Abstract
Objectives
State governments increasingly mandate public reporting of central line-associated blood stream infections (CLABSIs). This study tests if hospitals located in states with state-mandated, facility-identified, pediatric-specific public CLABSI reporting have lower rates of CLABSIs as defined by the Agency for Healthcare Research and Quality’s Pediatric Quality Indicator 12 (PDI12).
Methods
Utilizing the Kids’ Inpatient Databases from 2000 to 2009, we compared changes in PDI12 rates across three groups of states: states with public CLABSI reporting begun by 2006, states with public reporting begun by 2009 and never-reporting states. In the baseline period (2000–2003), no states mandated public CLABSI reporting. A multivariable, hospital-level random intercept, logistic regression was performed comparing changes in PDI12 rates in states with public reporting to changes in PDI12 rates in never-reporting states.
Results
4,705,857 discharge records were eligible for PDI12. PDI12 rates significantly decreased in all reporting groups, comparing baseline to the post-public reporting time period (2009): Never Reporters 88% decrease (95% CI: 86%, 89%), Reporting Begun by 2006 90% decrease (95% CI: 83%, 94%), and Reporting Begun by 2009 74% decrease (95% CI: 72%, 76%). The Never Reporting Group had comparable decreases in PDI12 rates to the Reporting Begun by 2006 group (p=0.4) and significantly larger decreases in PDI12 rates compared to the Reporting Begun by 2009 group (p<0.001), despite having no states with public reporting.
Conclusions
Public CLABSI reporting alone appears to be insufficient to affect administrative data-based measures of pediatric CLABSI rates or children may be inadequately targeted in current public reporting efforts.
Introduction
Over the past decade significant strides have been made in reducing harmful medical errors and improving patient safety and quality.1–3 Despite this, healthcare-associated infections (HAI) in general and central line-associated blood stream infections (CLABSIs) in particular, continue to extract a significant toll in morbidity and mortality across pediatric populations.1,4–7 Many practitioners, hospitals, insurers and government organizations suggest public reporting of HAI rates informs healthcare consumers and consequently, motivates improved quality and decreased HAI rates.8–13 Supporters of public HAI reporting believe that increased consumer knowledge forces providers and healthcare institutions to improve practice or face lost revenue as patients move their healthcare to sites with lower infection rates.
State mandates for public HAI reporting in general, and CLABSIs in particular, increased rapidly in the past six years, with varying levels of penetrance, robustness and effectiveness.11,14–16 For example, while 34 states or territories in the United States (65%) had laws requiring HAI infection reporting on August 1, 2011, only 22 of them (42% total, 66% of those with laws) required facility identifiers in publically released reports.15 The evidence supporting improved quality of care for adults after state-wide institution of public reporting systems is limited at best.17–21 It is unclear if current public HAI reporting affects pediatric patients or pediatric CLABSI rates, and few states specifically mandate pediatric public reporting.
We hypothesized that states mandating pediatric, facility identified, public CLABSI reporting would be associated with lower pediatric state CLABSI rates from 2000 to 2009. We investigated this hypothesis using a retrospective, difference-in-differences natural experiment, which defined CLABSIs by the Agency for Healthcare Research and Quality’s (AHRQ) administrative data-based, Pediatric Quality Indicator 12 tool (PDI12) and compared states with and without pediatric, facility identified, public CLABSI reporting laws. To our knowledge, this is the first study examining the affect of public pediatric CLABSI reporting on pediatric HAIs.
Methods
Databases
A retrospective analysis of the Kids’ Inpatient Database (KID) from 2000–2009 was performed. The KID is released every three years (2000, 2003, 2006 and 2009) and comprises a national sample of pediatric hospital discharges (age less than 20). While the KID database was released in 1997, it did not include state level identifiers and therefore was excluded from this analysis. Patients in the KID database are sampled from discharges in all community, non-rehabilitation hospitals in states which participate in the Healthcare Cost and Utilization Project (HCUP). In 2000, 27 states and 2,784 hospitals participated in the KID. By 2009, 44 states and 4,121 hospitals participated in the KID. Each database utilizes a systematic random sampling method to select 10% of uncomplicated in-hospital births and 80% of other pediatric discharges and complicated in-hospital births. This method does not sample hospitals, but takes discharges from all participating hospitals, who voluntarily supply this data to HCUP for quality assurance and standardization. Data collected upon discharge includes patient and hospital level data. Each of the KID databases contain over 1.5 million discharge records.22
Public CLABSI Reporting Rates
CLABSIs were chosen as the HAI of interest because of the broad consensus that these types of infections are “never events.”23 State-mandated, facility identified public CLABSI reporting data was derived from previously published briefs and manuscripts based on analysis of state laws.16,24–27 Consistent with previous publications, we defined states as ‘CLABSI public reporters’ if they had pediatric specific, CLABSI public reporting laws that mandated facility identification in reports.15 For the years 2000 to 2003, there were no states that mandated public CLABSI reporting. Given the increased national focus on CLABSI prevention between 2003 and 2009,6 we separated states that began reporting before 2006 from those that began reporting before 2009. These states likely experienced different pressures at the onset of their reporting periods and are not comparable in event time analysis. Two states in the KID database mandated pediatric CLABSI reporting by 2006 and seven additional states mandated pediatric CLABSI reporting by 2009. These two groups (CLABSI Reporting Begun by 2006 and CLABSI Reporting Begun by 2009) were compared to states that never mandated adult or pediatric CLABSI reporting in order to provide the cleanest possible contrast between reporters and non-reporters. Eighteen states never mandated public CLABSI reporting and these states served as our control group. Table 1.
Table 1.
States in the KID Database with State-Mandated, Facility Identified, Central Line Associated Blood Stream Infection (CLABSI) Reporting
|
Never CLABSI Reporters (Control Group: No CLABSI Reporting Before December 31, 2009): AZ, FL, GA, HI, IA, IN, KS, KY, LA, MI, MN, MT, NC, NE, SD, TX, WI, WY |
|
CLABSI Reporting Begun by 2006 (Exposed Group: Pediatric CLABSI Reporting with Facility Identifiers Required in Reporting Begun by 2006): MO, VT |
|
CLABSI Reporting Begun by 2009 (Exposed Group: Pediatric CLABSI Reporting with Facility Identifiers Required in Reporting Begun by 2009): CA, MD, NY, PA, RI, SC, WA |
State-mandated, public CLABSI reporting rates were derived from previously published briefs and manuscripts based on analysis of state laws.16,24–27 Excluded states with pediatric CLABSI reporting without facility identifiers required or law is silent/unclear on need for facility identifiers: AR, CT, MA, NV, TN, UT, VA. Excluded states with adult only, voluntary CLABSI reporting only begun by January 2009, or pediatric CLABSI reporting begun during 2009: CO, IL, ME, NH, NJ, OH, OK, OR, NM, WV.
We defined three time periods for analysis: baseline (2000–2003), early reporting period (2006) and later reporting period (2009). We pursued a natural experiment, comparing changes in CLABSI rates (baseline versus early or later reporting periods) in states with facility identified, public, pediatric CLABSI reporting to changes in CLABSI rates (baseline versus early or later reporting periods) in states that never mandated public CLABSI reporting.
AHRQ Pediatric Quality Indicators
CLABSIs were defined by AHRQ’s administrative data-based, PDI12 tool. In response to the national focus on medical errors, AHRQ developed a set of patient safety indicators designed to identify inpatient adverse events from discharge billing data.28 These indicators, 13 of which are pediatric specific, are meant to identify errors from easily accessible, administrative data.29 They have been previously used to track longitudinal trends in patient safety events over time.30 Much research has been done with the Pediatric Quality Indicators (PDIs), illustrating increased length of stays, increased risks of mortality and increased total charges for patients with an identified PDI.3,31–34
The PDI12 indicator is defined as ‘Selected infections due to medical care’ and checks discharge records for ICD-9-CM codes 99662 (Infection due to other vascular device, implant, and graft) and 9993 (Other infection). In October 2007, ICD-9-CM code 99931 indicating ‘Infection due to central venous catheter,’ was created and added to PDI12. The PDI12 indicator excludes all newborns born in-hospital, acute care facility neonatal transfers, obstetric patients and patients with length of stay less than two days. PDI12 was chosen as an administrative databased marker for CLABSIs because PDI12 is focused on identifying CLABSIs, although before 2007 other infections may have been included. PDI12 has the highest calculated positive predictive value of the AHRQ PDI indicators (80%).35 Detailed information on this tool and its uses can be found on AHRQ’s quality indicators website.28
Each KID database was analyzed by the AHRQ Quality Indicators WinQI software program version 3.2.28 The 2009 KID database was also analyzed by the AHRQ Quality Indicators WinQI software program version 4.1b in order to capture the new ICD-9-CM code (99931) created in 2007.28 The software generated an output file noting which records contained a PDI12 and which records were eligible for this type of infection. Patients who had selected infectious diagnoses present on admission would not be eligible for a ‘Selected infection due to medical care,’ because it would be impossible to determine if infections identified at discharge were due to medical care or to the original infection. Additionally, discharges with missing data or discharges excluded as noted above, were also not eligible for PDI12.
Statistical Analysis
Utilizing Stata 11 (Stata Corp, College Station, TX), estimates of PDI12 rates and patient and hospital demographics were calculated for the three analysis groups (CLABSI Reporting Begun by 2006, CLABSI Reporting Begun by 2009 and Never CLABSI Reporting). Pearson Chi squared statistics were used to compare patient and hospital characteristics between groups for categorical variables and the F-test was used to compare means for continuous variables.
A multivariable logistic regression model was used to estimate the odds of PDI12 during 2000–2003, 2006 and 2009 separately for each reporting group. A random intercept for hospital was included to account for the correlation in PDI12 within a hospital over time, as each hospital’s culture may interpret and implement state mandated pediatric CLABSI reporting laws differently. The model included adjustment for variables suggesting higher acuity and therefore higher underlying risk of CLABSI: patient age in years, gender, expected primary payer (Medicare, Medicaid, Private including health maintenance organization, self-pay, no charge, other), number of procedures, number of diagnoses, hospital bed size (small, medium, large), hospital location (rural, urban) and hospital teaching status.
Within each reporting group, the change in PDI12 rates over time was estimated by the odds ratios comparing PDI12 rates in 2006 or 2009 to the baseline period (2000–2003). The odds ratios of PDI12 over time for the two reporting groups (Reporting begun by 2006 and Reporting Begun by 2009) were subsequently compared to the Never Reporting group to estimate the outcome of interest, a relative odds ratio. The sensitivity of our findings were evaluated by 1) quantifying the effects of the above potential confounders by fitting unadjusted models, 2) investigating different definitions for CLABSI events by fitting models where the 2009 KID database was analyzed with WinQI software version 3.2 only and with WinQI software version 4.1b only and 3) evaluating whether clustering at the state level instead of the hospital level was important to the findings by fitting models with a random intercept for state, models with state fixed effects and marginal models clustered on state or hospital levels. Additional model descriptions, rationale and results are presented in the Appendix. All results from sensitivity analyses were similar except as discussed below. This study of publicly-available, de-identified data was deemed exempt by the Johns Hopkins University School of Medicine Institutional Review Board.
Results
There were 12,039,446 discharge records in the 2000–2009 KID databases and 6,414,595 (53%) discharge records were eligible for PDI12. 2,595,638 discharges were not eligible for PDI12 (22%) because they had lengths of stays less than two days. 4,705,857 (39%) discharge records were eligible for PDI12 and within a state included in one of the three reporting groups. There were 18 states and 2,066 hospitals in the Never Reporting group, two states and 135 hospitals in the CLABSI Reporting Begun by 2006 group, and seven states and 1,006 hospitals in the CLABSI reporting Begun by 2009 group. Complete demographics for the discharge records within each reporting group are presented in Table 2. There were statistically significant differences between the reporting groups for all analyzed demographic variables (p<0.05), likely due to large numbers of records analyzed.
Table 2.
Demographics of Discharges Eligible for PDI12 in 2000–2009 KID Databases
| Never CLABSI Reporters N (%) |
CLABSI Reporting Begun by 2006 N (%) |
CLABSI Reporting Begun by 2009 N (%) |
|
|---|---|---|---|
| Number of Discharges | 2,580,621 | 179,322 | 1,945,914 |
| Number of States | 18 | 2 | 7 |
| Number of Hospitals | 2,066 | 135 | 1,006 |
|
| |||
| Hospital Level Characteristics | |||
| Hospital Location: Urban | 2,187,602 (88) | 140,257 (84) | 1,844,731 (96) |
| Hospital Teaching Status: Teaching | 1,292,034 (52) | 91,819 (55) | 1,279,475 (58) |
| Hospital Bed Size: | |||
| Large | 1,632,289 (66) | 87,368 (52) | 1,160,009 (60) |
| Medium | 570,186 (23) | 30,907 (18) | 565,528 (29) |
| Small | 285,138 (11) | 49,656 (30) | 205,275 (11) |
|
| |||
| Patient Level Characteristics | |||
| Gender: Female | 1,180,251 (46) | 82,721 (46) | 882,419 (45) |
| Expected Primary Payer: | |||
| Medicare | 6,255 (0.2) | 363 (0.2) | 4,237 (0.2) |
| Medicaid | 1,223,412 (48) | 85,854 (48) | 894,863 (46) |
| Private including HMO | 1,129,596 (44) | 83,375 (46) | 915,011 (47) |
| Self-pay | 107,993 (4) | 4,622 (3) | 66,278 (3) |
| No charge | 11,221 (0.4) | 111 (0.1) | 440 (0.02) |
| Other | 97,040 (4) | 4,780 (3) | 61,615 (3) |
|
| |||
| Mean Age in Years (s.d.) | 3.5 (5.5) | 4.4 (6) | 3.6 (5.6) |
| Mean Number of Diagnoses (s.d.) | 4.1 (2.8) | 4.3 (3.4) | 4.0 (3) |
| Mean Number of Procedures (s.d.) | 1.1 (1.8) | 1.2 (2.4) | 1.4 (2.1) |
|
| |||
| Unadjusted PDI12 Rates | |||
| 2000–2003 (Baseline):** | |||
| Total Discharges with PDI12 | 2,449 | 196 | 2,861 |
| Total PDI12 Eligible Discharges | 1,174,344 | 75,593 | 967,754 |
| PDI12 Rate per 1,000 Discharges | 2.1 | 2.6 | 3.0 |
| 2006: | |||
| Total Discharges with PDI12 | 1,606 | 168 | 1,402 |
| Total PDI12 Eligible Discharges | 680,873 | 51,851 | 445,796 |
| PDI12 Rate per 1,000 Discharges | 2.4 | 3.2 | 3.1 |
| 2009: | |||
| Total Discharges with PDI12 | 698 | 23 | 1,063 |
| Total PDI12 Eligible Discharges | 725,404 | 51,878 | 532,364 |
| PDI12 Rate per 1,000 Discharges | 1.0 | 0.4 | 2.0 |
Due to missing data, not all rows add to the total N for each group.
Data in these cells combines discharges from the 2000 and 2003 KID databases.
All hospital and patient characteristics had statistically significant differences across reporting groups with p<0.05.
Each database year (2000, 2003, 2006, and 2009) had over 2,000 discharges categorized as PDI12s (range 2,413–4,262) and over 1.3 million discharges eligible for PDI12 (range 1.35 million–1.82 million). Reporting groups within a given year had between 23 and 1,606 discharges categorized as PDI12 and between 25,158 and 724,706 discharges eligible for PDI12. The large ranges between groups are likely due to the variance in the number of states in each group. Unadjusted, baseline (2000–2003) PDI12 rates per 1,000 discharges for the three reporting groups were: Never Reporting group 2.1, Reporting Begun by 2006 group 2.6 and Reporting Begun by 2009 group 3.0. By 2009, the unadjusted PDI12 rates per 1,000 discharges decreased for all three groups: Never Reporting group 1.0, Reporting Begun by 2006 group 0.4 and Reporting Begun by 2009 group 2.0. Table 2.
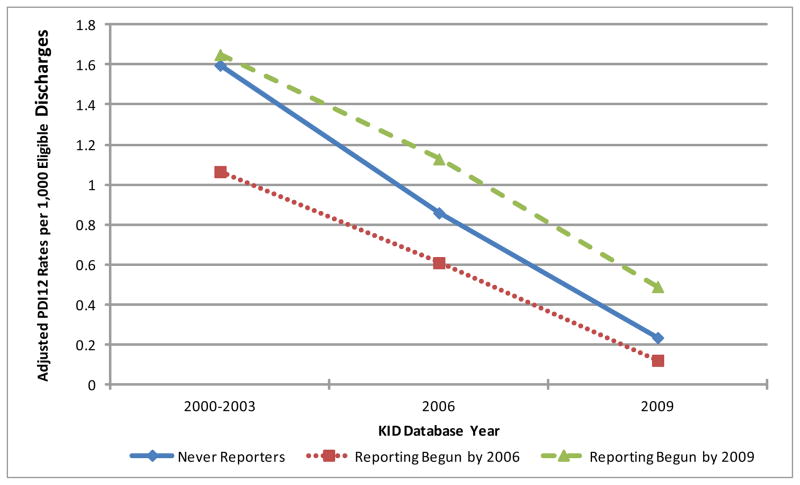
Using a multivariable, hospital-level random intercept logistic regression model, the estimated adjusted PDI12 rates per 1,000 discharges during the baseline period (2000–2003) for the three reporting groups were: Never Reporting group 1.6, Reporting Begun by 2006 group 1.1 and Reporting Begun by 2009 group 1.7. Table 3. The adjusted PDI12 rates significantly decreased over time in each group comparing 2006 and 2009 to the baseline period. Figure 1. Specifically, the Never Reporting hospitals had a 50% (95% Confidence Interval (CI): 46%, 54%) and 88% (95% CI: 86%, 89%) decrease in PDI12 rates during 2006 and 2009 compared to the baseline period, respectively. For those states who began reporting in 2006, the PDI12 rates decreased by46% (95% CI: 31%, 68%) and 90% (95% CI: 83%, 94%) comparing 2006 and 2009 to the baseline period, respectively. For those states who began reporting by 2009 the PDI12 rates decreased by 35% (95% CI: 30%, 39%) and 74% (95% CI: 72%, 76%) in 2006 and 2009 compared to the baseline period, respectively. Table 3.
Table 3.
Adjusted odds ratios comparing the odds of PDI12 in 2006 or 2009 to the baseline period (2000–2003) in each reporting group; Relative Odds Ratios of PDI12 over time for the Begun by 2006 and Begun by 2009 Reporters versus the Never Reporters
| Never CLABSI Reporters | CLABSI Reporting Begun by 2006 | CLABSI Reporting Begun by 2009 | |
|---|---|---|---|
| Adjusted PDI12 Rates per 1,000 Discharges | |||
| 2000–2003 (Baseline) | 1.6 (1.4, 1.8) | 1.1 (0.64, 1.5) | 1.7 (1.5, 1.8) |
| 2006 | 0.86 (0.75, 0.97) | 0.61 (0.36, 0.86) | 1.1 (1.0, 1.3) |
| 2009 | 0.24 (0.20, 0.27) | 0.12 (0.05, 0.20) | 0.49 (0.43, 0.56) |
|
| |||
| Adjusted Odds Ratio of PDI12 for Each Reporting Group: | |||
| Comparing 2006 versus 2000–2003 | 0.5 (0.46, 0.54) | 0.54 (0.42, 0.69) | 0.65 (0.61, 0.7) |
| Comparing 2009 versus 2000–2003 | 0.12 (0.11, 0.14) | 0.10 (0.06, 0.17) | 0.26 (0.24, 0.28) |
|
| |||
| Relative Odds Ratio of PDI12 Comparing Odds Ratios of PDI12 in Exposed to Control Groups:* | |||
| CLABSI Reporting Groups versus Never | ref | 1.1 (0.84, 1.4) | N/A |
| CLABSI Reporting Group in 2006 | |||
| CLABSI Reporting Groups versus Never | ref | 0.79 (0.47, 1.4) | 2.1 (1.9, 2.4) |
| CLABSI Reporting Group in 2009 | |||
Values are presented with (95% Confidence Interval). Model used hospital-level random intercept logistic regression and was adjusted for patient age in years, gender, expected primary payer, number of procedures, number of diagnoses, hospital bed size, hospital location and hospital teaching status. Bolded Odds Ratios are statistically significant (p<0.05).
The relative odds ratio of PDI12 compares the change in PDI12 rates (Odds Ratios) for the exposed groups (CLABSI Reporting Begun by 2006 or CLABSI Reporting Begun by 2009) to the change in PDI12 rates (Odds Ratio) in the Never CLABSI Reporters group in the KID database year listed. For example: the OR of PDI12 for the CLABSI Reporting Begun by 2009 group comparing 2009 versus 2000–2003 was 0.26. The OR of PDI12 for the Never CLABSI Reporters group comparing 2009 versus 2000–2003 was 0.12. The relative odds ratio of PDI12 comparing the Reporting Begun by 2009 group and the Never CLABSI Reporters group in 2009 is 0.26/0.12 = 2.1, suggesting the Never CLABSI Reporting group had a greater decrease in CLABSI rates than the Reporting Begun by 2009 group in 2009.
Figure 1. Adjusted PDI12 Rates per 1,000 Eligible Discharges by State-Mandated Public Reporting Groups (Never Reporters, Reporting Begun by 2006 and Reporting Begun by 2009).
Healthcare-associated infections were defined by the Agency for Healthcare Research and Quality’s administrative data based Pediatric Quality Indicator Tool (PDI12).28. Model was adjusted for patient age in years, gender, expected primary payer, number of procedures, number of diagnoses, hospital bed size, hospital location and hospital teaching status. Model assumed random effects at the hospital level.
*Adjusted PDI12 rates statistically significantly decreased for all three groups comparing their respective baseline rates to their 2006 or 2009 rates (p<0.001).
Despite having no states with public reporting, the Never Reporting Group had comparable decreases in adjusted PDI12 rates to the Reporting Begun by 2006 group during both the early and later reporting periods (early period: 50% decrease versus 46% respectively, Relative Odds Ratio: 1.1, p=0.55; later period: 88% decrease versus 90% respectively, Relative Odds Ratio 0.79, p=0.4). Additionally, the Never Reporting Group had significantly greater decreases in PDI12 rates during the later reporting period when compared to the Reporting Begun by 2009 group (later period: 88% decrease versus 74% respectively, Relative Odds Ratio 2.1, p<0.001). Table 3.
The Appendix presents associations between hospital and patient characteristics and the odds of PDI12 after accounting for group and time. All investigated confounders, except gender and medium hospital bed size, were significantly associated with odds of PDI12 (p<0.05). After accounting for the patient and hospital characteristics included in the model, we estimated that 22% of the total variation in the odds of PDI12 was attributable to differences between hospitals.
All sensitivity analyses resulted in qualitatively similar results except for the unadjusted models, which showed a statistically significant greater decrease in PDI12 rates in the Reporting Begun by 2006 group when compared to the Never Reporters in the later reporting period (2009) only (p<0.001). Appendix.
Discussion
To our knowledge, this is the first study in pediatric patients to examine the association between public, facility identified CLABSI reporting and administrative data-based infection rates, meant to approximate CLABSI rates. In all three groups, Reporting Begun by 2006, Reporting Begun by 2009 and Never Reporters, rates of PDI12, a measure of CLABSI, decreased between 2000–2003 and 2009. This result mirrors national trends in decreasing CLABSI and HAI rates.1–3 A multifactorial approach, including collaborative learning,7,36 intense national attention and continued advancements in patient safety and quality of care,37 likely contributed to this decline. Unfortunately, public reporting, often seen as a key component to reducing HAIs and CLABSIs,8,10 was not associated with greater decreases in PDI12 rates in children. Despite having state mandated, facility identified, pediatric CLABSI reporting for at least three years, the Reporting Begun by 2006 group did not experience significantly greater decreases in PDI12 rates when compared to the Never Reporters in 2009. Additionally, the Reporting Begun by 2009 group had significantly smaller decreases in PDI12 rates when compared to the Never Reporters.
Contrary to our original hypothesis, these findings suggest that public CLABSI reporting alone may not reduce rates of pediatric CLABSIs above and beyond other national efforts. Previous adult-focused studies have called into question the effect of public reporting on HAI rates.17–20 As states and federal agencies continue to emphasize public reporting of infection rates, it is important to consider whether these efforts are having an impact on all segments of the population, including pediatric patients.10,11,13 Public HAI reporting has multiple potential purposes besides improving HAI rates, such as increasing consumer knowledge, improving health purchaser choice and improving policy decisions at local and federal levels. It is possible that state-mandated, public CLABSI reporting has improved these areas for pediatric patients, although adult reviews on the topic suggest otherwise.18 The authors would caution against the conclusion that public, pediatric HAI reporting should no longer be pursued: public reporting has likely contributed to national decreases in HAI rates in ways that cannot be measured. Public reporting may have been an influential component driving the recent decrease in national CLABSI rates and not have produced a signal that could be detected above other concomitant interventions. Further research is needed to determine if domains such as consumer knowledge and purchaser choice have been improved by state-mandated, public HAI reporting. Additionally, some research suggests that quality improvement interventions, such as those spurred by state mandated public reporting, discover cases of harm that would not have otherwise been identified.38 It is possible that states with public reporting mandates actually had greater decreases in CLABSI rates but these were not observed in our data because of the increase in rates accompanying quality improvement case finding. This could explain why the Reporting Begun by 2009 group had smaller decreases in CLABSI rates than the Never Reporting group. Finally, if institutions are already collecting data on CLABSIs and other HAIs, reporting them to centralized agencies for public consumption adds little additional cost, especially when compared to the total net revenue of most healthcare institutions. Investigations on how to best operationalize public reporting, including data presentation, public education, and ways to prevent data overload, need to be studied with clearly measured outcomes, before policy makers further mandate the application of public reporting in other states and to other medical error categories. It is crucial that pediatric patients are also considered as these policy decisions are made.
This study suggests that pediatric CLABSI rates declined across the US and across all three reporting groups comparing 2000–2003 to 2006 and 2009. One reason for this decline could be due to institutional reductions in infection reporting unrelated to actual changes in CLABSI rates. As institutions and providers prepared themselves for future reporting mandates, such as those codified in the Affordable Care Act of 2010,13 and for changes in reimbursement practices for HAIs,39 they could be purposefully or inadvertently altering coding behaviors to reflect fewer HAIs. If coding changes have altered based on reimbursements concerns, this would imply the observed decrease in national pediatric CLABSI rates is actually only a change in reported infections. It is unclear if institutional “gaming the system” efforts would be increased in states with public reporting mandates, and we are unable to ascertain whether these practices contributed to decreases in PDI12 rates.
This study has a number of limitations. First, there is potential for misclassification bias when using the AHRQ PDI12 tool on discharge data. This tool is dependent on medical coders and administrative data and may not accurately designate every CLABSI. Nonetheless, PDI12 does have the highest positive predictive value of all the AHRQ PDIs (80%).35 Additionally, we have no reason to believe this misclassification bias varied by the three groups of interest. We attempted to lessen the risk for misclassification bias in the 2009 KID data by performing sensitivity analyses using three possible PDI12 definitions as described above. These different definitions account for the impact of new ICD-9 codes instituted in 2007 and we believe any bias due to definition change would affect the odds ratios across all three groups to a similar magnitude. Our study has only two post-public CLABSI reporting time points and they may not be representative of all years or of future changes in pediatric CLABSI rates. As with all studies, there exists potential for Type II error given the Reporting Begun by 2006 group included only two states. This study focused on public CLABSI reporting and not non-public CLABSI reporting, where hospitals anonymously report infection rates to state databases and internally compare their results to aggregated baselines. It is possible states with non-public reporting, or reporting without facility identifiers, may be different and cause greater decreases in CLABSI rates. Many of the state laws cited in this manuscript focus on preventing CLABSIs in intensive care units. The KID database does not provide markers for intensive care unit admission and it is possible a larger effect size would be noted if we could limit our analysis to intensive care unit patients. Additionally, each state’s CLABSI reporting law is unique16 and we are unable to speak to the effect different pediatric CLABSI reporting laws have on state specific CLABSI rates; specific states may have decreased their CLASBI rates because of their specific law but the effect was overshadowed due to data from other states. Providers in the Never Reporting group may have anticipated future public reporting mandates and therefore worked to lower CLABSI rates. We believe this concern is less likely given prior adult studies suggesting no association between public reporting and HAI rates17–21, and recent research suggesting that the Center for Medicare and Medicaid Services reimbursement limitations for CLABSIs did not decrease CLABSI rates.39 This analysis uses KID data from a non-random sample of hospitals within each state. We feel comfortable with this limitation because it is unlikely that the non-random selection of hospitals is associated with our outcome of interest: CLABSIs. Finally, our unadjusted models showed a greater decrease in PDI12 rates in the Reporting Begun by 2006 group for the later reporting time period. It is likely that this result is confounded by the variables indicating patient acuity that we define in Table 2.
Conclusion
Adjusted administrative data-based PDI12 rates, approximating CLABSIs, decreased in pediatric discharges from 2000–2009. This decrease was not significantly greater in states with state-mandated, facility-identified, pediatric-specific public CLABSI reporting. Additional research is needed to determine if public HAI reporting is useful in other domains and how to best operationalize public reporting for future efforts.
Supplementary Material
Acknowledgments
Source of Funding:
Dr. Rinke was supported by Grant Number 5KL2RR025006 from the National Center for Research Resources, a component of the National Institutes of Health and the Roadmap for Medical Research. Dr. Miller and Dr. Bundy are supported by the Children’s Hospital Association for their work.
Footnotes
Conflicts of Interest:
For the remaining authors, none were declared.
References
- 1.Centers for Disease Control and Prevention. Vital signs: central line--associated blood stream infections --- United States, 2001, 2008, and 2009. MMWR Morbidity and mortality weekly report. 2011;60:243–8. [PubMed] [Google Scholar]
- 2. [Accessed December 2, 2011];2010 National Healthcare Quality Report. 2010 at http://www.ahrq.gov/qual/nhqr10/nhqr10.pdf.)
- 3.Friedman B, Berdahl T, Simpson LA, et al. Annual report on health care for children and youth in the United States: focus on trends in hospital use and quality. Acad Pediatr. 2011;11:263–79. doi: 10.1016/j.acap.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Klevens RM, Edwards JR, Gaynes RP. The impact of antimicrobial-resistant, health care-associated infections on mortality in the United States. Clin Infect Dis. 2008;47:927–30. doi: 10.1086/591698. [DOI] [PubMed] [Google Scholar]
- 5.Klevens RM, Edwards JR, Richards CL, Jr, et al. Estimating health care-associated infections and deaths in U.S hospitals, 2002. Public Health Rep. 2007;122:160–6. doi: 10.1177/003335490712200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. The New England Journal of Medicine. 2006;355:2725–32. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
- 7.Miller MR, Griswold M, Harris JM, 2nd, et al. Decreasing PICU catheter-associated bloodstream infections: NACHRI’s quality transformation efforts. Pediatrics. 2010;125:206–13. doi: 10.1542/peds.2009-1382. [DOI] [PubMed] [Google Scholar]
- 8.Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- 9.Pronovost PJ, Marsteller JA, Goeschel CA. Preventing bloodstream infections: a measurable national success story in quality improvement. Health Aff (Millwood) 2011;30:628–34. doi: 10.1377/hlthaff.2011.0047. [DOI] [PubMed] [Google Scholar]
- 10.Leape LL. Transparency and Public Reporting Are Essential for a Safe Health Care System. The Commonwealth Fund. 2010:4. [Google Scholar]
- 11.Halpin HA, Milstein A, Shortell SM, Vanneman M, Rosenberg J. Mandatory public reporting of hospital-acquired infection rates: a report from California. Health Aff (Millwood) 2011;30:723–9. doi: 10.1377/hlthaff.2009.0990. [DOI] [PubMed] [Google Scholar]
- 12.First State-Specific Healthcare-Associated Infections Summary Data Report. [Accessed December 2, 2011];CDC’s National Healthcare Safety Network. 2009 at http://www.cdc.gov/hai/pdfs/stateplans/SIR_05_25_2010.pdf.)
- 13.Kocher R, Emanuel EJ, DeParle NA. The Affordable Care Act and the future of clinical medicine: the opportunities and challenges. Ann Intern Med. 2010;153:536–9. doi: 10.7326/0003-4819-153-8-201010190-00274. [DOI] [PubMed] [Google Scholar]
- 14.Murphy DJ, Needham DM, Goeschel C, Fan E, Cosgrove SE, Pronovost PJ. Monitoring and reducing central line-associated bloodstream infections: a national survey of state hospital associations. Am J Med Qual. 2010;25:255–60. doi: 10.1177/1062860610364653. [DOI] [PubMed] [Google Scholar]
- 15.Reagan J, Hacker C. Laws pertaining to healthcare-associated infections: a review of 3 legal requirements. Infect Control Hosp Epidemiol. 2012;33:75–80. doi: 10.1086/663204. [DOI] [PubMed] [Google Scholar]
- 16.Reagan JK. The Movement Toward Patient Safety: State Action Related to Reporting and Disclosure of Healthcare-Associated Infections. Houston: The University of Texas; 2010. [Google Scholar]
- 17.Fung CH, Lim YW, Mattke S, Damberg C, Shekelle PG. Systematic review: the evidence that publishing patient care performance data improves quality of care. Ann Intern Med. 2008;148:111–23. doi: 10.7326/0003-4819-148-2-200801150-00006. [DOI] [PubMed] [Google Scholar]
- 18.Ketelaar NA, Faber MJ, Flottorp S, Rygh LH, Deane KH, Eccles MP. Public release of performance data in changing the behaviour of healthcare consumers, professionals or organisations. Cochrane Database Syst Rev. 2011;11:CD004538. doi: 10.1002/14651858.CD004538.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKibben L, Fowler G, Horan T, Brennan PJ. Ensuring rational public reporting systems for health care-associated infections: systematic literature review and evaluation recommendations. Am J Infect Control. 2006;34:142–9. doi: 10.1016/j.ajic.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Kim HK, Black BS. Does Hospital Infection Reporting Affect Actual Infection Rates, Reported Rates, or Both? A Case Study of Pennsylvania Northwestern Law & Econ Research Paper 2011. (11–19) [Google Scholar]
- 21.Joynt KE, Blumenthal DM, Orav EJ, Resnic FS, Jha AK. Association of public reporting for percutaneous coronary intervention with utilization and outcomes among Medicare beneficiaries with acute myocardial infarction. JAMA. 2012;308:1460–8. doi: 10.1001/jama.2012.12922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. [Accessed December 2, 2011];Introduction to the HCUP KIDS’ Inpatient Database. 2011 at http://www.hcup-us.ahrq.gov/db/nation/kid/kid_2009_introduction.jsp.)
- 23. [Accessed May 16, 2011];“Never Events”: Medicare’s and Health Plan’s Policies on Providing Payment for Serious and Preventable Hospital Errors. 2008 at http://www.calhealthplans.org/documents/DH03_NeverEventsPoliciesProvidingPmt.pdf.)
- 24.Health-care-associated infections in hospitals: an overview of state reporting programs and individual hospital initiatives to reduce certain infections. [Accessed December 2, 2011];2008 at http://www.gao.gov/new.items/d08808.pdf.)
- 25.Healthcare-associated infections homepage. [Accessed December 2, 2011];2010 at http://www.ncsl.org/default.aspx?tabid=14084.)
- 26.State legislation and initiatives for healthcare-associated infections. [Accessed December 2, 2011];2011 at http://www.hospitalinfection.org/legislation.shtml.)
- 27.The states take action: hospital infection reporting and control. [Accessed December 2, 2011];2007 at http://www.rwjf.org/files/research/extendcurebriefnov2007.pdf.)
- 28.Agency for Healthcare Research and Quality. [Accessed May 17, 2011];Quality Indicators. 2011 at http://www.qualityindicators.ahrq.gov/.)
- 29.Miller MR, Elixhauser A, Zhan C, Meyer GS. Patient Safety Indicators: using administrative data to identify potential patient safety concerns. Health Serv Res. 2001;36:110–32. [PMC free article] [PubMed] [Google Scholar]
- 30.Downey JR, Hernandez-Boussard T, Banka G, Morton JM. Is patient safety improving? National trends in patient safety indicators: 1998–2007. Health Serv Res. 2012;47:414–30. doi: 10.1111/j.1475-6773.2011.01361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Camp M, Chang DC, Zhang Y, Chrouser K, Colombani PM, Abdullah F. The Agency for Healthcare Research and Quality (AHRQ) pediatric quality indicators (PDIs): accidental puncture or laceration during surgery in children. Ann Surg. 2010;251:165–70. doi: 10.1097/SLA.0b013e3181b977c4. [DOI] [PubMed] [Google Scholar]
- 32.Miller MR, Elixhauser A, Zhan C. Patient safety events during pediatric hospitalizations. Pediatrics. 2003;111:1358–66. doi: 10.1542/peds.111.6.1358. [DOI] [PubMed] [Google Scholar]
- 33.Miller MR, Zhan C. Pediatric patient safety in hospitals: a national picture in 2000. Pediatrics. 2004;113:1741–6. doi: 10.1542/peds.113.6.1741. [DOI] [PubMed] [Google Scholar]
- 34.McDonald KM, Davies SM, Haberland CA, Geppert JJ, Ku A, Romano PS. Preliminary assessment of pediatric health care quality and patient safety in the United States using readily available administrative data. Pediatrics. 2008;122:e416–25. doi: 10.1542/peds.2007-2477. [DOI] [PubMed] [Google Scholar]
- 35.Scanlon MC, Harris JM, 2nd, Levy F, Sedman A. Evaluation of the agency for healthcare research and quality pediatric quality indicators. Pediatrics. 2008;121:e1723–31. doi: 10.1542/peds.2007-3247. [DOI] [PubMed] [Google Scholar]
- 36.Rinke ML, Chen AR, Bundy DG, et al. Implementation of a Central Line Maintenance Care Bundle in Hospitalized Pediatric Oncology Patients. Pediatrics. 2012;130:e996–e1004. doi: 10.1542/peds.2012-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valentine KM. Ethanol lock therapy for catheter-associated blood stream infections in a pediatric intensive care unit. Pediatr Crit Care Med. 2011;12:e292–6. doi: 10.1097/PCC.0b013e318219267c. [DOI] [PubMed] [Google Scholar]
- 38.Pierce CA, Haut ER, Kardooni S, et al. Surveillance bias and deep vein thrombosis in the national trauma data bank: the more we look, the more we find. J Trauma. 2008;64:932–6. doi: 10.1097/TA.0b013e318166b808. discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 39.Lee GM, Kleinman K, Soumerai SB, et al. Effect of nonpayment for preventable infections in U.S. hospitals. N Engl J Med. 2012;367:1428–37. doi: 10.1056/NEJMsa1202419. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.