Abstract
Background
This study was conducted to investigate an outbreak caused by imipenem-resistant Acinetobacter baumannii (IRAB) in a medical intensive care unit (ICU) in a regional hospital.
Methods
In response to an IRAB outbreak from October 2012 to February 2013, we developed several infection control measures, including an extensive review process of environmental cleaning and disinfection, and used molecular methods to identify each clinical and environmental IRAB isolate.
Results
During this five-month period, 22 patients were colonized with IRAB and 18 patients had IRAB infections. The in-hospital mortality rate was significantly higher among patients with infections rather than colonizations (44.4% vs 9.1%, p = 0.028). Additionally, nine environmental specimens, including five specimens collected after terminal disinfection, were positive for IRAB. 12 environmental isolates and 28 of 36 available clinical isolates belonged to one unique pulsotype A, which was confirmed by molecular methods. We found the concentration of disinfectant, 0.08% sodium hypochlorite, was inadequate. After correcting the environmental cleansing methods, the surveillance study showed no further IRAB isolates on the control panel surfaces of the medical equipment or in patients in the ICU. Additionally, an in vitro study of IRAB immersed in different concentrations of sodium hypochlorite showed that 0.5% sodium hypochlorite eradicates IRAB after 30 seconds of inoculation, but 0.08% sodium hypochlorite only reduces the bacterial load.
Conclusions
This study highlights the importance of the preparation of disinfectants to adequately achieve environmental disinfection in the control of IRAB outbreaks in the ICU.
Introduction
Acinetobacter baumannii, a non-fermenting Gram-negative coccobacillus has become an important nosocomial pathogen, especially in intensive care units (ICUs). Moreover, the increasing appearance of multiple drug resistance in this pathogen, especially carbapenem-resistance, limits the therapeutic antibiotic options for patients infected with A. baumannii. Most importantly, this multi-drug resistant (MDR) pathogen can cause healthcare-associated infections and can increase mortality and the length of stay in the ICU [1], [2].
Because A. baumannii has a great ability to colonize humans and environmental surfaces [3], [4], it is difficult to eradicate this pathogen from the environment. In addition to colonization, it can cause life-threatening human infections, especially in immunocompromised and critically ill patients. Therefore, MDR A. baumannii (MDRAB) remains a global issue in public health despite aggressive infection control measures to avoid nosocomial acquisition and further dissemination.
Recently, we noted an outbreak of imipenem-resistant A. baumannii (IRAB) in an ICU at a regional hospital in southern Taiwan. To prevent further outbreaks and their accompanying risks, we extensively reviewed our infection control policy and designed a care bundle for restricting the colonization and spread of IRAB.
Materials and Methods
Setting
Chi Mei Medical Center, Liouying campus is a 900-bed regional hospital located in southern Taiwan with a 16-bed medical ICU. In October 2012, a computer-based infection control system for the analysis of microbiologic and clinical data detected an outbreak of IRAB. All of the case with IRAB was identified by microbiology department initially and the information was transmitted to infection control nurses. After checking electrical chart for collecting the clinical information, the outbreak of IRAB was confirmed by the committee of infection control. Case definitions for infection or colonizations followed the guidelines published by the Centers for Disease Control and Prevention [5]. To investigate this outbreak, we conducted active surveillance and molecular characterization of IRAB isolates from the environment and patients who were either colonized or infected. An ethics approval was obtained from Institutional Review Board of Chi Mei Medical Center after the investigation of the outbreak.
Microbiological investigation
A. baumannii isolates were identified by conventional biochemical tests and by two commercial identification kits, Api20NE (bioMerieux, Marcy I’Etoile, France) and the Phoenix System (Becton Dickson, Sparks, MD). Isolates were classified as susceptible or resistant (including an intermediate category) by broth microdilution methods according to Clinical and Laboratory Standards Institute (CLSI) guidelines [6]. IRAB was defined as A. baumannii isolates resistant to imipenem. Environmental specimens (including bedrails, monitors, respirators, bedside desks, and bedside sinks) were collected on moistened gauze, incubated overnight in tryptic soy broth and then subcultured onto blood agar and MacConkey agar. The hands of personnel caring for these patients were randomly sampled during work and before hand washing with a cotton swab moistened with brain heart infusion (BHI) broth [7]. To avoid the concern that the staffs will clean their hands vigorously before the sampling, infection control nurses were responsible for surveillance culture and they must perform the sampling culture without informing the staff.
Infection control intervention
After the detection of the outbreak, a team including intensivists, ICU nurses, microbiologists, house-keeping staff and infectious control specialists was organized. The team developed a plan regarding the control of the outbreak based on the consensus of infection control team. We developed several infection control measures, including (1) enhancing contact isolation of all IRAB patients and empirical contact isolation of patients at high risk for acquiring IRAB (including hospitalization in the preceding 90 days, residency in a nursing home, extended-care facility, and receiving broad-spectrum antimicrobial therapy in the preceding 90 days); (2) enhancing contact precautions to interrupt transmission, including hand washing and the use of disposable gloves and gowns; (3) monitoring isolation and hand hygiene adherences; (4) reviewing the process of environmental cleaning and disinfection; (5) instituting an educational program for healthcare workers; (6) establishing periodic environmental cultures to identify contaminated surfaces that might constitute a source of indirect A. baumannii spread; (7) using molecular methods to identify available IRAB clinical and environmental isolates; and (8) discontinue isolation when there were three successive negative cultures from the original site of clinical specimen positive culture for IRAB such as respiratory specimen, urine, or wounds, if available during the follow-up period.
Pulsed-field gel electrophoresis analysis
The genetic relatedness studies of IRAB isolates were performed by pulsed-field gel electrophoresis (PFGE) using ApaI (New England Biolabs, Ipswich, MA, USA) as previously described with slight modifications. [8] Briefly, the bacteria were grown at 37°C overnight on Trypticase soy agar with 2% sheep blood (BBL) and suspended in 2 mL of cell suspension buffer (100 mM Tris, 100 mM EDTA, pH 8.0) to a concentration of 109 CFU/mL. Then, the bacterial suspension was mixed with an equal volume of 1% Pulsed Field Certified Agarose (Bio-Rad Laboratories, Hercules, CA, USA) and allowed to solidify in a 100-µL plug mold (Bio-Rad Laboratories, Hercules, CA, USA). The gel plugs were then lysed, washed, and digested with the restriction enzyme ApaI. PFGE was performed with a CHEF Mapper system (Bio-Rad Laboratories, Hercules, CA, USA) at 14°C and a field strength of 6 V/cm with a pulse time of 1–25 seconds for 22 hours. A bacteriophage lambda ladder PFGE marker (New England Biolabs, Ipswich, MA, USA) was used as a reference marker. Cluster analyses were performed using BioNumerics software, version 3.5 (Applied Maths, Sint-Martens-Latem, Belgium). DNA relatedness was calculated using the Dice coefficient with a tolerance of 1%. PFGE profiles were interpreted as epidemiological relatedness according to the criteria suggested by Tenover et al. [9] Strains with more than 85% similarity values of PFGE profiles were considered as closely related strains in this study.
In vitro study of the effect of sodium hypochlorite on IRAB isolates
In this in vitro study, 6 isolates were randomly select from different pulsed-field gel electrophoresis (PFGE) samples. After overnight culture in tryptic soy broth (TSB) (Difco Co; Becton Dickinson, Sparks, MD), the concentration was adjusted using the 0.5 Mc Farland standard (1×108 CFU/mL). The culture was pelleted in 1 mL bacterial suspension by centrifugation, and the supernatant was replaced with normal saline or 0.5%, 0.2%, 0.1%, 0.08% or 0.05% sodium hypochlorite. The cultures were then incubated for 30 and 60 seconds at 20°C. After each period of time, 9 mL of neutralizing broth (D/E Neutralizing Broth, Difco Co) was added to terminate the antimicrobial action of the test agents. Ten-fold serial dilutions were performed in reduced transport fluid. Each dilution was plated onto TSA plates. The plates were then incubated for 24 hours at 37°C, and bacterial numbers were calculated. Controls were exposed to sterile saline for the same periods. Three replicates were performed for each antimicrobial agent and control.
Drug Definition
Extended-spectrum cephalosporins included ceftriaxone, flomoxef, ceftazidime, and cefpirome. Extended-spectrum β-lactam-β-lactamase inhibitor combinations included piperacillin and piperacillin-tazobactam. Carbapenems included imipenem, meropenem, and ertapenem, and fluoroquinolones included ciprofloxacin, moxifloxacin and levofloxacin.
Statistical analysis
Continuous variables are expressed as the mean±standard deviation. Continuous variables were compared using the Wilcoxon rank-sum test or Student’s independent t test, as appropriate. Categorical variables were compared using the chi-square test or Fisher’s exact test. All statistical analyses were conducted using the statistical package SPSS for Windows (Version 11.0, SPSS, Chicago, Il, USA).
Results
Clinical characteristics of patients with IRAB colonization and infection
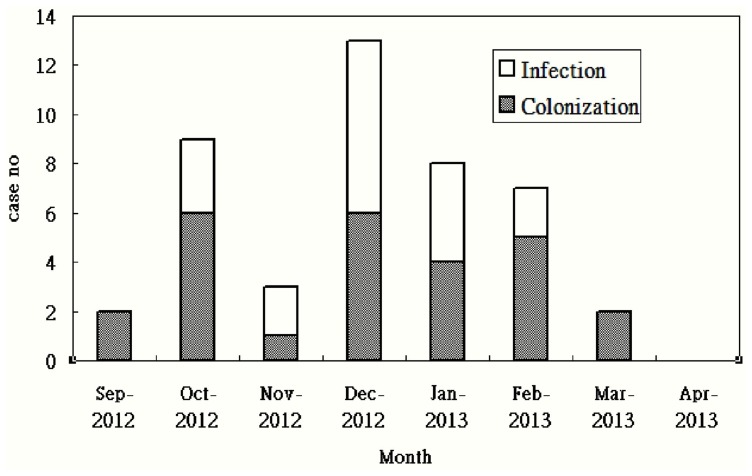
During a five-month period, a total of 40 patients yielded clinical specimens that were positive for IRAB isolates. The clinical characteristics of these 40 patients are summarized in table 1. The mean age was 69.2 years, and 62.5% (n = 25) of patients were ≥65 years. Diabetes mellitus was the most common comorbidity, followed by malignancy. An endotracheal aspirate was the most common clinical specimen positive for IRAB, followed by the tip of a central venous catheter, blood, and catheterization urine. Except for one patient, most of the patients (n = 39) had received broad-spectrum antibiotics (such as extended-spectrum cephalosporins, extended-spectrum β-lactam-β-lactamase inhibitor combinations, carbapenems, and fluoroquinolones), especially fluoroquinolones. A total of 22 patients (55%) were defined as being colonized by IRAB, and the other 18 patients were defined as having IRAB infections (Figure 1). Overall in-hospital mortality was 25%.
Table 1. Demographic characteristics of patients with positive isolates for imipenem-resistant Acinetobacter baumannii (IRAB).
| Variable | No of patients (%) n = 40 |
| Age (years), mean ± SD | 69.2±16.3 |
| Male (%) | 24 (60.0) |
| Underlying disease | |
| Diabetes mellitus | 14 (35.0) |
| Cancer | 13 (32.5) |
| Stroke | 7 (17.5) |
| Chronic kidney disease | 5 (12.5) |
| Liver cirrhosis | 4 (10.0) |
| Connective tissue disease | 2 (5.0) |
| Use of steroid | 6 (15.0) |
| Use of immunosuppressant | 4 (10.0) |
| Site of isolates | |
| Endotracheal aspirate | 30 (75.0) |
| Catheter tip | 4 (10.0) |
| Blood | 3 (15.0) |
| Urine | 2 (5.0) |
| Wound | 2 (5.0) |
| Device | |
| Endotracheal tube | 37 (92.5) |
| Central venous catheter | 32 (80.0) |
| Port-A catheter | 7 (17.5) |
| Double lumen catheter | 4 (10.0) |
| AV shunt | 1 (2.5) |
| Abdominal drainage | 4 (10.0) |
| Pleural drainage | 2 (5.0) |
| Total parenteral nutrition | 3 (7.5) |
| Previous use of antibiotic in the preceding 90 days | |
| Fluoroquinolones | 27 (67.5) |
| Carbapenem | 17 (42.5) |
| Extended-spectrum cephalosporin | 16 (40.0) |
| Extended-spectrum β-lactam-β-lactamase inhibitor combinations | 7 (17.5) |
| Hospital stay before acquisition of IRAB (days) | 14.4±9.4 |
| ICU stay before acquisition of IRAB (days) | 10.1±5.5 |
| Clinical significance | |
| Colonization | 22 (55.0) |
| Ventilator associated pneumonia | 15 (30.0) |
| Central venous catheter related infection | 2 (10.0) |
| Skin and soft tissue infection | 1 (5.0) |
| Outcome | |
| In-hospital mortality | 10 (25.0) |
Figure 1. Case numbers of imipenem-resistant Acinetobacter baumannii colonization and infections over time.
Comparison between patients with IRAB colonization and infections
Among the 18 patients who had IRAB infections, ventilator-associated pneumonia was the most common type of infection. The comparison between patients with IRAB colonizations and infections is summarized in table 2. Although patients with infections were older than patients with colonizations, this difference was not significant (mean age in year, 74.3 vs 65.0, p = 0.0714). However, we noted that the patients with colonizations were more likely to have received 3rd or 4th generation cephalosporins than patients with infections (59.1% vs 16.7%, p = 0.0165). In contrast, the patients with infections had longer ICU stays before the acquisition of IRAB than patients who were colonized (mean duration in days, 11.2 vs 9.2, p = 0.0498). Importantly, the patients with infections had a higher in-hospital mortality rate than patients who were colonized (44.4% vs 9.1%, p = 0.0279).
Table 2. Comparison between patients with imipenem-resistant Acinetobacter baumannii (IRAB) colonization and infections.
| Variable | No of patients with colonization (n = 22) | No of patients with infections (n = 18) | P value |
| Age (years), mean ± SD | 65.0±17.4 | 74.3±13.5 | 0.0714 |
| Male (%) | 11 (50.0) | 13 (72.2) | 0.2707 |
| Underlying disease | |||
| Diabetes mellitus | 8 (36.4) | 6 (33.3) | 0.8976 |
| Cancer | 8 (36.4) | 5 (27.8) | 0.8116 |
| Stroke | 3 (13.6) | 4 (22.2) | 0.7687 |
| Chronic kidney disease | 3 (13.6) | 2 (11.1) | 0.8081 |
| Liver cirrhosis | 2 (9.1) | 2 (11.1) | 0.7490 |
| Connective tissue disease | 2 (9.1) | 0 (0.0) | 0.5590 |
| Use of steroid | 3 (13.6) | 3 (16.7) | 0.8635 |
| Use of immunosuppressant | 3 (13.6) | 1 (5.6) | 0.7571 |
| Device | |||
| Endotracheal tube | 19 (86.4) | 18 (100.0) | 0.3065 |
| Central venous catheter | 15 (68.2) | 17 (94.4) | 0.0962 |
| Port-A catheter | 4 (18.2) | 3 (16.7) | 0.7689 |
| Double lumen catheter | 2 (9.1) | 2 (11.1) | 0.7490 |
| AV shunt | 1 (4.5) | 0 (0.0) | 0.9112 |
| Abdominal drainage | 2 (9.1) | 2 (11.1) | 0.7490 |
| Pleural drainage | 1 (4.5) | 1 (5.6) | 0.5683 |
| Total parenteral nutrition | 1 (4.5) | 2 (11.1) | 0.8529 |
| Previous use of antibiotic | |||
| Fluoroquinolones | 15 (68.2) | 12 (66.7) | 0.8114 |
| Carbapenem | 11 (50.0) | 6 (33.3) | 0.4584 |
| Extended-spectrum cephalosporin | 13 (59.1) | 3 (16.7) | 0.0165 |
| Extended-spectrumβ-lactam-β-lactamase inhibitor combinations | 5 (13.6) | 2 (22.2) | 0.7687 |
| Hospital stay before acquisition of IRAB (days) | 14.7±11.1 | 13.9±7.2 | 0.7936 |
| ICU stay before acquisition of IRAB (days) | 9.2±4.8 | 11.2±5.5 | 0.0498 |
| Outcome | |||
| In-hospital mortality | 2 (9.1) | 8 (44.4) | 0.0279 |
Microbiologic investigations
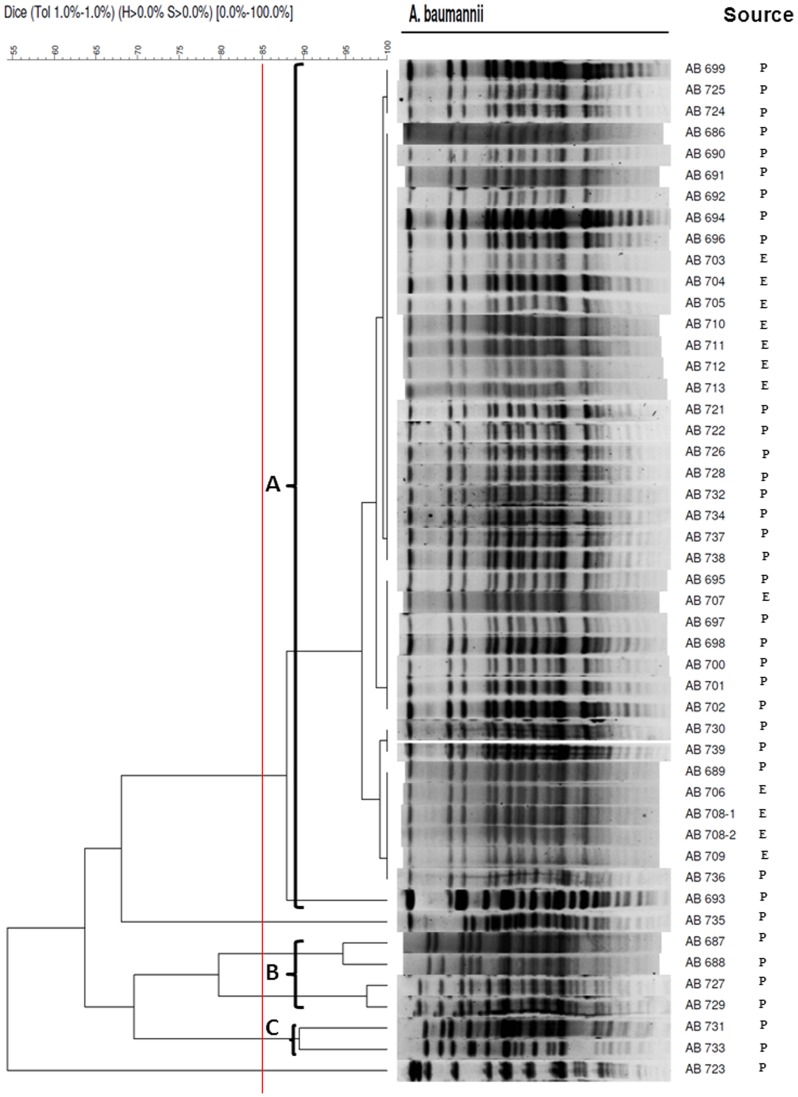
Of 22 environmental samplings during the outbreak investigation, a total of twelve environmental isolates which including five specimens collected after terminal disinfection were positive for IRAB. All environmental isolates and the 36 clinical isolates that were available from patients were sent for molecular study. We found that all of the environmental isolates and most of the clinical isolates (n = 28) belonged to one unique pulsotype A. Additionally, four isolates were belonged to pulsotype B, and two clinical isolates were belonged to pulsotype C (Figure 2).
Figure 2. Pulsed-field gel electrophoresis (PFGE) profiles of 48 imipenem-resistant A. baumannii isolates digested with ApaI.
Red line indicated the 85% similarity values of PFGE profiles. P: clinical isolate from patient; E: environmental isolate.
Infection control process
After the IRAB outbreak was identified, we performed environmental surveillance cultures. We found IRAB in isolates grown from sampling the bedside desks and the surface of a body weight scale, even after cleaning and terminal disinfection. This compelled us to extensively review the entire process of terminal disinfection. It appears that for some reason, a too low concentration was used. Therefore, we corrected the procedure for preparing disinfectant solutions and reinforced the need for adherence to disinfection protocols. After the surveillance study and the reinforcement of the correct process for environmental cleansing, further testing of 40 environmental specimens showed no IRAB isolates from samples on control panel surfaces of the medical equipment and of the patients in the ICU. Additionally, the hand hygiene compliance of the ICU staff increased from 48% in the pre-intervention period to 89% in the post-intervention period.
In vitro study of the effect of sodium hypochlorite on IRAB isolates
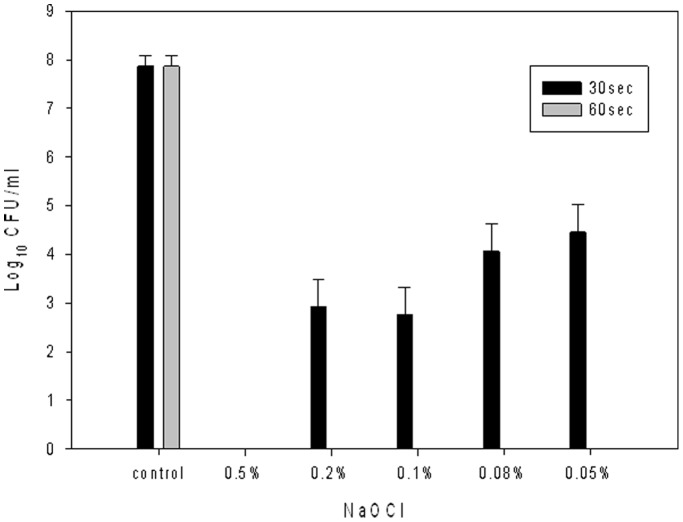
After 30 seconds of treatment, only 0.5% sodium hypochlorite eradicated all six isolates. 0.2%∼0.05% sodium hypochlorite can only reduce the bacterial number to 103∼105 CFU/ml. The bacteria number after 60 seconds in the various solutions of sodium hypochlorite was below detectable levels (Figure 3).
Figure 3. The number of imipenem-resistant Acinetobacter baumannii in different concentration of sodium hypochlorite after 30 and 60 seconds.
Discussion
Several significant findings arose from our investigation of an outbreak of IRAB in an ICU. First, we showed the presence of a large outbreak of IRAB that lasted for five months using a molecular approach. The study showed that this outbreak was strongly associated with the use of incorrect procedures during terminal disinfections. The wrong protocol for preparing the disinfectant, sodium hypochlorite, meant that the concentration of disinfectant (0.08%) was inadequate as a bactericidal agent. Several specimens from the environmental surveillance grew IRAB even after terminal disinfection. After extensively reviewing every step of the infection control protocol and correcting the process for terminal disinfection, no new IRAB cases were identified. In addition, an in vitro study of IRAB using different concentrations of sodium hypochlorite confirmed that only a 0.5% sodium hypochlorite solution eradicates IRAB after 30 seconds of exposure. Concentrations less than 0.5% cannot effectively kill all isolates unless the clinical isolates are exposed to the cleaning agent for more than 60 seconds. Therefore, although the outbreak of IRAB, causing 12 and 28 episodes of infection and colonization in the study unit may be multifactorial, both of the clinical and laboratory findings suggest that inadequate terminal disinfection should be one of most important factors. Such a costly mistake reminds us of the importance of infection control measures [10], including the preparation of disinfectant. As our experience is limited, we cannot recommend the appropriate concentration of sodium hypochlorite solution for environmental cleaning all of the important nosocomial pathogens. However, our finding may indicate that only sodium hypochlorite solution with the concentration of at least 0.5% can be useful for terminal disinfection for IRAB.
At the beginning of the intervention, the hand hygiene compliance of the ICU staff was less than 50%. It is difficult to totally exclude the possibility that IRAB was spread patient-to-patient by contaminated hands; however, we were unable to isolate IRAB on the hands of the ICU staff during the environmental surveillance despite we only sampled a very small portion of the actual patient contacts. Therefore, the impact of poor hand hygiene on this outbreak may be limited. In addition, hand hygiene compliance rapidly improved after education and monitoring of hand hygiene. By the end of the outbreak, the compliance was nearly 90%. This improvement in hand hygiene may be due to repeated education programs and the monitoring of the contact precautions.
In this study, the patients with IRAB had varying risk factors, including immunocompromised comorbidities, antecedent broad-spectrum antimicrobial therapy and recent invasive procedures. This finding is consistent with previous studies showing that the risk factors for the acquisition of multi-drug resistant A. baumannii include malignancy, recent exposure to antibiotics, mechanical ventilation, and higher disease severity measured by the Acute Physiology and Chronic Health Evaluation (APACHE) score [11]–[19]. This suggests that while caring for patients at high risk, healthcare workers should strictly follow the standard care procedures to prevent patients from acquiring multi-drug resistant organisms.
In addition, we were unable to find any significant differences in the underlying conditions, including age, gender, and comorbidities among patients with IRAB infections versus colonizations in the present study. However, we noted that patients with infections had longer ICU stays before the acquisition of IRAB than patients who were colonized. Furthermore, as previous reported in Spain [20], patients with IRAB infections had significantly higher mortality rates than patients who were colonized (44.4% vs 9.1%). Moreover, our findings and other recent studies suggest that multi-drug resistant A. baumannii infections are associated with high mortality [19]–[22].
In conclusion, this study highlights the importance of adequate environmental disinfection and the correct preparation of disinfectants in the control of IRAB outbreaks in ICUs. After eradicating environmental contamination through effective terminal disinfection, outbreaks of IRAB can be controlled, as we demonstrated. If outbreaks of IRAB are not well controlled, they are associated with high mortality.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
The authors have no support or funding to report.
References
- 1. Maragakis L, Perl TM (2008) Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis 46: 1254–1263. [DOI] [PubMed] [Google Scholar]
- 2. Falagas ME, Blizotis IA, Siempos I (2006) Attributable mortality of Acinetobacter baumannii infections in critically ill patients: a systemic review of matched cohort and case-control studies. Crit Care 10: R48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Playford EG, Craig JC, Iredell JR (2007) Carbapenem-resistant Acinetobacter baumannii in intensive care unit patients: risk factors for acquisition, infection and their consequences. J Hosp Infect 65: 204–211. [DOI] [PubMed] [Google Scholar]
- 4. García-Garmendia JL, Ortiz-Leyba C, Garnacho-Montero J, Jiménez-Jiménez FJ, Pérez-Paredes C, et al. (2001) Risk factors for Acinetobacter baumannii nosocomial bacteremia in critical ill patients: a cohort study. Clin Infect Dis 33: 939–946. [DOI] [PubMed] [Google Scholar]
- 5. Horan TC, Andrus M, Dudeck MA (2008) CDC/NHSN surveillance definition of healthcare-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 36: 309–332. [DOI] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute (2009) Performance Standards for Antimicrobial Susceptibility Testing; Eighteenth Informational Supplement. M100-S19. CLSI, Wayne, PA, USA.
- 7. Aygün G, Demirkiran O, Utku T, Mete B, Urkmez S, et al. (2002) Environmental contamination during a carbapenem-resistant Acinetobacter baumannii outbreak in an intensive care unit. J Hosp Infect 52: 259–262. [DOI] [PubMed] [Google Scholar]
- 8. Seifert H, Dolzani L, Bressan R, van der Reijden T, van Strijen B, et al. (2005) Standardization and interlaboratory reproducibility assessment of pulsed-field gel electrophoresis-generated fingerprints of Acinetobacter baumannii. J Clin Microbiol 43: 4328–4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, et al. (1995) Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 33: 2233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Valencia R, Arroyo LA, Conde M, Aldana JM, Torres MJ, et al. (2009) Nosocomial outbreak of infection with pan-drug-resistant Acinetobacter baumannii in a tertiary care university hospital. Infect Control Hosp Epidemiol 30: 257–263. [DOI] [PubMed] [Google Scholar]
- 11. Kim YJ, Kim SI, Kim YR, Hong KW, Wie SH, et al. (2012) Carbapenem-resistant Acinetobacter baumannii: diversity of resistant mechanisms and risk factors for infection. Epidemiol Infect 140: 137–145. [DOI] [PubMed] [Google Scholar]
- 12. Jung JY, Park MS, Kim SE, Park BH, Son JY, et al. (2010) Risk factors for multi-drug resistant Acinetobacter baumannii bacteremia in patients with colonization in the intensive care unit. BMC Infect Dis 10: 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sheng WH, Liao CH, Lauderdale TL, Ko WC, Chen YS, et al. (2010) A multicenter study of risk factors and outcome of hospitalized patients with infections due to carbapenem-resistant Acinetobacter baumannii. Int J Infect Dis 14: e764–769. [DOI] [PubMed] [Google Scholar]
- 14. Dent LL, Marshall DR, Pratap S, Hulette RB (2010) Multidrug resistant Acinetobacter baumannii: a descriptive study in a city hospital. BMC Infect Dis 10: 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lautenbach E, Synnestvedt M, Weiner MG, Bilker WB, Vo L, et al. (2009) Epidemiology and impact of imipenem resistance in Acinetobacter baumannii. Infect Control Hosp Epidemiol 30: 1186–1192. [DOI] [PubMed] [Google Scholar]
- 16. Shih MJ, Lee NY, Lee HC, Chang CM, Wu CJ, et al. (2008) Risk factors of multidrug resistance in nosocomial bacteremia due to Acinetobacter baumannii: a case-control study. J Microbiol Immunol Infect 41: 118–123. [PubMed] [Google Scholar]
- 17. Chang PY, Hsueh PR, Wu PS, Chan PC, Yang TT, et al. (2007) Multidrug-resistant Acinetobacter baumannii isolates in pediatric patients of a university hospital in Taiwan. J Microbiol Immunol Infect 40: 406–410. [PubMed] [Google Scholar]
- 18. Lai CC, Hsu HL, Tan CK, Tsai HY, Cheng A, et al. (2012) Recurrent bacteremia caused by the Acinetobacter calcoaceticus-Acinetobacter baumannii complex. J Clin Microbiol 50: 2982–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kuo LC, Lai CC, Liao CH, Hsu CK, Chang YL, et al. (2007) Multidrug-resistant Acinetobacter baumannii bacteraemia: clinical features, antimicrobial therapy and outcome. Clin Microbiol Infect 13: 196–198. [DOI] [PubMed] [Google Scholar]
- 20. Corbella X, Montero A, Pujol M, Domínguez MA, Ayats J, et al. (2000) Emergence and rapid spread of carbapenem resistance during a large and sustained hospital outbreak of multiresistant Acinetobacter baumannii. J Clin Microbiol38: 4086–4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Prata-Rocha ML, Gontijo-Filho PP, Melo GB (2012) Factors influencing survival in patients with multidrug-resistant Acinetobacter baumannii infection. Braz J Infect Dis16: 237–241. [PubMed] [Google Scholar]
- 22. Hernández-Torres A, García-Vázquez E, Gómez J, Canteras M, Ruiz J, et al. (2012) Multidrug and carbapenem-resistant Acinetobacter baumannii infections: Factors associated with mortality. Med Clin (Barc) 138: 650–655. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.