Abstract
Background
The sensitivity of rapid influenza diagnostic test (RIDT) of children with influenza-like illness (ILI) remains low.
Objective
We compare the parameters between pandemic A(H1N1) 2009 influenza with negative RIDT and ILI not H1N1 for improving the low sensitivity of RIDT for children with ILI.
Methods
In a cohort of consecutive laboratory-confirmed H1N1 influenza, we identified 150 H1N1 children with positive RIDT, 152 H1N1 children with negative RIDT, and 75 children with ILI not H1N1. Viral load in throat, complete blood count (CBC), and C-reactive protein (CRP) levels between H1N1 children with negative RIDT and children with ILI not H1N1 were assessed.
Results
The diagnostic sensitivity of the RIDT was 45·5%. An analysis of CBC and CRP levels indicated that H1N1 children with negative RIDT had lower total leukocyte, neutrophil, lymphocyte, and basophil counts, and serum CRP levels (P < 0·01). Lymphocyte counts less than 1500 cells/mm3 and CRP levels <15 mg/l, determined by a receiver operating characteristic curve, showed a diagnostic sensitivity of 52·5% and 80·7%, respectively. Combining the lymphocyte counts and CRP levels provided a diagnostic sensitivity of 91·5%. Moreover, H1N1 children with negative RIDT had a lower viral load than those with positive RIDT (3·33 versus 4·48 log10 copies/ml, P < 0·001); the viral load was negatively correlated to the lymphocyte count (P < 0·001).
Conclusions
A combination of a low lymphocyte count and a low CRP level could, in the early disease phase, provide a useful screening for H1N1 children with false-negative RIDT, potentially facilitating differential diagnoses.
Keywords: Children, diagnostic tests, pandemic A (H1N1) 2009 influenza, sensitivity and specificity
Introduction
The swine-origin pandemic A(H1N1) 2009 influenza virus (hereafter referred to simply as H1N1) was first recognized around the border area of Mexico and the United States in April 20091 and has since caused significant morbidity and mortality worldwide.2 The H1N1 pandemic began at the end of the annual human influenza A season; other respiratory viruses that cause influenza-like illness (ILI) also existed in the community. Rapidly distinguishing pandemic A(H1N1) influenza from other febrile illnesses is difficult.3 To control the spread of H1N1, the Taiwan Centers for Disease Control (CDC) provided antiviral medication, mainly oseltamivir, for all symptomatic patients with laboratory evidence of influenza, including a positive rapid influenza diagnostic test (RIDT). Under these circumstances, the RIDT was widely used in private and general clinics in Taiwan.4
A rapid influenza diagnostic test is mostly based on antigen detection and provides results within 15-30 minutes.5 This provides a screening test in a clinically relevant time frame that complements the use of antiviral medication and interrupts transmission. The US Food and Drug Administration approved more than 10 RIDTs. Most of them identify and distinguish between influenza A and B viruses.5 However, the RIDT frequently returns false negatives, and the sensitivity of these assays varies considerably (22–84%),6–8 with sensitivity dropping to below 20% in cases with severe pneumonia.9 These figures stress the necessity of obtaining a real-time reverse transcription polymerase chain reaction (rRT-PCR) diagnosis for all potential influenza patients, including those suspected of infection with H1N1. However, rRT-PCR test was unavailable in outpatient and private clinics, which hampered infection control efforts and the provision of appropriate recommendations for influenza precautions and antiviral regimens, stressing the necessity for the a means to obtain an early diagnosis of influenza for children with negative RIDT. This study attempts to establish a simplified set of laboratory clues for an early diagnosis of ILI children with negative RIDT.
Methods
Study design and subjects
As part of the preparedness for a possible nationwide epidemic of H1N1 in Taiwan in July 2009, our hospital launched a screening program in 2009 for H1N1 for patients with ILI, and patients with fever who lacked obvious localized symptoms that suggested an alternative diagnosis.10 ILI was defined according to the World Health Organization (WHO) guidelines as a fever (≧38·0°C), cough, and sore throat.11 A total of 856 children with ILI symptoms in fever clinic who had received both a RIDT and an rRT-PCR test between July and October of 2009 were included for retrospective analyses, to obtain the sensitivity and specificity of the RIDT for children with ILI. Respiratory specimens from throat swabs were collected by pediatricians. To obtain a specimen, the physician depressed the tongue with a tongue depressor, and a swab was inserted between the tonsillar pillars behind the uvula, which were swabbed back and forth across the posterior pharynx. The RIDT in a transport tube was performed immediately after sampling. A diagnosis of H1N1 was made when the H1N1-specific RNA was observed in the respiratory specimen (throat swab) of the patient, and the rRT-PCR test was positive, based on the recommendations by the CDC. During the epidemic containment period, all children with positive RIDTs or rRT-PCR were prescribed oseltamivir treatment for 5 days, which was provided by the CDC; these costs were underwritten by the Taiwan National Health Insurance program. The decision to perform laboratory testing, including a complete blood count (CBC) and C-reactive protein (CRP) test, was at the discretion of the treating physician.
Laboratory tests were performed on 337 (43%) of 781 rRT-PCR-confirmed children with H1N1, including 152 children with negative RIDTs and 185 children with positive RIDTs. In this study, we focus on the H1N1 children with negative RIDT. Therefore, we included overall 152 cases of H1N1 children with negative RIDT for whom a CBC and CRP had been performed. In term of having a homogeneous control group of patients to study, we used random-number generators to sample 150 age-matched H1N1 children with positive RIDT and 75 age-matched ILI children not H1N1 for controls because the CBC counts could vary among different age groups. Figure 1 presents the patient inclusion process. Various clinical features among the 377 H1N1 children who had performed the measurement of CBC and CRP levels were subjected to a comparison of H1N1 children with positive (n = 150), negative RIDT (n = 152), and ILI children not H1N1 (n = 75). Children confirmed to have H1N1 by rRT-PCR were classified into groups based on positive and negative RIDT, which were obtained using Puritan flu swabs (Puritan Medical Products Co., Guilford, Maine). The medical charts of the included patients were reviewed for their demographic, clinical, and laboratory information to conduct analyses.
Figure 1.
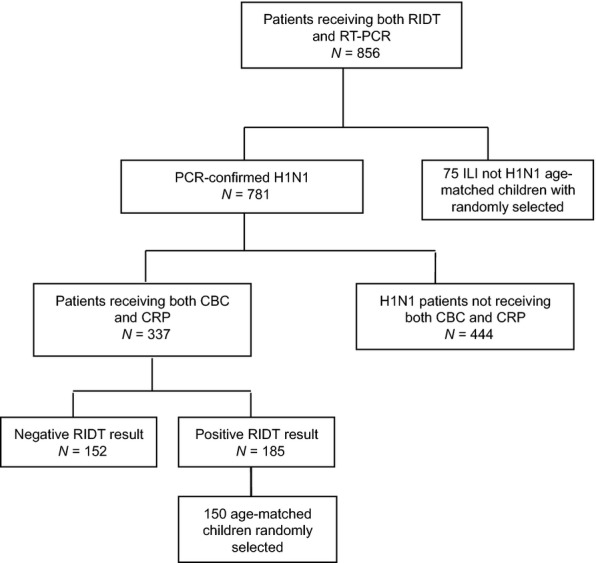
Flow chart of patients inclusion. RIDT, rapid influenza diagnostic test; RT-PCR, reverse transcription polymerase chain reaction.
Twenty-two children (11 boys and 11 girls; aged 6·7 ± 1·0 years) who had been admitted to the hospital and received 3 CBC tests were subjected to analysis for kinetic changes of CBC while they were ill with H1N1. This respective study used a waiver of patient consent that was approved by the Institutional Review Board (IRB) of Chang Gung Memorial Hospital, Taiwan (CGMH-IRB 98-2699B).
Confirmation and quantification of Pandemic A (H1N1) 2009 Influenza
Total nucleic acids from throat (tonsillopharyngeal) swabs suspended in 250 μl of a cultured medium were extracted using a MagNA Pure instrument (Roche Molecular Diagnostics, Mannheim, Germany) according to the manufacturer's external lysis protocol and with extraction reagents (total nucleic acid isolation kit; Roche Molecular Diagnostics) yielding 100 μl of the eluate. All PCR assays were performed using standard CDC-recommended procedures (CDC, Atlanta, GA, USA) for RT-PCR of H1N1, in which negative template controls (NTC) and human specimen controls (HSC) were conducted for negative controls, and various concentrations of positive template controls (PTC) were included as positive controls.9 As described,12 the RT-PCR was performed using a 7500 Sequence Detection System (PRISM, Applied Biosystems, Foster City, CA, USA) under the following conditions: reverse transcription at 50°C for 30 minutes and Taq inhibitor inactivation at 95°C for 2 minutes, followed by 45 cycles at 95°C for 15 seconds and 55°C for 30 seconds.
Calculation and statistics
Data were presented as the mean ± standard error. Statistical comparisons of CBC and CRP levels were tested using analysis of variance (anova) and were followed by post hoc tests for comparison between continuous variables; the χ2 test was used to assess differences between dichotomous variables. The Spearman's correlation was used to determine the association between the viral load and the differential count. Receiver operating characteristic (ROC) curves were plotted for single hematological and biochemical measurements. The diagnostic accuracy of single and combined measurements was assessed by calculating the area under ROC curves to obtain maximal sensitivity and specificity.
We calculated the sensitivity, specificity, positive predictive values (PPV), and negative predictive values (NPV) for positive and negative test results. A P value of <0·05 was considered statistically significant. All statistical tests were performed using spss statistical software, version 13.0 for Windows XP (SPSS Inc., Chicago, IL, USA).
Results
Low sensitivity and high specificity of RIDT in children with pandemic A(H1N1) influenza
In a series testing 856 children with ILI, 781 rRT-PCRs indicated H1N1 infection. Of the included children, the median age was 9·45 years (ranging from 0·08 to 18·0 years). More than half (61·5%) the ILI patients were boys (n = 527), and 37 (4·3%) children had at least one underlying disease or comorbid condition, including asthma (21 children), cardiac disease (10 children), and vesicoureteral reflux (2 children), hydronephrosis (1 child), and febrile convulsion (1 child). The mean interval from symptom onset to hospital presentation was 1·3 days. A total of 137 (16%) children were admitted to the hospital. Twelve (1·4%) children had pneumonia as defined by the presence of patchy alveolar opacities on a chest radiography. Thirteen children had received oseltamivir therapy before respiratory specimens were performed. Of all the children, 389 had positive RIDT results, and 467 had negative RIDT results. The overall sensitivity of the RIDT for H1N1 was 45·5%. The specificity of the RIDT was calculated as the number of patients without H1N1 with a negative RIDT result divided by the number of patients without H1N1 infection tested by rRT-PCR. The specificity was 96·5%. The capacity of a negative RIDT result to exclude H1N1 infection can be expressed by the NPV, which measures the probability that a patient with a negative test result is actually free of the disease. In our study during the epidemic containment period in Taiwan, the NPV of the RIDT in children was as low as 16·6%; however, the PPV was 99·1%, as shown in Table 1.
Table 1.
Diagnostic accuracy of pandemic A (H1N1) 2009 virus infection by RIDT using rRT-PCR as the reference
| Characteristic | n | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|
| Overall | 781 | 45·5 | 96·5 | 99·1 | 16·6 |
| Age (years) | |||||
| <3 | 60 | 43·9 | 100 | 100 | 46·3 |
| 3–6 | 113 | 47·9 | 100 | 100 | 27·9 |
| 6–12 | 375 | 44·1 | 89·5 | 98·7 | 7·9 |
| 12–18 | 233 | 48·6 | 95·7 | 99·0 | 16·9 |
PPV, positive predictive value; NPV, negative predictive value.
To evaluate the RIDT among infected children across different age groups, we separated our study population into 4 age groups. We found that the sensitivity of the RIDT was not significantly different among children of different age groups.
H1N1 children with negative RIDT have lower viral loads and higher lymphocyte counts than those with positive RIDT
Among 302 rRT-PCR determined H1N1 cases of children for whom CBC and CRP tests had been performed, the median age was lower than that of the overall cohort (8·10 versus 9·45 years), and 175 (57·9%) of the H1N1 children were boys and were more likely to have an underlying condition than the overall cohort (7·8% versus 4·3%). A total of 95 (32%) children were admitted to the hospital. The average time to confirm H1N1 diagnosis was 1·57 ± 0·07 days after disease onset. Among the 302 H1N1 children, the following coinfections were detected: parainfluenza (1 child), enterovirus (1 child), mycoplasma (2 children), pneumococcus (1 child), scarlet fever with group A streptococcus (1 child), and adenovirus (1 child).
To identify the possible mechanisms causing the low sensitivity of the RIDT, we analyzed the CBC, serum CRP levels in the blood and the viral load in the throat, as determined by the rRT-PCR in laboratory-confirmed H1N1 children with negative (n = 152) and positive RIDT (n = 150). We found that H1N1 children with negative RIDT had a significantly lower mean viral load than those with positive RIDT (3·25 versus 4·48 log10 copies/ml, P < 0·001; Figure 2) and had a significantly higher lymphocyte count than those with positive RIDT during the acute phase of the disease (1341 ± 77 versus 1667 ± 103 cells/mm3; P = 0·025). Further analysis indicated that lymphopenia was inversely correlated to the viral load (P < 0·001). However, no significant differences were observed between H1N1 children with negative RIDT and those with positive RIDT for age, sex, and other hematological or biological measurements, such as total leukocyte, neutrophil, monocyte, eosinophil, and basophil counts, and serum CRP levels.
Figure 2.
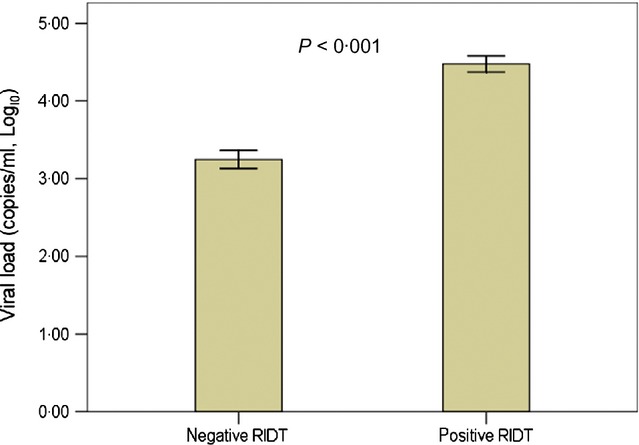
Viral load in pandemic A (H1N1) 2009 children with negative and positive RIDTs (data shown are mean and standard error)
Differences of laboratory testing between H1N1 children with negative RIDT and children with ILI not H1N1
In an attempt to identify the clinical characteristics of H1N1 children with negative RIDT, we included overall 152 H1N1 children with RIDT and analyzed the general hematological and biochemical examinations compared to 75 eligible children with ILI not H1N1 in the fever clinic on the same day. The demographic data, including age and sex, and underlying conditions were similar between H1N1 children with negative RIDT and those with ILI not H1N1, as shown in Table 2. H1N1 children with negative RIDT were more likely to have significantly lower total leukocyte (P < 0·001), neutrophil (P = 0·004), lymphocyte (P = 0·001), and basophil counts (P = 0·003), and serum CRP levels (P < 0·001) than children with ILI not H1N1 during the acute phase of the disease (Table 3).
Table 2.
Demographic characteristic and comorbidities in H1N1 children with negative RIDT and children with influenza-like illness (ILI) not H1N1 using rRT-PCR as the reference
| Negative RIDT n = 152, No. (%) | ILI not H1N1 n = 75, No. (%) | P-value | |
|---|---|---|---|
| Male gender | 87 (57) | 48 (64) | 0·329 |
| Age, year | 8·0 ± 0·4 | 7·1 ± 0·6 | 0·201 |
| Any one comorbid conditions | 12 (8) | 9 (12) | 0·315 |
| Asthma | 9 | 2 | |
| Cardiac disease | 2 | 5 | |
| Febrile convulsion | 0 | 1 | |
| Urologic anomalies | 1 | 1 | |
| Hospitalization | 43 (28) | 25 (33) | 0·435 |
rRT-PCR, real-time reverse transcriptase polymerase chain reaction.
Table 3.
Laboratory features presented by H1N1 children with negative RIDT and children with influenza-like illness (ILI) not H1N1 using rRT-PCR as the reference
| Negative RIDT (n = 152) | ILI not H1N1 (n = 75) | P-value | |
|---|---|---|---|
| Total leukocyte count, cells/mm3 | 7 228 ± 274 | 9 168 ± 512 | <0·001 |
| Neutrophil count, cells/mm3 | 4 978 ± 236 | 6 181 ± 470 | 0·004 |
| Lymphocyte count, cells/mm3 | 1 667 ± 103 | 2 244 ± 166 | 0·001 |
| Monocyte count, cells/mm3 | 571 ± 27 | 588 ± 42 | 0·706 |
| Eosinophil count, cells/mm3 | 61·1 ± 7·7 | 85·5 ± 23·8 | 0·230 |
| Basophil count, cells/mm3 | 15·5 ± 1·8 | 24·2 ± 3·1 | 0·003 |
| Serum CRP levels, mg/l | 10·6 ± 1·2 | 25·7 ± 4·6 | <0·001 |
rRT-PCR, real-time reverse transcriptase polymerase chain reaction.
Predictions of A(H1N1) 2009 influenza in children with negative RIDT based on laboratory testing
To determine a useful clinical marker for predicting H1N1 infection despite a false-negative RIDT, we further evaluated conventional hematological and biochemical measurement combinations, as well as the RIDT for the diagnosis of H1N1 infection by using an ROC curve in consideration of relative insensitively of RIDT (45·5%). As shown in Table 4, a leukocyte count lower than 6250 cells/mm3 was the single best variable for distinguishing ILI from H1N1 with a specificity of 79·7%; however, an NPV as low as 46·8% indicated that it could generate small changes in posttest probabilities. Although a lymphocyte count lower than 1500 cells/mm3 had a higher sensitivity at 52·5% than leukocyte counts lower than 6250 cells/mm3 (44·2%) for predicting H1N1 infection, the specificity was lower in H1N1 children with false-negative RIDT (58·9% versus 79·7%). CRP levels below 15 mg/l were the single most sensitive variable for screening for actual H1N1 in children, yielding a sensitivity of 80·7%, although the specificity was only 43·7%.
Table 4.
Diagnostic sensitivity and specificity of pandemic (H1N1) 2009 virus infection in children with negative RIDT according to CBC and CRP levels using rRT-PCR as the reference
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|
| Leukocyte <6250 cells/mm3 | 44·2 | 79·7 | 77·9 | 46·8 |
| Lymphocyte <1500 cells/mm3 | 52·5 | 58·9 | 67·4 | 43·4 |
| Basophil <12 cells/mm3 | 55·5 | 65·3 | 72·5 | 47·0 |
| CRP <15 mg/l | 80·7 | 42·3 | 70·1 | 56·6 |
| Lymphocyte <1500 cells/mm3 and CRP <15 mg/l | 91·5 | 21·4 | 66·0 | 60·0 |
rRT-PCR, real-time reverse transcriptase polymerase chain reaction; PPV, positive predictive value; NPV, negative predictive value.
The finding in combination of lymphocyte count less than 1500 cells/mm3 and CRP levels <15 mg/l generated a sensitivity of 91·5% and a specificity of 21·4% for differentiating H1N1 infection from ILI not H1N1. Moreover, the NPV for this combination in children with negative RIDT increased significantly over the NPV for RIDT alone (60·0% versus 16·6%). These results markedly improved the sensitivity and the NPV of diagnosis for H1N1 children with false-negative RIDT.
Kinetic changes of CBC during the course of A (H1N1) 2009 influenza infection
We performed an analysis on the kinetic CBC and differential count for 22 children with H1N1 by using 3 consecutive tests over 7 days.
As shown in Figure 3, the counts of total leukocyte, neutrophil, and monocyte decreased to the lowest level in 2–4 days after disease onset (Figures 3A,B, respectively). However, lymphocyte counts were lowest in the early phase of H1N1 infection and then returned to baseline after 5 to 7 days (P = 0·016; ANOVA; Figure 3B). A significant increase was observed in absolute eosinophil counts over the seven-day course of infection (P = 0·041; Figure 3C). However, the basophil counts and CRP levels of patients with H1N1 showed no significant changes during the entire monitoring process (P > 0·05).
Figure 3.
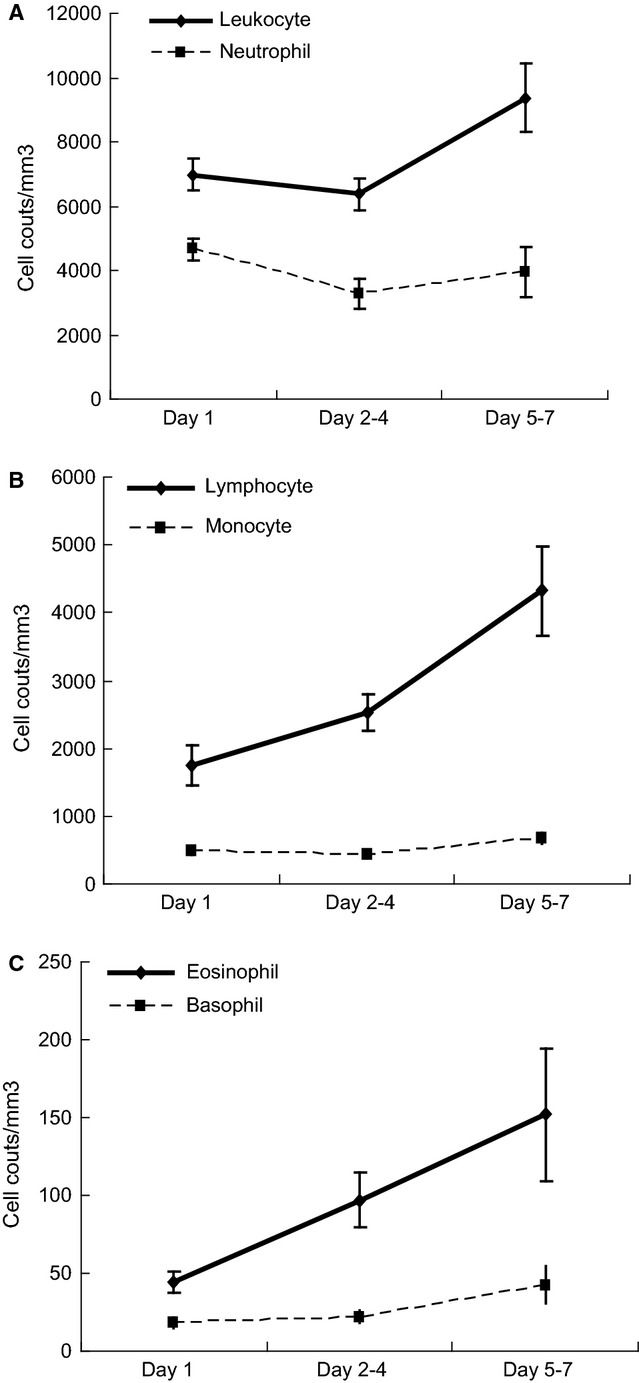
Kinetic changes in (A) total leukocyte and neutrophil counts (B) total lymphocyte and monocyte counts, (C) basophil and eosinophil counts in laboratory-confirmed H1N1 children. (data shown are mean and standard error)
Discussion
The sensitivity of RIDTs for the diagnosis of influenza in children is known to be less than 50%.6,13 Previous studies have demonstrated that RIDTs have a relatively low sensitivity in detecting severe cases of H1N1 infection; therefore, a cautious interpretation of negative RIDT results is particularly warranted in this patient population.14 This study confirmed that the sensitivity of RIDTs in a series of 856 throat swab samples from children was 45·5%, although the specificity was greater than 95%. We attempted to differentiate H1N1 from ILI not H1N1 by using CBC and CRP levels because they are routine tests for the early assessment of childhood febrile illnesses. Our analysis determined that a combination of 2 simple clinical laboratory clues, lymphopenia (<1500 cells/mm3) and lower CRP levels (<15 mg/l) provided a sensitivity of up to 91·5% and a specificity of 21·4% in ILI children; therefore, they are useful screening tools during influenza infection epidemics.
Numerous studies have demonstrated specific features in patients with influenza infections that differentiate them from patients with ILI. Symptomatic predictors of influenza have been examined using surveys and clinical trials and in practice settings. Brouqui et al.15 demonstrated that the absence of a cough distinguished a diagnosis of H1N1 infection in patients from those with ILI. Chan et al.16 indicated that patients with pandemic H1N1, compared to other viruses, were more likely to present with a fever, cough, sore throat, nausea, vomiting, abdominal pain, and a lower leukocyte count. Moreover, a history of contact with confirmed H1N1 patients, in addition to headaches, high body temperature, and coryza, were 4 predictors of positive rRT-PCR test results.17 In contrast, certain studies have indicated that common clinical symptoms of H1N1, including cough (present in 96% of patients), myalgia or arthralgia (present in 57% of patients), and sore throat (51%), are indistinguishable from those of other viral respiratory illnesses.18 The sensitivity of symptomatic predictors for influenza varies, depending on numerous factors, including the prevalence of the disease, age, underlying illnesses, the duration of symptoms prior to consultation, and the vaccination rate in the tested population. Thus, the use of symptomatic predictors should be limited.
In this study, we found that the RIDT had relatively a low sensitivity (45·5%) but excellent specificity (96·5%) for the detection of H1N1 infection; these results are consistent with other studies.6,7 Because of the low sensitivity and NPV (16·6%) of RIDT, we conducted additional evaluations to identify laboratory predictors for patients with negative results of RIDT. We found that a combination of the lymphocyte count and CRP level was more accurate than other laboratory tests or test combinations for predicting H1N1 infection in children with negative RIDT results (91·5% sensitivity). This combination also provides a better NPV for children with ILI than RIDT alone (60·0% versus 16·6%).
Factors that can impact the sensitivity of the RIDT include specimen collection techniques,11 age,19 and viral titers.20 Andresen et al.8 showed that specimen collection by nasopharyngeal aspirates had a higher RIDT sensitivity than those obtained by throat swabs (84% versus 66%). We were unable to compare the RIDT performance among specimen types because the specimens included in this study were all throat swabs. Although data show that RIDT sensitivity decreases significantly with increasing age,19 no significant differences were found among children of varying age in our study. Our results are consistent with other studies that have found children with H1N1 and negative RIDT to have lower viral loads compared to those with positive RIDT.20
Various viruses including the Epstein–Barr virus, cytomegalovirus, and certain types of respiratory viral infections cause lymphocytosis or neutropenia in children.21,22 However, lymphopenia is a common feature of H1N1 infection, according to the results of recent studies.23,24 This study demonstrated that lymphopenia could be a clue for the early diagnosis of H1N1 influenza, particularly among children with negative RIDT in the acute stages and that lymphopenia is transient and would turn to normal count 5 days after disease onset (Figure 3).
C-reactive protein (CRP) is an acute-phase protein, the levels of which rise rapidly in response to cytokines released by macrophage, including TNF-α and IL-6. It is believed to play an important role in innate immunity. Earlier reports have indicated that CRP levels are higher for bacterial infections and mild to moderate for viral infections.25 In this study, we determined that a low CRP level could differentiate H1N1 influenza from other respiratory virus infections, suggesting that CRP could not be adequately induced in the acute stage of H1N1 infection resulting from immunosuppression.26 This finding is interesting and unique, and this might be related to the early phases of the disease or cases of influenza without bacterial coinfection. Mixed bacterial and H1N1 pneumonia caused significantly higher CRP levels than pneumonia caused by H1N1 alone.27 Caution is necessary for patients with influenza-like symptoms presenting a high CRP level that does not exclude the diagnosis of H1N1 because influenza infection could be associated with bacterial coinfection.
Because this is a retrospective study, intrinsic limitations are present. First, we were unable to determine why some children had laboratory testing requested and others did not, which might have resulted from influenza cases presenting with severe symptoms, or a concept of blood collection more invasive than nasopharyngeal aspirates or throat swab collection. Thus, the results should be interpreted, as an opportunistic approach, in the context of ILI children with febrile symptomatic infections requiring blood collection, which captures the more severe and noteworthy cases. Furthermore, selection bias resulting from sample ascertainment is possible, and the impact of selection bias is unclear on the estimation of laboratory screening tool accuracy. In addition, these data are limited to cases observed in a medical center in Taiwan; laboratories in other settings might have the same or different characteristics of pandemic H1N1.
Although the influenza pandemic has presently passed, we must not ignore the impact of this disease. In our study, we used simple and rapid laboratory examinations to obtain early diagnoses of H1N1; this approach should be analyzed and tested in patients of other ethnic origins from the same and differing regions and must be tested prospectively to determine whether it can be applied to H3N2 or influenza B and to adult populations.28 Recently, Chen, et al.29 reported that victims with H7N9 infection also had lymphopenia. In conclusion, this study provides supporting evidence demonstrating that a combination of low lymphocyte count and low CRP levels could provide a useful clinical diagnostic marker that can improve predictions for children with influenza infection from the ILI children. These variables should be considered by physicians evaluating febrile children as information in addition to that provided by the RIDT in future pandemics. This can direct clinicians to undertake appropriate microbiological tests and initiate appropriate treatment.
Acknowledgments
The authors would like to thank the staffs of the Fever Clinic of the KCGMH-CGU for collecting throat swabs for the RIDT and rRT-PCR assays. This work was supported in part with grants CMRPG880643 and CMRPG8A0731 from Chang Gung Memorial Hospital, and Grant NMRP 100-2314-B-182A-051 from the National Science Council of Taiwan.
Authors' contributions
During this study period, Drs Wang, L. and Yang, K.D. designed the study; Drs You, H.L., Li, C.C., and Eng, H.L. gathered laboratory data; Drs Wang, L., Chang L.S., Tang, K.S., and Lee I.K. gathered the clinical data; Dr Yang, K.D. vouched for the analyses; and Drs Wang, L. and Chang, L.S. wrote the paper.
Conflict of interests
No potential conflict of interests are relevant to this article.
References
- 1.Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, et al. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361:680–689. doi: 10.1056/NEJMoa0904252. [DOI] [PubMed] [Google Scholar]
- 2.Libster R, Bugna J, Coviello S, et al. Pediatric hospitalizations associated with 2009 pandemic influenza A (H1N1) in Argentina. N Engl J Med. 2010;362:45–55. doi: 10.1056/NEJMoa0907673. [DOI] [PubMed] [Google Scholar]
- 3.Cunha BA. The clinical diagnosis of severe viral influenza A. Infection. 2008;36:92–93. doi: 10.1007/s15010-007-7255-9. [DOI] [PubMed] [Google Scholar]
- 4.Chen SY, Chen YC, Chiang WC, et al. Field performance of clinical case definitions for influenza screening during the 2009 pandemic. Am J Emerg Med. 2012;30:1796–1803. doi: 10.1016/j.ajem.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 5.Cunha BA, Syed U, Stroll S, et al. Winthrop-University Hospital Infectious Disease Division's swine influenza (H1N1) pneumonia diagnostic weighted point score system for hospitalized adults with influenza-like illnesses (ILIs) and negative rapid influenza diagnostic tests (RIDTs) Heart Lung. 2009;38:534–538. doi: 10.1016/j.hrtlng.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cruz AT, Demmler-Harrison GJ, Caviness AC, et al. Performance of a rapid influenza test in children during the H1N1 2009 influenza A outbreak. Pediatrics. 2010;125:e645–e650. doi: 10.1542/peds.2009-3060. [DOI] [PubMed] [Google Scholar]
- 7.Rashid H, Shafi S, Haworth E, et al. Value of rapid testing for influenza among Hajj pilgrims. Travel Med Infect Dis. 2007;5:310–313. doi: 10.1016/j.tmaid.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Andresen DN, Kesson AM. High sensitivity of a rapid immunochromatographic test for detection of influenza A virus 2009 H1N1 in nasopharyngeal aspirates from young children. J Clin Microbiol. 2010;48:2658–2659. doi: 10.1128/JCM.00229-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talbot HK, Williams JV, Zhu Y, et al. Failure of routine diagnostic methods to detect influenza in hospitalized older adults. Infect Control Hosp Epidemiol. 2010;31:683–688. doi: 10.1086/653202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu TP, Li CC, Wang L, et al. A surveillance system to reduce transmission of pandemic H1N1 (2009) influenza in a 2600-bed medical center. PLoS ONE. 2012;7:e32731. doi: 10.1371/journal.pone.0032731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim C, Ahmed JA, Eidex RB, et al. Comparison of nasopharyngeal and oropharyngeal swabs for the diagnosis of eight respiratory viruses by real-time reverse transcription-PCR assays. PLoS ONE. 2011;6:e21610. doi: 10.1371/journal.pone.0021610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li CC, Wang L, Eng HL, et al. Correlation of pandemic (H1N1) 2009 viral load with disease severity and prolonged viral shedding in children. Emerg Infect Dis. 2010;16:1265–1272. doi: 10.3201/eid1608.091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sutter DE, Worthy SA, Hensley DM, et al. Performance of five FDA-approved rapid antigen tests in the detection of 2009 H1N1 influenza A virus. J Med Virol. 2012;84:1699–1702. doi: 10.1002/jmv.23374. [DOI] [PubMed] [Google Scholar]
- 14.Wu UI, Wang JT, Chen YC, et al. Severity of pandemic H1N1 2009 influenza virus infection may not be directly correlated with initial viral load in upper respiratory tract. Influenza Other Respi Viruses. 2012;6:367–373. doi: 10.1111/j.1750-2659.2011.00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brouqui P, Vu Hai V, Nougairede A, et al. Improving the diagnostic efficiency of H1N1 2009 pandemic flu: analysis of predictive clinical signs through a prospective cohort. PLoS Curr. 2009;1:RRN1120. doi: 10.1371/currents.RRN1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan PA, Mermel LA, Andrea SB, et al. Distinguishing characteristics between pandemic 2009-2010 influenza A (H1N1) and other viruses in patients hospitalized with respiratory illness. PLoS ONE. 2011;6:e24734. doi: 10.1371/journal.pone.0024734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phungoen P, Sawanyawisuth K, Engchanil C, et al. Clinical factors predictive of PCR positive in pandemic H1N1 2009 influenza virus infection. Influenza Other Respi Viruses. 2011;5:e558–e562. doi: 10.1111/j.1750-2659.2011.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crum-Cianflone NF, Blair PJ, Faix D, et al. Clinical and epidemiologic characteristics of an outbreak of novel H1N1 (swine origin) influenza A virus among United States military beneficiaries. Clin Infect Dis. 2009;49:1801–1810. doi: 10.1086/648508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao F, Loring C, Laviolette M, et al. Detection of 2009 pandemic influenza A(H1N1) virus Infection in different age groups by using rapid influenza diagnostic tests. Influenza Other Respi Viruses. 2012;6:e30–e34. doi: 10.1111/j.1750-2659.2011.00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurt AC, Baas C, Deng YM, et al. Performance of influenza rapid point-of-care tests in the detection of swine lineage A(H1N1) influenza viruses. Influenza Other Respi Viruses. 2009;3:171–176. doi: 10.1111/j.1750-2659.2009.00086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bravender T. Epstein-Barr virus, cytomegalovirus, and infectious mononucleosis. Adolesc Med State Art Rev. 2010;21:251–264. ix. [PubMed] [Google Scholar]
- 22.Lindblom A, Bhadri V, Soderhall S, et al. Respiratory viruses, a common microbiological finding in neutropenic children with fever. J Clin Virol. 2010;47:234–237. doi: 10.1016/j.jcv.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao B, Li XW, Mao Y, et al. Clinical features of the initial cases of 2009 pandemic influenza A (H1N1) virus infection in China. N Engl J Med. 2009;361:2507–2517. doi: 10.1056/NEJMoa0906612. [DOI] [PubMed] [Google Scholar]
- 24.Cunha BA, Pherez FM, Schoch P. Diagnostic importance of relative lymphopenia as a marker of swine influenza (H1N1) in adults. Clin Infect Dis. 2009;49:1454–1456. doi: 10.1086/644496. [DOI] [PubMed] [Google Scholar]
- 25.Van der Meer V, Neven AK, van den Broek PJ, et al. Diagnostic value of C reactive protein in infections of the lower respiratory tract: systematic review. BMJ. 2005;331:26–31. doi: 10.1136/bmj.38483.478183.EB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu W, Zhang W, Booth JL, et al. Influenza A(H1N1)pdm09 virus suppresses RIG-I initiated innate antiviral responses in the human lung. PLoS ONE. 2012;7:e49856. doi: 10.1371/journal.pone.0049856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahn S, Kim WY, Kim SH, et al. Role of procalcitonin and C-reactive protein in differentiation of mixed bacterial infection from 2009 H1N1 viral pneumonia. Influenza Other Respi Viruses. 2011;5:398–403. doi: 10.1111/j.1750-2659.2011.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ngaosuwankul N, Noisumdaeng P, Komolsiri P, et al. Influenza A viral loads in respiratory samples collected from patients infected with pandemic H1N1, seasonal H1N1 and H3N2 viruses. Virol J. 2010;7:7–75. doi: 10.1186/1743-422X-7-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y, Liang W, Yang S, et al. Human infections with the emerging avian influenza A H7N9 virus from wet market poultry: clinical analysis and characterisation of viral genome. Lancet. 2013;381:1916–1925. doi: 10.1016/S0140-6736(13)60903-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


