Abstract
Erectile dysfunction (ED) and other forms of sexual dysfunction are highly prevalent among HIV+ men who have sex with men (MSM). Research has not previously identified the mechanisms by which depression may be associated with sexual dysfunction among HIV-positive and HIV-seronegative (HIV-negative) MSM. The present study examined the role of antidepressant use, stimulant use, and smoking as mediators of the relation between depression and sexual dysfunction among HIV-positive and HIV-negative MSM. Participants enrolled in the Multicenter AIDS Cohort Study (MACS), an ongoing prospective study of the natural and treated histories of HIV infection among MSM in the United States, completed a modified version of the International Index of Erectile Function for MSM. The study sample included 1,363 participants, with 619 HIV-positive men and 744 HIV-negative men. A structural equation model examined depression as a predictor of subsequent sexual dysfunction, mediated by antidepressant use, stimulant use, and smoking. Depression predicted subsequent sexual function among both HIV-negative and HIV-positive MSM. This effect appeared to be both a direct effect and an indirect effect via antidepressant use. Findings suggest that antidepressant medication use may partially explain sexual dysfunction among MSM.
Keywords: Depression, Sexual Dysfunction, SSRIs, Men Who Have Sex With Men, Erectile Dysfunction, sexual orientation
INTRODUCTION
Erectile dysfunction (ED) is more prevalent in HIV+ MSM than in the general population, with anywhere from 33%–74% of HIV+ MSM reporting ED (Asboe et al., 2007; Cove & Petrak, 2004; Ende, Re, DiNubile, & Mounzer, 2006) versus a prevalence ranging from 0–18% in general population samples (Selvin, Burnett, & Platz, 2007; Simons & Carey, 2001; Wessells, Joyce, Wise, & Wilt, 2007). ED is associated with lower quality of life and higher depression among men (Asboe et al., 2007; Fisher et al., 2005). Although several studies have examined ED among MSM, most studies of MSM or HIV+ persons have used small samples of less than 200 participants, which limit the statistical power and generalizability of the findings. The literature on other forms of sexual dysfunction among MSM, such as anorgasmia, or other indicators of sexual well-being, such as sexual satisfaction, is even more sparse. Examining sexual dysfunction among HIV+ MSM is relevant not only to understand how it affects quality of life, but also because sexual dysfunction is treatable. For example, 76% of HIV+ MSM receiving treatment for ED reported achieving erections and 14% reported some improvement in erectile functioning (Catalan & Meadows, 2000). A previous study using the MACS data showed the correlates of ED among HIV+ and HIV− MSM (Hart et al., 2012). This study offered significant new insights into predictors of ED among MSM and served as a basis for the present study about mechanisms of action. The present study goes beyond previous research by longitudinally examining how depression may predict ED via three potential mediators.
Although ED has received the most attention in the literature, other forms of sexual dysfunction are also highly common among MSM. In a study of 7,001 MSM, more than half (57%) reported low sexual desire and about a third (36%) reported anorgasmia or that sex was not pleasurable (37%) (Hirschfield et al., 2010). These statistics compare with 45% who reported erection problems. In a multivariable analysis controlling for demographic variables such as age and race, HIV+ MSM were more likely than HIV− MSM to report sexual dysfunction of all types except premature ejaculation. In this study, HIV+ MSM were 1.8 times more likely to report anorgasmia and 1.5 times more likely to report that sex was not pleasurable. However, little is known about the mechanisms linking HIV infection to sexual dysfunction among MSM.
Depression and Sexual Dysfunction
Several studies have shown an association between depression and ED and other forms of male sexual dysfunction, such as low sexual desire and delayed ejaculation (de Ryck et al., 2012; Laumann et al., 2005; Mathew & Weinman, 1982; Rosen, Lane, & Menza, 1999; Rosen et al., 2004; Waldinger, Hengeveld, Zwinderman, & Olivier, 1998). For example, among 1017 HIV+ men, lower sexual satisfaction was associated with increased report of depression (de Ryck et al., 2012). In general population samples, depression has also been shown to be associated with erectile difficulties, low sexual desire, and anorgasmia (Laumann et al., 2005). Depression may also be a reason why HIV-positive men have higher sexual dysfunction than HIV-negative men (Hirschfield et al., 2010; Lamba, Goldmeier, Mackie, & Scullard, 2004), as HIV-positive men also have higher depression rates than their HIV-negative counterparts (Ciesla & Roberts, 2001). However, the extant research has not indicated whether it is depression per se that causes sexual dysfunction or whether its effects are mediated through other variables. The literature has also not shown whether there are psychosocial mechanisms by which depression leads to sexual dysfunction among HIV+ and HIV− MSM. Some known covariates of sexual dysfunction may serve as mediators that explain why depression is associated with ED and other forms of male sexual dysfunction among MSM.
Potential Mediators
Several studies have documented deleterious effects on male sexual functioning after long-term use of antidepressants, especially selective serotonin reuptake inhibitors (Corona et al., 2009b; Montejo-Gonzalez et al., 1997; Rosen et al., 1999; Waldinger et al., 1998). A recent study also found that antidepressant use predicted ED among MSM in the MACS (Hart et al., 2012), with a 59% higher prevalence of ED per ten cumulative years of antidepressant use. However, this study only examined ED, and did not examine other types of sexual dysfunction. In addition, depression was not examined in this previous study, so it is not known if antidepressant use or how other mediators may explain how depression is associated with sexual dysfunction.
Among other possible mediators, depression has been linked with smoking behavior although the direction of this relationship is unclear. Depression may lead to smoking (Breslau, Peterson, Schultz, Chilcoat, & Andreski, 1998), but a recent meta-analysis found evidence that depression may also follow smoking (Chaiton, Cohen, O’Laughlin, & Rehm, 2009). Smoking is associated with ED in the general population (Selvin et al., 2007; Wessels et al., 2007). Smoking may also be associated with other forms of sexual dysfunction. Smoking’s effects on sexual dysfunction may be due to increases in atherosclerosis and specifically in plaque formation (Fowkes et al., 1992). Depression’s effects on sexual dysfunction may therefore be at least partially accounted for by the effects of smoking.
Depression is also associated with increased illicit drug use among MSM (McKirnan, Tolou-Shams, Turner, Dyslin, & Hope, 2006). Stimulant use may make achieving and maintaining an erection physiologically impossible (Frosch, Shoptaw, Huber, Rawson, & Ling, 1996). Less research has found specific effects of antidepressants, smoking, and stimulant use on other forms of sexual dysfunction, especially among MSM. It is, therefore useful, to disentangle the relationship between depression and sexual dysfunction among MSM, using a longitudinal study with these three mediators.
Demographic and Biomedical Covariates
Demographic variables
Because ED differs across demographic variables (Johannes et al., 2000), it is important to control for these variables in any study examining the mechanisms by which depression predicts sexual dysfunction. Age has been shown to be an important predictor of ED, with an annual incidence rate in the general population of 12.4 cases per 1000 man-years for men 40–49 versus 46.4 cases per 1000 man-years for men 60–69 years old (Johannes et al., 2000), and with similar findings regarding age and increased ED prevalence (Selvin et al., 2007; Wessells et al., 2007). Age is associated inconsistently with ED among HIV+ MSM (Asboe et al., 2007; Ende et al., 2006). Among men in the general population, age is associated with other forms of sexual dysfunction, such as increases in low sexual desire, low sexual satisfaction, and anorgasmia (Araujo, Mohr, & McKinlay, 2004; Laumann et al., 2005). Among MSM, higher age is also associated with anorgasmia, but was negatively associated with report of low sexual desire (Hirschfield et al., 2010). Risk for ED also increases among men with lower education and men with specific health problems, such as diabetes, heart disease, and hypertension (Johannes et al., 2000; Selvin et al., 2007). Studies have also demonstrated that the rate of ED was higher among Black men than White men (Wessells et al., 2007). However, Black men reported a lower rate of anorgasmia than White MSM in another study (Hirschfield et al., 2010).
Biomedical covariates
HIV and ED-related biomedical variables are also relevant factors. HIV-positive status is associated with higher rates of ED among MSM (Hart et al., 2012) although HIV clinical variables such as CD4+ T-cell count, viral load, and use of HIV medications were not associated with ED in multivariable models. Regarding other forms of sexual dysfunction, ED medication use may not be associated with decreased likelihood of reporting low sexual desire or anorgasmia (Hirschfield et al., 2010), which may be due to the positive effects of ED medications on sexual desire and ability to have an orgasm.
Hypotheses
The goal of the present study was to determine the mechanisms by which depression was associated with ED and other forms of sexual dysfunction among MSM. It was hypothesized that the relationship between depression and sexual dysfunction would be mediated by antidepressant use, stimulant use, and smoking. Consistent with previous research, it was also hypothesized that, compared to HIV- participants, HIV+ participants would have higher depression and sexual dysfunction.
METHOD
Participants
Participants were enrolled in the Multicenter AIDS Cohort Study (MACS), an ongoing prospective study of the natural and treated histories of HIV infection among homosexual and bisexual men in the United States. A total of 6,972 men were recruited (4954 in 1984–1985, 668 in 1987–1991, and 1350 in 2001–2003) at four centers: Baltimore/Washington, DC, Chicago, Los Angeles, and Pittsburgh. Among the total enrollment for all MACS participants (N = 6972), 2100 participants were deceased, 1704 HIV-seronegative participants were administratively censored from the MACS in 1993, and 888 participants were “inactive” by April 2010 (they moved out of the area or were lost to follow-up). Therefore, 2,282 MACS participants were “active” by April 2010. Among the 1,966 MACS participants who completed visits 46 and 47, three men did not complete the IIEF questionnaire and another 600 participants did not report having male sex partner(s). The final analytic sample included 1,363 participants.
The 2001–2003 cohort enrolled more young men, more Black and Hispanic men with higher median CD4+ cell counts. t-test or chi-square analysis tested the association of HIV status with age, race, and CD4+ cell count. As shown in Table 1, race was not associated with HIV status, but age and CD4+ cell count were. Thus, age and CD4 were included in the bivariate correlation matrix (Table 2) and were adjusted for in the final structural equation model (Fig. 1). This approach has been conducted and published in other longitudinal analyses of the MACS to account for any possible cohort effect (Shoptaw et al., 2012; Tsao, Stein, Ostrow, Stall, & Plankey, 2011).
Table 1.
Sample Characteristics
| HIV-Negative (n = 744) | HIV-Positive (n = 619) | |
|---|---|---|
|
| ||
| N (%) | ||
| Study Center | ||
| Baltimore | 192 (25.8) | 125 (20.2) |
| Chicago | 120 (16.1) | 157 (25.4) |
| Pittsburgh | 229 (30.8) | 155 (25.0) |
| Los Angeles | 203 (27.3) | 182 (29.4) |
| Race | ||
| White, non-Hispanic | 567 (76.2) | 354 (57.2) |
| Black, non-Hispanic | 97 (13.0) | 159 (25.7) |
| Hispanic | 64 (8.6) | 99 (16.0) |
| Other | 16 (2.1) | 7 (1.2) |
| Education | ||
| Less than HS graduate | 19 (2.5) | 26 (4.2) |
| HS graduate | 57 (7.7) | 93 (15.0) |
| Some college | 179 (24.1) | 191 (30.9) |
| Graduate 4 year college | 196 (26.3) | 140 (22.6) |
| Some graduate college | 90 (12.1) | 47 (7.6) |
| Post-graduate degree | 198 (26.6) | 103 (16.6) |
| Missing | 5 (0.7) | 19 (3.1) |
| Antidepressant Use* | 103 (13.8) | 132 (21.3) |
| Smoker* | 112 (15.1) | 159 (25.7) |
| Stimulant Use* | 51 (6.9) | 108 (17.4) |
| M (SD) | ||
| Age* | 50.8 (11.2) | 47.3 (8.9) |
| Depression | 8.7 (9.9) | 9.9 (9.9) |
| CD4 T-Cell Count* | 9.8 (0.5) | 9.0 (0.9) |
| Erectile Function* | 25.8 (5.3) | 24.7 (5.7) |
| Orgasmic Function* | 8.7 (2.1) | 8.3 (2.3) |
| Sexual Desire | 7.4 (1.8) | 7.3 (2.0) |
| Intercourse Satisfaction* | 11.2 (2.3) | 10.9 (2.5) |
| Overall Sexual Satisfaction | 7.4 (2.1) | 7.3 (2.1) |
Notes: HS = high school. CD4 cell count was log transformed to base 2. Depression was assessed using the Center for Epidemiologic Studies Depression Scale.
p < .05 between HIV− and HIV+ participants using a t-statistic for continuous variables and chi-square statistic for categorical variables.
Table 2.
Correlation Matrix
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Antidepressant Use | -- | 0.03 | 0.33** | 0.31** | − 0.16** | − 0.14** | −0.14* | −0.14* | −0.13 | 0.03 | − 0.15** |
| 2. Smoker | 0.15 | -- | 0.43** | 0.25** | −0.03 | 0.03 | −0.04 | −0.02 | −0.02 | − 0.22** | −0.03 |
| 3. Stimulant Use | −0.10 | 0.38** | -- | 0.22** | 0.08 | −0.10 | 0.01 | 0.04 | 0.00 | −0.14* | −0.08 |
| 4. Depression | 0.30** | 0.16** | 0.19** | -- | − 0.14** | − 0.13** | −0.06 | − 0.16** | − 0.19** | − 0.19** | −0.11* |
| 5. Erectile Function | −0.15* | −0.05 | −0.05 | − 0.12** | -- | 0.44** | 0.38** | 0.52** | 0.41** | − 0.19** | 0.11** |
| 6. Orgasmic Function | −0.15* | 0.05 | −0.09 | − 0.10** | 0.50** | -- | 0.27** | 0.35** | 0.26** | − 0.13** | 0.04 |
| 7. Sexual Desire | − 0.19** | −0.04 | −0.03 | 0.00 | 0.22** | 0.23** | -- | 0.42** | 0.42** | − 0.11** | 0.07 |
| 8. Intercourse Satisfaction | −0.15* | 0.00 | − 0.17** | − 0.13** | 0.48** | 0.32** | 0.33** | -- | 0.58** | −0.10* | 0.09* |
| 9. Overall Satisfaction | − 0.30** | 0.03 | 0.00 | − 0.26** | 0.35** | 0.21** | 0.29** | 0.59** | -- | −0.07 | 0.07 |
| 10. Age | 0.08 | − 0.29** | − 0.39** | − 0.14** | − 0.26** | − 0.20** | − 0.14** | −0.01 | −0.07 | -- | 0.03 |
| 11. CD4 T-Cell Count | 0.03 | 0.11 | 0.04 | −0.04 | 0.01 | 0.05 | −0.03 | 0.06 | −0.06 | −0.01 | -- |
Note: HIV− correlations are below the diagonal and HIV+ are above the diagonal
p < .01,
p < .001
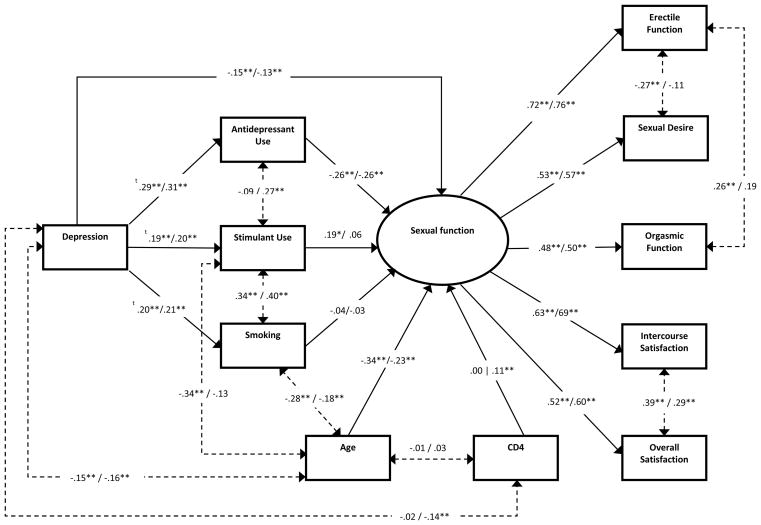
Figure 1.
Final Structural Equation Model with Standardized Estimates. Solid lines represent regression paths while dashed lines represent covariance relations. The HIV-negative group coefficients appear to the left of the slash while the HIV-positive appear to the right. ** p < .01, * p < .05, t probit coefficients
For the present study, ED data were collected at only visits 46 and 47 (October 1, 2006 through September 31, 2007). We therefore used data at the first visit of 46 and 47 with ED data available for analysis as index visit resulting in an analytic sample of 1,363 men. The sample was a primarily middle- to older-aged sample (M = 49.2 years, SD = 10.4) with a diverse ethnic and educational background. Table 1 shows these sample characteristics as well as descriptive statistics for all observed variables used in analyses stratified by HIV status.
Procedure
The MACS study design has been described elsewhere (Dudley et al., 1995; Kaslow et al., 1987). Only methods relevant to the present study are presented. MACS study protocols were approved by the institutional review boards of each of the participating centers, their community partners, and community advisory boards. Informed consent was obtained from all participants.
MACS participants return every six months for detailed interviews, physical examinations, and collection of blood for laboratory testing and storage in a central repository. The interview included questions about medical conditions, medical treatments, sexual behavior, illegal drug use (including methamphetamine, marijuana, alkyl nitrites, cocaine and crack), and cigarette and alcohol consumption since the previous visit. MACS questionnaires are available at http://www.statepi.jhsph.edu/macs/forms.html. Enzyme-linked immunosorbent assay with confirmatory Western blot tests were performed on all participants initially and at every semiannual visit thereafter for initially HIV-seronegative participants to confirm HIV-seronegative status. For all participants, T-lymphocyte subset levels were quantified by each MACS center using standardized flow cytometry (Asboe et al., 2007; Shindel, Horberg, Smith, & Breyer, 2011).
A 3-visit retrospective cohort design was used. In this design, the three mediators were assessed at the visit immediately preceding the index visit (visit 45 or 46), and depression, demographic, and biomedical variables (e.g., CD4 cell count) were assessed at two visits prior to the index visit (visit 44 or 35). For HIV-seropositive men, HAART use and CD4+ cell count were also included in separate models as biomedical variables.
Measures
Demographic variables
Age, educational level (less than high school graduation to post-graduate degree), and racial/ethnic background (e.g., non-Hispanic White, non-Hispanic Black, Hispanic, Asian, Native American), and HIV serostatus were assessed at baseline. Study center comprised the four cities of the MACS.
Biomedical variables
For HIV+ participants, use of highly active antiretroviral therapy (HAART) was assessed. For both HIV+ and HIV− participants, CD4 cell count, an important measure of cell-mediated immunity among HIV+ participants, was assessed. Since CD4 cell count was not normally distributed, this measure was log transformed to base 2, consistent with previous studies (Achhra et al., 2010; Guiguet et al., 2009).
Center for Epidemiologic Studies Depression Scale (CES-D)
The CES-D includes 20 items evaluating the frequency of depressive symptoms over the past week (Radloff, 1977). An example of an item is “I was bothered by things that don’t usually bother me.” Each item includes four response categories ranging from “rarely or none of the time (less than 1 day)” to “most or all of the time (5–7 days).” Higher scores indicate a greater degree of depression. This well-validated scale has been widely used in studies of MSM (e.g., Duggan & McCreary, 2004; Salomon et al., 2009) and HIV+ populations (Grov, Golub, Parsons, Brennan, & Karpiak, 2010). In the current study, Cronbach’s alpha = .77.
Behavioral mediators
For recent antidepressant use and stimulant use, participants reported the use of medications and non-prescription drugs since the last study visit. Antidepressants included selective serotonin reuptake inhibitors (e.g., escitalopram, paroxetine) or serotonin norepinerphrine reuptake inhibitors (e.g., venalafaxine). For stimulant use, participants reported their use of cocaine or “uppers” since the last study visit, with examples given such as crystal methamphetamine, crack, speed or ice. Recent smoking behavior was assessed with a single question asking participants: “Do you smoke cigarettes now (as of 1 month ago)?
International Index of Erectile Function Questionnaire (IIEF)
The following five domains of male sexual function were calculated as described in Rosen et al. (1997): erectile function, orgasmic function, sexual desire, intercourse satisfaction, and overall satisfaction. In this scale, higher scores characterize better functioning or satisfaction during sex. Erectile functioning was determined by the summed scores of a modified version of the IIEF-MSM (Coyne et al., 2010). The IIEF-MSM avoids assumptions in the original IIEF that all men engage in penetrative sexual intercourse during sexual activity. An example of an item from the Erectile Functioning scale is “Over the past 4 weeks, when you had erections with sexual stimulation, how often were your erections been hard enough for the sexual activity or activities in which you engaged?” Response items vary by item, but typically range from “1 = almost never or never” to “5 = almost always or always.” This measure was made available to the MACS investigators by the instrument’s creators before the scale was published. This measure has been used in our previous MSM study (Hart et al., 2012). Per this previous study, a modified scoring system was adopted where all responses representing lack of sexual intercourse or sexual activity were set to missing instead of zero. If more than 20% of the individual subscale items were missing, a score was not calculated. In the present study, Cronbach’s alphas for each scale ranged from .70–92.
Analytic Plan
Independent t-test analyses for continuous variables or chi-square analyses for dichotomous outcomes were conducted to compare the HIV-negative and HIV-positive groups on the five domains of male sexual function and depression. Structural equation modeling was performed to test if the effect of depression on sexual function was mediated by antidepressant use, stimulant use, and/or smoking status using Mplus 6.12. A multi-group analysis model was used to compare differences between HIV-negative and HIV-positive participants. The sexual functioning latent variable was measured using the previously mentioned five domains of male sexual function from the IIEF. Model fit was assessed using the chi-square statistic (χ2), the normed chi-square (χ2/df), root mean square error of approximation (RMSEA), Comparative Fit Index (CFI), and the Tucker-Lewis Index (TLI). As suggested by Kline (1998), both the CFI and TLI should be >.90, RMSEA <.08, and the chi-square statistic should be non-significant, or the normed chi-square value to be <3 for the model fit to be acceptable. The squared multiple correlation statistic (R2) was used to determine effectiveness of the model predictors in explaining variance in the latent sexual function variable. The weighted least squares means and variance (WLSMV) estimator was used because model variables were both categorical and non-normally distributed. Mplus’s weighted least squares procedure for analyzing datasets with missing values was used, which allowed for the use of all 1,363 cases without the need for data imputation (Asparouhov & Muthén, 2010).
Model development was performed sequentially. First, we established the fit of our measurement model and tested for measurement invariance across groups. Measurement invariance indicates that the relationship between the latent factor and observed variables is equal across groups (Widaman & Reise, 1997). Since all observed variables were continuous we tested our measurement model using maximum likelihood estimation. Invariance was checked by comparing the difference in chi-square values and degrees of freedom for two nested models (Reise, Widaman, & Pugh, 1993). Models were considered invariant if the change in chi-square statistic was non-significant, which indicated that constraining the model parameters did not worsen model fit. Second, structural portions of the model were added, including the mediation paths as well as age and CD4 cell count. To compare the groups on the structural portions of the model, in the HIV-negative group, the CD4 cell count path coefficient was set to zero, but was freely estimated in the HIV-positive group. Lastly, the mediation model (antidepressant use, stimulant use, and smoking as mediators of the relationship between depression and sexual dysfunction) and covariate invariance was checked across groups using the DIFFTEST command in Mplus. Since all mediators were categorical variables, WLSMV parameter estimation was used for the final models.
The Mplus “Model Indirect” command was used to test the indirect effect of the mediators on sexual function using the bootstrapping method which does not require normality of the sample distribution. Bootstrapping is a powerful method of measuring mediation and allows for the simultaneous examination of multiple mediators (Preacher & Hayes, 2008). For each mediator, three paths were calculated: (1) a, the effect of depression on each mediator, (2) b, the effect of each mediator on sexual function, and (3) c′, the direct effect of depression on sexual function. Estimation of standard errors of the path coefficients was conducted by using 1000 bootstrapped samples. Modification indices calculated by Mplus were utilized in combination with theoretical reasoning to improve model fit by allowing for observed variables’ residual errors to be correlated.
RESULTS
The mean score for the CES-D was 9.3 (SD = 9.9), which is below even the lowest suggested cutoff scores for depression of 15 (Radloff, 1977), and the mean score for Erectile Functioning was 25.3 (SD = 5.5), which is above the mean expected for men with ED both from the original IIEF (Rosen et al., 1997) and the adapted IIEF for MSM (Coyne et al., 2010). Between 10–20% used antidepressants, stimulants, or smoked. The HIV-negative group had significantly higher scores on the erectile function, t(1207) = 3.34, p < .01, orgasmic function, t (1257) = 2.96, p < .01, and intercourse satisfaction, t(1037) = 2.13, p < .05, domains compared to the HIV-positive group. Results for the sexual desire and overall satisfaction domains as well as depression were non-significant. The effect sizes for all five domains of the IIEF and depression were found to be small (Cohen’s d < 0.20) (Gravetter & Wallnau, 2005). The HIV-positive group reported significantly higher use of antidepressants, χ2(1, N = 1158) = 14.19, p < .001, stimulants, χ2(1, N = 1136) = 38.38, p < .001, and smoking, χ2(1, N = 1145) = 26.20, p < .001 compared to the HIV-negative group. The effect sizes for all three of these mediators were also found to be small (0.10 < phi coefficient < 0.30) (Gravetter & Wallnau, 2005).
The bivariate correlation matrix of all variables included in the final structural equation model is shown in Table 2. Values above the diagonal refer to the HIV-positive group and values below the diagonal refer to the HIV-negative group. The latent variable indicators were all significantly, positively intercorrelated for both groups with all correlations > 0.20. All bivariate correlations were > 0.85, which indicates the lack of multicollinearity or variable redundancy in the model (Kline, 2005). In the correlation matrix, depression predicted antidepressant use, smoking, and stimulant use, and predicted more sexual dysfunction for most of the domains of the IIEF for both HIV-positive and HIV-negative MSM. Antidepressant use predicted the five domains of the IIEF for both groups. Smoking did not predict sexual dysfunction. Stimulant use also did not predict sexual dysfunction, with the exception of one negative correlation with intercourse satisfaction for HIV-negative MSM.
In order to test for measurement model invariance across both groups, a completely unconstrained multi-group CFA model was fit to the data (see Model 1 in Table 3). Modification indices also suggested the addition of correlated residuals for the orgasmic function and erectile function indicators, which was deemed theoretically acceptable and therefore implemented. Next, a second CFA model with the loadings of the indicators of the latent factor constrained across groups and intercepts freely estimated was fit to the data and the difference in χ2 statistics was calculated (see Model 2 in Table 3). There was no significant change in χ2 statistics between the two models, indicating that model fit did not significantly deteriorate with these constraints. Lastly, a separate CFA model with factor loading and intercepts constrained across groups was compared to the previous model. However, the chi-square difference between these models was significant, indicating the model fit worsened (model not shown). Modification indices from Model 2 suggested that constraining the sexual desire, intercourse satisfaction, and overall sexual satisfaction intercepts would improve model fit. By doing this, the intercepts for each of these sexual function domains were set to be equal across the HIV-negative and HIV-positive groups. Restricting these intercepts and allowing the erection function and orgasmic function intercepts to be freely estimated resulted in a non-significant change in chi-square values from Model 2, indicating that constraining these parameters did not significantly change model fit (see Model 3 in Table 3). All model fit indices for Model 3 met acceptable standards.
Table 3.
Model Comparison and Fit Indices
| χ2 | Δ in χ2 | χ2/df | RMSEA | CFI | TLI | |||
|---|---|---|---|---|---|---|---|---|
| Value (df) | p-value | Value (df) | p-value | |||||
| Model 1 | 6.22 (4) | 0.18 | -- | -- | 1.56 | 0.03 | 1.00 | 0.99 |
| Model 2 | 7.67 (8) | 0.47 | 1.46 (4) | .80<p<.90 | 0.96 | 0.00 | 1.00 | 1.00 |
| Model 3 | 12.38 (11) | 0.34 | 4.71 (3) | .10<p<.20 | 1.13 | 0.01 | 1.00 | 1.00 |
| Model 4 | 139.10 (69) | 0.00 | -- | -- | 2.02 | 0.04 | 0.95 | 0.92 |
| Model 5 | 136.60 (76) | 0.00 | 5.27 (7) | 0.628 | 1.80 | 0.03 | 0.96 | 0.94 |
Notes: Model 1 is a completely unconstrained multi-group CFA model. Model 2 adds the loadings of the indicators of the latent factor constrained across groups and intercepts freely estimated. Model 3 restricted the intercepts and allowed the erection function and orgasmic function intercepts to be freely estimated. Model 4 used Model 3 to create a structural model, in which parameters were unconstrained across groups. Model 5 was the final structural equation model, with all structural regression coefficients constrained across groups, except for stimulant use and CD4 cell count, which were set to zero in the HIV-negative group. RMSEA = root mean square error of approximation; CFI = Comparative Fit Index (CFI); TLI = Tucker-Lewis Index.
Using Model 3 as the final measurement model with complete factor loading invariance and partial intercept invariance, the structural portion of the model was added. First, all structural model parameters were unconstrained across groups and model fit indices were good (see Model 4 in Table 3) except for the chi-square which was found to be significant. However, this fit statistic is sensitive to sample size and with large samples the power increases, which in turn makes it more likely to find a significant result (Henson, 2006). In situations like this, the normed chi-square (χ2/df ) has been substituted since it minimizes the effect of sample size (Wheaton, Muthen, Alwin, & Summers, 1977) and here it was found to be less than Kline’s reasonably accepted cut-off of three.
Next, a series of models were run that constrained individual regression coefficients across groups one-by-one to determine if they significantly worsened model fit. All regression coefficients other than stimulant use did not worsen model fit when they were constrained across groups. The final structural equation model has all structural regression coefficients constrained across groups, except for stimulant use and CD4 cell count, which we set to zero in the HIV-negative group. Final model fit indices were very good (see Model 5 in Table 3).
The final model along with the standardized coefficients is shown in Fig. 1. As hypothesized, a negative association (direct effect) between depression and sexual function was found for both groups (HIV−: β = −0.15, p < .001; HIV+: β = −0.13, p < .001). The indirect effect of depression on sexual function via antidepressant use was also significant and comprised 33.9% of the total effects for both groups (HIV−: β = −0.08, 95% CI = −0.12, −0.04; HIV+: β= −0.07, 95% CI = −0.10, −0.03); however, the indirect effects via stimulant use and smoking were non-significant. Though these indirect effects were non-significant, both stimulant use and smoking had significant direct relationships with depression and stimulant use also had a significant association with sexual functioning among the HIV-negative group. Overall, the final model explained 20.2% of the variance in sexual function in the HIV-negative group and 13.9% in the HIV-positive group.
Since HAART use could not be included in the final model due to the zero variance in the HIV-negative group, a replica of the final model including HAART use as a covariate was analyzed, but only using data from the HIV-positive sample. When taking HAART use into account as a covariate, no substantial differences in model parameters were found; however, HAART use had a significantly negative association with sexual function (β = −0.09, p = .038). Model fit statistics were strong (χ2 = 45.8, p = .18, χ2/df = 1.21, RMSEA = .02, CFI = .99, TLI = .98).
DISCUSSION
Our findings suggested that depression predicted subsequent sexual function among both HIV-negative and HIV-positive MSM. This effect appeared to be both a direct effect and an indirect effect via antidepressant use. Despite higher use of antidepressants and stimulants, and a greater likelihood of smoking among HIV-positive MSM, there did not appear to be significant differences in the pattern of predictions between HIV-positive and HIV-negative men. The present study extended beyond previous research by using a longitudinal model with temporal sequencing of depression and three behavioral mediators as predictors of sexual dysfunction among MSM using a multi-group structural equation model. The study also extended beyond research showing a cumulative effect of antidepressants on ED for HIV-positive MSM (Hart et al., 2012) by showing that antidepressants may partially explain why depression increases sexual dysfunction among MSM, regardless of HIV status.
The present findings were also consistent with previous research showing that depression is associated with smoking behavior and stimulant use among MSM (McKirnan et al., 2006). There was less evidence for an indirect effect of depression via stimulant use or smoking behavior in the present study. Smoking also did not have any effect on subsequent sexual dysfunction, inconsistent with previous studies showing an effect of smoking on ED (Selvin et al., 2007; Wessells et al., 2007). It may be that the effects of smoking on sexual dysfunction were specific to erectile dysfunction as opposed to other domains assessed in this study (e.g., sexual desire). Findings should not be interpreted as evidence against previous research showing an effect of stimulants (Frosch et al., 1996), as the present study did not demonstrate a lack of effect of stimulants on sexual dysfunction. Indeed, among HIV-negative men, there was still an effect of stimulant use on sexual dysfunction; however, among both groups, stimulant use did not appear to be the mechanism by which depression was associated with sexual dysfunction. Present findings regarding mediators of the relation between depression and sexual dysfunction broadly defined by the full IIEF should also be considered in the light of our past study using the MACS data showing no effect for cumulative stimulant use and smoking behavior on ED (Hart et al., 2012). Another possible explanation for the lack of findings in this study and Hart et al. may be underreporting of stimulant use or smoking behavior, both of which may be considered socially undesirable.
Limitations and Future Directions
Although the present study suggests that antidepressants may contribute to ED, it is still unknown to what extent ED medications may work in the presence of depression and the use of antidepressants. Future research should test the role of ED medications in reducing ED. The present study also did not demonstrate the cumulative effect of antidepressants prescribed for depression. Although the study benefited from historical data to predict sexual dysfunction, we were unable to examine sexual dysfunction over each timepoint, which may be useful in future longitudinal studies. Other mediators may important variables to consider in the relationship between depression and subsequent sexual dysfunction. Biochemical variables may be relevant, such as chronic cortisol release (Kobori et al., 2009), and abnormally high levels of prolactin among men taking antidepressants (Corona et al., 2009b) may also be relevant mediators. Low testosterone levels have also been implicated in sexual dysfunction and may have a larger role to play for men experiencing depression, especially for HIV-positive men (e.g., Corona et al., 2009a; Gray et al., 2006).
Although the MACS is a longitudinal study, the administration of the modified IIEF questionnaire to the two consecutive semiannual study visits limited us to a three time-point study design. We were, therefore, unable to assess the trajectories of sexual dysfunction using robust repeated measures. We were also unable to show how a reversed model in which sexual dysfunction could lead to depression. Other models could examine how chronic stimulant use leads to depression, and antidepressants could have preceded depression, especially if antidepressants were prescribed for a condition other than depression. This model also does not address other reasons for taking antidepressants that could also be related to sexual dysfunction, such as anxiety disorders or even anxiety specific to sexual situations.
The study sample may have influenced our findings, in that there was a low proportion of stimulant users (11.7%), which was slightly lower than the proportion of smokers or the proportion using antidepressants. The low proportions could have led to Type II error. The MACS cohort is also primarily middle aged so fewer people may be using stimulants like cocaine or crystal methamphetamine. However, another study using a similar design with a younger sample might have a greater proportion of stimulant use, but would also result in less report of sexual dysfunction, which may explain why stimulants may not always predict sexual dysfunction at a population level. In addition, sexual dysfunction and some of the co-factors were measured by self-report, as opposed to psychiatric diagnostic interviews or by biological markers. More objective assessments would have increased the validity of the definition of sexual dysfunction. Future research should attempt to rectify these limitations and retest the model among other male populations.
Future research should also consider the role of different substances in sexual dysfunction across time. It would be useful to explore not only cumulative effects of a given class of drugs, but also to examine shifts in use of substances among gay and bisexual men. For example, some data suggest that younger MSM are more likely to use multiple recreational drugs, including stimulants, than their older counterparts (Buchacz et al., 2005; Stall et al., 2001). For older MSM, if they are similar to other men as they age, there may instead be increasing use of prescription medications such as pain medications (Kaufman, Kelly, Rosenberg, Anderson, & Mitchell, 2002; Paulose-Ram et al., 2003). This research should consider the effects of these recreational and prescription medications on sexual dysfunction, and whether the low stimulant use prevalence in the present study was partially due to fears of sexual dysfunction in this primarily middle-aged sample.
Future research can also explore whether stimulants may have effects on sexual dysfunction more for certain groups of MSM than for others. For example, the present study did not examine motivations for stimulant use among MSM. Two major categories of motivations have previously been identified: use to enhance sex and self-medication to reduce negative affect (Semple, Patterson, & Grant, 2002). If stimulants are used to enhance sex, there is more likely to be an effect on sexual dysfunction than if one is using stimulants for energy or self-esteem. Both of these are relevant for depression. Future studies should therefore consider the role of stimulant use among MSM. Stimulant use, which has been associated with erectile dysfunction in other studies (McKirnan et al., 2006), may also play more of a role in erectile dysfunction among men preferring the insertive role, or tops, because of the need to maintain an erection during anal intercourse.
Clinical Implications
The present study and other research suggest that caution is in order for MSM using antidepressants for depression, especially for HIV-positive MSM (Hart et al., 2012). Antidepressants may also lead to sexual dysfunction even after discontinuation of antidepressant use (Csoka, Bahrick, & Mehtonen, 2008). However, as noted above, there are other reasons why men might have been prescribed antidepressants besides depression. Although a meta-analysis found that sexual dysfunction was commonly reported for many antidepressants (Gartlehner et al., 2011), antidepressants differ in their side effect profile, with some having a greater likelihood of leading to sexual dysfunction. For example, bupropion causes significantly less sexual dysfunction than escitalopram, fluoxetine, paroxetine, and sertraline. In addition, this analysis is not a treatment outcome study and was only observational, and therefore cannot contraindicate the use of antidepressant prescriptions. Despite the limitations of the present study, other depression treatments may be useful alternatives to antidepressants for MSM who wish to avoid sexual dysfunction. Cognitive behavior therapy and behavioral activation therapies have been found to be as effective as antidepressants, even in the treatment of moderate to severe depression (DeRubeis et al., 2005; Dimidjian et al., 2006). In addition, cognitive behavioral approaches are also available for HIV-positive MSM that integrate other concerns, such as promotion of HIV medication adherence (Safren et al., 2009). Finding effective treatments for depression that do not increase sexual dysfunction is important not only because of the negative effects of sexual dysfunction on quality of life (Asboe et al., 2007; Fisher et al., 2005) but also because concerns about erectile dysfunction specifically may be associated with increased risky sexual behavior among MSM. In one study, 38% of HIV-positive MSM reported ED, but 51% reported ED when trying to use condoms (Cove & Petrak, 2004). Most (90%) gay men who reported ED that was associated with condoms used condoms inconsistently during insertive anal intercourse, versus only 28% of gay men who reported no ED associated with condoms.
Summary
The present study was the first to determine, using a 3-timepoint design, that antidepressant use may partially explain why depression is associated sexual dysfunction among both HIV-positive and HIV-negative MSM. Depression had both a direct and indirect effect on sexual dysfunction. Stimulant use and smoking did not appear to mediate the relation between depression and sexual dysfunction. Clinicians should carefully consider the use of antidepressants among MSM who report dysfunction.
Acknowledgments
T. A. Hart is supported by a Career Scientist Award from the Ontario HIV Treatment Network. The Multicenter AIDS Cohort Study (MACS) includes the following: Baltimore: The Johns Hopkins University Bloomberg School of Public Health: Joseph B. Margolick (Principal Investigator), Haroutune Armenian, Barbara Crain, Adrian Dobs, Homayoon Farzadegan, Joel Gallant, John Hylton, Lisette Johnson, Shenghan Lai, Justin McArthur, Ned Sacktor, Ola Selnes, James Shepard, Chloe Thio. Washington, DC: Michael W. Plankey. Chicago: Howard Brown Health Center, Feinberg School of Medicine, Northwestern University, and Cook County Bureau of Health Services: John P. Phair (Principal Investigator), Joan S. Chmiel (Co-Principal Investigator), Sheila Badri, Bruce Cohen, Craig Conover, Maurice O’Gorman, David G Ostrow, Frank Palella, Daina Variakojis, Steven M. Wolinsky. Los Angeles: University of California, UCLA Schools of Public Health and Medicine: Roger Detels (Principal Investigator), Barbara R. Visscher (Co-Principal Investigator), Aaron Aronow, Robert Bolan, Elizabeth Breen, Anthony Butch, Thomas Coates, Rita Effros, John Fahey, Beth Jamieson, Otoniel Martínez-Maza, Eric N. Miller, John Oishi, James A. Peck, Paul Satz, Harry Vinters, Dorothy Wiley, Mallory Witt, Otto Yang, Stephen Young, Zuo Feng Zhang. Pittsburgh: University of Pittsburgh, Graduate School of Public Health: Charles R. Rinaldo (Principal Investigator), Lawrence Kingsley (Co-Principal Investigator), James T. Becker, Robert L. Cook, Robert W. Evans, John Mellors, Sharon Riddler, Anthony Silvestre, Ron Stall. Data Coordinating Center: The Johns Hopkins University Bloomberg School of Public Health: Lisa P. Jacobson (Principal Investigator), Alvaro Muñoz (Co-Principal Investigator), Haitao Chu, Stephen R. Cole, Christopher Cox, Stephen J. Gange, Janet Schollenberger, Eric C. Seaberg, Sol Su. NIH: National Institute of Allergy and Infectious Diseases: Robin E. Huebner; National Cancer Institute: Geraldina Dominguez; National Heart, Lung and Blood Institute: Cheryl McDonald. Website located at http://www.statepi.jhsph.edu/macs/macs.html. The authors wish to thank Amy C. Willis and the staff at the HIV Prevention Lab at Ryerson University for their technical assistance in the preparation of this manuscript.
References
- Achhra AC, Amin J, Law MG, Emery S, Gerstoft J, Gordin FM, Cooper DA. Immunodeficiency and the risk of serious clinical endpoints in a well-studied cohort of treated HIV-infected patients. AIDS. 2010;24:1877–1886. doi: 10.1097/QAD.0b013e32833b1b26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo AB, Mohr BA, McKinlay JB. Changes in sexual function in middle-aged and older men: Longitudinal data from the Massachusetts Male Aging Study. Journal of the American Geriatrics Society. 2004;52:1502–1509. doi: 10.1111/j.0002-8614.2004.52413.x. [DOI] [PubMed] [Google Scholar]
- Asboe D, Catalan J, Mandalia S, Dedes N, Florence E, Schrooten W, Colebunders R. Sexual dysfunction in HIV positive men is multi-factorial: A study of prevalence and associated factors. AIDS Care. 2007;19:955–965. doi: 10.1080/09540120701209847. [DOI] [PubMed] [Google Scholar]
- Asparouhov T, Muthén BO. Weighted least squares estimation with missing data. Technical Report. 2010 Retrieved from http://www.statmodel.com/download/GstrucMissingRevision.pdf.
- Breslau N, Peterson EL, Schultz LR, Chilcoat HD, Andreski P. Major depression and stages of smoking: A longitudinal investigation. Archives of General Psychiatry. 1998;55:161–166. doi: 10.1001/archpsyc.55.2.161. [DOI] [PubMed] [Google Scholar]
- Buchacz K, McFarland W, Kellogg TA, Loeb L, Holmberg SD, Dilley J, Klausner JD. Amphetamine use is associated with increased HIV incidence among men who have sex with men in San Francisco. AIDS. 2005;19:1423–1438. doi: 10.1097/01.aids.0000180794.27896.fb. [DOI] [PubMed] [Google Scholar]
- Catalan J, Meadows J. Sexual dysfunction in gay and bisexual men with HIV infection: Evaluation, treatment, and implications. AIDS Care. 2000;12:279–286. doi: 10.1080/09540120050042927. [DOI] [PubMed] [Google Scholar]
- Chaiton MO, Cohen JE, O’Laughlin J, Rehm J. A systematic review of longitudinal studies on the association between depression and smoking in adolescents. BMC Public Health. 2009;9:356. doi: 10.1186/1471-2458-9-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. American Journal of Psychiatry. 2001;158:725–730. doi: 10.1176/appi.ajp.158.5.725. [DOI] [PubMed] [Google Scholar]
- Corona G, Mannucci E, Ricca V, Lotti F, Boddi V, Bandini E, Maggi M. The age-related decline of testosterone is associated with different specific symptoms and signs in patients with sexual dysfunction. International Journal of Andrology. 2009a;32:720–728. doi: 10.1111/j.1365-2605.2009.00952.x. [DOI] [PubMed] [Google Scholar]
- Corona G, Ricca V, Bandini E, Mannucci E, Lotti F, Boddi V, Maggi M. Selective serotonin reuptake inhibitor-induced sexual dysfunction. Journal of Sexual Medicine. 2009b;6:1259–1269. doi: 10.1111/j.1743-6109.2009.01248.x. [DOI] [PubMed] [Google Scholar]
- Cove J, Petrak J. Factors associated with sexual problems in HIV-positive gay men. International Journal of STDs and AIDS. 2004;15:732–736. doi: 10.1258/0956462042395221. [DOI] [PubMed] [Google Scholar]
- Coyne K, Mandalia S, McCullough S, Catalan J, Noestlinger C, Colebunders R, Asboe D. The International Index of Erectile Function: Development of an adapted tool for use in HIV-positive men who have sex with men. Journal of Sexual Medicine. 2010;7:769–774. doi: 10.1111/j.1743-6109.2009.01579.x. [DOI] [PubMed] [Google Scholar]
- Csoka A, Bahrick A, Mehtonen OP. Persistent sexual dysfunction after discontinuation of selective serotonin reuptake inhibitors. Journal of Sexual Medicine. 2008;5:227–233. doi: 10.1111/j.1743-6109.2007.00630.x. [DOI] [PubMed] [Google Scholar]
- DeRubeis RJ, Hollon SD, Amsterdam JD, Shelton RC, Young PR, Salomon RM, Gallop R. Cognitive therapy vs medications in the treatment of moderate to severe depression. Archives of General Psychiatry. 2005;62:409–416. doi: 10.1001/archpsyc.62.4.409. [DOI] [PubMed] [Google Scholar]
- de Ryck I, Van Laeken D, Nostlinger C, Platteau T, Colebunders R Eurosupport Study Group. Sexual satisfaction among men living with HIV in Europe. AIDS and Behavior. 2012;16:225–230. doi: 10.1007/s10461-011-9987-x. [DOI] [PubMed] [Google Scholar]
- Dimidjian S, Hollon SD, Dobson KS, Schmaling KB, Kohlenberg RJ, Addis ME, Jacobson NS. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. Journal of Consulting and Clinical Psychology. 2006;74:658–670. doi: 10.1037/0022-006X.74.4.658. [DOI] [PubMed] [Google Scholar]
- Dudley J, Jin S, Hoover D, Metz S, Thackery R, Chmiel J. The Multicenter AIDS Cohort: Retention after 9 1/2 years. American Journal of Epidemiology. 1995;142:323–330. doi: 10.1093/oxfordjournals.aje.a117638. [DOI] [PubMed] [Google Scholar]
- Duggan SJ, McCreary DR. Body image, eating disorders, and the drive for muscularity in gay and heterosexual men: The influence of media images. Journal of Homosexuality. 2004;47:45–58. doi: 10.1300/J082v47n03_03. [DOI] [PubMed] [Google Scholar]
- Ende AR, Re VL, DiNubile MJ, Mounzer K. Erectile dysfunction in an urban HIV-positive population. AIDS Patient Care and STDs. 2006;20:75–78. doi: 10.1089/apc.2006.20.75. [DOI] [PubMed] [Google Scholar]
- Fisher WA, Rosen RC, Mollen M, Brock G, Karlin G, Pommerville P, Sand M. Improving the sexual quality of life of couples affected by erectile dysfunction: A double-blind, randomized, placebo-controlled trial of vardenafil. Journal of Sexual Medicine. 2005;2:699–708. doi: 10.1111/j.1743-6109.2005.00119.x. [DOI] [PubMed] [Google Scholar]
- Fowkes FGR, Housley E, Riemersma RA, Macintyre CCA, Cawood EHH, Prescott RJ, Ruckley CV. Smoking, lipids, glucose intolerance, and blood pressure as risk factors for peripheral atherosclerosis compared with ischemic heart disease in the Edinburgh Artery Study. American Journal of Epidemiology. 1992;135:331–340. doi: 10.1093/oxfordjournals.aje.a116294. [DOI] [PubMed] [Google Scholar]
- Frosch D, Shoptaw S, Huber A, Rawson RA, Ling W. Sexual HIV risk among gay and bisexual male methamphetamine abusers. Journal of Substance Abuse Treatment. 1996;13:483–486. doi: 10.1016/s0740-5472(96)00098-0. [DOI] [PubMed] [Google Scholar]
- Gartlehner G, Hansen RA, Morgan LC, Thaler K, Lux L, van Noord M, Lohr KN. Comparative risk for harms of second-generation antidepressants for treating major depressive disorder: An updated meta-analysis. Annals of Internal Medicine. 2011;155:722–785. doi: 10.7326/0003-4819-155-11-201112060-00009. [DOI] [PubMed] [Google Scholar]
- Gravetter FJ, Wallnau LB. Essentials of statistics for the behavioral sciences. 5. Belmont, CA: Thomson Learning; 2005. [Google Scholar]
- Gray PB, Singh AB, Woodhouse LJ, Storer TW, Casaburi R, Dzekov J, Bhasin S. Dose-dependent effects of testosterone on sexual function, mood, and visuospatial cognition in older men. Journal of Clinical Endocrinology and Metabolism. 2006;90:3838–3846. doi: 10.1210/jc.2005-0247. [DOI] [PubMed] [Google Scholar]
- Grov C, Golub SA, Parsons JT, Brennan M, Karpiak SE. Loneliness and HIV-related stigma explain depression among older HIV-positive adults. AIDS Care. 2010;22:630–639. doi: 10.1080/09540120903280901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiguet M, Boué F, Cadranel J, Lang JM, Rosenthal E, Costagliola D. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): A prospective cohort study. Lancet Oncology. 2009;10:1152–1159. doi: 10.1016/S1470-2045(09)70282-7. [DOI] [PubMed] [Google Scholar]
- Hart TA, Moskowitz D, Cox C, Li X, Ostrow DG, Stall RD, Plankey M. The cumulative effects of medication use, drug use, and smoking on erectile dysfunction among men who have sex with men. Journal of Sexual Medicine. 2012;9:1106–1113. doi: 10.1111/j.1743-6109.2011.02648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RK. Effect-size measures and meta-analytic thinking in counseling psychology research. The Counseling Psychologist. 2006;34:601–629. [Google Scholar]
- Hirshfield S, Chiasson MA, Wagmiller RL, Remien RH, Humberstone M, Scheinmann R, Grov C. Sexual dysfunction in an internet sample of U.S. men who have sex with men. Journal of Sexual Medicine. 2010;7:3104–3114. doi: 10.1111/j.1743-6109.2009.01636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes CB, Araujo AB, Feldman HA, Derby CA, Kleinman KP, McKinlay JB. Incidence of erectile dysfunction in men 40 to 69 years old: Longitudinal results from the Massachusetts Male Aging Study. Journal of Urology. 2000;163:460–463. [PubMed] [Google Scholar]
- Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR. The multicenter AIDS cohort study: Rationale, organization, and selected characteristics of the participants. American Journal of Epidemiology. 1987;126:310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: The Slone Survey. Journal of the American Medical Association. 2002;287:337–344. doi: 10.1001/jama.287.3.337. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. New York: Guilford; 1998. [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. 2. New York: Guilford; 2005. [Google Scholar]
- Kobori Y, Koh E, Sugimoto K, Izumi K, Narimoto K, Maeda Y, Namiki M. The relationship of serum and salivary cortisol levels to male sexual dysfunction as measured by the International Index of Erectile Function. International Journal of Impotence Research. 2009;21:207–212. doi: 10.1038/ijir.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba H, Goldmeier D, Mackie NE, Scullard G. Antiretroviral therapy is associated with sexual dysfunction and with increased serum oestradiol levels in men. International Journal of STD & AIDS. 2004;15:234–237. doi: 10.1258/095646204773557749. [DOI] [PubMed] [Google Scholar]
- Laumann EO, Nicolosi A, Glasser DB, Paik A, Gingell C, Moreira E, Wang T. Sexual problems among women and men aged 40–80 y: Prevalence and correlates identified in the Global Study of Sexual Attitudes and Behaviors. International Journal of Impotence Research. 2005;17:39–57. doi: 10.1038/sj.ijir.3901250. [DOI] [PubMed] [Google Scholar]
- Mathew R, Weinman M. Sexual dysfunctions in depression. Archives of Sexual Behavior. 1982;11:323–328. doi: 10.1007/BF01541593. [DOI] [PubMed] [Google Scholar]
- McKirnan DJ, Tolou-Shams M, Turner L, Dyslin K, Hope B. Elevated risk for tobacco use among men who have sex with men is mediated by demographic and psychosocial variables. Substance Use & Misuse. 2006;41:1197–1208. doi: 10.1080/10826080500514503. [DOI] [PubMed] [Google Scholar]
- Montejo-González AL, Liorca G, Izquierdo JA, Ledesma A, Bousoño M, Calcedo A, Vicens E. SSRI-induced sexual dysfunction: fluoxetine, paroxetine, sertraline, and fluvoxamine in a prospective, multicenter, and descriptive clinical study of 344 patients. Journal of Sex & Marital Therapy. 1997;23:176–194. doi: 10.1080/00926239708403923. [DOI] [PubMed] [Google Scholar]
- Paulose-Ram R, Hirsch R, Dillon C, Losonczy K, Cooper M, Ostchega Y. Prescription and non-prescription analgesic use among the US adult population: Results from the third National Health and Nutrition Examination Survery (NHANES III) Pharmacoepidemiology and Drug Safety. 2003;12:315–326. doi: 10.1002/pds.755. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Reise SP, Widaman KF, Pugh RH. Confirmatory factor analysis and item response theory: Two approaches for exploring measurement invariance. Psychological Bulletin. 1993;114:552–566. doi: 10.1037/0033-2909.114.3.552. [DOI] [PubMed] [Google Scholar]
- Rosen RC, Lane RM, Menza M. Effects of SSRIs on sexual function: A critical review. Journal of Clinical Psychopharmacology. 1999;19:67–85. doi: 10.1097/00004714-199902000-00013. [DOI] [PubMed] [Google Scholar]
- Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The International Index of Erectile Function (IIEF): A multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–830. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- Rosen RC, Seidman SN, Menza MA, Shabsigh R, Roose SP, Tseng LJ, Siegel RL. Quality of life, mood, and sexual function: a path analytic model of treatment effects in men with erectile dysfunction and depressive symptoms. International Journal of Impotence Research. 2004;16:334–340. doi: 10.1038/sj.ijir.3901197. [DOI] [PubMed] [Google Scholar]
- Safren SA, O’Cleirigh C, Tan JY, Raminani SR, Reilly LC, Otto MW, Mayer KH. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected individuals. Health Psychology. 2009;28:1–10. doi: 10.1037/a0012715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon E, Mimiaga M, Husnik M, Welles S, Manseau M, Montenegro AB, Mayer K. Depressive symptoms, utilization of mental health care, substance use and sexual risk among young men who have sex with men in EXPLORE: Implications for age-specific interventions. AIDS and Behavior. 2009;13:811–821. doi: 10.1007/s10461-008-9439-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the U.S. American Journal of Medicine. 2007;120:151–157. doi: 10.1016/j.amjmed.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Semple SJ, Patterson TL, Grant I. Motivations associated with methamphetamine use among HIV+ men who have sex with men. Journal of Substance Abuse Treatment. 2002;22:149–156. doi: 10.1016/s0740-5472(02)00223-4. [DOI] [PubMed] [Google Scholar]
- Shindel AW, Horberg MA, Smith JF, Breyer BN. Sexual dysfunction, HIV, and AIDS in men who have sex with men. AIDS Patient Care & STDs. 2011;25:341–349. doi: 10.1089/apc.2011.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoptaw S, Stall R, Bordon J, Kao U, Cox C, Li X, Ostrow DG, Plankey MW. Cumulative exposure to stimulants and immune function outcomes among HIV-positive and HIV-negative men in the Multicenter AIDS Cohort Study. International Journal of STDs and AIDS. 2012;23:576–580. doi: 10.1258/ijsa.2012.011322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Carey MP. Prevalence of sexual dysfunctions: Results from a decade of research. Archives of Sexual Behavior. 2001;30:177–219. doi: 10.1023/a:1002729318254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stall R, Paul JP, Greenwood G, Pollack LM, Bein E, Crosby GM, Catania JA. Alcohol use, drug use and alcohol-related problems among men who have sex with men: the Urban Men’s Health Study. Addiction. 2001;96:1589–1601. doi: 10.1046/j.1360-0443.2001.961115896.x. [DOI] [PubMed] [Google Scholar]
- Tsao JC, Stein JA, Ostrow D, Stall RD, Plankey MW. The mediating role of pain in substance use and depressive symptoms among Multicenter AIDS Cohort Study (MACS) participants. Pain. 2011;152:2757–2764. doi: 10.1016/j.pain.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldinger MD, Hengeveld MW, Zwinderman AH, Olivier B. Effect of SSRI antidepressants on ejaculation: A double-blind, randomized, placebo-controlled study with fluoxetine, fluvoxamine, paroxetine, and sertraline. Journal of Clinical Psychopharmacology. 1998;18:274–281. doi: 10.1097/00004714-199808000-00004. [DOI] [PubMed] [Google Scholar]
- Wessells H, Joyce GF, Wise M, Wilt TJ. Erectile dysfunction. Journal of Urology. 2007;177:1675–1681. doi: 10.1016/j.juro.2007.01.057. [DOI] [PubMed] [Google Scholar]
- Wheaton B, Muthen B, Alwin DF, Summers GF. Assessing reliability and stability in panel models. Sociological Methodology. 1977;8:84–136. [Google Scholar]
- Widaman KF, Reise SP. Exploring the measurement invariance of psychological instruments: Applications in the substance use domain. In: Bryant KJ, Windle M, West SB, editors. The science of prevention. Washington, DC: American Psychological Association; 1997. pp. 281–324. [Google Scholar]