252. doi: 10.1111/bph.12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ivanov AV, Smirnova OA, Ivanova ON, Masalova OV, Kochetkov SN, Isaguliants MG. Hepatitis C virus proteins activate NRF2/ARE pathway by distinct ROS-dependent and independent mechanisms in HUH7 cells. PLoS One. 2011;6:e24957. doi: 10.1371/journal.pone.0024957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carvajal-Yepes M, Himmelsbach K, Schaedler S, Ploen D, Krause J, Ludwig L, Weiss T, Klingel K, Hildt E. Hepatitis C virus impairs the induction of cytoprotective Nrf2 target genes by delocalization of small Maf proteins. J Biol Chem. 2011;286:8941–8951. doi: 10.1074/jbc.M110.186684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hou WH, Rossi L, Shan Y, Zheng JY, Lambrecht RW, Bonkovsky HL. Iron increases HMOX1 and decreases hepatitis C viral expression in HCV-expressing cells. World J Gastroenterol. 2009;15:4499–4510. doi: 10.3748/wjg.15.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi J, Lee KJ, Zheng Y, Yamaga AK, Lai MM, Ou JH. Reactive oxygen species suppress hepatitis C virus RNA replication in human hepatoma cells. Hepatology. 2004;39:81–89. doi: 10.1002/hep.20001. [DOI] [PubMed] [Google Scholar]
- 45.Chen WC, Wang SY, Chiu CC, Tseng CK, Lin CK, Wang HC, Lee JC. Lucidone suppresses hepatitis C virus replication by Nrf2-mediated heme oxygenase-1 induction. Antimicrob Agents Chemother. 2013;57:1180–1191. doi: 10.1128/AAC.02053-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paredes AH, Torres DM, Harrison SA. Nonalcoholic fatty liver disease. Clin Liver Dis. 2012;16:397–419. doi: 10.1016/j.cld.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 47.Krawczyk M, Bonfrate L, Portincasa P. Nonalcoholic fatty liver disease. Best Pract Res Clin Gastroenterol. 2010;24:695–708. doi: 10.1016/j.bpg.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 48.Smith BW, Adams LA. Non-alcoholic fatty liver disease. Crit Rev Clin Lab Sci. 2011;48:97–113. doi: 10.3109/10408363.2011.596521. [DOI] [PubMed] [Google Scholar]
- 49.Begriche K, Igoudjil A, Pessayre D, Fromenty B. Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion. 2006;6:1–28. doi: 10.1016/j.mito.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 50.Mantena SK, King AL, Andringa KK, Eccleston HB, Bailey SM. Mitochondrial dysfunction and oxidative stress in the pathogenesis of alcohol- and obesity-induced fatty liver diseases. Free Radic Biol Med. 2008;44:1259–1272. doi: 10.1016/j.freeradbiomed.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bataille AM, Manautou JE. Nrf2: a potential target for new therapeutics in liver disease. Clin Pharmacol Ther. 2012;92:340–348. doi: 10.1038/clpt.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nobili V, Pastore A, Gaeta LM, Tozzi G, Comparcola D, Sartorelli MR, Marcellini M, Bertini E, Piemonte F. Glutathione metabolism and antioxidant enzymes in patients affected by nonalcoholic steatohepatitis. Clin Chim Acta. 2005;355:105–111. doi: 10.1016/j.cccn.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 53.Videla LA, Rodrigo R, Orellana M, Fernandez V, Tapia G, Quiñones L, Varela N, Contreras J, Lazarte R, Csendes A, et al. Oxidative stress-related parameters in the liver of non-alcoholic fatty liver disease patients. Clin Sci (Lond) 2004;106:261–268. doi: 10.1042/CS20030285. [DOI] [PubMed] [Google Scholar]
- 54.Cui Y, Wang Q, Li X, Zhang X. Experimental nonalcoholic fatty liver disease in mice leads to cytochrome p450 2a5 upregulation through nuclear factor erythroid 2-like 2 translocation. Redox Biol. 2013;1:433–440. doi: 10.1016/j.redox.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yachi R, Muto C, Ohtaka N, Aoki Y, Koike T, Igarashi O, Kiyose C. Effects of tocotrienol on tumor necrosis factor-α/d-galactosamine-induced steatohepatitis in rats. J Clin Biochem Nutr. 2013;52:146–153. doi: 10.3164/jcbn.12-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pak W, Takayama F, Mine M, Nakamoto K, Kodo Y, Mankura M, Egashira T, Kawasaki H, Mori A. Anti-oxidative and anti-inflammatory effects of spirulina on rat model of non-alcoholic steatohepatitis. J Clin Biochem Nutr. 2012;51:227–234. doi: 10.3164/jcbn.12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chartoumpekis DV, Kensler TW. New player on an old field; the keap1/Nrf2 pathway as a target for treatment of type 2 diabetes and metabolic syndrome. Curr Diabetes Rev. 2013;9:137–145. doi: 10.2174/1573399811309020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shin S, Wakabayashi J, Yates MS, Wakabayashi N, Dolan PM, Aja S, Liby KT, Sporn MB, Yamamoto M, Kensler TW. Role of Nrf2 in prevention of high-fat diet-induced obesity by synthetic triterpenoid CDDO-imidazolide. Eur J Pharmacol. 2009;620:138–144. doi: 10.1016/j.ejphar.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tanaka Y, Ikeda T, Yamamoto K, Ogawa H, Kamisako T. Dysregulated expression of fatty acid oxidation enzymes and iron-regulatory genes in livers of Nrf2-null mice. J Gastroenterol Hepatol. 2012;27:1711–1717. doi: 10.1111/j.1440-1746.2012.07180.x. [DOI] [PubMed] [Google Scholar]
- 60.Tanaka Y, Aleksunes LM, Yeager RL, Gyamfi MA, Esterly N, Guo GL, Klaassen CD. NF-E2-related factor 2 inhibits lipid accumulation and oxidative stress in mice fed a high-fat diet. J Pharmacol Exp Ther. 2008;325:655–664. doi: 10.1124/jpet.107.135822. [DOI] [PubMed] [Google Scholar]
- 61.Sugimoto H, Okada K, Shoda J, Warabi E, Ishige K, Ueda T, Taguchi K, Yanagawa T, Nakahara A, Hyodo I, et al. Deletion of nuclear factor-E2-related factor-2 leads to rapid onset and progression of nutritional steatohepatitis in mice. Am J Physiol Gastrointest Liver Physiol. 2010;298:G283–G294. doi: 10.1152/ajpgi.00296.2009. [DOI] [PubMed] [Google Scholar]
- 62.Wang C, Cui Y, Li C, Zhang Y, Xu S, Li X, Li H, Zhang X. Nrf2 deletion causes “benign” simple steatosis to develop into nonalcoholic steatohepatitis in mice fed a high-fat diet. Lipids Health Dis. 2013;12:165. doi: 10.1186/1476-511X-12-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gupte AA, Lyon CJ, Hsueh WA. Nuclear factor (erythroid-derived 2)-like-2 factor (Nrf2), a key regulator of the antioxidant response to protect against atherosclerosis and nonalcoholic steatohepatitis. Curr Diab Rep. 2013;13:362–371. doi: 10.1007/s11892-013-0372-1. [DOI] [PubMed] [Google Scholar]
- 64.Kay HY, Kim WD, Hwang SJ, Choi HS, Gilroy RK, Wan YJ, Kim SG. Nrf2 inhibits LXRα-dependent hepatic lipogenesis by competing with FXR for acetylase binding. Antioxid Redox Signal. 2011;15:2135–2146. doi: 10.1089/ars.2010.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wakabayashi N, Slocum SL, Skoko JJ, Shin S, Kensler TW. When NRF2 talks, who’s listening? Antioxid Redox Signal. 2010;13:1649–1663. doi: 10.1089/ars.2010.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Endo H, Niioka M, Kobayashi N, Tanaka M, Watanabe T. Butyrate-producing probiotics reduce nonalcoholic fatty liver disease progression in rats: new insight into the probiotics for the gut-liver axis. PLoS One. 2013;8:e63388. doi: 10.1371/journal.pone.0063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oh CJ, Kim JY, Min AK, Park KG, Harris RA, Kim HJ, Lee IK. Sulforaphane attenuates hepatic fibrosis via NF-E2-related factor 2-mediated inhibition of transforming growth factor-β/Smad signaling. Free Radic Biol Med. 2012;52:671–682. doi: 10.1016/j.freeradbiomed.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 68.Shimozono R, Asaoka Y, Yoshizawa Y, Aoki T, Noda H, Yamada M, Kaino M, Mochizuki H. Nrf2 activators attenuate the progression of nonalcoholic steatohepatitis-related fibrosis in a dietary rat model. Mol Pharmacol. 2013;84:62–70. doi: 10.1124/mol.112.084269. [DOI] [PubMed] [Google Scholar]
- 69.Pereira AF, Sá LL, Reis FH, Cardoso FC, Alberici RM, Prado IM, Eberlin MN, Uyemura SA, Curti C, Alberici LC. Administration of a murine diet supplemented with conjugated linoleic acid increases the expression and activity of hepatic uncoupling proteins. J Bioenerg Biomembr. 2012;44:587–596. doi: 10.1007/s10863-012-9463-y. [DOI] [PubMed] [Google Scholar]
- 70.Shepard BD, Tuma DJ, Tuma PL. Chronic ethanol consumption induces global hepatic protein hyperacetylation. Alcohol Clin Exp Res. 2010;34:280–291. doi: 10.1111/j.1530-0277.2009.01091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ronis MJ, Korourian S, Blackburn ML, Badeaux J, Badger TM. The role of ethanol metabolism in development of alcoholic steatohepatitis in the rat. Alcohol. 2010;44:157–169. doi: 10.1016/j.alcohol.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kang L, Chen X, Sebastian BM, Pratt BT, Bederman IR, Alexander JC, Previs SF, Nagy LE. Chronic ethanol and triglyceride turnover in white adipose tissue in rats: inhibition of the anti-lipolytic action of insulin after chronic ethanol contributes to increased triglyceride degradation. J Biol Chem. 2007;282:28465–28473. doi: 10.1074/jbc.M705503200. [DOI] [PubMed] [Google Scholar]
- 73.Diehl AM. Obesity and alcoholic liver disease. Alcohol. 2004;34:81–87. doi: 10.1016/j.alcohol.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 74.Wang Y, Kou Y, Wang X, Cederbaum A, Wang R. Multifactorial comparative proteomic study of cytochrome P450 2E1 function in chronic alcohol administration. PLoS One. 2014;9:e92504. doi: 10.1371/journal.pone.0092504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bae SH, Sung SH, Cho EJ, Lee SK, Lee HE, Woo HA, Yu DY, Kil IS, Rhee SG. Concerted action of sulfiredoxin and peroxiredoxin I protects against alcohol-induced oxidative injury in mouse liver. Hepatology. 2011;53:945–953. doi: 10.1002/hep.24104. [DOI] [PubMed] [Google Scholar]
- 76.Van de Wiel A. The effect of alcohol on postprandial and fasting triglycerides. Int J Vasc Med. 2012;2012:862504. doi: 10.1155/2012/862504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bessembinders K, Wielders J, van de Wiel A. Severe hypertriglyceridemia influenced by alcohol (SHIBA) Alcohol Alcohol. 2011;46:113–116. doi: 10.1093/alcalc/agq088. [DOI] [PubMed] [Google Scholar]
- 78.Dey A, Cederbaum AI. Alcohol and oxidative liver injury. Hepatology. 2006;43:S63–S74. doi: 10.1002/hep.20957. [DOI] [PubMed] [Google Scholar]
- 79.Mani M, Khaghani S, Gol Mohammadi T, Zamani Z, Azadmanesh K, Meshkani R, Pasalar P, Mostafavi E. Activation of Nrf2-Antioxidant Response Element Mediated Glutamate Cysteine Ligase Expression in Hepatoma Cell line by Homocysteine. Hepat Mon. 2013;13:e8394. doi: 10.5812/hepatmon.8394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lu SC. Glutathione synthesis. Biochim Biophys Acta. 2013;1830:3143–3153. doi: 10.1016/j.bbagen.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Harvey CJ, Thimmulappa RK, Singh A, Blake DJ, Ling G, Wakabayashi N, Fujii J, Myers A, Biswal S. Nrf2-regulated glutathione recycling independent of biosynthesis is critical for cell survival during oxidative stress. Free Radic Biol Med. 2009;46:443–453. doi: 10.1016/j.freeradbiomed.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu D, Cederbaum AI. Oxidative stress and alcoholic liver disease. Semin Liver Dis. 2009;29:141–154. doi: 10.1055/s-0029-1214370. [DOI] [PubMed] [Google Scholar]
- 83.Gong P, Cederbaum AI. Nrf2 is increased by CYP2E1 in rodent liver and HepG2 cells and protects against oxidative stress caused by CYP2E1. Hepatology. 2006;43:144–153. doi: 10.1002/hep.21004. [DOI] [PubMed] [Google Scholar]
- 84.Lamlé J, Marhenke S, Borlak J, von Wasielewski R, Eriksson CJ, Geffers R, Manns MP, Yamamoto M, Vogel A. Nuclear factor-eythroid 2-related factor 2 prevents alcohol-induced fulminant liver injury. Gastroenterology. 2008;134:1159–1168. doi: 10.1053/j.gastro.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 85.Lu Y, Zhang XH, Cederbaum AI. Ethanol induction of CYP2A5: role of CYP2E1-ROS-Nrf2 pathway. Toxicol Sci. 2012;128:427–438. doi: 10.1093/toxsci/kfs164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Choi MK, Han JM, Kim HG, Lee JS, Lee JS, Wang JH, Son SW, Park HJ, Son CG. Aqueous extract of Artemisia capillaris exerts hepatoprotective action in alcohol-pyrazole-fed rat model. J Ethnopharmacol. 2013;147:662–670. doi: 10.1016/j.jep.2013.03.065. [DOI] [PubMed] [Google Scholar]
- 87.Bardag-Gorce F, Oliva J, Lin A, Li J, French BA, French SW. Proteasome inhibitor up regulates liver antioxidative enzymes in rat model of alcoholic liver disease. Exp Mol Pathol. 2011;90:123–130. doi: 10.1016/j.yexmp.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Z, Dou X, Li S, Zhang X, Sun X, Zhou Z, Song Z. Nuclear factor (erythroid-derived 2)-like 2 activation-induced hepatic very-low-density lipoprotein receptor overexpression in response to oxidative stress contributes to alcoholic liver disease in mice. Hepatology. 2014;59:1381–1392. doi: 10.1002/hep.26912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shen GM, Zhao YZ, Chen MT, Zhang FL, Liu XL, Wang Y, Liu CZ, Yu J, Zhang JW. Hypoxia-inducible factor-1 (HIF-1) promotes LDL and VLDL uptake through inducing VLDLR under hypoxia. Biochem J. 2012;441:675–683. doi: 10.1042/BJ20111377. [DOI] [PubMed] [Google Scholar]
- 90.Jo H, Choe SS, Shin KC, Jang H, Lee JH, Seong JK, Back SH, Kim JB. Endoplasmic reticulum stress induces hepatic steatosis via increased expression of the hepatic very low-density lipoprotein receptor. Hepatology. 2013;57:1366–1377. doi: 10.1002/hep.26126. [DOI] [PubMed] [Google Scholar]
- 91.Ramachandran R, Kakar S. Histological patterns in drug-induced liver disease. J Clin Pathol. 2009;62:481–492. doi: 10.1136/jcp.2008.058248. [DOI] [PubMed] [Google Scholar]
- 92.Lee WM. Acetaminophen and the U.S. Acute Liver Failure Study Group: lowering the risks of hepatic failure. Hepatology. 2004;40:6–9. doi: 10.1002/hep.20293. [DOI] [PubMed] [Google Scholar]
- 93.Lee WM. Acetaminophen toxicity: changing perceptions on a social/medical issue. Hepatology. 2007;46:966–970. doi: 10.1002/hep.21926. [DOI] [PubMed] [Google Scholar]
- 94.Jaeschke H, Bajt ML. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol Sci. 2006;89:31–41. doi: 10.1093/toxsci/kfi336. [DOI] [PubMed] [Google Scholar]
- 95.Han D, Shinohara M, Ybanez MD, Saberi B, Kaplowitz N. Signal transduction pathways involved in drug-induced liver injury. Handb Exp Pharmacol. 2010;(196):267–310. doi: 10.1007/978-3-642-00663-0_10. [DOI] [PubMed] [Google Scholar]
- 96.Reid AB, Kurten RC, McCullough SS, Brock RW, Hinson JA. Mechanisms of acetaminophen-induced hepatotoxicity: role of oxidative stress and mitochondrial permeability transition in freshly isolated mouse hepatocytes. J Pharmacol Exp Ther. 2005;312:509–516. doi: 10.1124/jpet.104.075945. [DOI] [PubMed] [Google Scholar]
- 97.Ghosh A, Sil PC. Protection of acetaminophen induced mitochondrial dysfunctions and hepatic necrosis via Akt-NF-kappaB pathway: role of a novel plant protein. Chem Biol Interact. 2009;177:96–106. doi: 10.1016/j.cbi.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 98.Jaeschke H, McGill MR, Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev. 2012;44:88–106. doi: 10.3109/03602532.2011.602688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kale VM, Hsiao CJ, Boelsterli UA. Nimesulide-induced electrophile stress activates Nrf2 in human hepatocytes and mice but is not sufficient to induce hepatotoxicity in Nrf2-deficient mice. Chem Res Toxicol. 2010;23:967–976. doi: 10.1021/tx100063z. [DOI] [PubMed] [Google Scholar]
- 100.Enomoto A, Itoh K, Nagayoshi E, Haruta J, Kimura T, O’Connor T, Harada T, Yamamoto M. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol Sci. 2001;59:169–177. doi: 10.1093/toxsci/59.1.169. [DOI] [PubMed] [Google Scholar]
- 101.Chan K, Han XD, Kan YW. An important function of Nrf2 in combating oxidative stress: detoxification of acetaminophen. Proc Natl Acad Sci USA. 2001;98:4611–4616. doi: 10.1073/pnas.081082098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reisman SA, Buckley DB, Tanaka Y, Klaassen CD. CDDO-Im protects from acetaminophen hepatotoxicity through induction of Nrf2-dependent genes. Toxicol Appl Pharmacol. 2009;236:109–114. doi: 10.1016/j.taap.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mobasher MA, González-Rodriguez A, Santamaría B, Ramos S, Martín MÁ, Goya L, Rada P, Letzig L, James LP, Cuadrado A, et al. Protein tyrosine phosphatase 1B modulates GSK3β/Nrf2 and IGFIR signaling pathways in acetaminophen-induced hepatotoxicity. Cell Death Dis. 2013;4:e626. doi: 10.1038/cddis.2013.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.O’Connor MA, Koza-Taylor P, Campion SN, Aleksunes LM, Gu X, Enayetallah AE, Lawton MP, Manautou JE. Analysis of changes in hepatic gene expression in a murine model of tolerance to acetaminophen hepatotoxicity (autoprotection) Toxicol Appl Pharmacol. 2014;274:156–167. doi: 10.1016/j.taap.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Domitrović R, Jakovac H, Vasiljev Marchesi V, Vladimir-Knežević S, Cvijanović O, Tadić Z, Romić Z, Rahelić D. Differential hepatoprotective mechanisms of rutin and quercetin in CCl(4)-intoxicated BALB/cN mice. Acta Pharmacol Sin. 2012;33:1260–1270. doi: 10.1038/aps.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu S, Yue Y, Tian H, Li Z, Li X, He W, Ding H. Carthamus red from Carthamus tinctorius L. exerts antioxidant and hepatoprotective effect against CCl(4)-induced liver damage in rats via the Nrf2 pathway. J Ethnopharmacol. 2013;148:570–578. doi: 10.1016/j.jep.2013.04.054. [DOI] [PubMed] [Google Scholar]
- 107.Krajka-Kuźniak V, Paluszczak J, Szaefer H, Baer-Dubowska W. Betanin, a beetroot component, induces nuclear factor erythroid-2-related factor 2-mediated expression of detoxifying/antioxidant enzymes in human liver cell lines. Br J Nutr. 2013;110:2138–2149. doi: 10.1017/S0007114513001645. [DOI] [PubMed] [Google Scholar]
- 108.Chen P, Zeng H, Wang Y, Fan X, Xu C, Deng R, Zhou X, Bi H, Huang M. Low dose of oleanolic acid protects against lithocholic acid-induced cholestasis in mice: potential involvement of nuclear factor-E2-related factor 2-mediated upregulation of multidrug resistance-associated proteins. Drug Metab Dispos. 2014;42:844–852. doi: 10.1124/dmd.113.056549. [DOI] [PubMed] [Google Scholar]
- 109.Lu YF, Wan XL, Xu Y, Liu J. Repeated oral administration of oleanolic acid produces cholestatic liver injury in mice. Molecules. 2013;18:3060–3071. doi: 10.3390/molecules18033060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tomasi ML, Ryoo M, Yang H, Iglesias Ara A, Ko KS, Lu SC. Molecular mechanisms of lipopolysaccharide-mediated inhibition of glutathione synthesis in mice. Free Radic Biol Med. 2014;68:148–158. doi: 10.1016/j.freeradbiomed.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]


