Abstract
Our ability to learn and control the motor aspects of complex laryngeal behaviors, such as speech and song, is modulated by the laryngeal motor cortex (LMC), which is situated in the area 4 of the primary motor cortex and establishes both direct and indirect connections with laryngeal motoneurons. In contrast, the LMC in monkeys is located in the area 6 of the premotor cortex, projects only indirectly to laryngeal motoneurons and its destruction has essentially no effect on production of species-specific calls. These differences in cytoarchitectonic location and connectivity may be a result of hominid evolution that led to the LMC shift from the phylogenetically “old” to “new” motor cortex in order to fulfill its paramount function, i.e., voluntary motor control of human speech and song production.
Introduction
The larynx participates in a wide range of vital behaviors, such as breathing, swallowing and voice production, all of which are indispensible for our existence and communication. While breathing and swallowing are innate behaviors, the ability to produce voice for speaking and singing involves intensive learning and requires a proper integration between several brain networks for the motor output of an uttered word. The ability to control laryngeal muscles voluntarily is most remarkable in actors and singers, who are able, on demand, to raise and lower the larynx, regulate the amount of airflow through the vocal folds, tense and relax the vocal folds, and even move each vocal fold separately in order to modulate their speaking or singing voice.
Voluntary voice production in humans is under the direct control of the laryngeal motor cortex (LMC), which gives rise to a final common cortical motor pathway descending via the corticobulbar tract and communicating with laryngeal motoneurons in the brainstem to innervate the laryngeal muscles. In regard to the central motor control, the open question is what (neurologically) makes us humans unique in our ability to learn and produce voice for speech and song as oppose to other primate species, which have limited, if any, capacity for vocal learning and voluntary voice production [1,2]••. A possible candidate brain region that appears to have grossly similar but importantly distinct topology and connectivity in humans compared to other mammals is the LMC itself.
The laryngeal motor cortex: location
In contrast to other body part representations within the primary motor cortex, the exact LMC location in humans remained largely unknown until recently. Based on the seminal work by Penfield and colleagues in 1930s-50s [3]••, the LMC was assumed to be located somewhere within the vocalization area in the inferior portion of the precentral gyrus, just above the swallowing and below the face representations (Fig. 1A). Using direct electrical stimulation, the LMC was also identified in the chimpanzee, rhesus monkey, and squirrel monkey but its location was far rostrally within the precentral gyrus [4,5]• compared to Penfiled's vocalization area in humans [3]. The existence of a motor cortical region specialized for isolated vocal fold movements was questioned in other mammals, such as the dog and cat [6]. A recent study reported that the laryngeal motor cortical representation might exist in mice and is possibly involved in the modulations of pitch of ultrasound vocalizations [7], although these findings and their homology with the human and non-human primate LMC require further investigation.
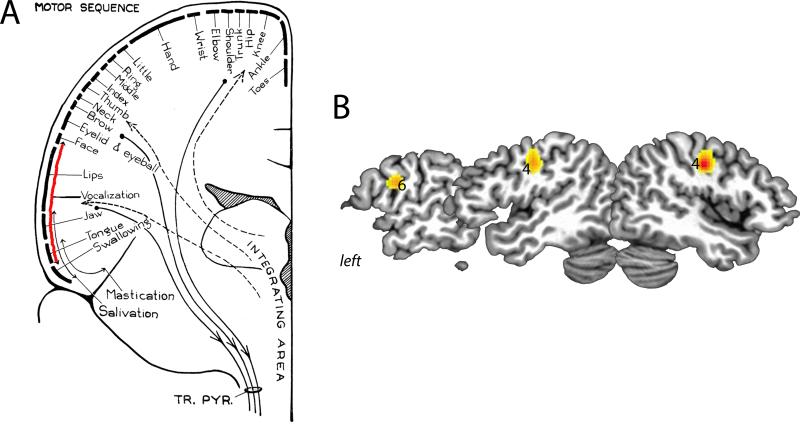
Figure 1.
(A) The “Motor sequence” within the primary motor cortex with the extensive vocalization region in the inferior portion of the precentral gyrus [62]. (B) Meta-analysis of 19 fMRI studies between 2000-2013 using activation likelihood estimation (ALE) of brain function during voice production (GingerALE software). Bilateral peaks of LMC activation were found in the area 4p with an additional peak of activation in the left area 6 [15]. Data are presented on a series of sagittal slices in the standard Talairach-Tournoux space.
The LMC regions in humans and non-human primates are considered to be homologues [2,8] because, while stimulated, both yield an approximation (or adduction) of vocal folds to the midline of the larynx, which is independent from the movement of the other facial or upper body muscles [4,9-13]. Physiologically, vocal fold adduction is necessary for the majority of laryngeal behaviors, such as voice production, coughing, sneezing, stabilizing thorax for lifting heavy weights, etc. A recent series of neuroimaging studies suggested that the LMC in humans is located more caudally within the precentral gyrus compared to the LMC of non-human primates [4,5] and more dorsally from the Sylvian fissure than originally thought based on the vocalization mapping studies by Penfield and colleagues [3]. We conducted a meta-analysis of 19 functional MRI (fMRI) studies between 2000 and 2013 in healthy humans during production of meaningful and meaningless syllables, vowels, glottal stops, and phonation with and without articulatory movements and identified that the bilateral peaks of activation corresponding to the LMC are located in the primary motor cortex (area 4 of Brodmann [14]) [15] (Fig. 1B). This finding is in line with high-resolution multi-electrode cortical recording study during syllable production [16] and transcranial magnetic stimulation (TMS) study of the motor cortex during resting and voice production [17-19], which reported the laryngeal muscle representation in the dorsal portion of the ventral primary motor cortex. Furthermore, the location of this region corresponds to the motor cortical area where left hemisphere lateralized brain activity during reading is associated with FOXP2 polymorphism [20]. The peak of activity within the LMC, as identified in our meta-analysis study, was located in the posterior part of area 4 (i.e., area 4p of Geyer et al. [21,22]). It has been shown that the area 4p is involved in initiation and execution of motor commands as well as modulation of movement-related attention as oppose to the area 4a (the anterior part of area 4), which functionally resembles the secondary motor cortex by requiring higher-order sensory feedback for motor execution [23-25].
The meta-analysis of neuroimaging literature has also showed an additional peak of activation in the left premotor cortex (area 6 of Brodmann) [15] (Fig. 1B), which is similar to the location of monkey LMC, as described below. Studies using direct electrical stimulation of the motor strip in the macaque have identified the laryngeal muscle representation only between the inferior branch of the arcuate sulcus rostrally and the subcentral dimple caudally [4,11,26] (Fig. 2B). A similar location of LMC was also described in the squirrel monkey [12,27,28]. The LMC region in the rhesus monkey was shown to contain vocalization-related neurons [26]. Cytoarchitectonically, this region falls within the premotor cortex (area 6 of Brodmann [14], area 6bα of Vogt and Vogt [29], area FCBm of von Bonin and Bailey [30], area F5 of Matelli et al. [31], area 6VR(F5)/ProM of Paxinos et al. [32], or area F5(6Va/Vb) by Saleem and Logothetis [33]). However, extensive explorations of the precentral gyrus with direct electrical stimulation in non-human primates have failed, so far, to identify a region within the primary motor cortex (i.e., area 4), which would elicit isolated bilateral laryngeal muscle movements [11,26,27].
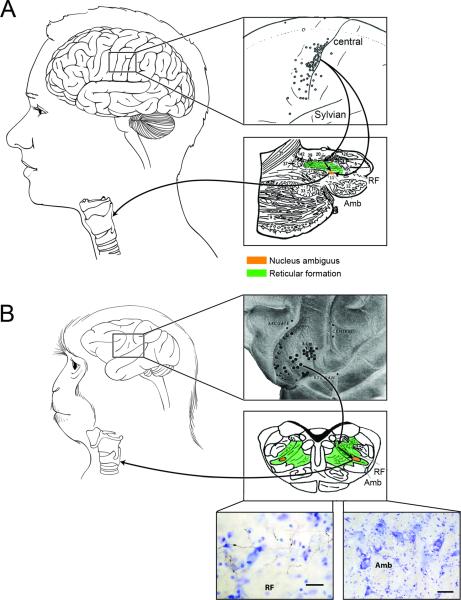
Figure 2.
(A) Schematic drawing of the human brain and larynx (left) with the insets showing (top) the sites of vocalization elicitation during direct electrical stimulation of the primary motor cortex [63] and (bottom) the sagittal section of the brainstem depicting the distribution of degenerating fibers (small dots) in the nucleus ambiguus (Amb) and surrounding reticular formation [38]. The arrows represent the direct (monosynaptic) connections from the LMC to the reticular formation and nucleus ambiguus, the site of laryngeal motoneurons, which project to the laryngeal muscles (B) Schematic drawing of the macaque brain and larynx (left) with the insets illustrating (top) topographic representation of the intrinsic and extrinsic laryngeal muscle in the premotor cortex [4]. Sca –subcentral dimple; right-angled triangle – cricothyroid muscle; circle – thyroarytenoid muscle; encircled right-angled triangle - combination of the cricothyroid and thyroarytenoid muscles; square – extrinsic laryngeal muscles. (bottom) The crosssection of the brainstem and photomicrographs show terminal fields of the laryngeal motor cortical projections in the reticular formation (RF) but not nucleus ambiguus in the rhesus monkey, which was injected with the anterograde tracer, biotin dextranamine, into the LMC [36]. The arrows show indirect connection of the LMC with the nucleus ambiguus via the surrounding reticular formation. The scale bar corresponds to 50 μm.
The laryngeal motor cortex: connectivity
Another distinct feature of the LMC organization between humans and non-human primates is its projection network. Both species have largely comparable LMC structural connectivity with numerous cortical and subcortical brain regions [8,15,34]. Human LMC in the area 4 appears to have a more refined and dense projection network with the parietal cortex, supporting a more active sensorimotor integration for voluntary voice control. Conversely, monkey's LMC in the area 6 has greater connectivity with the anterior cingulate cortex, which is potentially important for voluntary initiation of genetically pre-programmed species-specific calls [2,35].
The only connection that is exclusively present in humans but not in monkeys is the direct (monosynaptic) projection from the LMC to nucleus ambiguus, a site of laryngeal motoneurons in the brainstem [27,36-38]•• (Fig. 1A-III, 1A-II). Instead, the monkey LMC is connected with the nucleus ambiguus via the dorsal and parvicellular nuclei of the reticular formation of the brainstem [27,39], which is known to be involved in vocal motor coordination of vocalizations [28] (Fig. 1B-III). It is, therefore, not surprising that electrophysiological experiments in humans and monkeys have demonstrated significant differences in onset latencies of cortical motor evoked potentials (MEPs) from the laryngeal muscles. Studies using transcranial magnetic stimulation (TMS) in healthy humans were successful in identifying the topographic representation of different laryngeal muscles within the primary motor cortex with the onset latencies of corticobulbar MEPs ranging from 7.3 ms to 14.1 ms [17-19,40-42]. Similarly, distinct cortical topography of laryngeal muscles was described in non-human primates, but with a location in the premotor cortex and MEP onset latencies more than twice as long at 20-40 ms [4]. Direct LMC-ambigual projections in humans allow the LMC to bypass the relay station in the reticular formation and thus directly modulate the activity of brainstem laryngeal motoneurons.
The laryngeal motor cortex: function
The existence of direct projections descending from the LMC to laryngeal motoneurons of the brainstem in humans only may explain an important observation that LMC stimulation elicits vocalizations in humans but not monkeys [3,9]. Physiologically, the direct monosynaptic pathway in humans facilitates the ability of nucleus ambiguus to control the production of complex voluntary learned laryngeal movements during speaking and singing [36]. In non-human primates, the indirect access of the LMC to nucleus ambuguus via reticular formation limits production of voluntary learned laryngeal patterns. The role of LMC in these species may primarily be related to the control of other (by and large innate) laryngeal behaviors, such as coughing, swallowing, breathing, etc. [12]. Moreover, while monkeys lack a full coordination between the laryngeal and other articulators [2,26], they are still able to have control the basic aspects of their innate vocalizations, e.g., tuning the amplitude and duration of calls to environmental parameters [43-45]. This may be possible through the direct connections that the LMC establishes with the other cortical and subcortical brain regions involved in voice control, such as the anterior cingulate cortex, ventrolateral reticular formation, and motor and sensory pools of neurons in the brainstem controlling orofacial articulators [11,27,36]. Based on recordings from the premotor LMC in the macaque, a recent study defined a population of neurons, two-thirds of which discharged before the sound onset and one third were time locked with the sound onset [26]. However, these premotor neurons fired only during conditioned but not spontaneous vocalizations, suggesting that they have limited role in voluntary control of monkey species-specific calls. Another study using single-cell recording in vocalizing monkeys identified neurons in the monkey homologue of human's Broca area (areas 44 and 45) in the ventrolateral prefrontal cortex, which predominantly fired before conditioned vocal onset, suggesting the involvement of this region in motor selection and voluntary call initiation [46].
The „disadvantages” of direct LMC-ambigual connectivity are reflected in inability to control different aspects of voluntary voice production (e.g., modulation of pitch, intensity and harmonious quality of voice) when the LMC is lesioned bilaterally in humans [3,39,47,48]. However, human innate vocalizations, such as laughter and cry, remain generally unaffected due, in part, to independent control by other cortical (e.g., anterior cingulate cortex) and subcortical (e.g., periaqueductal gray) structures [2,8]. On the other hand, bilateral LMC lesions in non-human primates appear to have little, if any, effect on acoustic structure of their vocalizations [49-51], again pointing to a limited role of this region in the control of complex voluntary voice production in these species.
The laryngeal motor cortex: the hypothesis
The absence of laryngeal representation in the primary motor cortex and of direct (monosynaptic) motor cortical connections with laryngeal motoneurons in the brainstem in non-human primates has been puzzling for several years [2,52-55]. We proposed earlier “the fact that monkeys, in contrast to humans, lack a direct connection of the motor cortex with the laryngeal motoneurons suggests that this connection has evolved in the last few million years and might represent one of the factors that made speech evolution possible” (p. 43, [36]). These crucial differences between the two species may be explained in the light of an emerging view of the organization of the motor cortex. Based on differential distribution of cortico-motoneuronal cells that make monosynaptic connections with motoneurons innervating arm and hand muscles, Rathelot and Strick defined two subdivisions within the primary motor cortex, the rostral “old” and the caudal “new” [56]••. The rostral “old” region on the crest of the precentral gyrus appears to be a standard structure for many mammals, such as the monkey, rodent, cat, and opossum, and contains neurons influencing arm motoneurons indirectly via at least a disynaptic pathway [57,58]. It is thought to function through the integrative mechanisms of the spinal cord to generate motor behaviors. Conversely, neurons in the caudal “new” region within the primary motor cortex are located within the anterior bank of the central sulcus and establish monosynaptic connections with motoneurons. Phylogenetically, this is a relatively new development, which is observed only in cebus monkeys, macaques, and great apes along with humans [57]. The existence of a monosynaptic motor cortex to motoneuron connection enables the generation of more complex patterns of arm/hand muscle activity for the performance of highly skilled movements [56]. Interestingly, in macaques, the descending projections of the “old” primary motor cortex are present at birth, whereas the connections of the “new” motor cortex are formed over the first few months of life and mature at around 2 years of age [57,59-61]. The latter coincides with the monkey's ability to produce fine finger movements for skilled hand tasks.
It is highly conceivable that the differences in the LMC cytoarchitectonic location and its brainstem connectivity between humans and non-human primates may explain the differences in functional ability of the LMC to control fine voluntary laryngeal movements. It is plausible to suggest that, in non-human primates, the rostral LMC representation in the premotor cortex, with its indirect access to laryngeal motoneurons, may correspond to phylogenetically and ontogenetically “old” motor cortex with the basic function to control laryngeal movements during innate behaviors. During the course of hominid evolution, the LMC representation appears to be „shifted” caudally to the “new” motor cortex, establishing the direct access to laryngeal motoneurons and providing our ability to voluntarily control laryngeal movements for complex learned behaviors, such as human speech and song. Furthermore, the “new” LMC in humans allows for better integration of incoming sensorimotor information from auditory, parietal and prefrontal cortices, which is critical for normal speech and language control. In addition, humans preserved the “older” LMC in the premotor cortex (at least in the left hemisphere, Fig. 1B) for additional indirect control of laryngeal motoneurons via the reticular formation, similar to monkeys.
As in case of maturity of “old” and “new” arm and hand motor regions, newborn's cry at birth is an innate behavior not requiring either vocal learning or voluntary manipulation of laryngeal movements. However, even very young children are able to modulate the pitch and duration of their cries or imitate sounds to gain attention at around 4 months of age, well before they are able to produce their first word. As speech development is a gradual process with the ability to speak in complete multi-word sentences typically at around 3 years of age, it may coincide with the establishment of full connectivity between the “new” LMC and laryngeal motoneurons in the brainstem. In addition, left representation of both “old” and “new” LMC as oppose to only “new” LMC in the right hemisphere may point to some clues for understanding the intrinsically left-hemisphere dominant speech and language networks. Modern neuroanatomical tract tracing using rabies virus in non-human primates combined with high- and ultra-high field structural and functional brain imaging in humans are promising methods to provide a more definite answer and shed light on the evolutionary differences of functional importance of the LMC in these closely related species.
Highlights.
Laryngeal motor cortex is indispensible for human but not monkey vocal motor control
It is located in the area 4 in humans but in the area 6 in non-human primates
It establishes direct (monosynaptic) connection with laryngeal motoneurons in humans only
A shift of laryngeal representation from the primary motor to premotor cortex may be a result of hominid evolution
Acknowledgments
I would like to thank Jill K. Gregory, MFA, CMI, from Mount Sinai's Instructional Technology Group for help with illustrations. Supported by the National Institute on Deafness and Other Communication Disorders, National Institutes of Health (R01DC011805, R01DC012545, and R00DC009629) to K. Simonyan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure/Conflict of Interest: None
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
• of outstanding interest
- 1.Simonyan K, Horwitz B, Jarvis ED. Dopamine regulation of human speech and bird song: a critical review. Brain Lang. 2012;122:142–150. doi: 10.1016/j.bandl.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2••.Jürgens U. Neural pathways underlying vocal control. Neurosci Biobehav Rev. 2002;26:235–258. doi: 10.1016/s0149-7634(01)00068-9. [A compehensive review of literature and hypotheses on the central and peripheral organization of voice control in humans and non-human primates.] [DOI] [PubMed] [Google Scholar]
- 3••.Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443. [Seminal work on the mapping of the cerebral cortex in human patients, which revealed a somatotopic organization of the motor cortex.] [Google Scholar]
- 4•.Hast MH, Fischer JM, Wetzel AB, Thompson VE. Cortical Motor Representation of Laryngeal Muscles in Macaca-Mulatta. Federation Proceedings. 1974;33:399–399. doi: 10.1016/0006-8993(74)91046-4. [A direct electrical stimulation study of the brain in the rhesus monkey, which described the localization of individual laryngeal muscles in this species.] [DOI] [PubMed] [Google Scholar]
- 5.Woolsey CN, Settlage PH, Meyer DR, Sencer W, Hamuy TP, Travis AM. Patterns of Localization in Precentral and Supplementary Motor Areas and Their Relation to the Concept of a Premotor Area. Research Publications- Association for Research in Nervous and Mental Disease. 1950;30:238–264. [PubMed] [Google Scholar]
- 6.Milojevi B, Hast MH. Cortical Motor Centers of Laryngeal Muscles in Cat and Dog. Annals of Otology Rhinology and Laryngology. 1964;73:979. doi: 10.1177/000348946407300411. [DOI] [PubMed] [Google Scholar]
- 7.Arriaga G, Zhou EP, Jarvis ED. Of mice, birds, and men: the mouse ultrasonic song system has some features similar to humans and song-learning birds. PLoS One. 2012;7:e46610. doi: 10.1371/journal.pone.0046610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simonyan K, Horwitz B. Laryngeal motor cortex and control of speech in humans. Neuroscientist. 2011;17:197–208. doi: 10.1177/1073858410386727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foerster o. Motorische Felder und Bahnen. In: Bumke O, Foerster O, editors. Hanbuch der Neurologie. VI. Springer; 1936. [Google Scholar]
- 10.Leyton ASF, Sherrington CS. Observations on the excitable cortex of the chimpanzee, orang-utan and gorilla. Quarterly Journal of Experimental Physiology. 1917;11:135–222. [Google Scholar]
- 11.Simonyan K, Jürgens U. Cortico-cortical projections of the motorcortical larynx area in the rhesus monkey. Brain Res. 2002;949:23–31. doi: 10.1016/s0006-8993(02)02960-8. [DOI] [PubMed] [Google Scholar]
- 12.Jürgens U. Afferents to the cortical larynx area in the monkey. Brain Res. 1982;239:377–389. doi: 10.1016/0006-8993(82)90516-9. [DOI] [PubMed] [Google Scholar]
- 13.Jürgens U. On the elicitability of vocalization from the cortical larynx area. Brain Res. 1974;81:564–566. doi: 10.1016/0006-8993(74)90853-1. [DOI] [PubMed] [Google Scholar]
- 14.Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrinde. Barth; Leipzig: 1909. [Google Scholar]
- 15.Croxson PL, Simonyan K. Society for Neuroscience. San Diego: 2013. A comparison of the connections of laryngeal motor cortex in humans and macaque monkey using diffusion tractography. Online. [Google Scholar]
- 16.Bouchard KE, Mesgarani N, Johnson K, Chang EF. Functional organization of human sensorimotor cortex for speech articulation. Nature. 2013;495:327–332. doi: 10.1038/nature11911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Espadaler J, Rogic M, Deletis V, Leon A, Quijada C, Conesa G. Representation of cricothyroid muscles at the primary motor cortex (M1) in healthy subjects, mapped by navigated transcranial magnetic stimulation (nTMS). Clin Neurophysiol. 2012;123:2205–2211. doi: 10.1016/j.clinph.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Rodel RM, Olthoff A, Tergau F, Simonyan K, Kraemer D, Markus H, Kruse E. Human cortical motor representation of the larynx as assessed by transcranial magnetic stimulation (TMS). Laryngoscope. 2004;114:918–922. doi: 10.1097/00005537-200405000-00026. [DOI] [PubMed] [Google Scholar]
- 19.Deletis V, Fernandez-Conejero I, Ulkatan S, Costantino P. Methodology for intraoperatively eliciting motor evoked potentials in the vocal muscles by electrical stimulation of the corticobulbar tract. Clin Neurophysiol. 2009;120:336–341. doi: 10.1016/j.clinph.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Pinel P, Fauchereau F, Moreno A, Barbot A, Lathrop M, Zelenika D, Le Bihan D, Poline JB, Bourgeron T, Dehaene S. Genetic variants of FOXP2 and KIAA0319/TTRAP/THEM2 locus are associated with altered brain activation in distinct language-related regions. J Neurosci. 2012;32:817–825. doi: 10.1523/JNEUROSCI.5996-10.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geyer S, Ledberg A, Schleicher A, Kinomura S, Schormann T, Burgel U, Klingberg T, Larsson J, Zilles K, Roland PE. Two different areas within the primary motor cortex of man. Nature. 1996;382:805–807. doi: 10.1038/382805a0. [DOI] [PubMed] [Google Scholar]
- 22.Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 23.Terumitsu M, Ikeda K, Kwee IL, Nakada T. Participation of primary motor cortex area 4a in complex sensory processing: 3.0-T fMRI study. Neuroreport. 2009;20:679–683. doi: 10.1097/WNR.0b013e32832a1820. [DOI] [PubMed] [Google Scholar]
- 24.Nakada T, Suzuki K, Fujii Y, Matsuzawa H, Kwee IL. Independent component- cross correlation-sequential epoch (ICS) analysis of high field fMRI time series: direct visualization of dual representation of the primary motor cortex in human. Neurosci Res. 2000;37:237–244. doi: 10.1016/s0168-0102(00)00122-x. [DOI] [PubMed] [Google Scholar]
- 25.Johansen-Berg H, Matthews PM. Attention to movement modulates activity in sensori-motor areas, including primary motor cortex. Exp Brain Res. 2002;142:13–24. doi: 10.1007/s00221-001-0905-8. [DOI] [PubMed] [Google Scholar]
- 26.Coude G, Ferrari PF, Roda F, Maranesi M, Borelli E, Veroni V, Monti F, Rozzi S, Fogassi L. Neurons controlling voluntary vocalization in the macaque ventral premotor cortex. PLoS One. 2011;6:e26822. doi: 10.1371/journal.pone.0026822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jürgens U. Projections from the cortical larynx area in the squirrel monkey. Exp Brain Res. 1976;25:401–411. doi: 10.1007/BF00241730. [DOI] [PubMed] [Google Scholar]
- 28.Jürgens U, Ehrenreich L. The descending motorcortical pathway to the laryngeal motoneurons in the squirrel monkey. Brain Res. 2007;1148:90–95. doi: 10.1016/j.brainres.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 29.Vogt C, Vogt O. Allgemeinere Ergebnisse unserer Hirnforschung. J Psychol Neurol. 1919;25:279–462. [Google Scholar]
- 30.von Bonin G, Bailey P. The neocortex of Macaca mulatta. University of Illinois; Urbana/Illinois: 1947. [Google Scholar]
- 31.Matelli M, Luppino G, Rizzolatti G. Patterns of Cytochrome-Oxidase Activity in the Frontal Agranular Cortex of the Macaque Monkey. Behavioural Brain Research. 1985;18:125–136. doi: 10.1016/0166-4328(85)90068-3. [DOI] [PubMed] [Google Scholar]
- 32.Paxinos G, Huang XF, Toga AW. The rhesus monkey brain in stereotaxic coordinates. Academic Press; San Diego, CA: 2000. [Google Scholar]
- 33.Saleem KS, Logothetis N. A combined MRI and histology atlas of the rhesus monkey brain in stereotaxic coordinates. Academic; London ; Burlington, MA: 2007. [Google Scholar]
- 34.Simonyan K, Ostuni J, Ludlow CL, Horwitz B. Functional but not structural networks of the human laryngeal motor cortex show left hemispheric lateralization during syllable but not breathing production. J Neurosci. 2009;29:14912–14923. doi: 10.1523/JNEUROSCI.4897-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ploog D. Neurobiology of primate audio-vocal behavior. Brain Res. 1981;228:35–61. doi: 10.1016/0165-0173(81)90011-4. [DOI] [PubMed] [Google Scholar]
- 36••.Simonyan K, Jürgens U. Efferent subcortical projections of the laryngeal motorcortex in the rhesus monkey. Brain Res. 2003;974:43–59. doi: 10.1016/s0006-8993(03)02548-4. [A neuroanatomical tract tracing study, which provided evidence for the absence of direct monosynaptic connections between the laryngeal motor cortex and nucleus ambiguus of the brainstem in the rhesus monkey.] [DOI] [PubMed] [Google Scholar]
- 37.Iwatsubo T, Kuzuhara S, Kanemitsu A, Shimada H, Toyokura Y. Corticofugal projections to the motor nuclei of the brainstem and spinal cord in humans. Neurology. 1990;40:309–312. doi: 10.1212/wnl.40.2.309. [DOI] [PubMed] [Google Scholar]
- 38••.Kuypers HG. Corticobular connexions to the pons and lower brain-stem in man: an anatomical study. Brain. 1958;81:364–388. doi: 10.1093/brain/81.3.364. [One of the few existing studies in human patients, which traced the direct monosynaptic projections from the laryngeal/orofacial motor cortex to the nucleus ambiguus.] [DOI] [PubMed] [Google Scholar]
- 39.Alajouanine T, Thurel R. Cerebral facial diplegia - Cortical form of the pseudobulbar paralysis (Contribution to the study on the dissociation of voluntary and reflex activities). Revue Neurologique. 1933;60:441–458. [Google Scholar]
- 40.Amassian VE, Anziska BJ, Cracco JB, Cracco RQ, Maccabee PJ. Focal magnetic excitation of frontal cortex activates laryngeal muscles in man. Proc Physiol Soc. 1987;41 [Google Scholar]
- 41.Ertekin C, Turman B, Tarlaci S, Celik M, Aydogdu I, Secil Y, Kiylioglu N. Cricopharyngeal sphincter muscle responses to transcranial magnetic stimulation in normal subjects and in patients with dysphagia. Clin Neurophysiol. 2001;112:86–94. doi: 10.1016/s1388-2457(00)00504-6. [DOI] [PubMed] [Google Scholar]
- 42.Khedr EM, Aref EE. Electrophysiological study of vocal-fold mobility disorders using a magnetic stimulator. Eur J Neurol. 2002;9:259–267. doi: 10.1046/j.1468-1331.2002.00394.x. [DOI] [PubMed] [Google Scholar]
- 43.Sinnott JM, Stebbins WC, Moody DB. Regulation of voice amplitude by the monkey. J Acoust Soc Am. 1975;58:412–414. doi: 10.1121/1.380685. [DOI] [PubMed] [Google Scholar]
- 44.Brumm H, Voss K, Kollmer I, Todt D. Acoustic communication in noise: regulation of call characteristics in a New World monkey. J Exp Biol. 2004;207:443–448. doi: 10.1242/jeb.00768. [DOI] [PubMed] [Google Scholar]
- 45.Egnor SER, Hauser MD. Noise-induced vocal modulation in cotton-top tamarins (Saguinus oedipus). Am J Primatol. 2006;68:1183–1190. doi: 10.1002/ajp.20317. [DOI] [PubMed] [Google Scholar]
- 46.Hage SR, Nieder A. Single neurons in monkey prefrontal cortex encode volitional initiation of vocalizations. Nat Commun. 2013;4:2409. doi: 10.1038/ncomms3409. [DOI] [PubMed] [Google Scholar]
- 47.Groswasser Z, Korn C, Groswasserreider I, Solzi P. Mutism Associated with Buccofacial Apraxia and Bihemispheric Lesions. Brain and Language. 1988;34:157–168. doi: 10.1016/0093-934x(88)90129-0. [DOI] [PubMed] [Google Scholar]
- 48.Mao CC, Coull BM, Golper LAC, Rau MT. Anterior Operculum Syndrome. Neurology. 1989;39:1169–1172. doi: 10.1212/wnl.39.9.1169. [DOI] [PubMed] [Google Scholar]
- 49.Jürgens U, Kirzinger A, von Cramon D. The effects of deep-reaching lesions in the cortical face area on phonation. A combined case report and experimental monkey study. Cortex. 1982;18:125–139. doi: 10.1016/s0010-9452(82)80024-5. [DOI] [PubMed] [Google Scholar]
- 50.Kirzinger A, Jürgens U. Cortical lesion effects and vocalization in the squirrel monkey. Brain Res. 1982;233:299–315. doi: 10.1016/0006-8993(82)91204-5. [DOI] [PubMed] [Google Scholar]
- 51.Sutton D, Larson C, Lindeman RC. Neocortical and limbic lesion effects on primate phonation. Brain Res. 1974;71:61–75. doi: 10.1016/0006-8993(74)90191-7. [DOI] [PubMed] [Google Scholar]
- 52.Myers RE. Comparative neurology of vocalization and speech: proof of a dichotomy. Ann N Y Acad Sci. 1976;280:745–760. doi: 10.1111/j.1749-6632.1976.tb25537.x. [DOI] [PubMed] [Google Scholar]
- 53.Jarvis ED. Learned birdsong and the neurobiology of human language. Ann N Y Acad Sci. 2004;1016:749–777. doi: 10.1196/annals.1298.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuypers HGJM. The anatomical organization of the descending pathways and their contributions to motor control especially in primates. In: Desmedt JE, editor. New developments in electromyography and clinical neurophysiology. Karger; 1973. [Google Scholar]
- 55.Fitch WT, Huber L. Bugnyar T: Social cognition and the evolution of language: constructing cognitive phylogenies. Neuron. 2010;65:795–814. doi: 10.1016/j.neuron.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56••.Rathelot JA, Strick PL. Subdivisions of primary motor cortex based on cortico motoneuronal cells. Proc Natl Acad Sci U S A. 2009;106:918–923. doi: 10.1073/pnas.0808362106. [A classical study using rabies virus in a primate model to differentiate the existance of “new” and “old” regions within the motor cortex controlling hand and arm movements.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuypers HGJM. In: The nervous system II, Handbbook of Physiology. Brookhart JM, Mountcastle VB, editors. Am Physiol Soc; Bethesda: 1981. [Google Scholar]
- 58.Nudo RJ, Masterton RB. Descending pathways to the spinal cord: a comparative study of 22 mammals. J Comp Neurol. 1988;277:53–79. doi: 10.1002/cne.902770105. [DOI] [PubMed] [Google Scholar]
- 59.Armand J, Olivier E, Edgley SA, Lemon RN. Postnatal development of corticospinal projections from motor cortex to the cervical enlargement in the macaque monkey. J Neurosci. 1997;17:251–266. doi: 10.1523/JNEUROSCI.17-01-00251.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Olivier E, Edgley SA, Armand J, Lemon RN. An electrophysiological study of the postnatal development of the corticospinal system in the macaque monkey. J Neurosci. 1997;17:267–276. doi: 10.1523/JNEUROSCI.17-01-00267.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lawrence DG, Hopkins DA. The development of motor control in the rhesus monkey: evidence concerning the role of corticomotoneuronal connections. Brain. 1976;99:235–254. doi: 10.1093/brain/99.2.235. [DOI] [PubMed] [Google Scholar]
- 62.Penfield W, Jasper HH. Epilepsy and the functional anatomy of the human brain edn 1st. Little; Boston: 1954. [Google Scholar]
- 63.Penfield W, Rasmussen T. The cerebral cortex of man; a clinical study of localization of function. Macmillan; New York: 1950. [Google Scholar]