Abstract
Copy number variations (CNVs) are an important cause of ASD and those located at 15q11-q13, 16p11.2 and 22q13 have been reported as the most frequent. These CNVs exhibit variable clinical expressivity and those at 15q11-q13 and 16p11.2 also show incomplete penetrance. In the present work, through multiplex ligation-dependent probe amplification (MLPA) analysis of 531 ethnically admixed ASD-affected Brazilian individuals, we found that the combined prevalence of the 15q11-q13, 16p11.2 and 22q13 CNVs is 2.1% (11/531). Parental origin could be determined in 8 of the affected individuals, and revealed that 4 of the CNVs represent de novo events. Based on CNV prediction analysis from genome-wide SNP arrays, the size of those CNVs ranged from 206 kb to 2.27 Mb and those at 15q11-q13 were limited to the 15q13.3 region. In addition, this analysis also revealed 6 additional CNVs in 5 out of 11 affected individuals. Finally, we observed that the combined prevalence of CNVs at 15q13.3 and 22q13 in ASD-affected individuals with epilepsy (6.4%) was higher than that in ASD-affected individuals without epilepsy (1.3%; p<0.014). Therefore, our data show that the prevalence of CNVs at 15q13.3, 16p11.2 and 22q13 in Brazilian ASD-affected individuals is comparable to that estimated for ASD-affected individuals of pure or predominant European ancestry. Also, it suggests that the likelihood of a greater number of positive MLPA results might be found for the 15q13.3 and 22q13 regions by prioritizing ASD-affected individuals with epilepsy.
Introduction
Autism Spectrum Disorder (ASD) is a complex genetic disorder characterized by impaired social interaction and communication, and restricted, repetitive and stereotyped behavior patterns. ASD affects about 1% of the world population [1]–[3] and it occurs four times more commonly in males than in females [4]. In Brazil, a lower prevalence of ASD (0.27%) has been estimated, which was attributed to misdiagnosis [5]. In addition to the core symptoms, over 60% of the ASD-affected individuals can present other clinical conditions, such as epilepsy (∼30%), gastrointestinal problems (9–70%), attention deficit and hyperactivity disorder – ADHD – (∼30%) and sleep disturbance (∼50%) [6]–[8].
Genome-wide screenings for copy number variations (CNVs) have revealed their occurrence in 10 to 20% of ASD individuals [9]–[12], in which the great majority of CNVs is usually rare and private. Exceptions to this rule are CNVs at 15q11-q13, 16p11.2 and 22q13, which, combined, have been found in roughly 3 to 5% of ASD-affected individuals [10], [13]–[15]. While most CNVs at 16p11.2 are about 600 kb [14], CNV sizes in the other two regions vary widely. The 15q11-q13 region is particularly complex, with five breakpoint clusters (BP1-BP5) that define chromosome segments more prone to genomic rearrangements. Of these, the most recurrent chromosomal abnormality among ASD-affected individuals is a 15q11-q13 duplication (between BP2 and BP3, and about 4.95 Mb) and CNVs at 15q13.2-q13.3 (between BP4 and BP5 and ranging in size from 500kb to 2 Mb) [16]–[19]. The size of the 22q13 CNVs ranges from 100 kb to 9 Mb and always involves SHANK3 [20], [21].
CNVs at 15q11-q13, 16p11.2 and 22q13 have also been associated with other neurological conditions, such as epilepsy, schizophrenia and ADHD [22]–[26]. Apart from the variable clinical expressivity, these CNVs may exhibit incomplete penetrance [11], [23], [27]. The mechanism underlying the incomplete penetrance and the variable expressivity is not fully understood and it seems to depend on multiple hits [28]. Furthermore, the prevalence of these CNVs in distinct ASD subgroups (for instance, in ASD-affected individuals with epilepsy as compared to those without epilepsy) is unknown. Establishing clinical criteria to increase the likelihood of positive results for these alterations is important to prioritize genetic testing resources.
Whole-genome screening of CNVs in populations around the world have shown that their frequencies vary according to the ethnic background, allowing the distinction of populations of European, African and Asian ancestries [29], [30]. Studies of CNVs at 15q11-q13, 16p11.2 and 22q13 have mostly been conducted in populations of pure or predominant European ancestry. It is not known whether they are also prevalent among ASD-affected individuals in populations of other ancestries, such as the Brazilian population, which is tri-hybrid, with important African and Amerindian contributions in addition to the European ancestry [31].
Thus, we conducted the present study to estimate the combined frequency of CNVs at 15q11-q13, 16p11.2 and 22q13 within a group of 531 Brazilian ASD-affected individuals, and we also sought to determine the frequency of CNVs in those regions by taking into account the epileptic and non-epileptic subgroups. Finally, we aimed at investigating whether the individuals with CNVs at the 15q11–13, 16p11.2 and 22q13 regions harbor additional CNVs, through a genome-wide SNP-array analysis.
Materials and Methods
Subjects
This study was approved by the Ethics Committee of the Instituto de Biociencias (IB) – Universidade de Sao Paulo (USP). Written informed consent was obtained from all patients’ caregivers upon receiving information about the study.
Five hundred and thirty one Brazilian ASD-affected individuals were recruited for this study and ascertained at the “Centro de Pesquisa sobre o Genoma Humano e Células Tronco” (CEGH-Cel), IB-USP, following previously standardized criteria [32]–[34], which included a detailed anamnesis – pregnancy history, development history, age at onset, and weight, height and head circumference measurements – and a pedigree analysis. All probands were diagnosed according to Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria by psychiatrists from Instituto de Psiquiatria, Hospital das Clinicas - Universidade de Sao Paulo (IPq-USP). Whenever possible, an interview based on Autism Diagnostic Interview-Revised (ADI-R) and Childhood-Autism Rating Scale (CARS) evaluation was applied, as previously reported [32]. Epilepsy diagnosis was based on the occurrence of at least two unprovoked seizure episodes occurring more than 24 hours apart. Whenever possible, additional neurological and laboratorial tests were used to complement the diagnosis.
Blood samples from probands and parents were obtained for genomic DNA isolation, which was performed using the Autopure LS automated workstation, following manufacturer’s procedures (Gentra Systems, Minneapolis, US). All the affected boys tested negative for Fragile X Syndrome [35].
Multiplex Ligation-dependent Probe Amplification (MLPA) analysis
MLPA probe sets targeting the chromosomal regions 15q11-q13, 16p11.2 and 22q13, SALSA MLPA kits P343-B1 and P343-C1 that contain 52 probes (9 control probes, 3 covering chr22∶51,115,059–51,160,754, 11 covering chr16∶28,997,152–30,365,260, and 29 covering chr15∶25,297,217–32,988,875). They were purchased from MRC-Holland (Amsterdam, Netherlands) and used according to the manufacturer’s protocol. The amplification products were identified and quantified by capillary electrophoresis on an ABI 3730 DNA analyzer (Applied Biosystems, Forster City, CA, US). The data were analyzed using GeneMarker software v1.95 (SoftGenetics, State College, PA, US). Threshold values for the peak height ratio were set at 0.75 and 1.3 for deletions and duplications, respectively.
Microsatellite genotyping
Seven microsatellite markers spanning the 15q11-q13 region were genotyped to identify the parental origin of the duplication of patient 2. The markers D15S1002, D15S1007 and D15S1012, ABI PRISM Linkage Mapping Set version 2.0 (Applied Biosystems, Forster City, CA, US) were genotyped following the manufacturer’s protocol. The other primer sequence pairs, D15S1043, D15S976, D15S1031 and D15S1010, were obtained from the UCSC human genome browser (http://genome.ucsc.edu/cgi-bin/hgGateway, Feb 2009 GRCh37/hg19) and a M13 tail was added to the 5′-end of each forward primer [36]. Microsatellite genotyping was performed by means of an ABI DNA Analyzer (Applied Biosystems, Forster City, CA, US). Analysis of the results was performed with the GeneMarker v1.95 software (SoftGenetics, State College, PA, US).
CNV prediction analysis from genome-wide SNP arrays
CNV prediction analysis from genome-wide SNP arrays was carried out using the Affymetrix platform (Affymetrix, Santa Clara, CA, US): GeneChip Human Mapping 100K for ASD-affected individuals 10, as described in [37], and 11, GeneChip Human Mapping 500K Array Set for families of ASD-affected individuals 2 and 3, and Genome-Wide Human SNP Array 6.0 for the remaining ASD-affected individuals and parents. Protocols were performed according to the manufacturer’s recommendations.
Data analyses were carried out with Affymetrix Genotyping Console (Affymetrix, Santa Clara, CA, US) and PennCNV (http://www.openbioinformatics.org/penncnv/), using the hg19 assembly (Genome Reference Consortium GRCh37). In both analyses we used the default parameters recommended by the manufacturers. A deletion or duplication was considered for further analyses only when detected by both methods.
We considered a CNV as potentially pathogenic according to two criteria: 1) they contained genes previously associated with ASD and/or other neuropsychiatric and neurological disorders (e.g. ADHD, global developmental delay, intellectual disability, schizophrenia and epilepsy/seizure); and/or 2) they exhibited a minimum overlap of 50% in length with CNVs previously associated with these conditions. For this, we searched Simons Foundation Autism Research Initiative (SFARI – https://gene.sfari.org/autdb/Welcome.do) and Decipher (https://decipher.sanger.ac.uk/) databases. Even though we analyzed, whenever possible, if the CNVs had been inherited, we did not include this information in the classification criteria. The CNVs considered as potentially pathogenic were not found or occurred with a frequency <1% in the Database of Genomic Variants (DGV - http://dgv.tcag.ca/dgv/app/home) [38], [39].
Genome-wide SNP-array data are available in the ArrayExpress database (www.ebi.ac.uk/arrayexpress) under accession numbers E-MTAB-2818, E-MTAB-2819, E-MTAB-2820, E-MTAB-2821 and E-MTAB-2823.
Ancestry Analysis
We analyzed the ancestry of 9 out of 11 Brazilian ASD-affected individuals in whom high density genotyping (GeneChip Human Mapping 500K Array Set and Genome-Wide Human SNP Array 6.0) was carried out. The PLINK tool set (http://pngu.mgh.harvard.edu/purcell/plink/) [40] was used to merge the Brazilian dataset with the Human Genome Diversity Project (HGDP) [41] and HapMap project [42] datasets, and to select SNPs with a missing call inferior to 1% (geno option to 0.01), which yielded 84,805 SNPs. Next, we used Ancestry Mapper package from R, to produce AMids [43]. Admixture was used to produce ancestral proportions for each individual [44]. The R statistical language and environment [45] was used in most of the analysis, including the visualization, plotting data and clustering algorithms. Python was used to parse data and in some of the analysis.
Statistical Analysis
To assess the differences in CNV prevalence between the two subgroups, ASD-affected individuals without (N = 453) and with epilepsy (N = 78), we conducted two-tailed Fisher’s exact tests. P-values below 0.05 were considered statistically significant.
Results
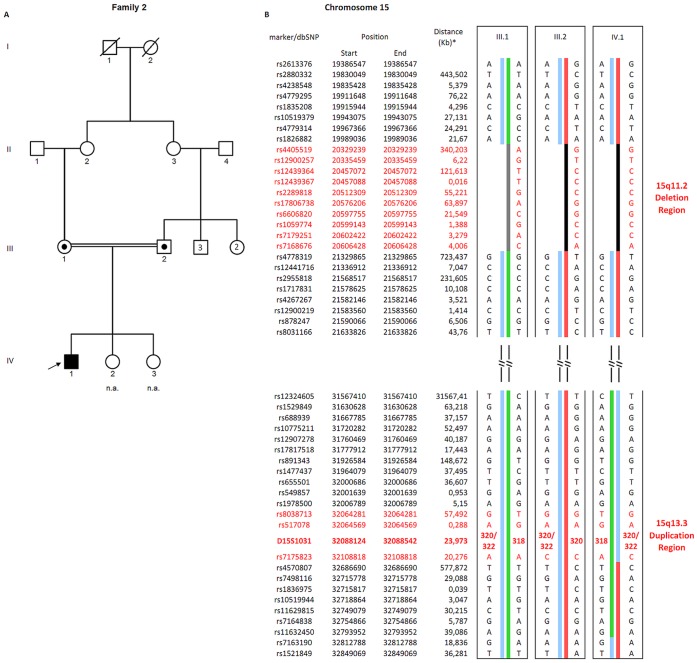
Through MLPA analysis, we identified CNVs at 15q11-q13 between BP4 and BP5 (15q13.3), and at 16p11.2 and 22q13, respectively, in three (0.6%), five (0.9%), and three (0.6%) of the 531 (423 boys and 108 girls) Brazilian ASD–affected individuals (Table 1; clinical characteristics in Table S1), which are ethnically admixed (Table S2), with a combined prevalence of 2.1% (11/531). In four of eight of those individuals, whose parents were available for genetic testing, the CNVs were found to be de novo (affected individuals 1, 6, 10 and 11). Among the other four individuals (affected individuals 2, 3, 4 and 7), only one CNV was maternally inherited (affected individual 7) (Table 1). The parents of affected individual 2 are consanguineous and both are carriers of the CNV at 15q13.3. The parents share a haplotype at this region, suggesting a common origin of the 15q13.3 duplication. Therefore, they probably inherited the CNV from their mothers, who are sisters, while the proband inherited it from his father (Figure 1A and 1B). None of the carrier parents reported behavioral or neurological issues.
Table 1. CNVs at the chromosomal regions 15q13.3, 16p11.2 and 22q13 in the Brazilian individuals with ASD.
| MLPA detected CNVs | CNVs detected by SNP-array | ||||||||||||
| AffectedIndividual | Region | Position (hg19) | Type | Size(Mb) | N Genes | Inheritance | Region | Position (hg19) | Type | Size (Mb) | Inheritance | Disease association | |
| Neurol. Dis. | ASD | ||||||||||||
| 1 | 15q13.2-q13.3 | chr15∶30,941,572–32,509,926 | del | 1.56 | 8 | de novo | 2q13 | chr2∶110,453,976–111,084,885 | dup | 0.631 | pat | + | + |
| 2* | 15q13.3 | chr15∶32,024,192–32,509,926 | dup | 0,486 | 1 | pat | 15q11.2 | chr15∶22,410,242–23,222,284 | del | 0.812 | mat | + | + |
| 3 | 15q13.3 | chr15∶31,956,036–32,511,581 | dup | 0,555 | 1 | pat | – | – | – | – | |||
| 4 | 16p11.2 | chr16∶29,696,973–30,191,907 | dup | 0,495 | 25 | Pat | 4q35.2 | chr4∶186,934,286–187,137,146 | dup | 0.203 | mat | + | + |
| 11p11.2 | chr11∶48,380,903–48,968,027 | del | 0.587 | pat | + | + | |||||||
| 5 | 16p11.2 | chr16∶29,517,699–30,191,895 | dup | 0,674 | 28 | father n.a. | – | – | – | – | – | ||
| 6 | 16p11.2 | chr16∶29,613,495–30,190,030 | dup | 0,576 | 27 | de novo | – | – | – | – | – | ||
| 7 | 16p11.2 | chr16∶29,402,301–30,226,931 | dup | 0,824 | 32 | Mat | – | – | – | – | – | ||
| 8 | 16p11.2 | chr16∶29,517,699–30,191,895 | del | 0.674 | 28 | father n.a. | 7p11.2 | chr7∶57,260,919–57,882,330 | dup | 0.621 | father n.a. | + | + |
| 9 | 22q13.3 | chr22∶51,027,581–51,234,443 | del | 0.206 | 6 | father n.a. | 17q11.2 | chr17∶25,974,257–26,075,524 | del | 0.101 | father n.a. | – | + |
| 10¥ | 22q13.3 | chr22∶50,282,986–51,304,566 | del | 1.02 | 35 | de novo | – | – | – | – | – | – | – |
| 11 | 22q13.3 | chr22∶49,033,233–51,193,680 | del | 2.27 | 37 | de novo | – | – | – | – | – | – | – |
Neurol. Dis. - Neurological Disorder; “–” – not reported in the literature; “+” reported in the literature; del – deletion; dup – duplication; n.a. – not available; pat - paternal; mat - maternal;
- ASD-affected individual 10 was described in [37]; * CNVs at 15q13.3 and 15q11.2 are present in both parents;
Figure 1. ASD-affected individual 2 pedigree and haplotypes.
A) Pedigree of the ASD-affected individual. B) Haplotype analysis of SNPs and microsatellite markers at 15q11-q13. ASD-affected individual – filled symbol; Carriers of 15q13.3 duplication and 15q11.2 deletion – symbols with a black dot in the middle; individuals unavailable or not affected – empty symbols; non-available individuals – n.a. * Distance (in kilobases) from anterior to posterior marker/dbSNP. The other microsatellite markers are not showed.
We conducted a CNV prediction analysis from genome-wide SNP arrays in the 11 ASD-affected individuals in whom we detected CNVs at 15q13.3, 16p11.2 and 22q13 regions to determine their sizes as well as to verify whether another potentially pathogenic CNV would be present. The 15q13.3, 16p11.2 and 22q13 CNVs sizes ranged from 206 kb to 2.27 Mb (Table 1). It is worth noting that the two 15q13.3 duplications were about 500 kb and included only the gene CHRNA7. Six additional CNVs (3 duplications and 3 deletions) were identified in 5 of those affected individuals (1, 2, 4, 8 and 9), two of the CNVs in individual 4 (Table 1). Four of these CNVs were inherited, while the parents of the other two affected individuals were unavailable for testing (Table 1). The 15q11.2 CNV in affected individual 2 was also present in both consanguineous parents and the maternal copy was transmitted to the affected proband (Figure 1B). Ancestry analysis was conducted in 9 out of 11 ASD-affected individuals (Table S2), which showed that they have the three main ancestral components commonly observed in the Brazilian population.
Next, we evaluated if CNVs at 15q13.3, 16p11.2 and 22q13 occurred more often among ASD-affected individuals with epilepsy. In our total sample, 78 (54 boys and 24 girls) of the 531 ASD-affected individuals had history of epilepsy (Table 2). We observed that 6 of the 453 ASD-affected individuals without epilepsy (1.3%) and 5 of the 78 ASD-affected individuals with epilepsy (6.4%) had CNVs in one of these regions. These frequencies were significantly different (p = 0.014; odds ratio = 5.1; 95% CI 1.19–20.55). CNVs at 15q13.3 and 22q13 among ASD-affected individuals with epilepsy were responsible for these differences (Table 2).
Table 2. Main findings in ASD Brazilian individuals with and without epilepsy.
| Total (N = 531) | ASD without epilepsy (N = 453) | ASD with epilepsy (N = 78) | |
| Gender | |||
| Male | 423 | 369 | 54 |
| Female | 108 | 84 | 24 |
| Ratio (m:f) | 4∶1 | 4.4∶1 | 2.3∶1 |
| Mean age, years (mean ± sd) | 10.2±6.6 | 13.2±6.5 | 9.7±6.5 |
| CNVs at 15q13.3, 16p11.2 and 22q13 (%) | 11 (2.1) | 6 (1.3) | 5 (6.4) * |
| CNVs at 15q13.3, 22q13 (%) | 6 (1.1) | 1 (0.02) | 5 (6.4) |
m – male; f – female; sd, standard deviation; * OR = 5.1; (p-value = 0.014).
Discussion
In the Brazilian sample of ASD-affected patients, the frequencies of CNVs at 15q13.3 (0.6%), 16p11.2 (0.9%), and 22q13 (0.6%) are consistent with those described in the literature [28], [38]–[42]. The Brazilian population is ethnically admixed, with around 56–62% of European contribution [31], [46]–[48]. In ASD-affected individuals positive for CNVs at 15q11-q13, 16p11.2 and 22q13, we observed that the contribution of European ancestry varied from 38 to 98%. This preliminary analysis suggests that the ethnic admixture of our population is not influencing the occurrence of these CNVs.
We identified both inherited and de novo CNVs at 15q11-q13 and 16p11.2, as previously described by other groups [49]–[52]. Indeed, it has been established that maternally and paternally inherited as well as de novo 16p11.2 microdeletions and microduplications contribute to the ASD phenotype [51], [53]–[55]. Additionally, 15q13.3 deletions and maternally inherited duplications at 15q11-q13 have been implicated in an increased risk of ASD [28], [56], [57]. However, the association between the 15q13.3 duplication, involving only CHRNA7, and neuropsychiatric disorders is still controversial [24], [49], [58]–[60]. In our sample, the two 15q13.3 duplications were inherited from the father, but both maternally and paternally inherited 15q13.3 duplications have been associated with the ASD phenotype [49], [58]. Finally, our data on the origin of the 22q13 deletions provide additional support to the hypothesis that they usually represent de novo mutations [21], [61], [62].
The size of the CNVs at 16p11.2 (600kb) and 15q13.3 (500kb or 1.6Mb), are within the range of those previously reported [49], [54], [57], as expected due to the presence of segmental duplications that flank these regions and mediate rearrangements through non-allelic homologous recombination [49], [54], [63]. At 22q13 the size of the CNVs were quite variable, but always included SHANK3. Loss-of-function mutations involving SHANK3 cause Phelan-McDermid syndrome, an autosomal dominant condition with full penetrance that presents ASD among other clinical features [21], [64]. In addition to the aforementioned CNVs, five ASD-affected individuals also carried at least another CNV with no correlation with the presence of CNVs at 15q13.3, 16p11.2 or 22q13. All those additional CNVs detected by the SNP-array platform overlap partially or completely CNVs previously associated with ASD or other neurological conditions [15], [65]–[74]. Therefore, these additional CNVs, which were found in nearly 50% of our affected individuals, might contribute to the penetrance of the ASD phenotype, in accordance with the two- or multiple hit hypotheses for ASD, that is, these CNVs are not the cause ASD alone and depend on the presence of at least a second mutation [58], [75]. Further studies will be necessary for testing their effect and specificity on the phenotype.
Our data suggest that CNVs at 15q13.3 and 22q13 are more prevalent among ASD-affected individuals with epilepsy than among those only with ASD. Indeed, it has been shown that 15q13.3 and 22q13 deletions represent strong genetic risk factors for ASD and epilepsy [76]–[79]. However, the contribution of 15q13.3 duplications, particularly those encompassing only CHRNA7, to both phenotypes is still uncertain [19], [49], [58]. Although the 15q13.3 duplication has been implicated in several psychiatric conditions [24], [60], [80], rare cases presented epilepsy as well [49], [81], [82]. Within this context, ASD-affected individual 2 deserves special attention: while his 15q13.3 duplication was paternally inherited, his parents, who do not have any history of epilepsy, probably inherited the duplication from their mothers (who are sibs). This individual also harbors a deletion at 15q11.2 (Table 1), which in turn was inherited from his mother, even though both parents carry this 15q11.2 deletion (Figure 1). De novo or inherited deletions at 15q11.2 have been associated with both epilepsy and ASD, however the relative risk that this CNV confers to ASD is low [70], [83], [84]. It is thus possible that CNVs both at 15q11.2 and 15q13.3 are causative factors of ASD and/or epilepsy, supporting the etiologic models that involve multiple genetic alterations in ASD, since both alterations have incomplete penetrance but have already been reported in ASD and other neuropsychiatric diseases.
None of our ASD individuals with epilepsy carry a 16p11.2 CNV. Even though CNVs at 16p11.2 have been associated with epilepsy, this finding is not unexpected, as the phenotype of patients with these CNVs is extremely variable and the overlap between ASD and epilepsy is not often observed among them [55], [85]–[88].
In summary, this work describes the combined prevalence of CNVs at 15q13.3, 16p11.2 and 22q13 as 2.1% in Brazilian ASD-affected individuals. CNVs at 15q13.3 and 22q13 were significantly more frequent in ASD-affected individuals with epilepsy in our sample; hence, these CNVs should be preferentially screened in ASD-affected individuals if resources are limited. Other potentially pathogenic CNVs were identified in 5 out of 11 ASD-affected individuals studied, thus highlighting the need for understanding how those and other genetic alterations interact to give rise to ASD and other clinical complications.
Supporting Information
Phenotypic characteristics of ASD individuals with CNVs at the 15q13.3, 16p11.2 and 22q13 regions.
(XLS)
Ancestral Contributions in Brazilian ASD-affected individuals.
(XLSX)
Acknowledgments
We are very grateful to the ASD-affected individuals and their family members who participated in this research. We would like to thank Cintia Ribeiro Marques for help in genetic evaluating the families, and MSc Gerson Shigeru Kobayashi for helping with manuscript revision.
Funding Statement
Support was provided by FAPESP-INCT - grant number: 2008/57899-7; FAPESP-CEPID - grant number: 2013/08028-1; CNPq [http://www.fapesp.br/]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kogan MD, Blumberg SJ, Schieve LA, Boyle CA, Perrin JM, et al. (2009) Prevalence of parent-reported diagnosis of autism spectrum disorder among children in the US, 2007. Pediatrics 124: 1395–1403. [DOI] [PubMed] [Google Scholar]
- 2. Lazoff T, Zhong L, Piperni T, Fombonne E (2010) Prevalence of pervasive developmental disorders among children at the English Montreal School Board. Can J Psychiatry 55: 715–720. [DOI] [PubMed] [Google Scholar]
- 3. Brugha TS, McManus S, Bankart J, Scott F, Purdon S, et al. (2011) Epidemiology of Autism Spectrum Disorders in Adults in the Community in England. Arch Gen Psychiatry 68: 459–466. [DOI] [PubMed] [Google Scholar]
- 4. Giarelli E, Wiggins LD, Rice CE, Levy SE, Kirby RS, et al. (2010) Sex differences in the evaluation and diagnosis of autism spectrum disorders among children. Disabil Health J 3: 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Paula C, Ribeiro S, Fombonne E, Mercadante M (2011) Brief report: Prevalence of pervasive developmental disorder in Brazil: A pilot study. J Autism Dev Disord 41: 1738–1742. [DOI] [PubMed] [Google Scholar]
- 6. Gillberg C, Billstedt E (2000) Autism and Asperger syndrome: coexistence with other clinical disorders. Acta Psychiatr Scand 102: 321–330. [DOI] [PubMed] [Google Scholar]
- 7. Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, et al. (2008) Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry 47: 921–929. [DOI] [PubMed] [Google Scholar]
- 8. Xue Ming, Brimacombe M, Chaaban J, Zimmerman-Bier B, Wagner GC (2008) Autism spectrum disorders: concurrent clinical disorders. J Child Neurol 23: 6–13. [DOI] [PubMed] [Google Scholar]
- 9. Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, et al. (2007) Strong association of de novo copy number mutations with autism. Science 316: 445–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Betancur C (2011) Etiological heterogeneity in autism spectrum disorders: more than 100 genetic and genomic disorders and still counting. Brain Res 1380: 42–77. [DOI] [PubMed] [Google Scholar]
- 11. Carter MT, Scherer SW (2013) Autism spectrum disorder in the genetics clinic: a review. Clin Genet 83: 399–407. [DOI] [PubMed] [Google Scholar]
- 12.Pinto D, Delaby E, Merico D, Barbosa M, Merikangas A, et al. (2014) Convergence of Genes and Cellular Pathways Dysregulated in Autism Spectrum Disorders. Am J Hum Genet: 677–694. [DOI] [PMC free article] [PubMed]
- 13. Walsh KM, Bracken MB (2011) Copy number variation in the dosage-sensitive 16p11.2 interval accounts for only a small proportion of autism incidence: A systematic review and meta-analysis. Genet Med 13: 377–384. [DOI] [PubMed] [Google Scholar]
- 14. Marshall CR, Noor A, Vincent JB, Lionel AC, Feuk L, et al. (2008) Structural variation of chromosomes in autism spectrum disorder. Am J Hum Genet 82: 477–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sanders SJ, Ercan-Sencicek AG, Hus V, Luo R, Murtha MT, et al. (2011) Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron 70: 863–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Menashe I, Larsen EC, Banerjee-Basu S (2013) Prioritization of Copy Number Variation Loci Associated with Autism from AutDB-An Integrative Multi-Study Genetic Database. PLoS One 8: e66707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Depienne C, Moreno-De-Luca D, Heron D, Bouteiller D, Gennetier A, et al. (2009) Screening for genomic rearrangements and methylation abnormalities of the 15q11-q13 region in autism spectrum disorders. Biol Psychiatry 66: 349–359. [DOI] [PubMed] [Google Scholar]
- 18. Rosenfeld JA, Stephens LE, Coppinger J, Ballif BC, Hoo JJ, et al. (2011) Deletions flanked by breakpoints 3 and 4 on 15q13 may contribute to abnormal phenotypes. Eur J Hum Genet 19: 547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miller DT, Shen Y, Weiss LA, Korn J, Anselm I, et al. (2009) Microdeletion/duplication at 15q13.2q13.3 among individuals with features of autism and other neuropsychiatric disorders. J Med Genet 46: 242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Soorya L, Kolevzon A, Zweifach J, Lim T, Dobry Y, et al. (2013) Prospective investigation of autism and genotype-phenotype correlations in 22q13 deletion syndrome and SHANK3 deficiency. Mol Autism 4: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bonaglia MC, Giorda R, Beri S, De Agostini C, Novara F, et al. (2011) Molecular mechanisms generating and stabilizing terminal 22q13 deletions in 44 subjects with Phelan/McDermid syndrome. PLoS Genet 7: e1002173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. International T, Consortium S (2008) Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature 455: 237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stefansson H, Meyer-Lindenberg A, Steinberg S, Magnusdottir B, Morgen K, et al. (2014) CNVs conferring risk of autism or schizophrenia affect cognition in controls. Nature 505: 361–366. [DOI] [PubMed] [Google Scholar]
- 24. Williams NM, Franke B, Mick E, Anney RJL, Freitag CM, et al. (2012) Genome-Wide Analysis of Copy Number Variants in Attention Deficit Hyperactivity Disorder: The Role of Rare Variants and Duplications at 15q13.3. Am J Psychiatry 169: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Galizia EC, Srikantha M, Palmer R, Waters JJ, Lench N, et al. (2012) Array comparative genomic hybridization: results from an adult population with drug-resistant epilepsy and co-morbidities. Eur J Med Genet 55: 342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Striano P, Coppola A, Paravidino R, Malacarne M, Gimelli S, et al. (2012) Clinical significance of rare copy number variations in epilepsy: a case-control survey using microarray-based comparative genomic hybridization. Arch Neurol 69: 322–330. [DOI] [PubMed] [Google Scholar]
- 27. Miles JH (2011) Autism spectrum disorders–a genetics review. Genet Med 13: 278–294. [DOI] [PubMed] [Google Scholar]
- 28. Marshall CR, Scherer SW (2012) Genomic Structural Variants. 838: 115–135. [Google Scholar]
- 29. Redon R, Ishikawa S, Fitch KR, Feuk L, Perry GH, et al. (2006) Global variation in copy number in the human genome. Nature 444: 444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jakobsson M, Scholz SW, Scheet P, Gibbs JR, VanLiere JM, et al. (2008) Genotype, haplotype and copy-number variation in worldwide human populations. Nature 451: 998–1003. [DOI] [PubMed] [Google Scholar]
- 31. Giolo SR, Soler JMP, Greenway SC, Almeida MAA, de Andrade M, et al. (2012) Brazilian urban population genetic structure reveals a high degree of admixture. Eur J Hum Genet 20: 111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Griesi-Oliveira K, Moreira DP, Davis-Wright N, Sanders S, Mason C, et al. (2012) A complex chromosomal rearrangement involving chromosomes 2, 5, and X in autism spectrum disorder. Am J Med Genet Part B, Neuropsychiatr Genet 159B: 529–536. [DOI] [PubMed] [Google Scholar]
- 33. Griesi-Oliveira K, Sunaga DY, Alvizi L, Vadasz E, Passos-Bueno MR (2013) Stem cells as a good tool to investigate dysregulated biological systems in autism spectrum disorders. Autism Res 6: 354–361. [DOI] [PubMed] [Google Scholar]
- 34. Orabona GM, Griesi-Oliveira K, Vadasz E, Bulcão VLS, Takahashi VNVO, et al. (2009) HTR1B and HTR2C in autism spectrum disorders in Brazilian families. Brain Res 1250: 14–19. [DOI] [PubMed] [Google Scholar]
- 35. Haddad L, Mingroni-Netto R, Vianna-Morgante A, Pena S (1996) A PCR-based test suitable for screening for fragile X syndrome among mentally retarded males. Hum Genet 97: 808–812. [DOI] [PubMed] [Google Scholar]
- 36. Schuelke M (2000) An economic method for the fluorescent labeling of PCR fragments. Nat Biotechnol 18: 233–234. [DOI] [PubMed] [Google Scholar]
- 37. Jehee FS, Takamori JT, Medeiros PFV, Pordeus ACB, Latini FRM, et al. (2011) Using a combination of MLPA kits to detect chromosomal imbalances in patients with multiple congenital anomalies and mental retardation is a valuable choice for developing countries. Eur J Med Genet 54: e425–32. [DOI] [PubMed] [Google Scholar]
- 38. Heil KM, Schaaf CP (2013) The genetics of Autism Spectrum Disorders–a guide for clinicians. Curr Psychiatry Rep 15: 334. [DOI] [PubMed] [Google Scholar]
- 39. Schaefer GB, Mendelsohn NJ (2013) Clinical genetics evaluation in identifying the etiology of autism spectrum disorders: 2013 guideline revisions. Genet Med 15: 399–407. [DOI] [PubMed] [Google Scholar]
- 40. Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira M a R, et al. (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81: 559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cann HM, de Toma C, Cazes L, Legrand M-F, Morel V, et al. (2002) A Human Genome Diversity Cell Line Panel. Science (80- ) 296: 261b–262. [DOI] [PubMed] [Google Scholar]
- 42. Altshuler DM, Gibbs RA, Peltonen L, Dermitzakis E, Schaffner SF, et al. (2010) Integrating common and rare genetic variation in diverse human populations. Nature 467: 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Magalhães TR, Casey JP, Conroy J, Regan R, Fitzpatrick DJ, et al. (2012) HGDP and HapMap analysis by Ancestry Mapper reveals local and global population relationships. PLoS One 7: e49438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Alexander DH, Novembre J, Lange K (2009) Fast model-based estimation of ancestry in unrelated individuals. Genome Res 19: 1655–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.R Core Team (2013) R: A Language and Environment for Statistical Computing.
- 46. Santos NPC, Ribeiro-Rodrigues EM, Ribeiro-Dos-Santos AKC, Pereira R, Gusmão L, et al. (2010) Assessing individual interethnic admixture and population substructure using a 48-insertion-deletion (INSEL) ancestry-informative marker (AIM) panel. Hum Mutat 31: 184–190. [DOI] [PubMed] [Google Scholar]
- 47. Brito LA, Cruz LA, Rocha KM, Barbara LK, Silva CBF, et al. (2011) Genetic contribution for non-syndromic cleft lip with or without cleft palate (NS CL/P) in different regions of Brazil and implications for association studies. Am J Med Genet A 155A: 1581–1587. [DOI] [PubMed] [Google Scholar]
- 48. Pena SDJ, Di Pietro G, Fuchshuber-Moraes M, Genro JP, Hutz MH, et al. (2011) The genomic ancestry of individuals from different geographical regions of Brazil is more uniform than expected. PLoS One 6: e17063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Szafranski P, Schaaf CP, Person RE, Gibson IB, Xia Z, et al. (2010) Structures and molecular mechanisms for common 15q13.3 microduplications involving CHRNA7: benign or pathological? Hum Mutat 31: 840–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Weiss LA, Arking DE, Daly MJ, Chakravarti A (2009) A genome-wide linkage and association scan reveals novel loci for autism. Nature 461: 802–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fernandez BA, Roberts W, Chung B, Weksberg R, Meyn S, et al. (2010) Phenotypic spectrum associated with de novo and inherited deletions and duplications at 16p11.2 in individuals ascertained for diagnosis of autism spectrum disorder. J Med Genet 47: 195–203. [DOI] [PubMed] [Google Scholar]
- 52. Pagnamenta AT, Wing K, Sadighi Akha E, Knight SJL, Bölte S, et al. (2009) A 15q13.3 microdeletion segregating with autism. Eur J Hum Genet 17: 687–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kumar RA, KaraMohamed S, Sudi J, Conrad DF, Brune C, et al. (2008) Recurrent 16p11.2 microdeletions in autism. Hum Mol Genet 17: 628–638. [DOI] [PubMed] [Google Scholar]
- 54. Weiss LA, Shen Y, Korn JM, Arking DE, Miller DT, et al. (2008) Association between microdeletion and microduplication at 16p11.2 and autism. N Engl J Med 358: 667–675. [DOI] [PubMed] [Google Scholar]
- 55. Ciuladaitė Z, Kasnauskienė J, Cimbalistienė L, Preikšaitienė E, Patsalis PC, et al. (2011) Mental retardation and autism associated with recurrent 16p11.2 microdeletion: incomplete penetrance and variable expressivity. J Appl Genet 52: 443–449. [DOI] [PubMed] [Google Scholar]
- 56. Vorstman J, Staal W, Daalen E, Engeland H, Hochstenbach P, et al. (2006) Identification of novel autism candidate regions through analysis of reported cytogenetic abnormalities associated with autism. Mol Psychiatry 11: 18–28. [DOI] [PubMed] [Google Scholar]
- 57. Ben-Shachar S, Lanpher B, German JR, Qasaymeh M, Potocki L, et al. (2009) Microdeletion 15q13.3: a locus with incomplete penetrance for autism, mental retardation, and psychiatric disorders. J Med Genet 46: 382–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Leblond CS, Heinrich J, Delorme R, Proepper C, Betancur C, et al. (2012) Genetic and functional analyses of SHANK2 mutations suggest a multiple hit model of autism spectrum disorders. PLoS Genet 8: e1002521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chilian B, Abdollahpour H, Bierhals T, Haltrich I, Fekete G, et al. (2013) Dysfunction of SHANK2 and CHRNA7 in a patient with intellectual disability and language impairment supports genetic epistasis of the two loci. Clin Genet 3: 9–14. [DOI] [PubMed] [Google Scholar]
- 60. Vu TH, Coccaro EF, Eichler EE, Girirajan S (2011) Genomic architecture of aggression: rare copy number variants in intermittent explosive disorder. Am J Med Genet Part B, Neuropsychiatr Genet 156B: 808–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nesslinger NJ, Gorski JL, Kurczynski TW, Shapira SK, Siegel-Bartelt J, et al. (1994) Clinical, cytogenetic, and molecular characterization of seven patients with deletions of chromosome 22q13.3. Am J Hum Genet 54: 464–472. [PMC free article] [PubMed] [Google Scholar]
- 62. Bonaglia MC, Giorda R, Mani E, Aceti G, Anderlid B-M, et al. (2006) Identification of a recurrent breakpoint within the SHANK3 gene in the 22q13.3 deletion syndrome. J Med Genet 43: 822–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lupski JR, Stankiewicz P (2005) Genomic disorders: molecular mechanisms for rearrangements and conveyed phenotypes. PLoS Genet 1: e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Phelan K, McDermid HE (2011) The 22q13.3 Deletion Syndrome (Phelan-McDermid Syndrome). Mol Syndromol: 186–201. [DOI] [PMC free article] [PubMed]
- 65. Bisgaard A-M, Kirchhoff M, Nielsen JE, Brandt C, Hove H, et al. (2007) Transmitted cytogenetic abnormalities in patients with mental retardation: pathogenic or normal variants? Eur J Med Genet 50: 243–255. [DOI] [PubMed] [Google Scholar]
- 66. Xu B, Roos JL, Levy S, van Rensburg EJ, Gogos JA, et al. (2008) Strong association of de novo copy number mutations with sporadic schizophrenia. Nat Genet 40: 880–885. [DOI] [PubMed] [Google Scholar]
- 67. Qiao Y, Tyson C, Hrynchak M, Lopez-Rangel E, Hildebrand J, et al. (2013) Clinical application of 2.7M Cytogenetics array for CNV detection in subjects with idiopathic autism and/or intellectual disability. Clin Genet 83: 145–154. [DOI] [PubMed] [Google Scholar]
- 68. Wu DJ, Wang NJ, Driscoll J, Dorrani N, Liu D, et al. (2009) Autistic disorder associated with a paternally derived unbalanced translocation leading to duplication of chromosome 15pter-q13.2: a case report. Mol Cytogenet 2: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kaminsky EB, Kaul V, Paschall J, Church DM, Bunke B, et al. (2011) An evidence-based approach to establish the functional and clinical significance of copy number variants in intellectual and developmental disabilities. Genet Med 13: 777–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Doornbos M, Sikkema-Raddatz B, Ruijvenkamp CAL, Dijkhuizen T, Bijlsma EK, et al. (2009) Nine patients with a microdeletion 15q11.2 between breakpoints 1 and 2 of the Prader-Willi critical region, possibly associated with behavioural disturbances. Eur J Med Genet 52: 108–115. [DOI] [PubMed] [Google Scholar]
- 71. Girirajan S, Brkanac Z, Coe BP, Baker C, Vives L, et al. (2011) Relative burden of large CNVs on a range of neurodevelopmental phenotypes. PLoS Genet 7: e1002334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Elia J, Gai X, Xie HM, Perin JC, Geiger E, et al. (2010) Rare structural variants found in attention-deficit hyperactivity disorder are preferentially associated with neurodevelopmental genes. Mol Psychiatry 15: 637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rosenfeld JA, Ballif BC, Torchia BS, Sahoo T, Ravnan JB, et al. (2010) Copy number variations associated with autism spectrum disorders contribute to a spectrum of neurodevelopmental disorders. Genet Med 12: 694–702. [DOI] [PubMed] [Google Scholar]
- 74.Wang L, Hranilovic D, Wang K, Lindquist IE, Yurcaba L, et al. (2010) Population-based study of genetic variation in individuals with autism spectrum disorders from Croatia. BMC Med Genet: 9–13. [DOI] [PMC free article] [PubMed]
- 75. Girirajan S, Rosenfeld JA, Cooper GM, Antonacci F, Siswara P, et al. (2010) A recurrent 16p12.1 microdeletion supports a two-hit model for severe developmental delay. Nat Genet 42: 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sharp AJ, Mefford HC, Li K, Baker C, Skinner C, et al. (2008) A recurrent 15q13.3 microdeletion syndrome associated with mental retardation and seizures. Nat Genet 40: 322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Helbig I, Mefford HC, Sharp AJ, Guipponi M, Fichera M, et al. (2009) 15q13.3 microdeletions increase risk of idiopathic generalized epilepsy. Nat Genet 41: 160–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mulley JC, Scheffer IE, Desai T, Bayly MA, Grinton BE, et al. (2011) Investigation of the 15q13.3 CNV as a genetic modifier for familial epilepsies with variable phenotypes. Epilepsia 52: e139–42. [DOI] [PubMed] [Google Scholar]
- 79. Phelan MC, Rogers RC, Saul RA, Stapleton GA, Sweet K, et al. (2001) 22q13 deletion syndrome. Am J Med Genet 101: 91–99. [DOI] [PubMed] [Google Scholar]
- 80.Melchior L, Bertelsen B, Debes NM, Groth C, Skov L, et al. (2013) Microduplication of 15q13.3 and Xq21.31 in a family with tourette syndrome and comorbidities. Am J Med Genet Part B, Neuropsychiatr Genet: 1–7. [DOI] [PubMed]
- 81.Beal JC (2013) Case Report: Neuronal Migration Disorder Associated With Chromosome 15q13.3 Duplication in a Boy With Autism and Seizures. J Child Neurol: 13–16. [DOI] [PubMed]
- 82. Wiśniowiecka-Kowalnik B, Kastory-Bronowska M, Bartnik M, Derwińska K, Dymczak-Domini W, et al. (2013) Application of custom-designed oligonucleotide array CGH in 145 patients with autistic spectrum disorders. Eur J Hum Genet 21: 620–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chaste P, Sanders SJ, Mohan KN, Klei L, Song Y, et al. (2014) Modest impact on risk for autism spectrum disorder of rare copy number variants at 15q11.2, specifically breakpoints 1 to 2. Autism Res 7: 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. De Kovel CGF, Trucks H, Helbig I, Mefford HC, Baker C, et al. (2010) Recurrent microdeletions at 15q11.2 and 16p13.11 predispose to idiopathic generalized epilepsies. Brain 133: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Degenhardt F, Priebe L, Herms S, Mattheisen M, Mühleisen TW, et al. (2012) Association between copy number variants in 16p11.2 and major depressive disorder in a German case-control sample. Am J Med Genet Part B, Neuropsychiatr Genet 159B: 263–273. [DOI] [PubMed] [Google Scholar]
- 86. McCarthy SE, Makarov V, Kirov G, Addington AM, McClellan J, et al. (2009) Microduplications of 16p11.2 are associated with schizophrenia. Nat Genet 41: 1223–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bedoyan JK, Kumar RA, Sudi J, Silverstein F, Ackley T, et al. (2010) Duplication 16p11.2 in a child with infantile seizure disorder. Am J Med Genet A 152A: 1567–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Schaaf CP, Goin-Kochel RP, Nowell KP, Hunter JV, Aleck KA, et al. (2011) Expanding the clinical spectrum of the 16p11.2 chromosomal rearrangements: three patients with syringomyelia. Eur J Hum Genet 19: 152–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phenotypic characteristics of ASD individuals with CNVs at the 15q13.3, 16p11.2 and 22q13 regions.
(XLS)
Ancestral Contributions in Brazilian ASD-affected individuals.
(XLSX)