Abstract
The Notch signaling pathway plays a critical role in maintaining the balance between cell proliferation, differentiation and apoptosis, and is a highly conserved signaling pathway that regulates normal development in a context- and dose-dependent manner. Dysregulation of Notch signaling has been suggested to be key events in a variety of hematological malignancies. Notch1 signaling appears to be the central oncogenic trigger in T cell acute lymphoblastic leukemia (T-ALL), in which the majority of human malignancies have acquired mutations that lead to constitutive activation of Notch1 signaling. However, emerging evidence unexpectedly demonstrates that Notch signaling can function as a potent tumor suppressor in other forms of leukemia. This minireview will summarize recent advances related to the roles of activated Notch signaling in human lymphocytic leukemia, myeloid leukemia, stem cells and stromal microenvironment, and we will discuss the perspectives of Notch signaling as a potential therapeutic target as well.
Introduction
The Notch signaling pathway is highly conserved from Drosophila to human and plays an important role in the regulation of cell proliferation, differentiation and apoptosis [1]. Moreover, it has been suggested that Notch signaling may be responsible for the development and progression of human malignancies, including leukemia.
Notch signaling pathway
Notch and the ligands
Four members of Notch proteins have been identified to date in mammals, including Notch1-4 [2-5]. The Notch proteins are single-pass transmembrane receptors, which are composed of extracellular, transmembrane and intracellular domains. The extracellular domain of all Notch proteins contain epidermal growth-factor-like repeats (EGFLR) and three LIN Notch (LNR) repeats, whereas the intracellular domain consists of the RAM23 domain (RAM) and seven Ankyrin/CDC10 repeats (ANK), necessary for protein-protein interactions. Moreover, five canonical Notch ligands have been found in mammals: Dll1 (Delta-like 1), Dll3 (Delta-like 3), Dll4 (Delta-like 4), Jagged1 and Jagged2 [2-5]. Notch ligands are transmembrane proteins of which the extracellular domain contains a characteristic number of EGF-like repeats and a cysteine rich N-terminal DSL domain, responsible for the interaction with Notch receptors.
Notch signaling activation
Notch signaling has been shown to be initiated by binding of the Notch transmembrane receptors with their specific ligands between two neighboring cells [6]. Upon activation, Notch receptors undergo a cascade of metalloprotease tumor necrosis factor-α-converting enzyme (TACE) and γ-secretase complex proteolytic cleavages, releasing the Notch intracellular domain (NICD). Subsequently, the NICD translocates into the nucleus and interacts with the DNA binding protein CSL to regulate gene expression. To date, only a few target genes have been identified. The best-known Notch target genes are two families of basic helixloop helix transcription factors: Hes (Hairy enhance of split) and Hey (Hairy/enhancer of spit related with YRPW motif) family [7]. Hes and Hey proteins are helix-loop-helix transcription factors that function as transcriptional repressors. Additionally, target genes of the Notch signaling pathways also include cyclin D1, c-myc, p21, p27, Akt, mTOR, VEGF, etc., some of which are dependent on Notch signaling in multiple tissues, while others are tissue specific [8-21] (Table 1). Nevertheless, many target genes of Notch signaling remain to be determined [8].
Table 1.
Target genes of the Notch signaling pathways
| Gene | Role | Tissues | Comments |
|---|---|---|---|
| cyclin D1, cyclin A , p21, p27, |
Cell cycle regulators |
Hepatocellular cancer, renal cancer |
[9,10] |
| c-myc, NF-κB2 , Akt, mTOR, |
Cell proliferation and survival |
Keratinocytes, liver, T-ALL, |
[11-16] |
| Hes1, Hes6 |
Embryonic development |
Embryonic neural progenitor cell, human pluripotent stem cells |
[17,18] |
| VEGF, VEGFR-2 |
Angiopoiesis |
Osteosarcoma, endothelial and neural cells. |
[19,20] |
| MMP-9, MMP-2 | Invasion and metastasis | Osteosarcoma, pancreatic cancer | [19,21] |
Notch signaling in lymphocytic leukemia
T cell lymphocytic leukemia
It has been shown that Notch signaling is abnormally regulated in many human malignancies [22,23]. Notch1 mutations causing Notch signaling continuously activated have been found in nearly 60% of T cell acute lymphoblastic leukemia (T-ALL) patients, making Notch1 the most prominent oncogene specifically involved in the pathogenesis of T-ALL [24,25]. The characterize mutations occur mostly in the heterodimerization (HD) domain and proline, glutamic acid, serine, threonine-rich (PEST) domain of the Notch1 receptor. HD domain mutation leads to a COOH-terminally truncated NICD, whereas PEST domain mutation results in loss of the negative regulatory domain, escaping from FBXW7-mediated degradation and prolongation of the half-life of NICD [26]. Notch1 mutations have been shown to be an early, prenatal genetic event in T-ALL patients [27]. In murine models of T-ALL, Notch1 activation is responsible for directly inducing leukemia and collaborating with other initiating genetic events to perpetuate leukemic growth [28,29]. Moreover, our previous study has shown that Notch1 signaling is also required for hypoxia-induced proliferation, invasion and chemoresistance in T-ALL, suggesting that pharmacological inhibitors of Notch1 signaling may be attractive interventions for T-ALL treatment [30].
Additionally, other Notch signaling and target genes are also involved in the initiation and progression of T-ALL. It has been reported that Notch3 and Hes1 are highly expressed by T-ALL cells, as well as dramatically reduced or absent in remission [31]. Downregulation of Notch3 by small hair RNA (shRNA) has been found to suppress the activity of Notch signaling, leading to growth inhibition and apoptosis induction of T-ALL cells [32].
B cell lymphocytic leukemia
Interestingly, the function of Notch signaling in leukemogenesis has been shown to be either oncogenic or tumor suppressive, and it could be context dependent [33,34]. Notch signaling and target genes have been demonstrated to be tumor suppressive rather than oncogenic in a limited number of leukemia types, including B-ALL (Table 2). It has been reported that in contrast to T-ALL, Notch3, Jagged1, Hes2, Hes4 and Hes5 were frequently hypermethylated in B-ALL, associated with gene silencing [33]. Furthermore, restoration of Hes5 expression by lentiviral transduction could give rise to growth arrest and apoptosis in Hes5 negative B-ALL cells but not in Hes5 expressing T-ALL cells [33]. Other investigators confirmed the fact and showed that activated forms of the 4 mammalian Notch receptors (NICD1-4) or hes1 was responsible for growth inhibition and apoptosis enhancement in both murine and human B-ALL [35-37].
Table 2.
Notch in B cell Lymphocytic leukemia
In contrast with B-ALL, Notch signaling could maintain B cell chronic lymphoblastic leukemia (B-CLL) cell survival and apoptosis resistance, undoubtedly indicating an oncogenic role in B-CLL. Emerging evidence suggests that the Notch signaling network is frequently deregulated in human B-CLL with up-regulated expression of Notch1 and Notch2 as well as their ligands Jagged1 and Jagged2 [42]. Moreover, Notch signaling inhibition by the gamma-secretase inhibitors (GSIs) and the specific Notch2 down-regulation using small interfering RNA (siRNA) could promote B-CLL cell apoptosis [38,42]. It has been also reported that Notch2 is not only overexpressed in B-CLL cells but also might be related to the failure of apoptosis-oriented treatment for this disease and deregulation of Notch2 signaling is involved in the aberrant expression of CD23 in B-CLL [39-41]. Taken together, these results suggest that Notch signaling is constitutively activated in B-CLL cells, and can sustain the survival of these cells.
Notch signaling in myeloid leukemia
Knowledge about the role of Notch signaling in acute myeloid leukemia (AML) is equally poorly understood. Very recently, Jagged1 and Dll1 were shown to be expressed at significantly higher levels in acute promyelocytic leukemia (APL) samples compared with all other subtypes, as well as normal myeloid populations [43]. Inhibition of Notch signaling by GSIs could reduce self-renewal and colony formation of Kit+Lin-Sca1+ cells from pre-leukemic Ctsg-PML-RARA mice [43]. Our previous study has also demonstrated that Dll4 and Notch1 expression were significantly higher in untreated AML patients than in the normal controls, and provides evidence that the activation of Notch signaling may indicate an unfavorable prognosis in AML [44]. These data suggest that Notch signaling can promot AML development [45]; however, other studies have shown opposite function of Notch signaling in AML (Table 3). A significant decrease in the levels of the Notch ligand and activated receptors as well as target genes was reported to be lower in AML samples than in normal hematopoietic stem cells (HSCs), suggesting that Notch signaling is not activated in AML [46-48]. Kannan et al. have found that all four Notch homologues and Hes1 were sufficient to inhibit the growth and induced caspase-dependent apoptosis of AML, which were associated with B cell lymphoma 2 (BCL2) loss and enhanced p53/p21 expression [45]. Additionally, the dnMAML (a pan-Notch inhibitor) could not affect AML proliferation in vitro but lead to dramatic increases in leukemia burden in two xenograft mouse models, which was associated with p53 dysregulation [45]. The 17-aa peptide with Notch agonist activity was able to activate Notch signaling to induce apoptosis of AML cells [45,49,50]. Besides inducing apoptosis, the recombinant Notch ligand proteins, Dll1 and Dll4 could alter AML blast cells into macrophage-like cells morphologically and increase the expression of differentiation markers such as CD13 or CD14 [51]. Tohda et al. also found that the Notch ligands tended to induce differentiation under the specific conditions rather than promoted the self-renewal capacity of AML cells [52]. Overall, different researchers and experiment methods come to different conclusions, illustrating the highly context-dependent nature of the pathway. Due to the complexity of the Notch pathway and limited tools to specifically modulate the this pathway, the function of this signaling is still unclear, and additional studies are needed to clarify the role of various Notch receptors in AML.
Table 3.
Notch in myeloid leukemia
| Leukemia types | Role | Mechanism | Comments |
|---|---|---|---|
| Non-APL AML |
Tumor suppressor |
B cell lymphoma 2 (BCL2) loss and enhanced p53/p21 expression |
[45] |
| APL |
Oncogene |
Jagged1 and Dll1 were overexpressed. GSIs could reduce self-renewal and colony formation of Kit + Lin-Sca1+ cell |
[43] |
| CMML |
Tumor suppressor |
Notch1–3−/− or Ncstn−/− mice developed CMML-like disease |
[53] |
| CML | Tumor suppressor | Inhibition of proliferation | [54,55] |
Notch signaling appears to play a tumor suppressive role in chronic myeloid leukemia (CML). It is reported that overexpression of the active form of Notch1 or Notch2 in K562 cells resulted in the inhibition of proliferation, accompanied by increased Hes1 mRNA level [54,55]. On the other hand, attenuation of Notch signaling by overexpression of a dominant-negative RBP-J calledRBP-JR218H led to the increased proliferation of K562 cells. Moreover, activation of Notch signaling was found to inhibit the colony-forming activity of K562 cells while repression of Notch signaling played the opposite role [55]. These results provide evidence that Notch signaling might play a role as a tumor suppressor in CML.
Notch signaling in leukemia stem cells
Leukemia stem cells (LSCs) arise either from corrupted HSCs or from more differentiated and committed progenitors that acquire self-renewal potential [56-58]. Therefore, targeting this unique property of LSCs—self-renewal capacity—is thought to be a promising way to eradicate disease if one can determine which pathways are critical for LSC, but not HSC. Notch signaling is active in HSCs in vivo and downregulated as HSCs differentiated. Inhibition of Notch signaling could lead to accelerated differentiation of HSCs in vitro and depletion of HSCs in vivo[59,60]. Furthermore, Notch1 drives cell fate decision (the choice between TCRγ/δ orα/β and between CD4+ or CD8+) by inductive interactions from thymic stromal cells [61,62], suggesting that Notch1 expression is finely regulated during T-cell lineage development [63]. Notch1 is also reported to plays a role in rescuing T cells from apoptosis [64].
To date, the role of Notch signaling in LSCs has not yet been examined adequately and seems to be context dependent. Notch signaling was shown to be silenced in CD34+/CD38- stem/multipotential progenitor populations from AML patients compared to normal CD34+ stem cells. Recently, inactivating mutations of Notch signaling have been described in patients with chronic myelomonocytic leukemia (CMML) [53]. In vivo studies have also revealed both oncogenic and tumor suppressive functions for Notch signaling (Figure 1). In an MLL-AF9–induced mouse AML model, Notch signaling was inactive in CD34+/CD38- stem/progenitor cells and upregulation of Notch signaling using genetic Notch gain of function models could result in the proliferation inhibition of this populations. Moreover, in vitro activation of Notch signaling using synthetic Notch ligand led to rapid cell cycle arrest, differentiation, and apoptosis of AML-initiating cells [65]. Notch1–3−/− or Ncstn−/− mice was also found to develop an aberrant accumulation of granulocyte/monocyte progenitors (GMP), extramedullary hematopoieisis and the induction of CMML-like disease. Furthermore, ectopic expression of Notch1-IC or Hes1 could suppress the expression of key GM commitment genes such as Cebpα and Pu.1, and the CMML-like disease developing in the Ncstn−/− animals [53]. However, an oncogenic role for Notch signaling has been identified by other groups. Grieselhuber et al. reported that Jagged1 was higher in Kit+Lin-Sca1+ cells from pre-leukemic Ctsg-PML-RARA mice, and both genetic and pharmacologic inhibition of Notch signaling abrogated the enhanced self-renewal seen in hematopoietic stem/progenitor cells [43]. Taken together, increasing evidence suggests that Notch signaling is involved in the regulation of self-renewal capacity of LSCs in murine models and human disease, and point out where Notch signaling be uniquely required in leukemia.
Figure 1.
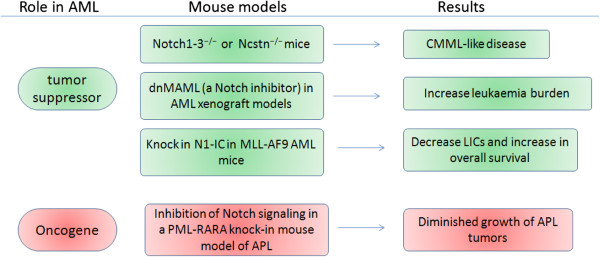
Mouse models reveal dual roles for Notch receptors. Mouse models reveal that Notch receptors either promote or inhibit AML development depending on the context.
Activation of Notch signaling by stromal microenvironment
Leukemia cell survival relies on leukemic microenvironment, which is composed of bone marrow stromal cells (BMSCs), endothelial cells and other factors. Accumulating evidence emphasized the importance of Notch signaling in the cross-talk between leukemia cells and their stromal microenvironment. BMSCs were shown to induce upregulation of Notch signaling molecules, such as Notch1, Notch3 and4 or Jagged1/2 and Dll1 [40,66]. Moreover, activation of Notch signaling by stromal microenvironment were necessary for leukemia cell survival by preventing blast cell apoptosis and favoring their reciprocal interactions and cross-talk with bone marrow microenvironment [66-68]. Our previous study reported that Notch-1 activation was induced by coculture with BMSCs and down-regulation of Notch-1 increased cocultured Jurkat cell sensitivity to chemotherapy [40,66]. Florence et al. also found that coculture of primary human T-ALL with a mouse stromal cell line expressing the Dll1 reproducibly allowed maintenance of T-LiC and long-term growth of blast cells through rescuing from apoptosis [69]. The molecular mechanisms of apoptosis resistance may be associated with a variety of cytokines, such as IL-7 [70,71], lymphocyte function-associated antigen-1 (LFA-1) and intercellular adhesion molecule-1 (ICAM-1) [71]. Inactivation of Notch signaling resulted in the decrease of leukemia cell survival, either cultured alone or cocultured in presence of stromal cells from normal donors and leukemia patients [40]. In addition, previous in vitro studies have demonstrated that endothelial cells enhance proliferation and survival of AML cells [72]. Our study showed a bidirectional cross-talk between endothelial and AML cells that had a promoting effect on endothelial cell function, and elucidated a novel mechanism by which the interplay between AML and endothelial cells promotes angiogenesis through VEGF activation of the Notch/Dll4 pathway [67].
Inhibitors of Notch signaling and the potential clinical application
The specific and profound involvement of Notch signaling in various leukemic types makes it an ideal target for pharmacological intervention. Several strategies have been proposed to inhibit or modulate this signaling [73,74]. The most widely used drug to globally inhibit Notch signaling is GSIs, which block the cleavage of Notch at the cell membrane, inhibiting release of the transcriptionally active Notch intracellular domain (NICD) subunit. A lot of clinical research or preclinical testing have focused on testing GSIs in the treatment of leukemia, but the results were initially disappointing (Table 4). It has been reported that RO4929097, one of GSIs, could induce insignificant differences in event free survival distribution compared to control in 0 of 8 (0%) of the evaluable ALL xenografts mice [75]. A phase I clinical trial also showed that MK-0752, another GSIs, had limited antitumor activity in relapsed T-ALL patients [76]. What is more, GSIs are nonspecific and can inhibit Notch signaling in the gut, leading to gastrointestinal toxicity, which also limit its application. However, in an attempt to the clinical application of GSIs, dexamethasone was found to abrogate GSI-induced toxicity in the gut and as well GSIs treatment could reverse glucocorticoid resistance in T-ALL patients [77]. Therefore, these results supported a role for combination therapy with GSIs plus glucocorticoids in the treatment T-ALL. In another attempt to remedy this issue, inhibitory antibodies have recently been synthesized for all Notch receptors. A Notch1-specific antibody significantly induced cell cycle arrest and reduced cell proliferation in T-ALL cells. Moreover, in mouse xenograft T-ALL and colon cancer models, the Notch1-specific antibody could induce significant tumor regression and slowing of growth [74], which would pave the way for new clinical trials to evaluate the efficacy of more selective and less toxic antibody-based therapies. The overwhelming potential of Notch-based cancer treatments cannot be ignored.
Table 4.
Clinical research of GSIs in the treatment of leukemia
| Test | Preclinical study | Phase I |
|---|---|---|
| Drug |
RO4929097 |
MK-0752 |
| Methods |
8 mice (leukemia models) were used in each control or treatment group. |
Six adult and two pediatric patients with leukemia (seven with T-ALL and one with AML) received MK-0752 |
| Percentages of human CD45+ cells were determined | ||
| Results |
No significance in event-free survival [53] |
Limited antitumor activity and major gastrointestinal toxicity |
| Comments | [75] | [76] |
Conclusions
Controversy will remain, as we do not understand the complexity of the Notch pathway and tools to specifically modulate the Notch pathway are still limited. Further studies assessing the levels of Notch activation and inhibition in leukemia still need to be carried out. Further advancement in understanding the molecular events of Notch signaling can potentially lead to further clinical benefit.
Competing interests
The authors declare no competing financial interests.
Authors’ contributions
All authors have contributed to data preparation, drafting and revising the manuscripts. All authors have read and approved the final manuscript.
Contributor Information
Na Liu, Email: liun2005@163.com.
Jingru Zhang, Email: zhang-jing-ru@163.com.
Chunyan Ji, Email: jichunyan@sdu.edu.cn.
References
- Capaccione KM, Pine SR. The Notch signaling pathway as a mediator of tumor survival. Carcinogenesis. 2013;34(7):1420–1430. doi: 10.1093/carcin/bgt127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Amo FF, Smith DE, Swiatek PJ, Gendron-Maguire M, Greenspan RJ, McMahon AP, Gridley T. Expression pattern of Motch, a mouse homolog of Drosophila Notch, suggests an important role in early postimplantation mouse development. Development. 1992;115(3):737–744. doi: 10.1242/dev.115.3.737. [DOI] [PubMed] [Google Scholar]
- Weinmaster G, Roberts VJ, Lemke G. Notch2: a second mammalian Notch gene. Development. 1992;116(4):931–941. doi: 10.1242/dev.116.4.931. [DOI] [PubMed] [Google Scholar]
- Lardelli M, Dahlstrand J, Lendahl U. The novel Notch homologue mouse Notch 3 lacks specific epidermal growth factor-repeats and is expressed in proliferating neuroepithelium. Mech Dev. 1994;46(2):123–136. doi: 10.1016/0925-4773(94)90081-7. [DOI] [PubMed] [Google Scholar]
- Uyttendaele H, Marazzi G, Wu G, Yan Q, Sassoon D, Kitajewski J. Notch4/int-3, a mammary proto-oncogene, is an endothelial cell-specific mammalian Notch gene. Development. 1996;122(7):2251–2259. doi: 10.1242/dev.122.7.2251. [DOI] [PubMed] [Google Scholar]
- Fehon RG, Kooh PJ, Rebay I, Regan CL, Xu T, Muskavitch MA, Artavanis-Tsakonas S. Molecular interactions between the protein products of the neurogenic loci Notch and Delta, two EGF-homologous genes in Drosophila. Cell. 1990;61(3):523–534. doi: 10.1016/0092-8674(90)90534-L. [DOI] [PubMed] [Google Scholar]
- Iso T, Kedes L, Hamamori Y. HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol. 2003;194(3):237–255. doi: 10.1002/jcp.10208. [DOI] [PubMed] [Google Scholar]
- Borggrefe T, Oswald F. The Notch signaling pathway: transcriptional regulation at Notch target genes. Cellular and molecular life sciences: CMLS. 2009;66(10):1631–1646. doi: 10.1007/s00018-009-8668-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi R, An H, Yu Y, Zhang M, Liu S, Xu H, Guo Z, Cheng T, Cao X. Notch1 signaling inhibits growth of human hepatocellular carcinoma through induction of cell cycle arrest and apoptosis. Cancer Res. 2003;63(23):8323–8329. [PubMed] [Google Scholar]
- Sjolund J, Johansson M, Manna S, Norin C, Pietras A, Beckman S, Nilsson E, Ljungberg B, Axelson H. Suppression of renal cell carcinoma growth by inhibition of Notch signaling in vitro and in vivo. J Clin Invest. 2008;118(1):217–228. doi: 10.1172/JCI32086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald F, Liptay S, Adler G, Schmid RM. NF-kappaB2 is a putative target gene of activated Notch-1 via RBP-Jkappa. Mol Cell Biol. 1998;18(4):2077–2088. doi: 10.1128/mcb.18.4.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickoloff BJ, Qin JZ, Chaturvedi V, Denning MF, Bonish B, Miele L. Jagged-1 mediated activation of notch signaling induces complete maturation of human keratinocytes through NF-kappaB and PPARgamma. Cell Death Differ. 2002;9(8):842–855. doi: 10.1038/sj.cdd.4401036. [DOI] [PubMed] [Google Scholar]
- Hales EC, Orr SM, Larson Gedman A, Taub JW, Matherly LH. Notch1 regulates AKT activation loop (T308) dephosphorylation through modulation of the PP2A phosphatase in PTEN-null T-cell acute lymphoblastic leukemia cells. J Biol Chem. 2013. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- Pajvani UB, Qiang L, Kangsamaksin T, Kitajewski J, Ginsberg HN, Accili D. Inhibition of Notch uncouples Akt activation from hepatic lipid accumulation by decreasing mTorc1 stability. Nat Med. 2013. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- Allen TD, Rodriguez EM, Jones KD, Bishop JM. Activated Notch1 induces lung adenomas in mice and cooperates with Myc in the generation of lung adenocarcinoma. Cancer Res. 2011;71(18):6010–6018. doi: 10.1158/0008-5472.CAN-11-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng AP, Millholland JM, Yashiro-Ohtani Y, Arcangeli ML, Lau A, Wai C, Del Bianco C, Rodriguez CG, Sai H, Tobias J. et al. c-Myc is an important direct target of Notch1 in T-cell acute lymphoblastic leukemia/lymphoma. Genes Dev. 2006;20(15):2096–2109. doi: 10.1101/gad.1450406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JB, Werbowetski-Ogilvie TE, Lee JH, McIntyre BA, Schnerch A, Hong SH, Park IH, Daley GQ, Bernstein ID, Bhatia M. Notch-HES1 signaling axis controls hemato-endothelial fate decisions of human embyronic and induced pluripotent cells. Blood. 2013. [Epub ahead of print] [DOI] [PubMed]
- Murai K, Philpott A, Jones PH. Hes6 is required for the neurogenic activity of neurogenin and NeuroD. PLoS One. 2011;6(11):e27880. doi: 10.1371/journal.pone.0027880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang J, Zhang L, Si M, Yin H, Li J. Diallyl trisulfide inhibits proliferation, invasion and angiogenesis of osteosarcoma cells by switching on suppressor microRNAs and inactivating of Notch-1 signaling. Carcinogenesis. 2013;34(7):1601–1610. doi: 10.1093/carcin/bgt065. [DOI] [PubMed] [Google Scholar]
- Thomas JL, Baker K, Han J, Calvo C, Nurmi H, Eichmann AC, Alitalo K. Interactions between VEGFR and Notch signaling pathways in endothelial and neural cells. Cellular and molecular life sciences: CMLS. 2013;70(10):1779–1792. doi: 10.1007/s00018-013-1312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Banerjee S, Li Y, Rahman KM, Zhang Y, Sarkar FH. Down-regulation of notch-1 inhibits invasion by inactivation of nuclear factor-kappaB, vascular endothelial growth factor, and matrix metalloproteinase-9 in pancreatic cancer cells. Cancer Res. 2006;66(5):2778–2784. doi: 10.1158/0008-5472.CAN-05-4281. [DOI] [PubMed] [Google Scholar]
- Zang S, Chen F, Dai J, Guo D, Tse W, Qu X, Ma D, Ji C. RNAi-mediated knockdown of Notch-1 leads to cell growth inhibition and enhanced chemosensitivity in human breast cancer. Oncol Rep. 2010;23(4):893–899. doi: 10.3892/or_00000712. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Nakanishi M, Rosenberg DW. Suppression of colon carcinogenesis by targeting Notch signaling. Carcinogenesis. 2013. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- Weng AP, Ferrando AA, Lee W, Morris JP, Silverman LB, Sanchez-Irizarry C, Blacklow SC, Look AT, Aster JC. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306(5694):269–271. doi: 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- Sulis ML, Williams O, Palomero T, Tosello V, Pallikuppam S, Real PJ, Barnes K, Zuurbier L, Meijerink JP, Ferrando AA. NOTCH1 extracellular juxtamembrane expansion mutations in T-ALL. Blood. 2008;112(3):733–740. doi: 10.1182/blood-2007-12-130096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neil J, Grim J, Strack P, Rao S, Tibbitts D, Winter C, Hardwick J, Welcker M, Meijerink JP, Pieters R. et al. FBW7 mutations in leukemic cells mediate NOTCH pathway activation and resistance to gamma-secretase inhibitors. J Exp Med. 2007;204(8):1813–1824. doi: 10.1084/jem.20070876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi-Ishimae M, Eguchi M, Kempski H, Greaves M. NOTCH1 mutation can be an early, prenatal genetic event in T-ALL. Blood. 2008;111(1):376–378. doi: 10.1182/blood-2007-02-074690. [DOI] [PubMed] [Google Scholar]
- van den Brandt J, Kwon SH, McPherson KG, Petrovic S, Zettl A, Muller-Hermelink HK, Reichardt HM. Unexpected features of acute T lymphoblastic lymphomas in Notch1IC transgenic rats. Eur J Immunol. 2006;36(8):2223–2234. doi: 10.1002/eji.200535791. [DOI] [PubMed] [Google Scholar]
- Lin YW, Nichols RA, Letterio JJ, Aplan PD. Notch1 mutations are important for leukemic transformation in murine models of precursor-T leukemia/lymphoma. Blood. 2006;107(6):2540–2543. doi: 10.1182/blood-2005-07-3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Li P, Lu F, Liu N, Dai J, Ye J, Qu X, Sun X, Ma D, Park J. et al. Notch1 is required for hypoxia-induced proliferation, invasion and chemoresistance of T-cell acute lymphoblastic leukemia cells. J Hematol Oncol. 2013;6:3. doi: 10.1186/1756-8722-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellavia D, Campese AF, Checquolo S, Balestri A, Biondi A, Cazzaniga G, Lendahl U, Fehling HJ, Hayday AC, Frati L. et al. Combined expression of pTalpha and Notch3 in T cell leukemia identifies the requirement of preTCR for leukemogenesis. Proc Natl Acad Sci U S A. 2002;99(6):3788–3793. doi: 10.1073/pnas.062050599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang J, Ouyang Y, Cui Y, Lin F, Ren J, Long M, Chen X, Wei J, Zhang H. Silencing of Notch3 using shRNA driven by survivin promoter inhibits growth and promotes apoptosis of human T-cell acute lymphoblastic leukemia cells. Clin Lymphoma Myeloma Leuk. 2012;12(1):59–65. doi: 10.1016/j.clml.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Kuang SQ, Fang Z, Zweidler-McKay PA, Yang H, Wei Y, Gonzalez-Cervantes EA, Boumber Y, Garcia-Manero G. Epigenetic inactivation of notch-hes pathway in human B-cell acute lymphoblastic leukemia. PLoS One. 2013;8(4):e61807. doi: 10.1371/journal.pone.0061807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothert JR, Brake RL, Smeets M, Duhrsen U, Begley CG, Izon DJ. NOTCH1 pathway activation is an early hallmark of SCL T leukemogenesis. Blood. 2007;110(10):3753–3762. doi: 10.1182/blood-2006-12-063644. [DOI] [PubMed] [Google Scholar]
- Zweidler-McKay PA, He Y, Xu L, Rodriguez CG, Karnell FG, Carpenter AC, Aster JC, Allman D, Pear WS. Notch signaling is a potent inducer of growth arrest and apoptosis in a wide range of B-cell malignancies. Blood. 2005;106(12):3898–3906. doi: 10.1182/blood-2005-01-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwabo Kamdje AH, Mosna F, Bifari F, Lisi V, Bassi G, Malpeli G, Ricciardi M, Perbellini O, Scupoli MT, Pizzolo G. et al. Notch-3 and Notch-4 signaling rescue from apoptosis human B-ALL cells in contact with human bone marrow-derived mesenchymal stromal cells. Blood. 2011;118(2):380–389. doi: 10.1182/blood-2010-12-326694. [DOI] [PubMed] [Google Scholar]
- Kannan S, Fang W, Song G, Mullighan CG, Hammitt R, McMurray J, Zweidler-McKay PA. Notch/HES1-mediated PARP1 activation: a cell type-specific mechanism for tumor suppression. Blood. 2011;117(10):2891–2900. doi: 10.1182/blood-2009-12-253419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubmann R, Hilgarth M, Schnabl S, Ponath E, Reiter M, Demirtas D, Sieghart W, Valent P, Zielinski C, Jager U. et al. Gliotoxin is a potent NOTCH2 transactivation inhibitor and efficiently induces apoptosis in chronic lymphocytic leukaemia (CLL) cells. Br J Haematol. 2013;160(5):618–629. doi: 10.1111/bjh.12183. [DOI] [PubMed] [Google Scholar]
- Hubmann R, Schwarzmeier JD, Shehata M, Hilgarth M, Duechler M, Dettke M, Berger R. Notch2 is involved in the overexpression of CD23 in B-cell chronic lymphocytic leukemia. Blood. 2002;99(10):3742–3747. doi: 10.1182/blood.V99.10.3742. [DOI] [PubMed] [Google Scholar]
- Duechler M, Shehata M, Schwarzmeier JD, Hoelbl A, Hilgarth M, Hubmann R. Induction of apoptosis by proteasome inhibitors in B-CLL cells is associated with downregulation of CD23 and inactivation of Notch2. Leukemia. 2005;19(2):260–267. doi: 10.1038/sj.leu.2403592. [DOI] [PubMed] [Google Scholar]
- Hubmann R, Duchler M, Schnabl S, Hilgarth M, Demirtas D, Mitteregger D, Holbl A, Vanura K, Le T, Look T. et al. NOTCH2 links protein kinase C delta to the expression of CD23 in chronic lymphocytic leukaemia (CLL) cells. Br J Haematol. 2010;148(6):868–878. doi: 10.1111/j.1365-2141.2009.08024.x. [DOI] [PubMed] [Google Scholar]
- Rosati E, Sabatini R, Rampino G, Tabilio A, Di Ianni M, Fettucciari K, Bartoli A, Coaccioli S, Screpanti I, Marconi P. Constitutively activated Notch signaling is involved in survival and apoptosis resistance of B-CLL cells. Blood. 2009;113(4):856–865. doi: 10.1182/blood-2008-02-139725. [DOI] [PubMed] [Google Scholar]
- Grieselhuber NR, Klco JM, Verdoni AM, Lamprecht T, Sarkaria SM, Wartman LD, Ley TJ. Notch signaling in acute promyelocytic leukemia. Leukemia. 2013;27(7):1548–1557. doi: 10.1038/leu.2013.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ma D, Ye J, Zang S, Lu F, Yang M, Qu X, Sun X, Ji C. Prognostic impact of delta-like ligand 4 and Notch1 in acute myeloid leukemia. Oncol Rep. 2012;28(4):1503–1511. doi: 10.3892/or.2012.1943. [DOI] [PubMed] [Google Scholar]
- Kannan S, Sutphin RM, Hall MG, Golfman LS, Fang W, Nolo RM, Akers LJ, Hammitt RA, McMurray JS, Kornblau SM. et al. Notch activation inhibits AML growth and survival: a potential therapeutic approach. J Exp Med. 2013;210(2):321–337. doi: 10.1084/jem.20121527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaramonte R, Basile A, Tassi E, Calzavara E, Cecchinato V, Rossi V, Biondi A, Comi P. A wide role for NOTCH1 signaling in acute leukemia. Cancer Lett. 2005;219(1):113–120. doi: 10.1016/j.canlet.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Chen PM, Yen CC, Wang WS, Lin YJ, Chu CJ, Chiou TJ, Liu JH, Yang MH. Down-regulation of Notch-1 expression decreases PU.1-mediated myeloid differentiation signaling in acute myeloid leukemia. Int J Oncol. 2008;32(6):1335–1341. doi: 10.3892/ijo_32_6_1335. [DOI] [PubMed] [Google Scholar]
- Palomero T, McKenna K, ON J, Galinsky I, Stone R, Suzukawa K, Stiakaki E, Kalmanti M, Fox EA, Caligiuri MA. et al. Activating mutations in NOTCH1 in acute myeloid leukemia and lineage switch leukemias. Leukemia. 2006;20(11):1963–1966. doi: 10.1038/sj.leu.2404409. [DOI] [PubMed] [Google Scholar]
- Etchin J, Sanda T, Mansour MR, Kentsis A, Montero J, Le BT, Christie AL, McCauley D, Rodig SJ, Kauffman M. et al. KPT-330 inhibitor of CRM1 (XPO1)-mediated nuclear export has selective anti-leukaemic activity in preclinical models of T-cell acute lymphoblastic leukaemia and acute myeloid leukaemia. Br J Haematol. 2013;161(1):117–127. doi: 10.1111/bjh.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakowski LA, Garagiola DD, Li CM, Decker M, Caruso S, Jones M, Kuick R, Cierpicki T, Maillard I, Chiang MY. Convergence of the ZMIZ1 and NOTCH1 pathways at C-MYC in acute T lymphoblastic leukemias. Cancer Res. 2013;73(2):930–941. doi: 10.1158/0008-5472.CAN-12-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatim A, Benne C, Sobhian B, Laurent-Chabalier S, Deas O, Judde JG, Lelievre JD, Levy Y, Benkirane M. NOTCH1 nuclear interactome reveals key regulators of its transcriptional activity and oncogenic function. Mol Cell. 2012;48(3):445–458. doi: 10.1016/j.molcel.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Nakamachi Y, Yakushijin K, Miyata Y, Okamura A, Kawano S, Matsuoka H, Minami H. A novel TRB@/NOTCH1 fusion gene in T-cell lymphoblastic lymphoma with t(7;9)(q34;q34) Eur J Haematol. 2013;90(1):68–75. doi: 10.1111/ejh.12019. [DOI] [PubMed] [Google Scholar]
- Klinakis A, Lobry C, Abdel-Wahab O, Oh P, Haeno H, Buonamici S, van De Walle I, Cathelin S, Trimarchi T, Araldi E. et al. A novel tumour-suppressor function for the Notch pathway in myeloid leukaemia. Nature. 2011;473(7346):230–233. doi: 10.1038/nature09999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Yang C, Zhang S, Li Y, Chen J. Notch2 inhibits proliferation of chronic myeloid leukemia cells. Oncology letters. 2013;5(4):1390–1394. doi: 10.3892/ol.2013.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin DD, Fan FY, Hu XB, Hou LH, Zhang XP, Liu L, Liang YM, Han H. Notch signaling inhibits the growth of the human chronic myeloid leukemia cell line K562. Leuk Res. 2009;33(1):109–114. doi: 10.1016/j.leukres.2008.06.023. [DOI] [PubMed] [Google Scholar]
- Jenkinson S, Koo K, Mansour MR, Goulden N, Vora A, Mitchell C, Wade R, Richards S, Hancock J, Moorman AV. et al. Impact of NOTCH1/FBXW7 mutations on outcome in pediatric T-cell acute lymphoblastic leukemia patients treated on the MRC UKALL 2003 trial. Leukemia. 2013;27(1):41–47. doi: 10.1038/leu.2012.176. [DOI] [PubMed] [Google Scholar]
- Hannon MM, Lohan F, Erbilgin Y, Sayitoglu M, O'Hagan K, Mills K, Ozbek U, Keeshan K. Elevated TRIB2 with NOTCH1 activation in paediatric/adult T-ALL. Br J Haematol. 2012;158(5):626–634. doi: 10.1111/j.1365-2141.2012.09222.x. [DOI] [PubMed] [Google Scholar]
- Ma W, Gutierrez A, Goff DJ, Geron I, Sadarangani A, Jamieson CA, Court AC, Shih AY, Jiang Q, Wu CC. et al. NOTCH1 signaling promotes human T-cell acute lymphoblastic leukemia initiating cell regeneration in supportive niches. PLoS One. 2012;7(6):e39725. doi: 10.1371/journal.pone.0039725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buontempo F, Chiarini F, Bressanin D, Tabellini G, Melchionda F, Pession A, Fini M, Neri LM, McCubrey JA, Martelli AM. Activity of the selective IkappaB kinase inhibitor BMS-345541 against T-cell acute lymphoblastic leukemia: involvement of FOXO3a. Cell Cycle. 2012;11(13):2467–2475. doi: 10.4161/cc.20859. [DOI] [PubMed] [Google Scholar]
- Tzoneva G, Ferrando AA. Recent advances on NOTCH signaling in T-ALL. Curr Top Microbiol Immunol. 2012;360:163–182. doi: 10.1007/82_2012_232. [DOI] [PubMed] [Google Scholar]
- Hasserjian RP, Aster JC, Davi F, Weinberg DS, Sklar J. Modulated expression of notch1 during thymocyte development. Blood. 1996;88(3):970–976. [PubMed] [Google Scholar]
- Washburn T, Schweighoffer E, Gridley T, Chang D, Fowlkes BJ, Cado D, Robey E. Notch activity influences the alphabeta versus gammadelta T cell lineage decision. Cell. 1997;88(6):833–843. doi: 10.1016/S0092-8674(00)81929-7. [DOI] [PubMed] [Google Scholar]
- Haydu JE, De Keersmaecker K, Duff MK, Paietta E, Racevskis J, Wiernik PH, Rowe JM, Ferrando A. An activating intragenic deletion in NOTCH1 in human T-ALL. Blood. 2012;119(22):5211–5214. doi: 10.1182/blood-2011-10-388504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelly LL, Fuchs C, Miele L. Notch-1 inhibits apoptosis in murine erythroleukemia cells and is necessary for differentiation induced by hybrid polar compounds. J Cell Biochem. 1999;73(2):164–175. doi: 10.1002/(SICI)1097-4644(19990501)73:2<164::AID-JCB3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Lobry C, Ntziachristos P, Ndiaye-Lobry D, Oh P, Cimmino L, Zhu N, Araldi E, Hu W, Freund J, Abdel-Wahab O. et al. Notch pathway activation targets AML-initiating cell homeostasis and differentiation. J Exp Med. 2013;210(2):301–319. doi: 10.1084/jem.20121484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Ye J, Li L, Dai J, Ma D, Ji C. Down-regulation of Notch-1 increases co-cultured Jurkat cell sensitivity to chemotherapy. Leuk Lymphoma. 2009;50(2):270–278. doi: 10.1080/10428190802553257. [DOI] [PubMed] [Google Scholar]
- Zhang J, Ye J, Ma D, Liu N, Wu H, Yu S, Sun X, Tse W, Ji C. Cross-talk between leukemic and endothelial cells promotes angiogenesis by VEGF activation of the Notch/Dll4 pathway. Carcinogenesis. 2013;34(3):667–677. doi: 10.1093/carcin/bgs386. [DOI] [PubMed] [Google Scholar]
- Nefedova Y, Cheng P, Alsina M, Dalton WS, Gabrilovich DI. Involvement of Notch-1 signaling in bone marrow stroma-mediated de novo drug resistance of myeloma and other malignant lymphoid cell lines. Blood. 2004;103(9):3503–3510. doi: 10.1182/blood-2003-07-2340. [DOI] [PubMed] [Google Scholar]
- Armstrong F, Brunet de la Grange P, Gerby B, Rouyez MC, Calvo J, Fontenay M, Boissel N, Dombret H, Baruchel A, Landman-Parker J. et al. NOTCH is a key regulator of human T-cell acute leukemia initiating cell activity. Blood. 2009;113(8):1730–1740. doi: 10.1182/blood-2008-02-138172. [DOI] [PubMed] [Google Scholar]
- Scupoli MT, Perbellini O, Krampera M, Vinante F, Cioffi F, Pizzolo G. Interleukin 7 requirement for survival of T-cell acute lymphoblastic leukemia and human thymocytes on bone marrow stroma. Haematologica. 2007;92(2):264–266. doi: 10.3324/haematol.10356. [DOI] [PubMed] [Google Scholar]
- Winter SS, Sweatman JJ, Lawrence MB, Rhoades TH, Hart AL, Larson RS. Enhanced T-lineage acute lymphoblastic leukaemia cell survival on bone marrow stroma requires involvement of LFA-1 and ICAM-1. Br J Haematol. 2001;115(4):862–871. doi: 10.1046/j.1365-2141.2001.03182.x. [DOI] [PubMed] [Google Scholar]
- Hatfield K, Ryningen A, Corbascio M, Bruserud O. Microvascular endothelial cells increase proliferation and inhibit apoptosis of native human acute myelogenous leukemia blasts. International journal of cancer Journal international du cancer. 2006;119(10):2313–2321. doi: 10.1002/ijc.22180. [DOI] [PubMed] [Google Scholar]
- Sharma A, Rangarajan A, Dighe RR. Antibodies against the extracellular domain of human Notch1 receptor reveal the critical role of epidermal-growth-factor-like repeats 25–26 in ligand binding and receptor activation. Biochem J. 2013;449(2):519–530. doi: 10.1042/BJ20121153. [DOI] [PubMed] [Google Scholar]
- Wu Y, Cain-Hom C, Choy L, Hagenbeek TJ, de Leon GP, Chen Y, Finkle D, Venook R, Wu X, Ridgway J. et al. Therapeutic antibody targeting of individual Notch receptors. Nature. 2010;464(7291):1052–1057. doi: 10.1038/nature08878. [DOI] [PubMed] [Google Scholar]
- Kolb EA, Gorlick R, Keir ST, Maris JM, Lock R, Carol H, Kurmasheva RT, Reynolds CP, Kang MH, Wu J. et al. Initial testing (stage 1) by the pediatric preclinical testing program of RO4929097, a gamma-secretase inhibitor targeting notch signaling. Pediatr Blood Cancer. 2012;58(5):815–818. doi: 10.1002/pbc.23290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deangelo DJ, Stone RM, Silverman LB, Stock W, Attar EC, Fearen I, Dallob A, Matthews C, Stone J, Freedman SJ, Aster J. A phase I clinical trial of the notch inhibitor MK-0752 in patients with T-cell acute lymphoblastic leukemia/lymphoma (T-ALL) and other leukemias. J Clin Oncol. 2006;24(18S) Abstr #6585. [Google Scholar]
- Real PJ, Tosello V, Palomero T, Castillo M, Hernando E, de Stanchina E, Sulis ML, Barnes K, Sawai C, Homminga I. et al. Gamma-secretase inhibitors reverse glucocorticoid resistance in T cell acute lymphoblastic leukemia. Nat Med. 2009;15(1):50–58. doi: 10.1038/nm.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]


