Abstract
We sought to examine whether there are patterns of oral hypoglycemic agent adherence among primary care patients with type 2 diabetes that are related to patient characteristics and clinical outcomes. Longitudinal analysis via growth curve mixture modeling was carried out to classify 180 patients who participated in an adherence intervention according to patterns of adherence to oral hypoglycemic agents across 12 weeks. Three patterns of change in adherence were identified: adherent, increasing adherence, and nonadherent. Global cognition and intervention condition were associated with pattern of change in adherence (p<0.05). Patients with an increasing adherence pattern were more likely to have an Hemoglobin A1c (HbA1c) < 7% (adjusted odds ratio = 14.52, 95% CI [2.54, 82.99]) at 12 weeks in comparison with patients with the nonadherent pattern. Identification of patients with type 2 diabetes at risk of nonadherence is important for clinical prognosis and the development and delivery of interventions.
Keywords: adherence, diabetes mellitus, primary health care, randomized controlled trials, glycemic control
Achieving optimal glycemic control is a cornerstone in reducing risk for micro-vascular and macro-vascular complications in type 2 diabetes.1 Despite the development of effective diabetes therapies and interventions to prevent both macrovascular and microvascular complications and adverse events, diabetes control remains suboptimal.2-5 Oral hypoglycemic adherence is a critical component of diabetes treatment regimens with guideline concordant adherence being associated with improved glycemic control, lower disease-related health-care expenditures, and reduced mortality.6,7 However, adherence estimates generally range between 36% and 93% of prescribed medication regimens for adherence to oral hypoglycemic agents, with a significant proportion of patients failing to meet recommended adherence targets.8
Cramer8 and others9-11 reviewed factors associated with adherence to diabetes medicines. Identified factors associated with adherence include age, ethnicity, depression, regimen complexity, dosing frequency of medications, adverse effects, medication costs, patient education and beliefs, and social support. However, these reviews have not specifically considered the issue of oral hypoglycemic agent adherence in primary care and have not examined factors associated with adherence in the context of an intervention. Education alone is insufficient to produce significant adherence behavior change.12 Interventions tailored to the individual focusing on multiple factors inhibiting adherence have been found to be more effective than interventions that are not individually-focused or focus on a single adherence barrier.13,14 Understanding which type 2 diabetes patients are at greatest risk for nonadherence and subsequently poor clinical outcomes based on clinically identifiable patient characteristics would help to identify patients who might benefit from interventions in real world clinical settings.
Investigations attempting to examine individuals at risk for poor adherence share one major shortcoming, they treat adherence as a discrete outcome despite the varying nature of adherence over time. Adherence to oral hypoglycemic agents has been assessed through proportions at singular point(s) in time with little evaluation of variation over time and group classification. We used an innovative technique for longitudinal data analysis, the general growth curve mixture model (GGCMM), in order to study individual differences in adherence and change over time. This technique identifies different subgroups of patients based on their underlying trends and allows for the examination of membership in the subgroups in relation to baseline patient characteristics and subsequent clinical outcomes. Prior work examining adherence to oral hypoglycemic agents has also been limited by recruitment from insurance entities, subjective adherence assessments, and/or primarily Caucasian samples (e.g.15-18). We used an objective assessment of adherence, electronic monitoring data, and our sample is drawn from primary care practices serving socioeconomically and ethnically diverse patients.
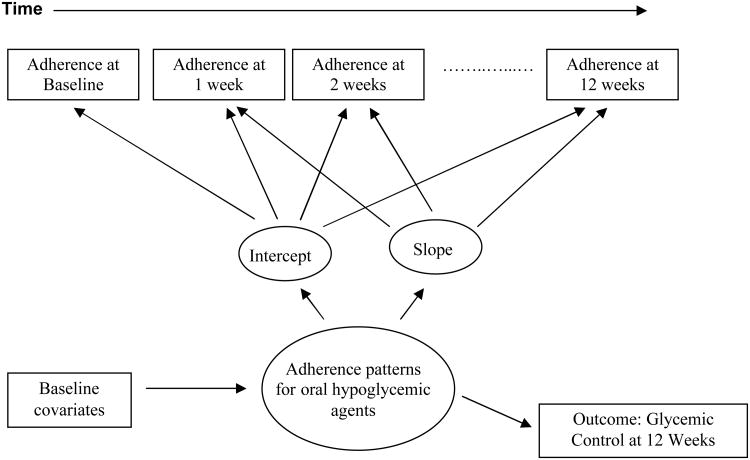
Our investigation used longitudinal adherence patterns to identify subgroups of type 2 diabetes patients at risk for nonadherence and poor glycemic control at 12 weeks. Our purpose was to further understand (1) the course of oral hypoglycemic agent adherence patterns over 12 weeks among type 2 diabetes patients in primary care, (2) whether such patterns are related to patient characteristics; and, (3) whether patterns predict glycemic control at 12 weeks (Figure 1). We employed data from a primary care randomized controlled trial in which the study intervention was implemented at the patient level and involved an integrated care specialist working with physicians to provide care.19 We hypothesized that type 2 diabetes patients would have distinct patterns of adherence over the 12 week period. We hypothesized based on the literature that patients who were younger, male, non-white, depressed, on a dosing frequency greater than 1 per day, or had a greater medical burden would be more likely than others to have a pattern of nonadherence.15,20-23 In addition, we hypothesized that patients in usual care would be less likely to adhere than patients in the intervention condition. Finally, we hypothesized that patients who were adherent or had increasing adherence across 12 weeks would be more likely to have Hemoglobin A1c (HbA1c) in comparison with patients who were nonadherent. Identifying patterns of adherence to oral hypoglycemic agents over 12 weeks linked to the outcome of glycemic control at 12 weeks will set the stage for interventions targeting resources for persons most at risk for nonadherence.
Figure 1.
Conceptual framework of baseline covariates, patterns of adherence, and glycemic control. Note: Patterns of adherence based on Medication Event Monitoring System (MEMS) data. Glycemic control at 12 weeks based on Hemoglobin A1c (HbA1c).
To our knowledge, no study has examined patient characteristics, pattern of time-varying adherence, and outcome in a primary care trial of diabetes management among type 2 diabetes with the ultimate goal of translating the knowledge into improving clinical outcomes. The Global Burden of Disease Study 2010 and the World Health Organization (WHO) list diabetes as a leading cause of disability.24 According to the WHO, the effectiveness of adherence interventions may have a far greater impact on the health of the population than any improvement in specific medical treatments.25 Identifying type 2 diabetes patients at risk for poor adherence is key to identifying groups of patients at greatest need for intervention and follow-up.
Methods
Recruitment
A Brief Intervention to Improve Adherence through Integrated Management of type 2 Diabetes Mellitus and Depression Treatment was a randomized controlled trial designed to assess whether an intervention in primary care improved glycemic control and depressive symptoms in type 2 diabetes patients. Patients were recruited from three primary care practices in Philadelphia, Pennsylvania. Patients were randomly assigned to the integrated care intervention or usual care. Electronic medical records were screened between April 2010 to April 2011 for patients with a diagnosis of type 2 diabetes and a prescription for an oral hypoglycemic agent within the past year. The study is described in detail elsewhere.19
Study Design
The purpose of the 2-week run-in phase was to assess the feasibility of use of Medication Event Monitoring System (MEMS) caps and to allow for the collection of pre-intervention adherence rates. Data collected during this phase also included baseline demographics and glycated hemoglobin (HbA1c) assays to measure glycemic control. After the two-week run-in phase, phase 2 of the study began in which patients were randomized to the integrated care intervention or usual care.
Intervention and Usual Care
The integrated care manager worked individually with patients to address factors resulting in nonadherence including depression, chronic medical conditions, function, cognition, social support, cost of medications, side effects, and past experiences with medications. Through in person sessions and telephone conversations the integrated care manager provided education about depression and type 2 diabetes, emphasizing the importance of controlling depression to manage diabetes; help to identify target symptoms; explanations for the rationale for antidepressant and oral hypoglycemic agent use; assessment for side-effects and assistance in their management; assessment for progress (e.g. reduction in depressive symptoms and improvement in finger stick results); assistance with referrals; and monitoring and response to life-threatening symptoms (e.g. chest pain, suicidal thoughts and actions).
The intervention was presented to patients as a supplement to, rather than a replacement for, existing primary care treatment. Over a three month period patients had three 30 minute in-person sessions (baseline, 6 weeks and 12 weeks) and two 15-minute telephone monitoring contacts. Usual care patients underwent in-person assessments at the same time points as the intervention. Research assistants conducted all assessments blinded to patient's randomization status.
Adherence to Oral Hypoglycemic Agents
Adherence to oral hypoglycemic agents was measured using electronic monitoring data obtained from the Medication Event Monitoring System (MEMS) Caps. MEMS caps on pill bottles record the precise date and time of container opening. Electronic drug monitoring data were assessed as the proportion of medication MEMS cap openings in a given week relative to the prescribed doses for the week. Our adherence calculation did not include excessive bottle openings or openings that exceeded the number of prescribed doses for the week.
Glycemic control
In accordance with American Diabetes Association Guidelines1 blood glycemic control was assessed at baseline and 12 weeks. The in2it A1C Analyzer provides point of care testing and was used to obtain Hemoglobin A1c (HbA1c) assays. This device has acceptable precision and agreement in comparison with laboratory services.26
Baseline Covariates
Standard questions were employed to obtain patient information on age, gender, ethnicity, marital status, and education. Consistent with prior investigations age was dichotomized as > 60 years and less than 60 years.27 Medical comorbidity was assessed by self-report at baseline. The Mini-Mental State Examination (MMSE) assessed cognitive status and has been widely employed for clinical and research purposes.28 The MMSE is a short standardized instrument that assesses orientation to time and place, registration, memory, attention and concentration, praxis, and constructional and language capacity.29 As in prior work, MMSE scores were analyzed as a dichotomous variable with a clinical threshold of 27.30,31 Functional status was measured using the Medical Outcomes Study Short Form (SF-36).32 Depressive symptoms were assessed using the nine-item Patient Health Questionnaire (PHQ-9). The PHQ-9 is a self-administered version of the PRIME-MD diagnostic instrument for mental disorders.33
Analytic strategy
In the first phase of this analysis, general growth curve mixture models (GGCMMs) were applied to indicators of adherence to oral hypoglycemic medication over the 12-week period to identify different adherence patterns.34 Binary indicators were adherence measurements assessed by MEMS caps at weekly intervals over a 12-week period. For each adherence assessment patients were categorized as adherent if they took at least 80% of their pills in the interval. Otherwise, patients were considered to be nonadherent. Greater or equal to 80% adherence was used because the 80% cut-point has been used as a standard to which other measures are compared.35 The model for the adherence patterns included random intercepts and slopes to account for within-subject correlations and separate fixed effect intercepts and fixed effects slopes for time within each latent class or pattern of adherence. We compared the results of this longitudinal linear model to models that accommodated nonlinear changes in adherence across time. The classifications into different patterns of adherence were similar across the different models, so we based our results on the simpler linear model. Number of classes was determined through examination of fit indices (AIC and BIC) as well as for clinically interpretable results. The Rubin-Lo-Mendell test was employed to determine if additional classes added further information to the model.36
In the second phase of this analysis, we examined differences in baseline patient characteristics across adherence profile types (adherent (n=67), increasing adherence (n=52), and nonadherence (n=61)) using χ2 for binary variables and analysis of variance (ANOVA) for continuous variables. We added patient characteristics and practice indicators to the GGCMM, one at a time to evaluate their association with class membership. Covariates that were significant at the p<0.10 level were included in the final model. Next, the relationship between the baseline covariates significant at the p<.10 level and latent classes or patterns of adherence was simultaneously assessed. The results were reported as odds ratios and corresponding confidence intervals. The baseline covariates were selected on the basis of previous literature on patient characteristics associated with adherence to oral hypoglycemic agents. We also examined interaction terms for which we had an a priori hypothesis: intervention by cognition and an intervention by education.
In the third phase, patients were classified into categories of longitudinal adherence profile types based on the largest posterior probability of membership in a certain class. Logistic regression related latent class variables to the clinical outcome of glycemic control at 12 weeks. Glycemic control was examined both continuously and categorically. As recommended by clinical guidelines, the categorical outcome was assessed using a cutoff of HbA1c < 7% at 12 weeks.1 The results of the categorical outcome are presented in the form of odds ratios and 95% confidence intervals. Consistent with prior work, the model included terms to adjust for age, ethnicity, gender, marital status, educational attainment, functional status, frequency of medication administration, number of medications, number of medical conditions, cognitive status, intervention condition, pre-intervention adherence and baseline clinical outcome.37 We set α at 0.05, recognizing that tests of statistical significance are approximations that serve as aids to inference. The GGCMM was fitted using Mplus version 7 (Muthén & Muthén)38 and other analyses were conducted in STATA version 12 for Windows (STATA Corporation, College Station, TX).
Results
Study sample
The CONSORT flow diagram for the Brief Intervention to Improve Adherence through Integrated Management of type 2 Diabetes Mellitus and Depression Treatment trial has been published elsewhere.19 The protocol was approved by the University of Pennsylvania Institutional Review Board. In brief, out of 715 patients identified by electronic medical records with type 2 diabetes, 265 were eligible and were approached for screening. Among type 2 diabetes patients who were approached, 190 were enrolled (71.7% participation rate). Patients who participated and patients who refused were similar in terms of key demographic characteristics such as age, gender, and ethnicity. After consent was obtained patients were assessed over a 2-week run-in phase in which medication adherence was examined. At the two-week visit, six patients had their medication discontinued, two patients were lost to follow-up and 182 patients were randomized to the integrated care intervention or usual care. In addition, two patients in the integrated care intervention were lost to follow-up subsequent to randomization. The remaining 180 patients completed all study visits.
The mean age of our sample was 57.4 years (standard deviation (s.d.) 9.5 years, range 32 to 84 years), and 122 (67.8%) of the patients were women. The self-identified ethnicity of patients was 65 white (36.1%), 102 African-American (56.7%), 7 Hispanic (3.9%), and 6 (3.3%) who self-identified as ‘other.’ In all, 69 persons (38.33%) were married, and 29 persons (16.1%) had less than a high school education. The mean MMSE score was 28.2 (s.d. 2.3). Baseline patient characteristics across adherence profile types (adherent (n=67), increasing adherence (n=52), and nonadherence (n=61)) are displayed in Table 1. White respondents and patients assigned to the intervention condition were more likely to show an adherent or increasing adherence pattern (p<.05). Physical functioning SF-36 score and mini-mental state examination (MMSE) scores significantly differed by adherence profile type (p<.05).
Table 1. Baseline patient characteristics by adherence profile type (n=180).
| Sociodemographic characteristics | Adherent (n=67) | Increasing Adherence (n=52) | Non-adherent (n=61) | P-value |
|---|---|---|---|---|
| Age, mean in years (s.d.) | 56.7 (9.3) | 59.3 (8.9) | 56.7 (10.1) | .25 |
| White, n (%) | 33 (49.3) | 13 (25) | 19 (31.2) | .02 |
| Gender, women n (%) | 40 (59.7) | 42 (80.8) | 40 (65.6) | .05 |
| Married, n (%) | 30 (44.8) | 21 (40.4) | 18 (29.5) | .19 |
| Less than HS education, n (%) | 5 (7.5) | 11 (21.2) | 13 (21.3) | .05 |
|
| ||||
| Functional status (SF-36) | ||||
|
| ||||
| Physical function score, mean (s.d.) | 60.8 (30.1) | 44.6 (32) | 49.1 (32.6) | .02 |
|
| ||||
| Medications | ||||
|
| ||||
| Frequency of oral hypoglycemic agent per day, mean (s.d.) | 1.3 (.5) | 1.3 (.6) | 1.2 (.4) | .77 |
| Number of medications, mean (s.d.) | 9.5 (4.5) | 9.4 (4.4) | 11 (5.4) | .12 |
|
| ||||
| Health status | ||||
|
| ||||
| Medical conditions, mean (s.d.) | 6.6 (2.5) | 7.6 (3.4) | 7.8 (3.7) | .09 |
|
| ||||
| Cognitive status | ||||
|
| ||||
| MMSE, mean (s.d.) | 28.8 (1.6) | 28.1 (2.2) | 27.6 (2.8) | .01 |
|
| ||||
| Randomization assignment | ||||
|
| ||||
| Intervention, n (%) | 40 (59.7) | 44 (84.6) | 8 (13.1) <.001 | |
|
| ||||
| Depression status | ||||
|
| ||||
| PHQ-9, mean (s.d.) | 9.5 (7.2) | 11.5 (8.3) | 10.1 (7.3) | .35 |
|
| ||||
| Type 2 diabetes | ||||
|
| ||||
| HbA1c, mean (s.d.) | 7.1 (1.7) | 7.2 (1.9) | 7.1 (2.0) | .87 |
Abbreviations: s.d., standard deviation; HS, high school; SF-36, Medical Outcomes Study Short Form; MMSE, Mini-Mental State Examination; PHQ-9, nine-item Patient Health Questionnaire; Hb, hemoglobin. P-values represent comparisons according to χ2 for binary variables and analysis of variance (ANOVA) for continuous variables for categorical or continuous data, respectively.
Patterns of adherence
Corresponding to the first phase of the analysis, we examined patterns of oral hypoglycemic agent adherence over 12 weeks. In order to delineate these patterns a series of GGCMMs were fitted to the MEMS data. The three-pattern model presented in Figure 2 improved the model fit over the two- and four-pattern models. The Rubin-Lo-Mendell test indicated that the three-pattern model of longitudinal adherence trajectories improved the model fit over the two-pattern model (p<0.001), with the four-pattern model providing no additional improvement in fit (p>0.99). Entropy, a measure of classification uncertainty, was 96.7% indicating a clear delineation of classes.
Figure 2.
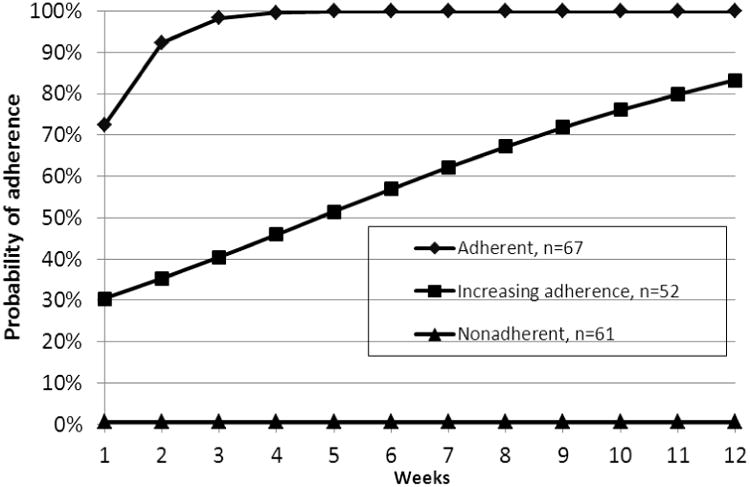
General growth curve mixture model analysis of adherence to oral hypoglycemic agents (number of patients in each class with plotted conditional probabilities) (n=180). Note: Data gathered from 2010-2011.
Figure 2 shows the patterns of adherence represented by the categorical indicators of adherence at weekly intervals over the 12-week interval. The number of persons assigned to each pattern is presented at the bottom of Figure 2. Patients were assigned to each pattern according to their maximal posterior probabilities in each pattern. The first pattern (n= 67, 37.0% of the entire sample, “adherent”) represents patients with a high probability of adherence at each time point. Patients with an “adherent” pattern had a probability of adherence at baseline of 72.1% and strongly increasing adherence over time (model slope: 1.54 (95% confidence interval (CI): 0.90, 2.17)), with adherence reaching near 100% by the 5th week.
The second pattern represents patients who have a low level of adherence at baseline and improve (“increasing adherence;” n = 52, 29.2% of the sample). Patients with “increasing adherence” had a modeled probability of adherence of 30.3% at baseline with a statistically significant increase in adherence over time (model slope of .22 (95% CI: 0.12, 0.32)). Modeled adherence reached over 80% by the end of the 12 week period. The third pattern (n=61, 33.8% of the entire sample, “nonadherent”) represents patients with a near zero probability of adherence at each time point. Patients with the nonadherent pattern had a near zero probability of adherence at baseline which remained near zero. To provide a more stable model, the slope parameters were fixed at zero for the third pattern of “nonadherent.”
Baseline Type 2 DM patient characteristics and patterns of adherence
Corresponding to the second phase of our analysis, we examined whether patient characteristics were associated with patterns of adherence to oral hypoglycemic agents. Odds ratios were employed to estimate the association of baseline patient characteristics with patterns of adherence. Two comparisons of patterns of adherence were examined: adherent vs. nonadherent and increasing adherence vs. nonadherent. Patients with MMSE score greater than or equal to 27 were more likely to have an adherent pattern compared to a nonadherent pattern (odds ratio (OR) = 1.29, 95% (CI) [1.06, 1.57]). Patients in the intervention condition were more likely to have an adherent pattern compared to a nonadherent pattern (OR = 11.6, 95% CI [4.08, 32.9]). In addition, patients in the intervention condition were more likely to have an increasing adherence pattern compared to a nonadherent pattern (OR= 41.31, 95% CI [13.87, 123.03]). In our final model, no associations between age, gender, ethnicity, education, depression status, dosing frequency, number of medications, functional status, or medical comorbidity and classes of adherence were found. We considered potential interaction terms: intervention by cognition and intervention by education, but none reached the level of significance of p < .05.
Clinical outcome of patterns of adherence: Hemoglobin A1c (HbA1c) at 12 weeks
Corresponding to the third phase of our analysis, we examined whether longitudinal profiles of adherence were associated with glycemic control as assessed by Hemoglobin A1c (HbA1c) at 12 weeks (Table 2). HbA1c at 12 weeks was strongly related to adherence patterns (χ2 (2)= 12.82, p<0.01). Patients with an increasing adherence pattern were significantly more likely to have an HbA1c < 7% (unadjusted OR = 4.39, 95% CI [1.91, 10.12]); adjusted OR = 14.52, 95% CI [2.54, 82.99]) at 12 weeks in comparison with patients with the nonadherent pattern. Patients with an adherent pattern were not significantly more likely to have an HbA1c < 7% (unadjusted OR = 1.86, 95% CI [0.92, 3.76]; adjusted OR = 2.24, 95% CI [0.51, 9.92]) at 12 weeks in comparison with the nonadherent pattern. The model included terms to adjust for baseline differences in age, ethnicity, gender, marital status, educational attainment, functional status, frequency of medication administration, number of medications, number of medical conditions, cognitive status, primary care practice, baseline HbA1c, pre-intervention adherence and intervention condition.
Table 2. Outcome glycemic control at 12 weeks for the three patterns of adherence (n=180).
| HbA1c < 7% vs. HbA1c ≥ 7% at 12 weeks | ||
|---|---|---|
| Patterns of adherence (0-12 weeks) | Unadjusted OR [95% CI] | Adjusted OR** [95% CI] |
| Adherent (n=67) | 1.86 [0.92, 3.76] | 2.24 [0.51, 9.92] |
| Increasing adherence (n=52) | 4.39* [1.91, 10.12] | 14.52* [2.54, 82.99] |
| Non adherent (n=61) | 1.00 | 1.00 |
Note: Hb = hemoglobin; OR = odds ratio; CI = confidence interval;
p< .05
adjusted for age, ethnicity, gender, marital status, educational attainment, functional status, frequency of medication administration, number of medications, number of medical conditions, cognitive status, practice, baseline HbA1c, pre-intervention adherence and intervention condition.
Discussion
Examination of adherence over 12 weeks revealed three patterns: adherent, increasing adherence, and nonadherent. Patients who had a MMSE score greater than or equal to 27 were more likely to have an adherent pattern than the nonadherent pattern compared to patients with a MMSE less than 27. Patients in the intervention condition were more likely to have an adherent pattern than a nonadherent pattern in comparison with patients in usual care. Similarly, patients in the intervention condition were more likely to have an increasing adherence pattern than a nonadherent pattern in comparison with patients in usual care. Patients with an increasing adherence pattern were also more likely to have an HbA1c < 7% at 12 weeks in comparison with patients with the nonadherent pattern. Our use of general growth curve mixture models allows us to assess distinct patterns of adherence over time instead of assessing adherence through proportions at singular point(s) in time with no assessment of variation over time. Our finding that patterns of adherence were differentially related to covariates of interest demonstrates the significance of delineating patterns of adherence over time.
Before discussing our findings, the results must first be considered in the context of some potential study limitations. First, we obtained our results from three primary care sites whose patients may not be representative of other primary care practices. However, the three practices were probably similar to other primary care practices in the region as they were diverse and varied in size. Second, there are limitations for all methods for assessing adherence. MEMS caps were our primary measure of adherence because as an objective measure of adherence they have a low failure rate and are more sensitive than other adherence measures.39 Any influence of MEMS caps on medication adherence would be experienced equally in both the intervention and usual care groups. Third, while research has assessed the 80% threshold for some medications,35 the clinical significance of this threshold has not been tested for many medications. Fourth, the length of follow-up for this trial was 12 weeks. However, significant intent-to-treat effects have been found in diabetes studies for a 12 week time period.14 Finally, even with confidence in our assessments of exposure, misspecification of the model relating adherence and blood glycemic control is still a possibility, such as when important variables have not been included in our model. We have tried to take care in adjusting our estimates of association for potentially influential characteristics that may relate to the outcome.
Despite these limitations, our results deserve attention because we attempted to characterize patterns of adherence over 12 weeks and the association of patient characteristics, intervention condition, and glycemic control with patterns of adherence. Assessment of a single point in time is insufficient to understand variations in adherence over time. The three patterns of adherence we observed suggest a possible need for more intensive interventions targeting individuals, particularly those with MMSE less than 27. Our results suggest that the intervention condition increased the number of patients with an increasing adherence pattern that might have otherwise have had a nonadherent pattern.
Our results were not wholly consistent with our initial hypotheses. We did not find an association of age, sex, ethnicity, dosing frequency or medical burden with patterns of adherence. The lack of significant associations of age, sex, ethnicity, dosing frequency, depression status, or medical burden in patients suggests that the effects of these variables did not appear to influence patterns of adherence. In terms of depression status, the onset and nature of clinical diagnosis of depression as well the influence of these diagnoses on functioning may be more important in adherence than symptom presentation. In the examination of medical burden more careful assessment of the type of medical conditions and everyday related tasks that include medication-taking related items may be necessary to find a clear association with medical co-morbidity. Given a lack of identification of clear risk profiles for nonadherence, physicians may only be able to suspect nonadherence during the course of treatment for diabetes. If nonadherence is suspected, reasons for nonadherence should be examined and addressed to mitigate poor health prognoses and adverse clinical outcomes.37
Patients with MMSE greater than or equal to 27 were more likely to have an adherent than a nonadherent pattern. Past work has demonstrated that individuals with type 2 diabetes show accelerated cognitive decline, particularly in information-processing speed and executive function, compared with individuals without diabetes.40 While the association between cognition and diabetes is supported by a number of biologically plausible mechanisms, successful diabetes management is largely determined by self-care behaviors such as adherence. Lower cognitive functioning is associated with reduced health literacy41 and understanding of diabetes management,42 and greater dependency43 in persons with diabetes. Furthermore, lower cognitive functioning is also linked to increased risk of poor glycemic control,44 severe hypoglycemia,45 and mortality46 in patients with type 2 diabetes. Patients with lower cognition scores on the MMSE are at risk for a pattern of nonadherence and may need special attention for adherence management.
Our results suggest that the intervention increased the number of patients with an adherent and increasing adherence pattern that might have otherwise have had a nonadherent pattern. The strong association between intervention assignment and adherence pattern substantiates evidence that intervention strategies targeting multiple adherence barriers and which are tailored to the needs of each patient are successful in improving adherence.14 The interventionist worked individually with patients to address the factors involved in adherence that related to their specific characteristics and medical co-morbidity. The high recruitment rates and retention of minorities in this intervention has particularly significance given the disportionate burden of diabetes among minority groups.47 This is aligned with prior work demonstrating that integrated interventions are more engaging and acceptable to minorities than other intervention approaches.48-50 Furthermore, this intervention was both brief and simple in comparison with other diabetes interventions suggesting it may be a sustainable strategy for improving patient adherence that can be implemented in primary care or other settings.51,52
Our findings hold particular clinical significance by demonstrating an increased likelihood of patients with HbA1c< 7% at 12 weeks among patients with an increasing adherence pattern. However, we did not find an association with the adherent pattern and glycemic control. As more is discovered regarding factors influencing adherence over time, we may be able to more accurately identify predictors of glycemic control. The lack of a significant association between the adherent pattern and glycemic control may be indicative of the severity of underlying disease or dosing inadequacy of oral hypoglycemic agents. Patients who are adherent to an inadequate dosage of oral hypoglycemic agents would be less likely to have glycemic control. In the future, longitudinal models may be used to link patient characteristics and patterns of adherence to the likelihood of adequate glycemic control in order to guide clinical treatment. Interventions tailored to the cognitive functioning of patients might have a substantial public health impact on glycemic control. A simple and brief adherence intervention was strongly related to adherence patterns and clinical outcome. Our results highlight the need for the development, implementation, and dissemination of clinical interventions to enhance medication adherence.
Contributor Information
Heather F. de Vries McClintock, Email: heather.devries@uphs.upenn.edu.
Knashawn H. Morales, Email: knashawn@mail.med.upenn.edu.
Dylan S. Small, Email: dsmall@wharton.upenn.edu.
Hillary R. Bogner, Email: hillary.bogner@uphs.upenn.edu.
References
- 1.American Diabetes Association. Clinical Practice Recommendations. Diabetes Care. 2014;37(Supplement 1) [PubMed] [Google Scholar]
- 2.Stark Casagrande S, Fradkin JE, Saydah SH, Rust KF, Cowie CC. The Prevalence of Meeting A1C, Blood Pressure, and LDL Goals Among People With Diabetes, 1988-2010. Diabetes Care. 2013 Aug;36(8):2271–2279. doi: 10.2337/dc12-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999;281(21):2005–2012. doi: 10.1001/jama.281.21.2005. [DOI] [PubMed] [Google Scholar]
- 4.UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998 Sep 12;352(9131):837–853. [PubMed] [Google Scholar]
- 5.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. Bmj. 2000 Aug 12;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stuart BC, Simoni-Wastila L, Zhao L, Lloyd JT, Doshi JA. Increased persistency in medication use by U.S. Medicare beneficiaries with diabetes is associated with lower hospitalization rates and cost savings. Diabetes Care. 2009 Apr;32(4):647–649. doi: 10.2337/dc08-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. Jama. 2007 Jan 10;297(2):177–186. doi: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- 8.Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004 May;27(5):1218–1224. doi: 10.2337/diacare.27.5.1218. [DOI] [PubMed] [Google Scholar]
- 9.Odegard PS, Capoccia K. Medication taking and diabetes: a systematic review of the literature. Diabetes Educ. 2007 Nov-Dec;33(6):1014–1029. doi: 10.1177/0145721707308407. discussion 1030-1011. [DOI] [PubMed] [Google Scholar]
- 10.Bailey CJ, Kodack M. Patient adherence to medication requirements for therapy of type 2 diabetes. Int J Clin Pract. 2011 Mar;65(3):314–322. doi: 10.1111/j.1742-1241.2010.02544.x. [DOI] [PubMed] [Google Scholar]
- 11.Rubin RR. Adherence to pharmacologic therapy in patients with type 2 diabetes mellitus. Am J Med. 2005 May;118(Suppl 5A):27S–34S. doi: 10.1016/j.amjmed.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 12.Mazzuca SA. Does patient education in chronic disease have therapeutic value? Journal of Chronic Disease. 1982;35:521–529. doi: 10.1016/0021-9681(82)90071-6. [DOI] [PubMed] [Google Scholar]
- 13.van Eijken M, Tsang S, Wensing M, de Smet PA, Grol RP. Interventions to improve medication compliance in older patients living in the community: a systematic review of the literature. Drugs Aging. 2003;20(3):229–240. doi: 10.2165/00002512-200320030-00006. [DOI] [PubMed] [Google Scholar]
- 14.Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;(2):CD000011. doi: 10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- 15.Kalsekar ID, Madhavan SS, Amonkar MM, et al. Impact of depression on utilization patterns of oral hypoglycemic agents in patients newly diagnosed with type 2 diabetes mellitus: a retrospective cohort analysis. Clin Ther. 2006 Feb;28(2):306–318. doi: 10.1016/j.clinthera.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Lamberts EJ, Nijpels G, Welschen LM, et al. Long term patterns of use after initiation of oral antidiabetic drug therapy. Pharmacoepidemiol Drug Saf. 2011 Apr;20(4):351–358. doi: 10.1002/pds.2089. [DOI] [PubMed] [Google Scholar]
- 17.van Dijk L, Heerdink ER, Somai D, et al. Patient risk profiles and practice variation in nonadherence to antidepressants, antihypertensives and oral hypoglycemics. BMC Health Serv Res. 2007;7:51–62. doi: 10.1186/1472-6963-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krapek K, King K, Warren SS, et al. Medication adherence and associated hemoglobin A1c in type 2 diabetes. Ann Pharmacother. 2004 Sep;38(9):1357–1362. doi: 10.1345/aph.1D612. [DOI] [PubMed] [Google Scholar]
- 19.Bogner HR, Morales KH, de Vries HF, Cappola AR. Integrated management of type 2 diabetes mellitus and depression treatment to improve medication adherence: a randomized controlled trial. Ann Fam Med. 2012 Jan-Feb;10(1):15–22. doi: 10.1370/afm.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sclar DA, Robison LM, Skaer TL, Dickson WM, Kozma CM, Reeder CE. Sulfonylurea pharmacotherapy regimen adherence in a Medicaid population: influence of age, gender, and race. Diabetes Educ. 1999 Jul-Aug;25(4):531–532. 535, 537–538. doi: 10.1177/014572179902500406. [DOI] [PubMed] [Google Scholar]
- 21.Hansen RA, Farley JF, Droege M, Maciejewski ML. A retrospective cohort study of economic outcomes and adherence to monotherapy with metformin, pioglitazone, or a sulfonylurea among patients with type 2 diabetes mellitus in the United States from 2003 to 2005. Clin Ther. 2010 Jul;32(7):1308–1319. doi: 10.1016/j.clinthera.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 22.Cohen HW, Shmukler C, Ullman R, Rivera CM, Walker EA. Measurements of medication adherence in diabetic patients with poorly controlled HbA(1c) Diabet Med. 2010 Feb;27(2):210–216. doi: 10.1111/j.1464-5491.2009.02898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paes AH, Bakker A, Soe-Agnie CJ. Impact of dosage frequency on patient compliance. Diabetes Care. 1997;20(10):1512–1517. doi: 10.2337/diacare.20.10.1512. [DOI] [PubMed] [Google Scholar]
- 24.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012 Dec 15;380(9859):2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. Adherence to long-term therapies: evidence for action. Geneva: World Health Organization; 2003. [Google Scholar]
- 26.Moridani MY, Verjee Z, Allen LC. Analytical evaluation of hemoglobin A(1c) dual kit assay on Bio-Rad Variant II: an automated HPLC hemoglobin analyzer for the management of diabetic patients. Clin Biochem. 2003;36(4):317–320. doi: 10.1016/s0009-9120(03)00013-4. [DOI] [PubMed] [Google Scholar]
- 27.Beverly EA, Fitzgerald S, Sitnikov L, Ganda OP, Caballero AE, Weinger K. Do older adults aged 60-75 years benefit from diabetes behavioral interventions? Diabetes Care. 2013 Jun;36(6):1501–1506. doi: 10.2337/dc12-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 29.Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992 Sep;40(9):922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 30.Spering CC, Hobson V, Lucas JA, Menon CV, Hall JR, O'Bryant SE. Diagnostic accuracy of the MMSE in detecting probable and possible Alzheimer's disease in ethnically diverse highly educated individuals: an analysis of the NACC database. J Gerontol A Biol Sci Med Sci. 2012 Aug;67(8):890–896. doi: 10.1093/gerona/gls006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Bryant SE, Humphreys JD, Smith GE, et al. Detecting dementia with the mini-mental state examination in highly educated individuals. Arch Neurol. 2008 Jul;65(7):963–967. doi: 10.1001/archneur.65.7.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stewart AL, Hays RD, Ware JE. The MOS Short-form General Health Survey: Reliability and validity in a patient population. Medical Care. 1988;26:724–735. doi: 10.1097/00005650-198807000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001 Sep;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCutcheon A. Latent Class Analysis. Beverly Hills: Sage University Press; 1987. [Google Scholar]
- 35.Winkler A, Teuscher AU, Mueller B, Diem P. Monotoring adherence to prescribed medication in type 2 diabetic patients treated with sulfonylureas. Swiss Medical Weekly. 2002;132(27-28):379–385. doi: 10.4414/smw.2002.10036. [DOI] [PubMed] [Google Scholar]
- 36.Lo Y, Mendell NR, Rubin DB. Testing the number of components in a normal mixture. Biometrika. 2001;88(3):767–778. [Google Scholar]
- 37.Bogner HR, de Vries HF, O'Donnell AJ, Morales KH. Measuring concurrent oral hypoglycemic and antidepressant adherence and clinical outcomes. Am J Manag Care. 2013 Mar;19(3):e85–92. [PMC free article] [PubMed] [Google Scholar]
- 38.Muthén LK, Muthén BO. Mplus users guide. Version 2. Los Angeles, CA: Muthén & Muthén;; Feb, 2001. 1998. [Google Scholar]
- 39.Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin Ther. 1999;21(6):1074–1090. doi: 10.1016/S0149-2918(99)80026-5. discussion 1073. [DOI] [PubMed] [Google Scholar]
- 40.Spauwen PJ, Kohler S, Verhey FR, Stehouwer CD, van Boxtel MP. Effects of type 2 diabetes on 12-year cognitive change: results from the Maastricht Aging Study. Diabetes Care. 2013 Jun;36(6):1554–1561. doi: 10.2337/dc12-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nguyen HT, Kirk JK, Arcury TA, et al. Cognitive function is a risk for health literacy in older adults with diabetes. Diabetes Res Clin Pract. 2013 Jun 24; doi: 10.1016/j.diabres.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hewitt J, Smeeth L, Chaturvedi N, Bulpitt CJ, Fletcher AE. Self management and patient understanding of diabetes in the older person. Diabet Med. 2011 Jan;28(1):117–122. doi: 10.1111/j.1464-5491.2010.03142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sinclair AJ, Girling AJ, Bayer AJ. Cognitive dysfunction in older subjects with diabetes mellitus: impact on diabetes self-management and use of care services. All Wales Research into Elderly (AWARE) Study. Diabetes Res Clin Pract. 2000 Dec;50(3):203–212. doi: 10.1016/s0168-8227(00)00195-9. [DOI] [PubMed] [Google Scholar]
- 44.Okura T, Heisler M, Langa KM. Association between cognitive function and social support with glycemic control in adults with diabetes mellitus. J Am Geriatr Soc. 2009 Oct;57(10):1816–1824. doi: 10.1111/j.1532-5415.2009.02431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Punthakee Z, Miller ME, Launer LJ, et al. Poor cognitive function and risk of severe hypoglycemia in type 2 diabetes: post hoc epidemiologic analysis of the ACCORD trial. Diabetes Care. 2012 Apr;35(4):787–793. doi: 10.2337/dc11-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McGuire LC, Ford ES, Ajani UA. The impact of cognitive functioning on mortality and the development of functional disability in older adults with diabetes: the second longitudinal study on aging. BMC geriatrics. 2006;6:8. doi: 10.1186/1471-2318-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.OMH. African-American profile: The Office of Minority Health. [Accessed December 17, 2013]; www.omhrc.gov.
- 48.Ayalon L, Arean PA, Linkins K, Lynch M, Estes CL. Integration of mental health services into primary care overcomes ethnic disparities in access to mental health services between black and white elderly. American Journal of Geriatric Psychiatry. 2007;15(10):906–912. doi: 10.1097/JGP.0b013e318135113e. [DOI] [PubMed] [Google Scholar]
- 49.Unutzer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. Jama. 2002 Dec 11;288(22):2836–2845. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- 50.Bogner HR, Dahlberg B, de Vries HF, Cahill EC, Barg FK. Older patients' views on the relationship between depression and heart disease. Family Medicine. 2008;40(9):652–657. [PMC free article] [PubMed] [Google Scholar]
- 51.Renders CM, Valk GD, Griffin SJ, Wagner EH, Eijk Van JT, Assendelft WJ. Interventions to improve the management of diabetes in primary care, outpatient, and community settings: a systematic review. Diabetes Care. 2001 Oct;24(10):1821–1833. doi: 10.2337/diacare.24.10.1821. [DOI] [PubMed] [Google Scholar]
- 52.Leeman J. Interventions to improve diabetes self-management: utility and relevance for practice. Diabetes Educ. 2006 Jul-Aug;32(4):571–583. doi: 10.1177/0145721706290833. [DOI] [PubMed] [Google Scholar]