Abstract
Long exposure of UV radiation increases risk of skin diseases such as cancer and photoallergic reactions. UV-B (280-320 nm) radiation is mainly responsible for inducing the skin problems. Skin protection is a suitable method against ultraviolet radiation-induced damage. Various synthetic agents have been used as photo protective but because of their potential toxicity in humans, they have limited usage. Natural substances have been recently considered as potential sunscreen resources due to their absorption in the UV region and their antioxidant activity. In the present study, the UV protective effects of 20 extracts from four common medicinal plants were evaluated. Their phenol and flavonoid contents and antioxidant activities were determined and correlation between SPF and these contents were evaluated. SPFs were between 0.102 and 24.470. The highest value was reached with ultrasonic extract of Crataegus pentagyna (SPF = 24.47) followed by methanolic extract of Feijoa sellowiana (SPF = 1.30). Good correlation was found between SPF and phenolic contents (Correlation Coefficient = 0.55 and p = 0.01) but no correlations were found between SPF and flavonoid contents or antioxidant activity. These extracts can be used alone or as additives in other sun screen formulations to enhance their SPF.
Key Words: Crataegus pentagyna, Feijoa sellowiana, Sambucus ebulus, Corn silk, Sun Protection factor
Introduction
Chronic exposure of human skin to solar ultraviolet (UV) radiation may cause several skin damages. These damages include sunburn, skin cancer and oxidative stress as well as photoaging depending on the amount and form of the UV radiation and on the type of the individual exposed (1). The ultraviolet region of the electromagnetic spectrum can be divided into three regions: UVA, from 320 to 400 nm; UVB, from 290 to 320 nm and UVC, from 200 to 290 nm (2). UVC radiation is filtered by the atmosphere before reaching the earth. UVB radiation is not completely filtered out by the ozone layer and is responsible for the damages due to sunburn. UVA radiation reaches the deeper layers of the epidermis and dermis and provokes the premature aging of the skin (3).
Sunscreens are now incorporated into products such as moisturizers, creams, lotions and other hair and skin preparations. The regular use of these products may help reduce the chance of the harmful effects of ultraviolet radiation. A sunscreen’s ability to block UV-B is more important for the prevention of negative effects of sun exposure (4). However, it is necessary that a very efficient sunscreen substance is used in the cosmetic formulation. The efficacy of a sunscreen is usually expressed by the sun protection factor (SPF), which is defined as the UV energy required for producing a minimal erythema dose (MED) on protected skin, divided by the UV energy required producing a MED on unprotected skin. MED is defined as the lowest time interval or dosage of UV light irradiation sufficient for producing a minimal, perceptible erythema on unprotected skin (3). The higher the SPF, the more protection a sunscreen offers against sunburn.
There is now an increasing body of evidence that the use of sunscreen is not entirely safe for sunscreen protection (5, 6). Natural products are therefore important sources for research in new active compounds. This offers the possibility of discovering new biological mechanisms, to obtain new active molecules, and to study their structure function relationships in order to develop more active drugs and to avoid unwanted side effects. Furthermore, if the natural substance sources are common and widely occurring, it is possible to produce a high quantity at a low price (7-9). Natural substances have been recently considered as potential sunscreen resources because of their absorption in the UV region (10) and their antioxidant activity (11). Green tea polyphenols, Aloe barbadensis extract, aromatic compounds isolated from lichens, glycosides of aesculin and Murrayakoenigii leaf essential oil are examples of natural substances evaluated for their sunscreen specifications (12-16). There is strong evidence that DNA-damaging ultraviolet (UV) light induces the accumulation of UV light-absorbing flavonoids and other phenolics in dermal tissue of the plant body. This suggests physiological function, yet speculative in light protection in plants and of course in human (17). There has been an increasing interest in the use of antioxidants in sunscreens to provide supplemental photo protective action activity. Antioxidants from natural sources may provide new possibilities for the treatment and prevention of UV-mediated diseases (18, 19).
The Iranian flora is rich in medicinal plants with a high potential for providing these antioxidants. Among them, Sambucus ebulus (traditional name: Palem), Zea maize, Feijoa sellowiana and Crataegus pentagyna (traditional name: Siah-Valik)are four medicinal plants which their high antioxidant activity has been reported recently (20-23). In spite of many reports of sun screen activity from medicinal plants, there are no data available about correlation between phenol/flavonoid contents or antioxidant activity and their SPF. The goals of this research are to evaluate: 1) SPF of 20 extracts from four medicinal plants; 2) their phenol and flavonoid contents; 3) their antioxidant activities and 4) correlation between SPF and these contents.
Experimental
Plant material and preparation of freeze-dried extract
Corn Silk (dried cut stigmata of Zea maysL,) and S. ebulus, F. sellowiana and C. pentagyna fruits were obtained in summer of 2011 from Sari, Iran. The sample was authenticated by Dr. Bahman Eslami (plant systematic specialist)and the voucher specimen (No. 275-278) has been deposited in the Sari School of Pharmacy herbarium. Plant material was dried under dark condition at room temperature for 2 weeks. The dry material was milled, obtaining 2-3 mm particles and then extracted by methanol (HPLC grade, Merk, Germany) and water (Deionized, Millipore, France), separately for 24 h at room temperature. The extracts were then separated from the sample residues by filtration through Whatman No. 1 filter paper and repeated three times. The resulting extracts were concentrated over a rotary vacuum at 35-40°C until a crude solid extract was obtained which was then freeze-dried (MPS-55 Freeze-drier, Cperon, Korea) for complete solvents removal (For yields see Table 1).
Table 1.
Sun protection factor, total phenol contents, total flavonoid contents and antioxidant activities of S. ebulus, Zea maize, F. sellowiana and C. pentagyna
| Plant name/Extraction method | Extraction yield (%) | Phenol contents (GAE /g of extract) | Flavonoid contents (QE /g of extract) | Antioxidant activity (IC 50 ) |
SPF
(at 2 mg /mL) |
|---|---|---|---|---|---|
| S. ebulus | |||||
| Aqueous extract | 20.5 | 355.17 | 21.31 | 95.56 | 0.715 |
| Methanolic extract | 34.2 | 585.51 | 83.75 | 82.73 | 0.202 |
| Polyphenol fraction | 4.9 | 1374.48 | 52.28 | 86.30 | 0.428 |
| Soxhlet extract | 41 | 166.50 | 31.25 | 88.76 | 0.165 |
| Ultrasonic extract | 21.8 | 90.00 | 27.91 | 142.24 | 0.224 |
| Zea maize | |||||
| Aqueous extract | 29.9 | 212.75 | 19.78 | 77.67 | 0.269 |
| Methanolic extract | 20.4 | 264.31 | 96.25 | 73.21 | 0.377 |
| Polyphenol fraction | 3 | 875.86 | 103.68 | 91.36 | 0.417 |
| Soxhlet extract | 40.28 | 45.50 | 19.58 | 179.67 | 0.335 |
| Ultrasonic extract | 24 | 38.00 | 14.33 | 481.15 | 0.166 |
| F. sellowiana | |||||
| Aqueous extract | 16.5 | 541.72 | 24.62 | 95.23 | 0.559 |
| Methanolic extract | 10 | 375.24 | 110.31 | 92.41 | 1.298 |
| Polyphenol fraction | 2.9 | 717.24 | 60.71 | 93.87 | 0.479 |
| Soxhlet extract | 16.7 | 274.50 | 22.08 | 427.91 | 0.187 |
| Ultrasonic extract | 4.86 | 216.50 | 22.5 | 697.89 | 0.222 |
| C. pentagyna | |||||
| Aqueous extract | 24.1 | 249.13 | 12.75 | 92.77 | 0.102 |
| Methanolic extract | 24 | 186.72 | 85.31 | 50.44 | 0.123 |
| Polyphenol fraction | 3.1 | 246.89 | 70.87 | 83.92 | 0.417 |
| Soxhlet extract | 28.3 | 137.00 | 37.08 | 17.48 | 0.152 |
| Ultrasonic extract | 16.26 | 136.50 | 56.66 | 22.81 | 24.47 |
GAE: Gallic acid equivalent; QE:Quercetin equivalent.
Preparation of polyphenol fraction
Samples were extracted according to our recently published paper (23). The extractions were performed twice at 20 °C in a shaking incubator. Extracting time was 30 min and extracting solvent was 100 mL of methanol/acetone/water (3.5/3.5/3) containing 1% formic acid. All extracts were collected and evaporated under vacuum at 35-40 °C to remove methanol and acetone. Lipophilic pigments were then eliminated from the aqueous phase by extraction with petroleum ether. The aqueous phase was collected and further extracted three times by ethyl acetate. Organic phases were collected and concentrated over a rotary vacuum until a crude solid extract was obtained, which was then freeze-dried for complete solvent removal and used as polyphenol (PP) fraction (For yields see Table 1).
Ultrasonically assisted extraction
Samples were extracted with methanol in an ultrasonic bath over 1 h through indirect sonication at a frequency of 100 kHz and a temperature of 25 ± 3°C for 1 h to yield ultrasonic extracts. The extracts were then separated from the samples residue by filtration. The resulting extracts were concentrated in a rotary evaporator until crude solid extracts were obtained which were freeze-dried for complete solvent removal and used as ultrasonic (US) extracts (23) (For yields see Table 1).
Soxhlet assisted extraction
The powders of samples were extracted exhaustively in a Soxhlet extractor with methanol. The extracts were then concentrated in a rotary evaporator until the solvent was completely removed. The methanol extract was kept in a well-closed container in refrigerator until use (24).
Determination of total phenolic and flavonoid contents
Total phenolic content was measured colorimetrically using the Folin-Ciocalteu reagent (25). Results were expressed as gallic acid equivalents (GAE). The standard curve was prepared by 25, 50, 100, 200, 250, 400 and 500 μg mL-1 solutions of gallic acid in methanol: water (50:50, v/v). Each sample was analyzed in triplicate (standard curve was Y=0.0058X, R2 = 0.989).Total flavonoids were estimated using aluminum chloride method (25). Total flavonoid contents were calculated as quercetin from a calibration curve.The calibration curve was prepared by preparing quercetin solutions at concentrations 15.62 to 250 μg ml-1 in methanol (standard curve was Y=0.0064X – 0.0076, R2 = 0.999). The experiment was repeated for three times.
DPPH radical-scavenging activity
1,1-diphenyl-2-picryl hydrazyl radical (DPPH) was used for determination of free radical-scavenging activity of the extracts (26). One mL of methanolic solution of DPPH (100 μM) was added to 1 mL of different concentrations (25-800 μg/mL) of each extract. After 15 min in dark at room temperature, the absorbance was recorded at 517 nm.
Calculation of in-vitro SPF
SPF by definition is determined in-vivo as the increase in exposure time required to induce erythema, i.e. SPF 4 means four times longer to induce erythema. The most common in-vitro technique involves measuring the spectral transmittance at UV wavelengths from 280 nm to 400 nm (27). Screening of sun protection activity was measured by determination of the in-vitro SPF. The extracts were dissolved in methanol. Scanning spectra of the samples in solution were obtained by running from 337.5 to 292.5 nm (at 5 nm intervals). The SPF determination model used in this study was based on the following equation proposed by Gharavi et al. (28).
Here, T (λ) is the measured sunscreen transmittance at λ; E (λ) is the spectral irradiance of terrestrial sunlight at λ; and ε (λ) is the erythemal action spectrum at λ. The values of the E (λ) and ε (λ) were calculated according to the report by Gharavi et al. (28). T (λ) was measured three times for each extract and the mean was used for calculation of SPF. At least, five different concentrations of each extract were used for obtaining the standard curve and calculating SPF in 2 mg/mL of solution.
Statistical analysis
Data were compared with Normal Density by Kolmogorov-Simonov test and their associations were analyzed by Spearman correlation coefficient. Data were analyzed statistically by SPSS 20.The experiment was repeated for three times.
Results
The SPF is a quantitative measurement of the effectiveness of a sunscreen formulation. To be effective in preventing sunburn and other skin damages, a sunscreen product should have a wide range of absorbance between 290 and 400 nm. Evaluation of the efficiency of a sunscreen has been assessed for a long time through in-vivo test which is performed on human volunteers. The in-vitro SPF is useful for screening test during the product development, as a supplement of the in-vivo SPS measure (3).
Most spectrophotometric techniques for transmittance measurements rely on preparing samples with a uniform and known thickness so that the optical path length through the sample is standardized. Many samples are dissolved in special solvents and placed into 10 mm path length cuvettes. The cuvettes for UV spectrophotometry are usually made from quartz which is transparent to UV wavelengths (29). In the solvent method, different concentrations of test products in methanol were prepared. Each sample’s transmittance was measured to evaluate the SPF value Sunscreens containing a wide variety of chemicals that have specific absorbance in some parts of the UV spectrum. Plant extracts, due to containing a wide range of natural compounds, usually cover full range of UV wavelengths. One approach to protecting the body from the harmful effects of UV irradiation is to use active photoprotectives.
In recent years, naturally occurring compounds have gained considerable attention as protective agents (30, 31). There is at least one literature review about the photoprotective effects of some naturally occurring herbal such as polyphenols (30). Phenolics are believed to be capable of acting in redox-sensitive signaling cascades to inhibit DNA damage. The phenolics may be beneficial in preventing UV-induced oxygen free radical generation and lipid peroxidation, i.e. events involved in pathological states such as photo aging and skin cancer (17). Antioxidant activity is important in UV protection (32). It is reported that high SPF values of D. moldavicaand V. tricolor (24.79 and 25.69 respectively) may be due to their high phenolic contents (32). High concentration of flavonoids such as rutin in plants may be used to prevent UV-induced oxygen free radical generation, too (33). On the other hand, UV is highly genotoxic and many studies have unanimously mentioned that the mutations generated in critical growth control genes by the UV component of sunlight, are the first stage in skin cancer development (32, 34). To prevent such DNA damage induced by UV that penetrates into skin cells, the anti mutagens having the potentials against UV is likely to be considered. To cut down UV radiation, one possible way is using anti-mutagens which are active towards UV-induced mutation. A number of herbs and their components have been found to have the anti-mutagenic potentials with their proposed mechanisms of action (32, 35-38). Although UV radiation has some benefits, its negative effect in human health is much more. Skin cancer is one of the serious results. UV is a strong physical mutagen. Its penetration into skin cells is likely to cause gene mutation and this is believed to be the first stage in skin cancer development (39).
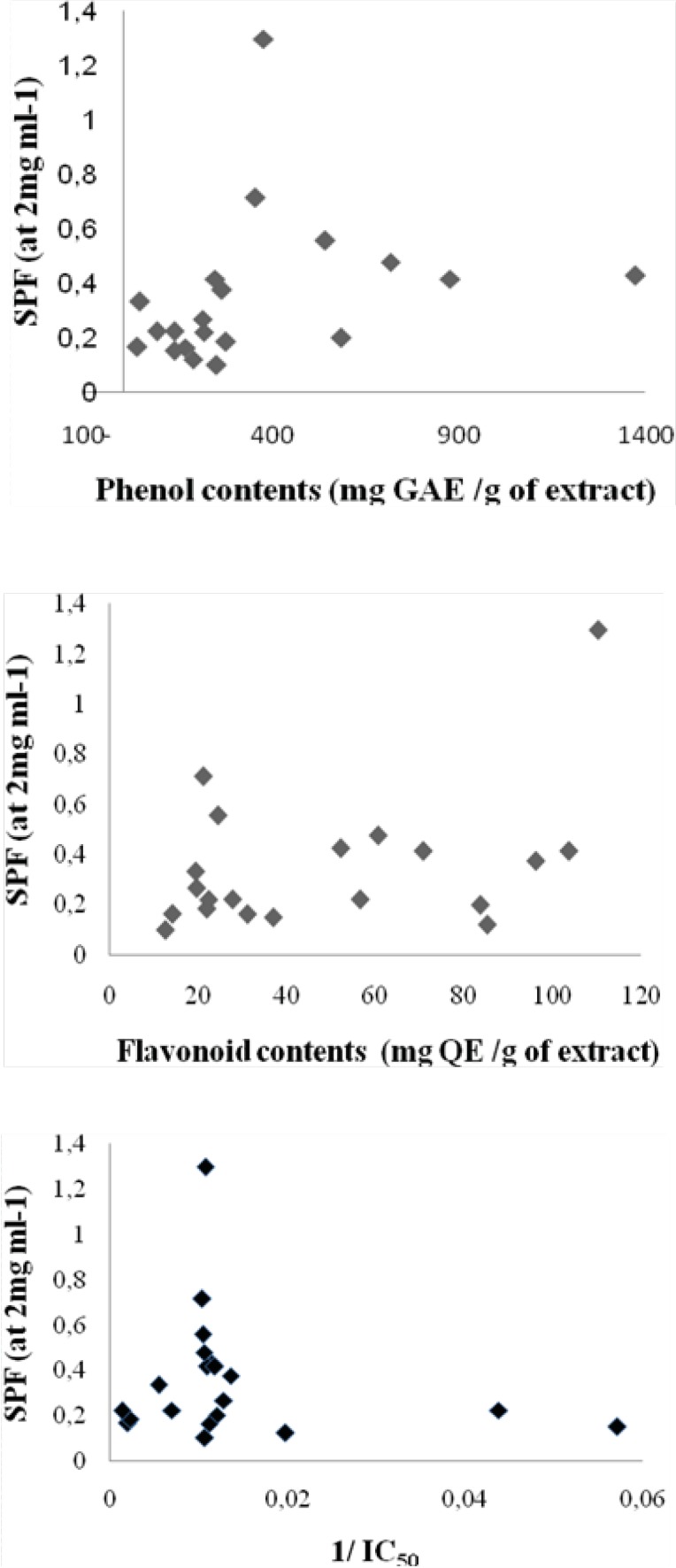
In this research, 20 extracts from four known antioxidant medicinal plants were evaluated for their SPF by UV spectrophotometry applying Gharavi mathematical equation (28). Table 1 shows the SPFs of extracts at 2 mg/mL concentrations in methanol. The protection factors were between 0.102 and 24.470. The highest value was reached using ultrasonic extract of Crataegus pentagyna (SPF = 24.47) followed by methanolic extract of Feijoa sellowiana (SPF = 1.30). Sun protective activities of the rest of the plants were low representing by their sun protection being lower than 1. Figure 1 shows correlations between SPF and phenolic contents, flavonoid contents and antioxidant activity (by DPPH test). Good correlation was found between SPF and phenolic contents (Correlation Coefficient = 0.55 and p = 0.01) but no correlations were found between SPF and flavonoid contents (Correlation Coefficient = 0.30 and p = 0.19) or antioxidant activity (Correlation Coefficient = 0.14 and p = 0.56) in tested extracts.
Figure 1.
Correlation between SPF and phenolic contents (upper), flavonoid contents (middle) and antioxidant activity (by DPPH test)(lower).
Conclusion
In this study, it was proved that ultrasonic extract of Crataegus pentagyna has high sun protection effect. This extract can be used as additives in other sun screen formulations to enhance their SPF. Good correlation was found between SPF and phenolic contents but no correlations were found between SPF and flavonoid contents or antioxidant activity.According to high SPF value of this extract, its anti-mutagenic activity should be considered.
Acknowledgment
This research was supported by a grant from Mazandaran university of Medical Sciences. Special thanks to Dr. BahmanEslami for authenticating the plant scientific name. This work was a Pharm. D. thesis.
References
- 1.Ichihashi M, Ueda M, Budiyanto A, Bito T, Oka M, Fukunaga M, Tsuru K, Horikawa T. UV-induced skin damage. Toxicol. 2003;189:21–39. doi: 10.1016/s0300-483x(03)00150-1. [DOI] [PubMed] [Google Scholar]
- 2.Mensah AY, Sampson J, Houghton PJ, Hylands PJ, Westbrook J, Dunn M. Effects of Buddlejaglobosaleaf and its constituents relevant to wound healing. J. Ethnopharmacol. 2001;77:216–221. doi: 10.1016/s0378-8741(01)00297-5. [DOI] [PubMed] [Google Scholar]
- 3.Dutra EA, Oliveira DA, Kedor-Hackmann ERM, Santoro MI. Determination of sun protection factor (SPF) of sunscreens by ultraviolet spectrophotometry. Braz. J. Pharm. Sci. 2004;40:381–385. [Google Scholar]
- 4.Thompson SC, Jolley D, Marks R. Reduction of solar keratoses by regular sunscreen use. N. Engl. J. Med. 1993;16:1147–1151. doi: 10.1056/NEJM199310143291602. [DOI] [PubMed] [Google Scholar]
- 5.Vainio H, Bianchini F. Cancer-preventive effects of sunscreens are uncertain. Scan. J. Work. Environ. Health . 2000;26:529–531. [PubMed] [Google Scholar]
- 6.Westerdahl J, Ingvar C, Masback A, Olsson H. Sunscreen use and malignant melanoma. Int. J. Cancer . 2000;87:145–150. doi: 10.1002/1097-0215(20000701)87:1<145::aid-ijc22>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Rancan F, Rosan S, Boehm K, Fernandez E, Hidalgo ME, Quihot W, Rubio C, Boehm F, Piazena H, Oltmanns U. Protection against UVB irradiation by natural filters extracted from lichens. J. Photochem. Photobiol. B . 2002;68:133–139. doi: 10.1016/s1011-1344(02)00362-7. [DOI] [PubMed] [Google Scholar]
- 8.Farasat M, Khavari-Nejad RA, Baghernabavi SM, Namjooyan F. Antioxidant activity, total phenolic and flavonoid contents of some edible green seaweeds from northen coasts of the persian gulf. Iran. J. Pharm. Res. 2014;13:163–170. [PMC free article] [PubMed] [Google Scholar]
- 9.Mirshafa SA, Azadbakht M, Ahangar N. Study of antidipressant and sedative-hypnotic activity of hydroalcholic extract of asperugo procumbens L. aerial parts in mice. Iran. J. Pharm. Res. 2013;12:529–535. [PMC free article] [PubMed] [Google Scholar]
- 10.Liu MC, Lin CT, Shau MD, Chen ZS, Chen MT. Studies on natural ultraviolet absorbers. J. Food Drug Anal. 1996;4:243–248. [Google Scholar]
- 11.Bonina F, Lanza M, Montenegro L, Puglisi C, Tomaino A, Trombetta D. Flavonoids as potential protective agents against photooxidative skin damage. Int. J. Pharm. 1996;145:87–91. [Google Scholar]
- 12.Urbach F. The historical aspects of sunscreens. J. Photochem. Photobiol. B . 2001;64:99–104. doi: 10.1016/s1011-1344(01)00202-0. [DOI] [PubMed] [Google Scholar]
- 13.Elmets CA, Young C. Sunscreens and photocarcinogenesis: An objective assessment. J. Photochem. Photobiol. B . 1996;63:435–440. doi: 10.1111/j.1751-1097.1996.tb03065.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang ZY, Agarwal R, Bickers DR, Mukhtar H. Protection against ultraviolet B radiation-induced photocarcinogenesis in hairless mice by green tea polyphenols. Carcinogenesis . 1991;68:133–139. doi: 10.1093/carcin/12.8.1527. [DOI] [PubMed] [Google Scholar]
- 15.Rancan F, Rosand S, Boehm K, Fernandez E, Hidalgo ME, Quihot W, Rubio C, Boehm F, Piazena H, Oltmanns U. Protection against UVB irradiation by natural filters extracted from lichens. J. Photochem. Photobiol. B . 2002;68:133–139. doi: 10.1016/s1011-1344(02)00362-7. [DOI] [PubMed] [Google Scholar]
- 16.Patil RB, Kale Sh, Badiyani DM, Yadav AV. Determination of invitrosun protection factor (SPF) of MurrayaKoenigiiL. (Rutaceae) essential oil formulation. Indian J. Pharm. Educ. Res. 2010;44:375–379. [Google Scholar]
- 17.Strack D. Phenolic Metabolism. In: Dey PM, Harborne JB, editors. Plant Biochemistry. London: Academic Press; 1997. pp. 388–392. [Google Scholar]
- 18.Bonina F, Lanza M, Montenegro L, Puglisi C, Tomaino A, Trombetta D, Castelli F, Saija A. Flavonoids as potential protective agents against photo-oxidative skin damage. Int. J. Pharm. 1996;145:87–91. [Google Scholar]
- 19.Fguyer S, Afaq F, Mukhtar H. Photochemoprevention of skin cancer by botanical agents. Photodermatol. Photoimmunol. Photomed. 2003;19:56–72. doi: 10.1034/j.1600-0781.2003.00019.x. [DOI] [PubMed] [Google Scholar]
- 20.Ebrahimzadeh MA, Hosseinimehr SJ, Hamidinia A, Jafari M. Antioxidant and free radical scavenging activity of Feijoa sallowiana fruits peel and leaves. Pharmacol. 2008;1:7–14. [Google Scholar]
- 21.Ebrahimzadeh MA, Pourmorad F, Hafezi S. Antioxidant activities of Iranian Corn Silk. Turk. J. Biol. 2008;32:43–49. [Google Scholar]
- 22.Ebrahimzadeh MA, Ehsanifar S, Eslami B. Sambucusebuluselburensis fruits: A good source for antioxidants. Pharmacog. Mag. 2009;4:213–218. [Google Scholar]
- 23.RabieiKh , Bekhradnia S, Nabavi SM, Nabavi SF, Ebrahimzadeh MA. Antioxidant activity of polyphenol and ultrasonic extracts from fruits of Crataeguspentagyna subsp. elburensis. Nat. Pro. Res. 2012;26:2353–2357. doi: 10.1080/14786419.2012.658799. [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Li X. Antioxidant activity of Durian (DuriozibethinusMurr.) shell in-vitro. Asian J. Pharm. Biol. Res. 2011;1:542–551. [Google Scholar]
- 25.Ghasemi K, Ghasemi Y, Ebrahimzadeh MA. Antioxidant activity, phenol and flavonoid contents of 13 Citrus specis peels and tissues. Pak. J. Pharm. Sci. 2009;22:277–281. [PubMed] [Google Scholar]
- 26.Dehpour AA, EbrahimzadehMA , Nabavi SF, Nabavi SM. Antioxidant activity of methanol extract of Ferula assafoetida and its Essential oil composition. Grasas Aceites . 2009;60:405–412. [Google Scholar]
- 27.Diffey BL, Robson J. A new substrate to measure sunscreen protection factors throughout the ultraviolet spectrum. J. Soc. Cosmet. Chem. 1989;40:127–133. [Google Scholar]
- 28.Gharavi SM, Tavakoli N, Pardakhti A, BaghaeiZadeh N. Determination of sun protection factor of sunscreens by two different in-vitro methods. J. Res. Med. Sci. 1999;2:53–54. [Google Scholar]
- 29.Springsteen A, Yurek R, Frazier M, Carr KF. In-vitro measurement of sun protection factor of sunscreens by diffuse transmittance. Anal. Chim. Acta . 1999;380:155–164. [Google Scholar]
- 30.Svobodov A, Psotov J, Walterov D. Natural phenolic in the prevention of UV-induced skin damage, a review. Biomed. Papers. 2003;147:137–145. [PubMed] [Google Scholar]
- 31.Khazaeli P, Mehrabani M. Screening of sun protective activity of the ethyl acetate extracts of some medicinal plants. Iran. J. Pharm. Res. 2008;7:5–9. [Google Scholar]
- 32.Kittiwannachot P, Borisut P, Wanasawas P, Ponpanich L, Rattanasuk O, Chulasiri M. Antimutagenic potentials of hydroalcoholicherbal extracts towards UV-induced mutation. Thai. J. Toxicol. 2008;23:27–34. [Google Scholar]
- 33.Leung AY, Foster S. Encyclopedia of Common Natural Ingredients, Used in Foods, Drugs, and Cosmetics. 2nd ed. New York: John Wiley & Sons Inc.; 1996. p. 452. [Google Scholar]
- 34.deGruijl FR. P53 mutations as a marker of skin cancer risk: comparison of UVA and UVB effects. Exp. Dermatol. 2002;11:37–39. doi: 10.1034/j.1600-0625.11.s.1.9.x. [DOI] [PubMed] [Google Scholar]
- 35.Grover IS, Bala S. Studies on antimutagenic effects of guava (Psidiumguajava) in Salmonella typhimurium. Mutat. Res. 1993;300:1–3. doi: 10.1016/0165-1218(93)90133-x. [DOI] [PubMed] [Google Scholar]
- 36.Miyazawa M, Okuno Y, Fukuyama M, Nakamura S, Kosaka H. Antimutagenic activity of polymethoxyflavanoids from Citrus aurantium. J. Agric. Food Chem. 1999;47:5239–5244. doi: 10.1021/jf990176o. [DOI] [PubMed] [Google Scholar]
- 37.Kinae N, Masuda S. Studies on antimutagenesis (a review) Environ. Mutagen. Res. 2002:129–144. [Google Scholar]
- 38.Matsuo T, Hanamura N, Shimoi K, Nakamura Y, Tomita I. Identification of (+) – gallocatechin as a bioantimutagenic compound in Psidium guava leaves. Phytochem. 1994;36:1027–1029. doi: 10.1016/s0031-9422(00)90484-9. [DOI] [PubMed] [Google Scholar]
- 39.Matsumura Y, Ananthaswamy HN. Toxic effects of ultraviolet radiation on the skin. Toxicol. Appl. Pharmacol. 2004;195:298–308. doi: 10.1016/j.taap.2003.08.019. [DOI] [PubMed] [Google Scholar]