Abstract
Objective
To compare the relative effectiveness of methotrexate and mycophenolate mofetil for non-infectious intermediate uveitis, posterior uveitis, or panuveitis.
Design
Multicenter, block-randomized, observer-masked clinical trial
Participants
Eighty patients with non-infectious intermediate, posterior or panuveitis requiring corticosteroid-sparing therapy at Aravind Eye Hospitals in Madurai and Coimbatore, India.
Intervention
Patients were randomized to receive 25mg weekly oral methotrexate or 1g twice daily oral mycophenolate mofetil and were monitored monthly for 6 months. Oral prednisone and topical corticosteroids were tapered.
Main Outcome Measures
Masked examiners assessed the primary outcome of treatment success, defined by achieving the following at 5 and 6 months: (1) ≤0.5+ anterior chamber cells, ≤0.5+ vitreous cells, ≤0.5+ vitreous haze and no active retinal/choroidal lesions in both eyes, (2) ≤ 10 mg of prednisone and ≤ 2 drops of prednisolone acetate 1% a day and (3) no declaration of treatment failure due to intolerability or safety. Additional outcomes included time to sustained corticosteroid-sparing control of inflammation, change in best spectacle-corrected visual acuity, resolution of macular edema, adverse events, subgroup analysis by anatomic location, and medication adherence.
Results
Forty-one patients were randomized to methotrexate and 39 to mycophenolate mofetil. A total of 67 patients (35 methotrexate, 32 mycophenolate mofetil) contributed to the primary outcome. Sixty-nine percent of patients achieved treatment success with methotrexate and 47% with mycophenolate mofetil (p=0.09). Treatment failure due to adverse events or tolerability was not significantly different by treatment arm (p=0.99). There were no statistically significant differences between treatment groups in time to corticosteroid-sparing control of inflammation (p=0.44), change in best spectacle-corrected visual acuity (p=0.68), and resolution of macular edema (p=0.31).
Conclusions
There was no statistically significant difference in corticosteroid-sparing control of inflammation between patients receiving methotrexate or mycophenolate mofetil. However, there was a 22% difference in treatment success favoring methotrexate.
The standard initial treatment for non-infectious uveitis is some form of corticosteroid therapy. However, corticosteroid therapy has well-documented local and systemic side effects, making long-term use undesirable.1 Thus, other immunosuppressive therapies are frequently used as corticosteroid-sparing agents when patients require ongoing treatment and are unable to taper to an acceptable long-term dose of oral prednisone (e.g. ≤10 mg a day).1 Currently there are no FDA approved systemic immunosuppressive therapies for non-infectious uveitis. Methotrexate and mycophenolate mofetil, two commonly used antimetabolites, are often used as initial corticosteroid-sparing treatments.2,3
Results from most non-comparative retrospective case series suggest that patients may be more likely to achieve controlled inflammation and tolerate treatment with mycophenolate mofetil compared to methotrexate.3–18 Furthermore, approximately half of the patients who fail treatment with methotrexate go on to successful treatment with mycophenolate mofetil.19 However, one small retrospective case series demonstrated that methotrexate had slightly higher success than mycophenolate mofetil.20 A recent survey reported that while the majority of uveitis specialists use methotrexate as their initial corticosteroid-sparing agent for all anatomical locations of uveitis, they would prefer to start with mycophenolate mofetil for intermediate and posterior/panuveitis if cost was not a factor.21
There has been a lack of prospective studies and randomized controlled trials to systematically determine which antimetabolite is more clinically efficacious as initial corticosteroid-sparing therapy for the treatment of non-infectious uveitis, making it difficult for clinicians to make informed, evidence-based decisions. The objective of this study was to compare the relative effectiveness of methotrexate and mycophenolate mofetil for non-infectious intermediate uveitis, posterior uveitis or panuveitis in patients requiring corticosteroid-sparing therapy.
METHODS
Study Design
This study was a multicenter, block-randomized, observer-masked comparative effectiveness trial (ClinicalTrials.gov: NCT01232920). Patients with non-infectious uveitis were enrolled at two Aravind Eye Hospital uveitis clinics located in Madurai and Coimbatore, South India. Institutional Review Board approval was obtained at the University of California, San Francisco and at Aravind Eye Hospitals. All patients provided written informed consent.
Eligibility Criteria
Eligible patients were 16 years or older and had non-infectious intermediate uveitis, posterior uveitis or panuveitis in at least one eye (active within the past 60 days defined by the presence of at least one of the following: ≥1+ anterior chamber cells, vitreous cells, vitreous haze and/or active retinal/choroidal lesions). Eligibility criteria also included a failed corticosteroid taper and/or a known chronic condition necessitating immunosuppressive therapy1 and no prior use of immunosuppressive drugs, other than corticosteroids. Complete eligibility criteria are listed in Figure 1.
Figure 1.
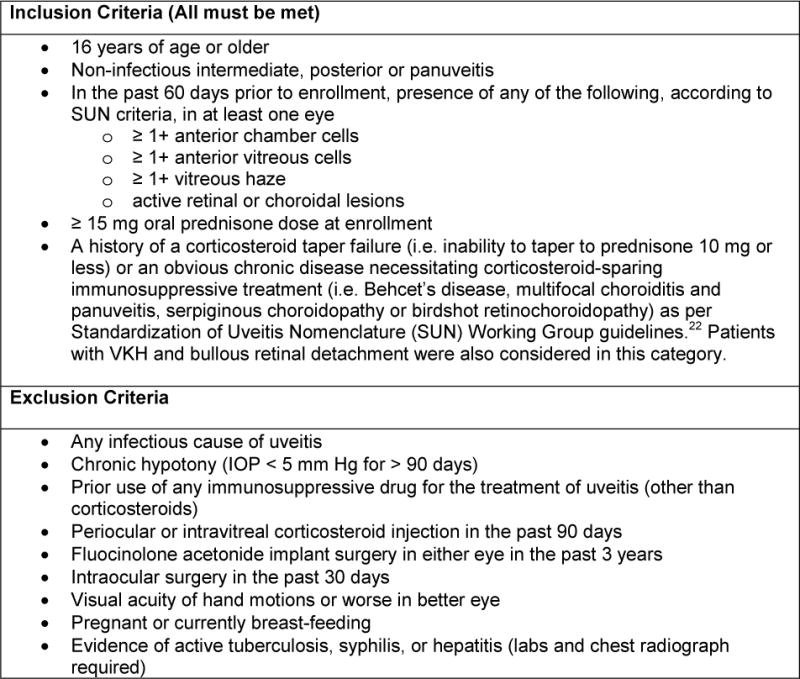
Trial Inclusion and Exclusion Criteria
Treatment Assignment
Patients were randomized (1:1 allocation ratio) to oral methotrexate or mycophenolate mofetil within clinic using permuted blocks of size 4 and 6. Assignments were generated by the principal statistician using the statistical software R (The R Project for Statistical Computing, version 2.11.1, Vienna, Austria, available at: http://www.r-project.org, accessed September 25, 2013). After eligibility was confirmed, the study coordinator contacted a designated individual who was otherwise uninvolved in the study to obtain the randomization assignment.
Study Timeline, Masking, and Assessments
Patients completed study visits at baseline, 2 weeks, and every month for up to 6 months. All personnel responsible for outcome measurements (i.e. ophthalmologists, visual acuity examiners, and optical coherence tomography (OCT) operators) were masked. Patients and study coordinators were unmasked.
Study ophthalmologists measured anterior chamber cells according to the Standardization of Uveitis Nomenclature (SUN) guidelines.22 Vitreous cells were graded using a scale derived from the Multicenter Uveitis Steroid Treatment (MUST) trial.23 Vitreous haze was assessed by the standardized National Eye Institute (NEI) scoring system24 which has been accepted by the SUN Working Group.22 Activity of retinal/choroidal lesions was judged by the treating ophthalmologist. Macular edema in isolation was not considered an active retinal/choroidal lesion.
Study-certified masked visual acuity examiners measured best spectacle-corrected visual acuity (BSCVA) using a logarithm of the minimum angle of resolution (logMAR) tumbling “E” chart at 4 meters. Acuities worse than logMAR 1.6 (~20/800) were recorded as: counting fingers (1.7), hand motion (1.8), light perception (1.9), and no light perception (2.0).25 Macular edema was measured by masked, study-certified OCT operators. Macular edema thresholds were derived from normative data on central subfield thickness (i.e. greater than 252μm for Stratus26 and greater than 308μm for Cirrus,27 Carl Zeiss Meditec, Dublin, CA).
Serious and non-serious ocular and systemic adverse events were ascertained at each visit by the coordinator and ophthalmologist. Blood samples were drawn at each visit to monitor values of CBC, AST, ALT and creatinine. Medication adherence was assessed using patient reports of missed doses.
Treatment
Patients received antimetabolite therapy at a reduced dose for the first 2 weeks to ensure tolerability (15mg a week oral methotrexate and 500mg twice daily oral mycophenolate mofetil). After two weeks, the dose was increased to a maintenance level for the remainder of the trial: 25mg a week oral methotrexate or 1g twice daily oral mycophenolate mofetil. Additionally, all patients on methotrexate were given 1mg of folic acid daily. The protocol provided guidelines regarding the administration of systemic and topical corticosteroids. Patients were placed on a minimum of 15mg of oral prednisone a day at enrollment and tapered according to SUN guidelines.22 If patients experienced intolerable symptoms or a non-serious adverse event, they were allowed to reduce their study dose by one or two levels, while the physician remained masked. The first dose reduction was to 20mg for methotrexate or 1g in the morning and 500mg in the evening for mycophenolate mofetil. Treatment could be further reduced to 15mg for methotrexate or 500mg twice daily for mycophenolate mofetil.
Outcomes
The primary outcome of treatment success was achieving corticosteroid-sparing control of inflammation in both eyes at the 5 and 6 month visits. This was defined by the following:
≤0.5+ anterior chamber cells, ≤0.5+ vitreous cells, ≤0.5+ vitreous haze, and no active retinal/choroidal lesions
≤10 mg of oral prednisone daily and ≤2 drops of prednisolone acetate 1% (or equivalent) a day
no declaration of treatment failure due to intolerability or safety concerns
Prespecified secondary outcomes included time to corticosteroid-sparing control of inflammation, change in best spectacle-corrected visual acuity, resolution of macular edema, frequency of adverse events, medication adherence, and treatment success by anatomic location.
Sample Size Determination
A target enrollment of 80 patients was deemed feasible and sufficient to detect a clinically relevant difference in treatment success between patients treated with methotrexate and mycophenolate mofetil. Specifically, a sample of 40 patients per arm was estimated to provide 80% power to detect a difference of approximately 30% in treatment success, assuming a dropout rate of 10%, and a two-tailed alpha of 0.05.
Statistical Analyses
The primary outcome was analyzed with a complete case analysis using a Fisher’s exact test, excluding patients who dropped out or were lost to follow-up. This outcome was evaluated at the patient level as success was defined as controlled inflammation in both eyes. An additional intent-to-treat analysis was conducted using regression-based multiple imputation using the following variables: treatment success, most severe vitreous haze score in either eye at baseline, most severe anterior chamber cell score in either eye at baseline, active retinal or choroidal lesions in either eye at baseline, sex, and dose of corticosteroids at the start of the study. All 80 patients were included. Imputation was conducted using the M.I.C.E. (Multiple Imputation by Chained Equations) package for R (mice, The R Project for Statistical Computing, version 2.14 for MacIntosh, Vienna, Austria).
Time to corticosteroid-sparing control of inflammation was compared by treatment arm using the Wilcoxon rank-sum test. Visual acuity and macular edema were analyzed at the eye level, and only included eyes with uveitis at enrollment. The effect of treatment on visual acuity was assessed using mixed-effects linear regression, controlling for the last visit at which visual acuity was assessed. Resolution of macular edema was modeled using a mixed-effects logistic regression. The occurrence of adverse events between arms was compared with a Fisher’s exact test. If a patient reported the same event more than once, it was only counted once. Additional analyses compared treatment success by anatomical type of uveitis (i.e. intermediate or posterior/pan) and by presence of activity in the posterior segment at enrollment using a Fisher’s exact test. Proportion of missed doses was compared by arm using Wilcoxon rank sum test. All statistical tests were two-sided, and p-values less than 0.05 were considered statistically significant. Reported p-values were not adjusted for multiple comparisons. Data were analyzed using the R version 2.12 (The R Project for Statistical Computing, Vienna, Austria).
RESULTS
Between October 2011 and June 2012, 80 patients with non-infectious uveitis were enrolled (41 randomized to methotrexate and 39 to mycophenolate). Among the 80 patients randomized, 67 (35 methotrexate and 32 mycophenolate mofetil) completed the study or were declared treatment failures prior to the 6- month visit. Figure 2 outlines the procession of patients through the trial and which patients were included in the analysis.
Figure 2.
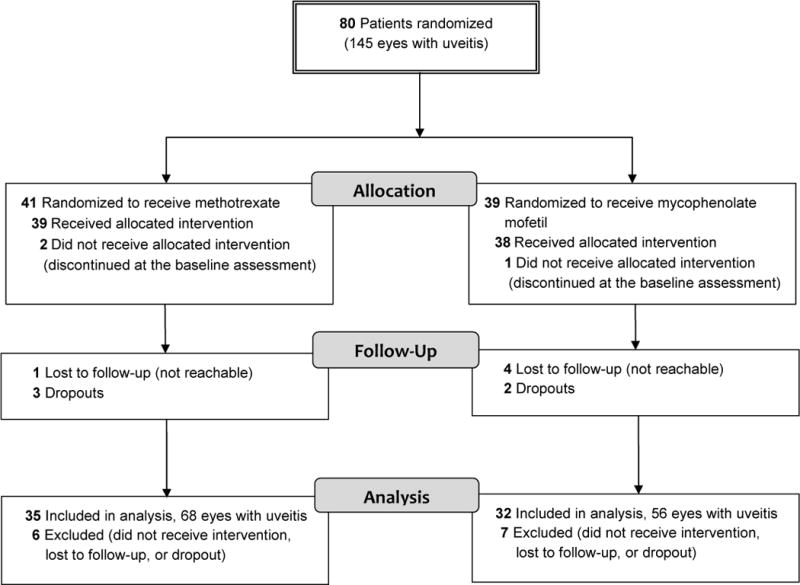
CONSORT diagram
Baseline demographic and clinical characteristics were distributed similarly between groups (Table 1) with the following exception: those taking methotrexate were more likely to have posterior/panuveitis (93% vs. 72% mycophenolate mofetil). The majority of patients had bilateral disease (90% methotrexate and 72% mycophenolate mofetil). The amount of corticosteroid exposure was similar between arms with a median of 40 mg at baseline for both. In the 90 days prior to enrollment, the highest oral prednisone (or equivalent) dose administered was similar between arms with a median of 60 mg in the methotrexate group and 53.3 mg in the mycophenolate mofetil group. Other than corticosteroids, all patients were immunosuppressant naïve, except for two who had briefly received methotrexate more than a year prior to enrollment and had stopped for financial reasons.Table 1 summarizes the highest level of inflammation in the 90 days prior to enrollment. Thirty percent of patients had active retinal/choroidal lesions in at least one eye at baseline and 65% in the past 90 days.
Table 1.
Baseline Characteristics of Patients by Treatment Assignment
| Methotrexate (N=41) |
Mycophenolate Mofetil (N=39) |
p-value | |
|---|---|---|---|
| Age mean years (SD) | 38.6 (10.3) | 40.2 (14.2) | 0.57 |
| Female N(%) | 26 (63) | 22 (56) | 0.65 |
| Occupation N(%) | 0.52 | ||
| Agricultural Worker | 4 (10) | 6 (15) | |
| Non-agricultural worker | 32 (78) | 27 (69) | |
| Student | 2 (5) | 4 (10) | |
| Retired | 0 (0) | 1 (3) | |
| Unemployed | 3 (7) | 1 (3) | |
| Uveitis Diagnosis N(%) | 0.09 | ||
| Vogt-Koyanagi-Harada disease | 27 (66) | 16 (41) | |
| Idiopathic | 3 (7) | 4 (10) | |
| Sympathetic Ophthalmia | 4 (10) | 4 (10) | |
| Pars Planitis | 0 (0) | 6 (15) | |
| Behcet’s Disease | 3 (7) | 3 (8) | |
| Retinal Vasculitis | 1 (2) | 1 (3) | |
| Sarcoidosis | 0 (0) | 2 (5) | |
| Non-Granulomotus Panuveitis | 2 (5) | 2 (5) | |
| Serpiginous Choroiditis | 0 (0) | 1 (3) | |
| Granulomotus Panuveitis | 1 (2) | 0 (0) | |
| Laterality of Uveitis N(%) | 0.05 | ||
| Bilateral | 37 (90) | 28 (72) | |
| Anatomic Location* N(%) | 0.02 | ||
| Anterior & Intermediate/Intermediate | 3 (7) | 11 (28) | |
| Posterior/Panuveitis | 38 (93) | 28 (72) | |
| Mean Visual Acuity** | |||
| logMAR (SD) | 0.42 (0.51) | 0.48 (0.55) | 0.59 |
| Location of Inflammation at Enrollment N(%) | 0.68 | ||
| Anterior chamber only | 4 (10) | 2 (5) | |
| Posterior segment (vitreous, retina, choroid) | 37 (90) | 37 (95) | |
| Macular Edema (in at least one eye) N(%) | 15 (37) | 18 (46) | 0.65 |
| Corticosteroids (prednisone mg) | |||
| Median at Baseline (IQR)*** | 40 (30–50) | 40 (27.5–50) | 0.94 |
| Median in past 90 days (IQR)**** | 60 (40–60) | 53.3 (3–60) | 0.73 |
| Highest Level of Inflammation past 90 days (either eye) N(%) | |||
| Anterior Chamber Cells | 0.93 | ||
| 0 | 6 (15) | 8 (21) | |
| 0.5+ | 6 (15) | 6 (15) | |
| 1+ | 10 (24) | 7 (18) | |
| ≥2 | 19 (46) | 18 (46) | |
| Vitreous Haze | 0.37 | ||
| 0 | 20 (49) | 13 (33) | |
| 0.5+ | 1 (2) | 4 (10) | |
| 1+ | 5 (12) | 9 (23) | |
| ≥2+ | 14 (34) | 13 (33) | |
| Could not access | 1 (2) | 0 (0) | |
| Anterior Vitreous Cells | 0.32 | ||
| 0 | 20 (49) | 14 (36) | |
| 0.5+ | 1 (2) | 4 (10) | |
| 1+ | 5 (12) | 8 (21) | |
| ≥2+ | 14 (34) | 13 (33) | |
| Could not access | 1 (2) | 0 (0) | |
| Active Retinal/Choroidal Lesions | 26 (63) | 26 (67) | 0.64 |
Anatomic location was assessed given all medical records available.
Best-corrected visual acuity of uveitic eyes only
Two protocol deviations occurred in which two patients on mycophenolate mofetil were placed on 10mg of prednisone, instead of the required ≥15mg at the start of the trial.
Corticosteroids in the past 90 days included oral, subcutaneous and intravenous and were adjusted to equivalent calculations of oral prednisone.
SD = standard deviation; logMAR = logarithm of the minimum angle of resolution; IQR = interquartile range
Treatment Success
In total, 39 patients (58%) had corticosteroid-sparing controlled inflammation at five and six months (15 with mycophenolate mofetil (47%) and 24 with methotrexate (69%)) (Table 2). There was no statistically significant difference in the proportion of patients achieving treatment success (p=0.09). Multiple imputation demonstrated similar results (p=0.13). Twenty-two patients (33%) did not achieve corticosteroid-sparing control at month five and six (15 with mycophenolate mofetil (47%) and 7 with methotrexate (20%)). In addition, 5 patients (8%) were declared treatment failures due to intolerability of side effects (3 with methotrexate and 2 with mycophenolate mofetil). One patient was declared a failure due to safety concerns after developing chicken pox while taking methotrexate.
Table 2.
Results from a Clinical Trial Comparing Methotrexate and Mycophenolate Mofetil for Non-Infectious Uveitis
| Patient Level | Methotrexate (N=35) |
Mycophenolate Mofetil (N=32) |
p-value |
|---|---|---|---|
| Treatment Success N(%) | 24 (69) | 15 (47) | 0.09 |
| Treatment Failure N(%) | 11 (31) | 17 (53) | |
| Reason for treatment failure N(%) | |||
| Lack of efficacy* | 7 (63) | 15 (88) | |
| Intolerability | 3 (27) | 2 (12) | |
| Safety concern | 1 (9) | 0 (0) | |
| Success by Anatomic Location N(%) | |||
| Anterior & Intermediate/Intermediate | 2/3 (67) | 5/10 (50) | 0.99 |
| Posterior/Panuveitis | 22/32 (69) | 10/22 (45) | 0.10 |
| Time to corticosteroid-sparing control of inflammation (days)** | |||
| Median (IQR) | 139 (62.5–142) | 124 (60–156) | 0.44 |
| Medication Compliance N(%) | |||
| Missed at least one dose | 13 (32) | 17 (44) | 0.97 |
|
| |||
| Eye Level | Methotrexate (E=68) |
Mycophenolate Mofetil (E=56) |
p-value |
|
| |||
| Mean change in visual acuity*** | 0.68 | ||
| logMAR (SD) | −0.26 (0.33) | −0.19 (0.36) | |
| Macular Edema Resolution N(%) | 17/22 (77) | 7/13 (54) | 0.31 |
Did not meet definition of corticosteroid-sparing controlled inflammation at 5 and 6 month visits
Time for those who met the definition of treatment success
Best-corrected visual acuity of uveitic eyes only
IQR = interquartile range; SD = standard deviation logMAR = logarithm of the minimum angle of resolution
Secondary Outcomes
Of those considered a treatment success, the median number of days to achieve corticosteroid-sparing control of inflammation was 139 days for methotrexate (Interquartile range (IQR) 62.5–142 days) and 124 days for mycophenolate mofetil (IQR 60–156 days), p=0.44. The mean change in BSCVA in the methotrexate group was −0.26 logMAR and in the mycophenolate mofetil group was −0.19 logMAR, p=0.68. Out of 35 eyes with macular edema (22 in methotrexate and 13 in mycophenolate mofetil), 17 eyes resolved with methotrexate (77%) and 7 eyes resolved with mycophenolate mofetil (54%), p=0.31. Twenty-two of the 32 patients with posterior/panuveitis (69%) achieved treatment success in the methotrexate group compared to 10 of the 22 (45%) in the mycophenolate mofetil group, p=0.10. Overall, 30 patients (38%) missed at least one medication dose: 13 in the methotrexate arm (32%) and 17 in the mycophenolate arm (44%), p=0.67. Of those who missed at least one dose, 67% missed no more than 5% of doses.
Adverse Events
One serious adverse event involving hospitalization due to hot water burns occurred but was deemed unrelated to the study drug. In total, 65 patients experienced at least one non-serious adverse event: 33 in the methotrexate arm (80%) and 32 in the mycophenolate mofetil arm (82%). For a description of all adverse events (serious and non-serious), see Table 3. There were no statistically significant differences by arm in the number of non-serious adverse events (p=0.38) or in the number of patients who experienced at least one adverse event (p=0.99). However, patients on mycophenolate mofetil were more likely to have a fever lasting over 12 hours (p=0.02). Two patients on methotrexate (6%) reduced their dose from the maintenance level of 25mg a week to 20mg a week due to tolerability issues.
Table 3.
Frequency of Adverse Events
| Methotrexate (N = 41) |
Mycophenolate Mofetil (N = 39) |
p-value | |
|---|---|---|---|
| N (%) | N (%) | ||
| Non-Serious | |||
| Ocular Events | |||
| Cataract | 5 (12) | 3 (8) | 0.81 |
| Ocular Hypertension | 4 (10) | 2 (5) | 0.68 |
| Glaucoma | 2 (5) | 2 (5) | 0.99 |
| Hypotony | 0 (0) | 2 (5) | 0.23 |
| Vitreous hemorrhage | 1 (2) | 0 (0) | 0.99 |
| Acute catarrhal conjunctivitis | 1 (2) | 0 (0) | 0.99 |
| Laboratory Events | |||
| Abnormal AST or ALT | 4 (10) | 2 (5) | 0.68 |
| Abnormal Hemoglobin | 0 (0) | 2 (5) | 0.23 |
| Systemic Events | |||
| Headache | 8 (20) | 12 (31) | 0.31 |
| Fever for 12 hours | 2 (5) | 9 (23) | 0.02 |
| Nausea | 6 (15) | 2 (5) | 0.26 |
| Diarrhea | 4 (10) | 4 (10) | 0.99 |
| Fatigue | 4 (10) | 4 (10) | 0.99 |
| Systemic infection | 3 (7) | 4 (10) | 0.71 |
| Vomiting | 3 (7) | 2 (5) | 0.99 |
| Allergic reaction | 0 (0) | 2 (5) | 0.23 |
| Dyspnea | 0 (0) | 1 (3) | 0.49 |
| Mood | 0 (0) | 1 (3) | 0.49 |
| Cardiac dysfunction | 0 (0) | 1 (3) | 0.49 |
| Serious | |||
| Hospitalization (not study related, burn wound) | 0 (0) | 1 (3) | 0.49 |
DISCUSSION
This randomized trial comparing methotrexate and mycophenolate mofetil for the treatment of non-infectious intermediate uveitis, posterior uveitis or panuveitis found that a higher, although not statistically significant, proportion of patients on methotrexate achieved treatment success (69% vs. 47%). The difference in treatment success was primarily due to efficacy in controlling inflammation; safety and tolerability issues were uncommon and did not differ between the treatment arms. Approximately half of the patients achieving treatment success had corticosteroid-sparing control of inflammation by 4.5 months, regardless of treatment assignment. Nearly 40% of patients had macular edema at baseline. Although not statistically significant, macular edema resolution was greatest in the methotrexate arm (77% vs. 54%). There was no difference in change in visual acuity between treatment arms, although overall patients on average improved by 10 letters.
Overall, both treatments were safe, well tolerated, and patients were able to adhere to the treatment regimens. Issues with tolerability and safety were uncommon, with only 6 patients (8%) stopping treatment. About 10% of patients in both groups suffered from gastrointestinal symptoms such as nausea, vomiting, and/or diarrhea. Abnormalities with hemoglobin and liver function tests were found in 10% or less of patients in both groups. Medication compliance was high in both treatment groups, with the majority of patients not missing any doses.
The results of this trial differ from most previously published retrospective studies2,11–19 and from physician opinion,21 raising questions on which immunosuppressant therapy should be used as first-line corticosteroid-sparing treatment. In a retrospective study by Galor et al., 42% of patients on methotrexate achieved treatment success after 6 months compared to 70% on mycophenolate mofetil.2 These results are similar to findings from other studies. Thorne et al. reported corticosteroid-sparing treatment success in 82% of patients on mycophenolate mofetil6 and Samson et al. reported success in 56% of patients on methotrexate.28 Studies published from the Systemic Immunosuppressive Therapy for Eye Diseases (SITE) cohort reported patients with posterior/panuveitis had corticosteroid-sparing success rates of 21% for methotrexate14 and 41% for mycophenolate mofetil.18
All of the above studies were retrospective and non-comparative, so their findings may differ from those of a clinical trial for a variety of reasons. Assessment protocols vary across studies, with different physicians measuring inflammation and visits occurring at inconsistent time points. In addition, the SUN classification was developed in 2005,22 so patients in studies before this date may have been assessed using different standards. Many patients in these studies had previously failed one or more immunosuppressive agents, in contrast to our trial. Dosing was also not standardized in these studies and patients on different doses were combined for analysis purposes. The average methotrexate dose ranged from 12mg to 20mg/wk,2,11,14,28 less than the 25mg/wk in this trial. This difference in dosing could have potentially affected outcomes. There has been one trial of 19 patients comparing methotrexate to interferon for uveitis-related macular edema.29 However, patients had very little inflammation in that study since the main focus was macular edema.
To our knowledge, this is the first randomized trial comparing the effectiveness of two frequently used antimetabolites for non-infectious uveitis. The major strength of this study is its prospective randomized design with standardized treatment and assessment regimens. Furthermore, this study was observer-masked to prevent bias. Beyond the comparison of treatments, this study provides detailed and unbiased information on patient outcomes. However, power and possible generalizability concerns may need consideration. The sample size was chosen for feasibility of enrollment in a single hospital system. Although we found a 22% difference, which could be considered clinically important, the study was underpowered to find statistical significance. As is common in many uveitis studies, it was not feasible to only enroll patients with a specific disease so a heterogeneous group of uveitis etiologies was included. Furthermore, all our patients were enrolled in India so there may be questions of generalizability to other populations. However, we do not know of any inherent biological difference that could affect treatment response.
Given the risk of damage from uncontrolled ocular inflammation and the severity of side effects related to long-term use of high dose corticosteroids, knowledge of the most effective initial corticosteroid-sparing treatment is essential for improving patient outcomes. There are also cost implications. Even though both medications are generic, a four week supply of maintenance dose methotrexate (25mg a week) costs $169 whereas an equivalent supply of mycophenolate mofetil (2g a day) costs $887.30
The large, but not significant, difference in efficacy favoring methotrexate necessitates further prospective investigation. These results are serving to inform a larger NEI-funded clinical trial currently underway at multiple international sites.
Acknowledgments
Financial Support: Funding for this trial was provided by That Man May See Foundation at UCSF and The South Asia Research Fund. Dr. Acharya is currently supported by an NEI U10 EY021125-01 grant. The UCSF Department of Ophthalmology is supported by the National Eye Institute and Research to Prevent Blindness Foundation.
The sponsor or funding organization had no role in the design or conduct of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting Presentation(s): This work was presented at the 12th International Symposium on Uveitis Meeting 2012 and at the American Uveitis Society Fall meeting at the American Academy of Ophthalmology Annual Meeting, November, 2012.
Conflict of Interest: No conflicting relationship exists for any author.
References
- 1.Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol. 2000;130:492–513. doi: 10.1016/s0002-9394(00)00659-0. [DOI] [PubMed] [Google Scholar]
- 2.Galor A, Jabs DA, Leder HA, et al. Comparison of antimetabolite drugs as corticosteroid-sparing therapy for noninfectious ocular inflammation. Ophthalmology. 2008;115:1826–32. doi: 10.1016/j.ophtha.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 3.Larson T, Nussenblatt RB, Sen HN. Emerging drugs for uveitis. Expert Opin Emerg Drugs. 2011;16:309–22. doi: 10.1517/14728214.2011.537824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siepmann K, Huber M, Stubiger N, et al. Mycophenolate mofetil is a highly effective and safe immunosuppressive agent for the treatment of uveitis: a retrospective analysis of 106 patients. Graefes Arch Clin Exp Ophthalmol. 2006;244:788–94. doi: 10.1007/s00417-005-0066-8. [DOI] [PubMed] [Google Scholar]
- 5.Teoh SC, Hogan AC, Dick AD, Lee RW. Mycophenolate mofetil for the treatment of uveitis. Am J Ophthalmol. 2008;146:752–60. doi: 10.1016/j.ajo.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Thorne JE, Jabs DA, Qazi FA, et al. Mycophenolate mofetil therapy for inflammatory eye disease. Ophthalmology. 2005;112:1472–7. doi: 10.1016/j.ophtha.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 7.Larkin G, Lightman S. Mycophenolate mofetil. A useful immunosuppressive in inflammatory eye disease. Ophthalmology. 1999;106:370–4. doi: 10.1016/S0161-6420(99)90078-7. [DOI] [PubMed] [Google Scholar]
- 8.Kilmartin DJ, Forrester JV, Dick AD. Rescue therapy with mycophenolate mofetil in refractory uveitis [letter] Lancet. 1998;352:35–6. doi: 10.1016/S0140-6736(05)79515-5. [DOI] [PubMed] [Google Scholar]
- 9.Baltatzis S, Tufail F, Yu EN, et al. Mycophenolate mofetil as an immunomodulatory agent in the treatment of chronic ocular inflammatory disorders. Ophthalmology. 2003;110:1061–5. doi: 10.1016/S0161-6420(03)00092-7. [DOI] [PubMed] [Google Scholar]
- 10.Choudhary A, Harding SP, Bucknall RC, Pearce IA. Mycophenolate mofetil as an immunosuppressive agent in refractory inflammatory eye disease. J Ocul Pharmacol Ther. 2006;22:168–75. doi: 10.1089/jop.2006.22.168. [DOI] [PubMed] [Google Scholar]
- 11.Bom S, Zamiri P, Lightman S. Use of methotrexate in the management of sight-threatening uveitis. Ocul Immunol Inflamm. 2001;9:35–40. doi: 10.1076/ocii.9.1.35.3983. [DOI] [PubMed] [Google Scholar]
- 12.Dev S, McCallum RM, Jaffe GJ. Methotrexate treatment for sarcoid-associated panuveitis. Ophthalmology. 1999;106:111–8. doi: 10.1016/S0161-6420(99)90011-8. [DOI] [PubMed] [Google Scholar]
- 13.Foeldvari I, Wierk A. Methotrexate is an effective treatment for chronic uveitis associated with juvenile idiopathic arthritis. J Rheumatol. 2005;32:362–5. [PubMed] [Google Scholar]
- 14.Gangaputra S, Newcomb CW, Liesegang TL, et al. Systemic Immunosuppressive Therapy for Eye Diseases Cohort Study. Methotrexate for ocular inflammatory diseases. Ophthalmology. 2009;116:2188–98. doi: 10.1016/j.ophtha.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holz FG, Krastel H, Breitbart A, et al. Low-dose methotrexate treatment in noninfectious uveitis resistant to corticosteroids. Ger J Ophthalmol. 1992;1:142–4. [PubMed] [Google Scholar]
- 16.Shah SS, Lowder CY, Schmitt MA, et al. Low-dose methotrexate therapy for ocular inflammatory disease. Ophthalmology. 1992;99:1419–23. doi: 10.1016/s0161-6420(92)31790-7. [DOI] [PubMed] [Google Scholar]
- 17.Taylor SR, Habot-Wilner Z, Pacheco P, Lightman SL. Intraocular methotrexate in the treatment of uveitis and uveitic cystoid macular edema. Ophthalmology. 2009;116:797–801. doi: 10.1016/j.ophtha.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 18.Daniel E, Thorne JE, Newcomb CW, et al. Mycophenolate mofetil for ocular inflammation. Am J Ophthalmol. 2010;149:423–32. doi: 10.1016/j.ajo.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sobrin L, Christen W, Foster CS. Mycophenolate mofetil after methotrexate failure or intolerance in the treatment of scleritis and uveitis. Ophthalmology. 2008;115:1416–21. doi: 10.1016/j.ophtha.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Myers TD, Smith JR, Wertheim MS, et al. Use of corticosteroid sparing systemic immunosuppression for treatment of corticosteroid dependent optic neuritis not associated with demyelinating disease. Br J Ophthalmol. 2004;88:673–80. doi: 10.1136/bjo.2003.028472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esterberg E, Acharya NR. Corticosteroid-sparing therapy: practice patterns among uveitis specialists. J Ophthalmic Inflamm Infect. 2012;2:21–8. doi: 10.1007/s12348-011-0047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Standardization of Uveitis Nomenclature (SUN) Working Group Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–16. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Multicenter Uveitis Steroid Treatment (MUST) Trial Research Group. Kempen JH, Altaweel MM, Holbrook JT, et al. Randomized comparison of systemic anti-inflammatory therapy versus fluocinolone acetonide implant for intermediate, posterior, and panuveitis: the Multicenter Uveitis Steroid Treatment Trial. Ophthalmology. 2011;118:1916–26. doi: 10.1016/j.ophtha.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nussenblatt RB, Palestine AG, Chan CC, Roberge F. Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology. 1985;92:467–71. doi: 10.1016/s0161-6420(85)34001-0. [DOI] [PubMed] [Google Scholar]
- 25.Barron BA, Gee L, Hauck WW, et al. Herpetic Eye Disease Study Group. Herpetic Eye Disease Study: a controlled trial of oral acyclovir for herpes simplex stromal keratitis. Ophthalmology. 1994;101:1871–82. doi: 10.1016/s0161-6420(13)31155-5. [DOI] [PubMed] [Google Scholar]
- 26.Chan A, Duker JS, Ko TH, et al. Normal macular thickness measurements in healthy eyes using Stratus optical coherence tomography. Arch Ophthalmol. 2006;124:193–8. doi: 10.1001/archopht.124.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu T, Hu AY, Kaines A, et al. A pilot study of normative data for macular thickness and volume measurements using Cirrus high-definition optical coherence tomography. Retina. 2011;31:1944–50. doi: 10.1097/IAE.0b013e31820d3f13. [DOI] [PubMed] [Google Scholar]
- 28.Samson CM, Waheed N, Baltatzis S, Foster CS. Methotrexate therapy for chronic noninfectious uveitis: analysis of a case series of 160 patients. Ophthalmology. 2001;108:1134–9. doi: 10.1016/s0161-6420(01)00576-0. [DOI] [PubMed] [Google Scholar]
- 29.Mackensen F, Jakob E, Springer C, et al. Interferon versus methotrexate in intermediate uveitis with macular edema: results of a randomized controlled clinical trial. Am J Ophthalmol. 2013;156:478–86. doi: 10.1016/j.ajo.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Truven Health Analytics Micromedex Clinical Knowledge [Internet] Truven Health Analytics; c2014 RED BOOK Drug References; c2014 [cited 2014 Feb 4] Available from: http://micromedex.com/redbook.


