Abstract
AIM: To establish a method for the reversible immortalization of human hepatocytes, which may offer a good and safe source of hepatocytes for practical applications.
METHODS: We successfully isolated primary human hepatocytes from surgically resected liver tissue taken from a patient with liver hemangiomas. The freshly isolated cells were then immortalized with retroviral vector SSR#69 expressing simian virus 40 large T antigen (SV40T) and hygromycin-resistance genes flanked by paired loxP recombination targets.
RESULTS: The freshly isolated hepatocytes with high viability (85%) were successfully immortalized using retroviral gene transfer of SV40T. SV40T in the immortalized cells was then excised by Cre/loxP site-specific recombination. This cell population exhibited the characteristics of differentiated hepatocytes.
CONCLUSION: We successfully established reversibly immortalized human hepatocytes, which will provide an unlimited supply of cells for practical applications.
Keywords: Hepatocyte, Primary human hepatocytes, Reversible immortalization, Hepatocyte isolation, SV40T
Core tip: It is meaningful to establish reversibly immortalized human hepatocytes which can be economically grown in tissue culture. Toward this goal, we successfully established a method for the reversible immortalization of human hepatocytes using Cre/loxP site-specific recombination, which may offer a good and safe source of hepatocytes for the bioartificial liver (BAL) system in the near future. If a sufficient number of human hepatocytes can be used, these extracorporeal devices will serve as successful “bridge-to-transplant”therapies. With the progress made in bioreactor development, the next-generation BAL system could reach the level of artificial kidney and save more patients.
INTRODUCTION
Acute liver failure is associated with high mortality. The current standard treatment for acute liver failure is orthotopic liver transplantation[1,2]. However, with the increasing shortage of organ donors, a considerable number of the patients die while still on the transplant waiting list. Among the liver assist therapies, bioartificial liver (BAL) therapy is considered the most promising solution to bridge them to liver regeneration or to liver transplantation[3,4]. Several BAL systems have been shown to result in significant improvements in survival in clinical applications[5]. In developing BAL therapy, normal human cells are an ideal cell source. However, the utility of primary cells is hampered by difficulties in timely obtaining the cells that have a limited lifespan in vitro. An attractive alternative source would be immortalized hepatocytes which would exhibit the characteristics of differentiated hepatocytes and make unlimited supplies of cells feasible[6]. Simian virus 40 large T antigen (SV40T) is a typical tool used for cellular immortalization. However, it is dangerous to use cells possessing oncogenes in the clinical setting. SV40T in immortalized cells would expose patients to tumorigenic risk. An attractive solution to such risk would be the use of the novel reversible immortalization strategy by using Cre/loxP site-specific recombination[7]. Cells derived from such a procedure would have the advantages of freedom from infectious pathogens, uniformity, and unlimited availability.
We have made significant efforts to establish liver cell lines for cell therapies[8-10]. We described a novel strategy for establishing porcine reversibly immortalized hepatocyte lines. However, when using xenogenic porcine cells, there would be a concern that species-specific pathogens may be transmitted to recipients (such as infection by endogenous retroviruses). Therefore, it is meaningful to establish reversibly immortalized human cells that can make unlimited supplies in tissue culture using the techniques of gene transfer. Toward this goal, we have focused on human hepatocyte reversible immortalization by using Cre/loxP site-specific recombination. We hope to establish reversible immortalization of human hepatocytes which may offer a good and safe source of hepatocytes for the BAL system in the near future.
MATERIALS AND METHODS
Primary human hepatocyte isolation
Following ethical and institutional guidelines and after obtaining informed consent from the tissue donor, a sample was collected from a patient undergoing liver surgery. The patient was suffering from liver hemangiomas and required partial hepatectomy. We successfully obtained 32 g of liver tissue (Figure 1A). Primary hepatocytes were isolated under sterile conditions using a modified four-step retrograde perfusion technique as previously reported[9]. Briefly, the resected sample was first cannulated using a suitable pipette in a visible blood vessel on the cut surface (Figure 1A). Then the selected liver tissue was continuously perfused with a pre-warmed (37 °C) digestion buffer solution (8.4 g/L Dispase II and 0.5 g/L Collagenase IV, Sigma, St. Louis, MO, USA). Following sufficient digestion, the liver capsule was mechanically disrupted (Figure 1B) and the emerging cell suspension was filtered through a 250 μm nylon mesh and centrifuged at 50 g and 4 °C for 2 min (Figure 1C-D). The resulting suspension then underwent several wash steps. The resulting cell clumps were finally resuspended in culture medium (William’s medium E, supplemented with 100 mU/mL penicillin, 100 μg/mL streptomycin and 10% fetal bovine serum). Hepatocyte yield and viability were determined using the traditional standard trypan blue exclusion technique. Finally, the freshly isolated hepatocytes were seeded at a concentration of 4-5 × 105/mL in culture flasks. The medium was changed every 24 h. The morphology of the cultured hepatocytes was assessed using a Nikon Diaphot inverted microscope.
Figure 1.

Hepatocyte isolation with a modified four-step retrograde perfusion technique. Perfusion was conducted by inserting a suitable pipette into vessels exposed on a cut surface of the sample (A). After sufficient digestion, the liver capsule was mechanically disrupted (B). The emerging cell suspension was filtrated through a 250 μm nylon mesh (C) and centrifuged (50 g, 2 min, 4 °C) (D).
Primary human hepatocyte immortalization
The retroviral vector SSR#69, which was kindly donated by Naoya Kobayashi, Okayama University, was cultured in DMEM medium (Sigma, St. Louis, MO, United States) supplemented with 10% newborn calf serum (Figure 2). SSR#69 contains SV40T and a gene resistant to hygromycin. Freshly isolated primary human hepatocytes were first cultured in T25 flasks. Then in each flask they were transduced with 2 mL of SSR#69 cell supernatant at 37 °C for 12 h each day for 5 d. Two days after the final transduction, a selection step was applied with 100 μg/mL hygromycin. After 2 wk of hygromycin selection, there were several colonies of transduced hepatocytes observed. These cells displayed the morphological characteristics of primary human hepatocytes. Then, the cells were isolated to culture by cloning rings. Finally, SV40T cDNA was excised by Cre/loxP site-specific recombination.
Figure 2.
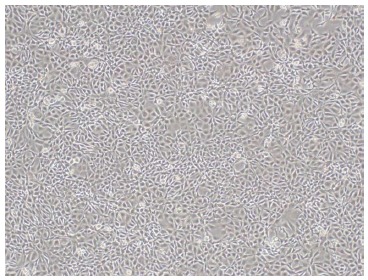
Morphology of SSR#69 cultured in DMEM medium supplemented with 10% newborn calf serum (× 100).
Immunofluorescent analysis of SV40T in immortalized human hepatocytes
Indirect immunofluorescent staining was performed to detect SV40Tag, using mouse monoclonal immunoglobulin G antibody to SV40Tag (Santa Cruz, CA, United States; Santa Cruz Biotechnology) and a secondary antibody, rhodamine (TRITC)-conjugated sheep anti-mouse IgG (Sigma, St. Louis, MO, United States). In order to stain nuclei (double-stranded DNA), DAPI (4’,6-diamidine-2’-phenylindole dihydrochloride, Roche, Cat. No. 10 236 276 001) blue-fluorescent dye was used.
Expression of liver-specific genes associated with liver function in immortalized human hepatocytes
Total RNA was extracted from immortalized human cells. According to the manufacturer’s protocol, reverse transcription-polymerase chain reaction (RT-PCR) was performed. Primers used were as follows: human albumin (576 bp), AAACCTCTTGTGGAAGAGCC (5’ primer) and CAAAGCAGGTCTCCTTATCG (3’ primer); human ASGPR (495 bp), TAGGAGCCAAGCTGGAGAAA (5’ primer), ACCTGCAGGCAGAAGTCATC (3’ primer) (Table 1); SV40T (422 bp), CAGGCATAGAGTGTCTGC (5’ primer), CAACAGCCTGTTGGCATATG (3’ primer).
Table 1.
Primers used in reverse transcription-polymerase chain reaction in this study
| Gene | Forward (Tm) | Reverse (Tm) | Product size |
| SV40T | CAGGCATAGAGTGTCTGC | CAACAGCCTGTTGGCATATG | 422 bp |
| Human ASGPR | TAGGAGCCAAGCTGGAGAAA | ACCTGCAGGCAGAAGTCATC | 495 bp |
| Human GST-II | GCCCTACACCGTGGTCTATT | GGCTAGGACCTCATGGATCA | 496 bp |
| Human GS | ATGCTGGAGTCAAGATTGCG | TCATTGAGAAGACACGTGCG | 535 bp |
| Human Albumin | AAACCTCTTGTGGAAGAGCC | CAAAGCAGGTCTCCTTATCG | 576 bp |
| Human HBCF-X | GTGCATGGAAGAGACCTGCT | GAAGTCAAGCAGGTCGAAGG | 493 bp |
| Human β-actin | TGACGGGGTCACCCACACTGTGCCCATCTA | AGAAGCATTTGCGGTGGACGATGGAGGG | 610 bp |
Human ASGPR: Human asialoglycoprotein receptor; GST-II: Glutathione-S-transferase II; GS: Glutamine synthetase; HBCF-X: Human blood coagulation factor X.
RESULTS
Successful isolation of primary human hepatocytes resulted in a cell yield of 6.56 × 106 cells/g liver tissue. The viability of cells immediately after isolation was 85%, as revealed by the trypan blue exclusion technique. The freshly isolated primary human cells attached to the plates showed the typical morphological appearance with granular cytoplasm, a polygonal shape and one or more nuclei (Figure 3).
Figure 3.
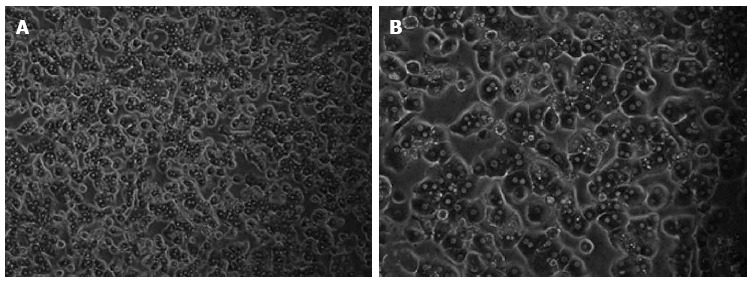
Morphology of freshly isolated primary human hepatocytes. The primary human hepatocytes attached to the plates showed the typical morphological appearance with the polygonal shape, granular cytoplasm and one or more nuclei.
After 100 μg/mL hygromycin selection within 2 wk, several clones (Figure 4B-C) grew steadily in the culture medium. These selected clones were then isolated by cloning rings. The selected cultured clones displayed the morphological characteristics of human cells featuring a large round nucleus with a few nucleoli and multiple granules in the cytoplasm. Treatment with Cre recombinase resulted in loss of proliferation of the expanded hepatocytes and they reverted to their pre-immortalized state. The SV40Tag in the nuclei of all immortalized cells and reverted cells was examined by immunofluorescent staining (Figure 5). Similar to normal primary human cells, immortalized and reverted human hepatocytes expressed the albumin gene, as shown by RT-PCR (Figure 6).
Figure 4.
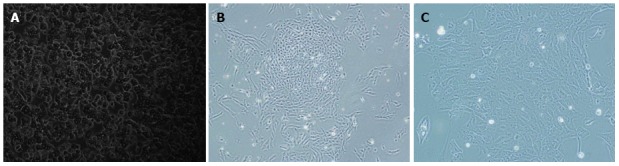
Morphology of primary and immortalized cells. Non-immortalized primary hepatocytes grow slowly with low plating efficiency and a short life span (A). Immortalized human hepatocytes grow rapidly as islands and had an extended life span (B, C). Magnifications: A and B: × 100; C: × 200.
Figure 5.
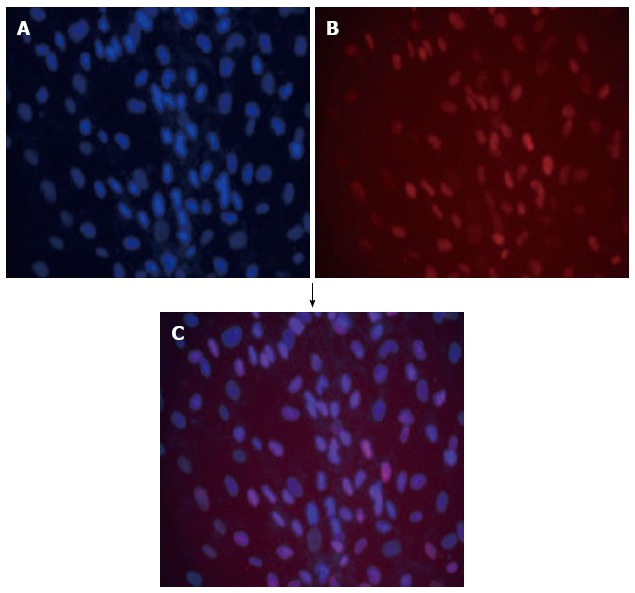
Double immunofluorescence of immortalized hepatocytes stained with DAPI (blue) (A) together with monoclonal antibody anti-SV40T revealed with texas red-antibody conjugate (red) (B); Blue and red fluorescence merged as purple (C).
Figure 6.
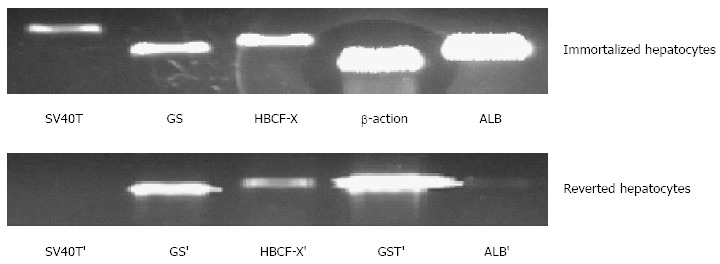
Expression of liver-specific genes associated with liver function in immortalized and reverted cells. Lanes 1 to 5, simian virus 40 large T antigen (SV40T) and SV40T’, glutamine synthetase) (GS and GS’, human blood coagulation factor X (HBCF-X) and HBCF-X’, β-action and GST’ (glutathione-S-transferase), human albumin (ALB) and ALB’, respectively.
DISCUSSION
Liver transplantation is the optimal therapy for end-stage hepatic failure. However, due to donor shortage, liver transplantation is not available for a great number of patients with liver failure[11]. Recently, there has been a need to develop extracorporeal liver assist systems that can be used as a bridge to liver transplantation or for curative treatment of acute liver failure[12,13], which would represent a significant alternative to resolving the donor shortage[14,15]. Among these liver assist therapies[16], BAL therapy is the most promising technique[17-19]. The treatment efficacy depends on the bioactivity of the hepatocytes in the bioreactor[20]. Bioactive mass and cell source or cell type play a key role in BAL treatment[21]. In order to be used in patients, the BAL system should have adequate hepatocytes to provide sufficient bioactive support. The BAL device consists of 5 to 20 × 109 hepatocytes.
About 20% of healthy liver mass would be needed, known from partial hepatectomy studies. It contains about 20 × 109 (approximately 200 g) hepatocytes. Thus, theoretically, approximately 20 × 109 well-functioning hepatocytes are needed to keep a patient alive[5]. In developing BAL therapy, normal human cells are an ideal cell source. However, the utility of cultured primary human hepatocytes is hampered by difficulties in timely obtaining populations which have a limited lifespan when cultured in vitro. Alternative sources of cells have been sought to overcome the limitation of primary human hepatocytes for BAL application.
To fulfill the requirements stated above, we attempted to establish easy-to-use human hepatocytes for BAL therapy. Theoretically, the ideal cells for a BAL system are normal human hepatocytes. However, there is a severe shortage of donated human livers for hepatocyte isolation. Furthermore, the cultured primary hepatocyte utility is hampered by difficulties in timely obtaining populations which cannot be expanded in vitro. Previously, we established an efficient procedure for primary porcine hepatocyte isolation using a modified four-step retrograde perfusion technique, which resulted in an improvement in cell viability and yield[9]. We then expanded the method to primary human hepatocyte isolation. The majority of previous researchers used tissue from surgically resected liver specimens of malignant tumors for human hepatocyte isolation. We explored the possibility of isolating human hepatocytes from resected normal liver tissue.
With regard to hepatocyte cryopreservation, several cryopreservation methods for hepatocytes have been reported[22]. Successful cryopreservation can be used for timely availability of hepatocytes[23]. The availability and logistics of BAL systems would be significantly improved if methods for the long-term hepatocyte preservation, without loss of cellular activity, were developed. As reported previously[9], we developed a modification with a pre-incubation step prior to density gradient centrifugation. The modification allows rapid separation of viable hepatocytes from dead cells, but with less viable cell loss. However, hepatocytes are very sensitive to freezing damage. Hepatocyte functional activities after thawing are still unsatisfactory. Another alternative is primary porcine hepatocytes. Porcine livers are easily obtained and can be available on demand. However, porcine hepatocytes produce xenogeneic proteins[24], making this technique controversial.
Another attractive alternative source of cells for BAL application would be immortalized hepatocytes which would exhibit the characteristics of differentiated hepatocytes and could make unlimited supplies of cells feasible[25]. However, the presence of the oncogene in the immortalized cells would expose humans to tumorigenic risk. An attractive solution to this question would be to use the novel strategy of reversible immortalization[26]. The reversible immortalization procedure was devised by an oncogene transfer which can be subsequently excised effectively (Figure 7)[7]. As reported previously[8], we established reversibly immortalized porcine hepatocytes by transfection with the retroviral vector SSR#69 (Figure 8). However, xenotransplantation has negative aspects. It was demonstrated that xenotransplantation associated viral infection could affect their families and attending physicians and nurses, not only the patients themselves[5]. To overcome possible immunologic reactions and zoonosis, BAL researchers have focused on the replacement of animal origin hepatocytes by human cells, either primary hepatocytes or immortalized cell lines.
Figure 7.
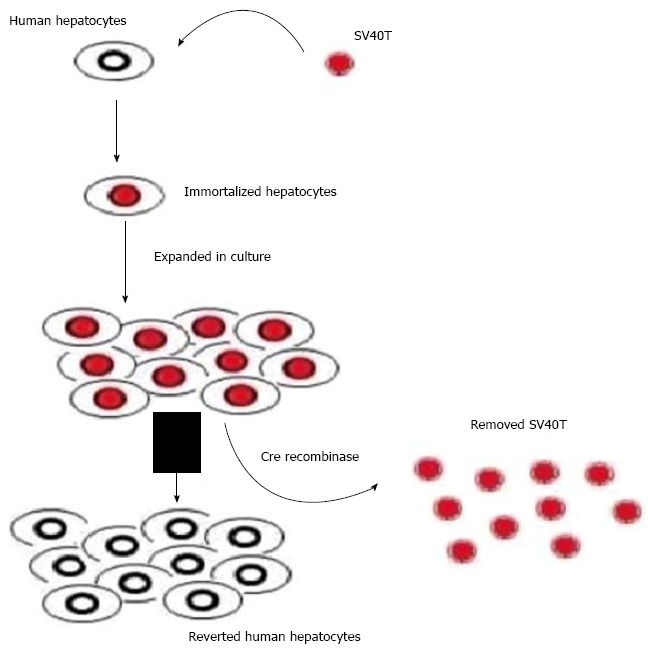
Scheme of reversible immortalization. The primary hepatocytes were immortalized by transfer of SV40Tag. After expansion of the immortalized cells, Cre/loxP recombination was performed to remove the oncogene (SV40T) and cells reverted to their preimmortalized state.
Figure 8.
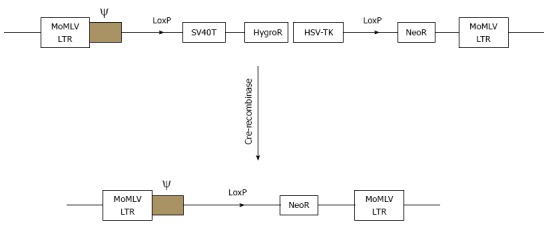
Schematic drawings of the integrating component of retrovitral vector SSR#69 before and after cre-recombination. SSR#69 contains hygromycin B resistance gene (Hyg R) as a positive selectable marker and the herpes simplex virus thymidine kinase gene (HSV-TK) as a negative selectable marker. The SV40T, Hyg R and HSV-TK genes are flanked by two LoxP sites.
To address these issues, in the present study we expanded the novel strategy of reversible immortalization from primary porcine hepatocytes to primary human hepatocytes. Although hepatocytes can be transduced with retroviral vectors, the transduction efficiency was significantly very low[27]. One of the major challenges of successful transduction is the difficulty in getting a sufficient amount of cells with high post-isolation viability. To effectively and successfully transduce retroviral vectors into hepatocytes, it is meaningful to establish an efficient technique of cell isolation. We here first established a modified four-step collagenase retrograde perfusion technique, which resulted in a hepatocyte yield of 6.56 × 106 cells/g liver. These freshly isolated hepatocytes with high viability (85%) were then successfully immortalized using retroviral gene transfer of SV40T. Finally, SV40T in the expanded hepatocytes was excised by Cre/loxP site-specific recombination. The population of reverted hepatocytes exhibited the characteristics of differentiated cells. The reversibly immortalized hepatocytes, by permitting temporary and controlled expansion of cell populations, may therefore be used for BAL application. If a sufficient amount of human hepatocytes can be used as a cell source, these extracorporeal devices will serve as successful “bridge-to-transplant” therapies. We hope that, with the corresponding progress in bioreactor development, next-generation BAL systems can reach the level of the artificial kidney and save more patients.
COMMENTS
Background
The authors have made significant efforts to establish liver cell lines for cell therapies. They previously described a strategy for establishing porcine reversibly immortalized cell lines which were intended for practical application. However, when using xenogenic porcine cells, there is a concern that species-specific pathogens could be transmitted to recipients. Thus, in the present study, they focused on establishing reversible immortalization of human hepatocytes which may offer a good and safe source of hepatocytes for practical application in the near future.
Research frontiers
Freshly isolated primary human hepatocytes were successfully immortalized by retrovirus-mediated transfer of an immortalizing oncogene (SV40T) which could be excised subsequently by Cre/loxP-mediated site-specific recombination.
Innovations and breakthroughs
The authors successfully immortalized primary human hepatocytes. The reversibly immortalized human hepatocytes could provide an unlimited supply of cells for practical application.
Applications
The established reversible immortalization of human hepatocytes may offer a good and safe source of hepatocytes for practical application in the near future. If a sufficient amount of human hepatocytes can be used as a cell source, these extracorporeal devices will serve as successful “bridge-to-transplant” therapies. With the progress in bioreactor development, next-generation BAL systems could reach the level of the artificial kidney and save more patients.
Terminology
Reversible immortalization: The reversible immortalization procedure was devised by a retrovirus-mediated oncogene transfer which could be subsequently excised effectively by site-specific recombination.
Peer review
It will be a significant advance to treat liver failure patients using immortalized cells if a functional hepatocyte line can be generated. This paper is a good presentation of the authors’ work and achieves good linearity. Reversible immortalization of primary human hepatocytes was successfully achieved using methods involving retrovirus-mediated transfer of the SV40 T antigen and subsequent excision by site specific recombination.
Footnotes
Supported by Major Scientific and Technological Project of Shandong Province, No. 201221019; and Cisco Clinical Oncology Research Fund and Bayer Schering Cancer Research Fund, No. Y-B2012-011
P- Reviewer: Smith SM, Wang XS, Wang WH S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Liu XM
References
- 1.Chen PX, Yan LN, Wang WT. Outcome of patients undergoing right lobe living donor liver transplantation with small-for-size grafts. World J Gastroenterol. 2014;20:282–289. doi: 10.3748/wjg.v20.i1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khaderi S, Khan R, Safdar Z, Stribling R, Vierling JM, Goss JA, Sussman NL. Long-term follow-up of portopulmonary hypertension patients after liver transplantation. Liver Transpl. 2014;20:724–727. doi: 10.1002/lt.23870. [DOI] [PubMed] [Google Scholar]
- 3.Ezzat T, Dhar DK, Malago M, Olde Damink SW. Dynamic tracking of stem cells in an acute liver failure model. World J Gastroenterol. 2012;18:507–516. doi: 10.3748/wjg.v18.i6.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gessner RC, Hanson AD, Feingold S, Cashion AT, Corcimaru A, Wu BT, Mullins CR, Aylward SR, Reid LM, Dayton PA. Functional ultrasound imaging for assessment of extracellular matrix scaffolds used for liver organoid formation. Biomaterials. 2013;34:9341–9351. doi: 10.1016/j.biomaterials.2013.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kobayashi N. Life support of artificial liver: development of a bioartificial liver to treat liver failure. J Hepatobiliary Pancreat Surg. 2009;16:113–117. doi: 10.1007/s00534-008-0022-1. [DOI] [PubMed] [Google Scholar]
- 6.MENG FY, ZHOU P. [Latest advances in immortalized human hepatocytes] Zhonghua Ganzangbing Zazhi. 2009;17:395–397. [PubMed] [Google Scholar]
- 7.Kobayashi N, Fujiwara T, Westerman KA, Inoue Y, Sakaguchi M, Noguchi H, Miyazaki M, Cai J, Tanaka N, Fox IJ, et al. Prevention of acute liver failure in rats with reversibly immortalized human hepatocytes. Science. 2000;287:1258–1262. doi: 10.1126/science.287.5456.1258. [DOI] [PubMed] [Google Scholar]
- 8.Meng FY, Chen ZS, Han M, Hu XP, He XX, Liu Y, He WT, Huang W, Guo H, Zhou P. Porcine hepatocyte isolation and reversible immortalization mediated by retroviral transfer and site-specific recombination. World J Gastroenterol. 2010;16:1660–1664. doi: 10.3748/wjg.v16.i13.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meng FY, Chen ZS, Han M, Hu XP, Zhou P. An improved purification approach with high cell viability and low cell loss for cryopreserved hepatocytes. Cryobiology. 2010;60:238–239. doi: 10.1016/j.cryobiol.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 10.He XX, Chang Y, Meng FY, Wang MY, Xie QH, Tang F, Li PY, Song YH, Lin JS. MicroRNA-375 targets AEG-1 in hepatocellular carcinoma and suppresses liver cancer cell growth in vitro and in vivo. Oncogene. 2012;31:3357–3369. doi: 10.1038/onc.2011.500. [DOI] [PubMed] [Google Scholar]
- 11.Chen ZS, Meng FY, Chen XP, Liu DG, Wei L, Jiang JP, Du DF, Zhang WJ, Ming CS, Gong NQ. Combined en bloc liver/pancreas transplantation in two different patients. World J Gastroenterol. 2009;15:2552–2555. doi: 10.3748/wjg.15.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uygun BE, Soto-Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, Milwid J, Kobayashi N, Tilles A, Berthiaume F, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16:814–820. doi: 10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Y, Li J, Pan X, Zhou P, Yu X, Cao H, Wang Y, Li L. Co-culture with mesenchymal stem cells enhances metabolic functions of liver cells in bioartificial liver system. Biotechnol Bioeng. 2013;110:958–968. doi: 10.1002/bit.24752. [DOI] [PubMed] [Google Scholar]
- 14.Lee JS, Shin J, Park HM, Kim YG, Kim BG, Oh JW, Cho SW. Liver extracellular matrix providing dual functions of two-dimensional substrate coating and three-dimensional injectable hydrogel platform for liver tissue engineering. Biomacromolecules. 2014;15:206–218. doi: 10.1021/bm4015039. [DOI] [PubMed] [Google Scholar]
- 15.Pi J, Fang B, Wang Y, Liu R. [3D liver vessel segmentation based on hessian matrix and GMM-EM algorithm] Shengwu Yixue Gongchengxue Zazhi. 2013;30:486–492. [PubMed] [Google Scholar]
- 16.Cristallini C, Cibrario Rocchietti E, Accomasso L, Folino A, Gallina C, Muratori L, Pagliaro P, Rastaldo R, Raimondo S, Saviozzi S, et al. The effect of bioartificial constructs that mimic myocardial structure and biomechanical properties on stem cell commitment towards cardiac lineage. Biomaterials. 2014;35:92–104. doi: 10.1016/j.biomaterials.2013.09.058. [DOI] [PubMed] [Google Scholar]
- 17.Ott HC, Mathisen DJ. Bioartificial tissues and organs: are we ready to translate? Lancet. 2011;378:1977–1978. doi: 10.1016/S0140-6736(11)61791-1. [DOI] [PubMed] [Google Scholar]
- 18.Hilal-Alnaqbi A, Mourad AH, Yousef BF. Effect of membranes on oxygen transfer rate and consumption within a newly developed three-compartment bioartificial liver device: Advanced experimental and theoretical studies. Biotechnol Appl Biochem. 2014;61:304–315. doi: 10.1002/bab.1173. [DOI] [PubMed] [Google Scholar]
- 19.Erro E, Bundy J, Massie I, Chalmers SA, Gautier A, Gerontas S, Hoare M, Sharratt P, Choudhury S, Lubowiecki M, et al. Bioengineering the liver: scale-up and cool chain delivery of the liver cell biomass for clinical targeting in a bioartificial liver support system. Biores Open Access. 2013;2:1–11. doi: 10.1089/biores.2012.0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humes HD, Buffington D, Westover AJ, Roy S, Fissell WH. The bioartificial kidney: current status and future promise. Pediatr Nephrol. 2014;29:343–351. doi: 10.1007/s00467-013-2467-y. [DOI] [PubMed] [Google Scholar]
- 21.Liu H, You S, Rong Y, Wu Y, Zhu B, Wan Z, Liu W, Mao P, Xin S. Newly established human liver cell line: a potential cell source for the bioartificial liver in the future. Hum Cell. 2013;26:155–161. doi: 10.1007/s13577-013-0068-5. [DOI] [PubMed] [Google Scholar]
- 22.Hutzler JM, Yang YS, Albaugh D, Fullenwider CL, Schmenk J, Fisher MB. Characterization of aldehyde oxidase enzyme activity in cryopreserved human hepatocytes. Drug Metab Dispos. 2012;40:267–275. doi: 10.1124/dmd.111.042861. [DOI] [PubMed] [Google Scholar]
- 23.Massie I, Selden C, Hodgson H, Fuller B. Cryopreservation of encapsulated liver spheroids for a bioartificial liver: reducing latent cryoinjury using an ice nucleating agent. Tissue Eng Part C Methods. 2011;17:765–774. doi: 10.1089/ten.TEC.2010.0394. [DOI] [PubMed] [Google Scholar]
- 24.Hoekstra R, Nibourg GA, van der Hoeven TV, Ackermans MT, Hakvoort TB, van Gulik TM, Lamers WH, Elferink RP, Chamuleau RA. The HepaRG cell line is suitable for bioartificial liver application. Int J Biochem Cell Biol. 2011;43:1483–1489. doi: 10.1016/j.biocel.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 25.Totsugawa T, Yong C, Rivas-Carrillo JD, Soto-Gutierrez A, Navarro-Alvarez N, Noguchi H, Okitsu T, Westerman KA, Kohara M, Reth M, et al. Survival of liver failure pigs by transplantation of reversibly immortalized human hepatocytes with Tamoxifen-mediated self-recombination. J Hepatol. 2007;47:74–82. doi: 10.1016/j.jhep.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 26.Chapdelaine P, Kang J, Boucher-Kovalik S, Caron N, Tremblay JP, Fortier MA. Decidualization and maintenance of a functional prostaglandin system in human endometrial cell lines following transformation with SV40 large T antigen. Mol Hum Reprod. 2006;12:309–319. doi: 10.1093/molehr/gal034. [DOI] [PubMed] [Google Scholar]
- 27.Adams RM, Soriano HE, Wang M, Darlington G, Steffen D, Ledley FD. Transduction of primary human hepatocytes with amphotropic and xenotropic retroviral vectors. Proc Natl Acad Sci USA. 1992;89:8981–8985. doi: 10.1073/pnas.89.19.8981. [DOI] [PMC free article] [PubMed] [Google Scholar]


