Abstract
While a coronary artery calcium score of 0 is associated with a very low 10-year risk for cardiac events, this risk is non-zero. A family history of CHD and has been associated with more subclinical atherosclerosis than individuals without a family history of CHD. The purpose of this study was to assess the significance of a family history for coronary heart disease (CHD) among individuals with a coronary artery calcium (CAC) score of 0. The MESA cohort includes 6,814 participants free of clinical CVD at baseline. Positive family history was defined as reporting a parent, sibling, or child who had a heart attack. Time to incident CHD or CVD event was modeled using multivariable Cox regression. 3,185 subjects were identified from the original MESA cohort as having a baseline CAC score of 0 (mean age 58 years, 37% male). Over a median follow-up of 10 years, 101 (3.2%) participants had CVD events and 56 (1.8%) had CHD events. In age and gender adjusted analyses, a family history of CHD was associated with an approximate 70% increase in CVD (HR 1.73, 95% CI 1.17-2.56) and CHD (HR 1.72, 95% CI 1.01-2.91) events. CVD events remained significant after further adjustment for ethnicity, risk factors and baseline medication use. In conclusion, asymptomatic individuals with a 0 CAC score and a positive family history of CHD are at increased risk for CVD and CHD events compared to those without a family history of CHD, although absolute event rates remain low.
Keywords: coronary calcium, family history, low risk
Introduction
While the presence and extent of coronary artery calcium (CAC) is directly proportional to subsequent cardiovascular events, a 0 CAC score is associated with a very low cardiac event rate of about 1% over a 10-year period (1,2). However, data from clinical trials and observational studies have documented the presence of non-calcified plaque by cardiac computed tomographic angiography (CCTA) with varying extent and severity in individuals with a 0 CAC score, with a prevalence ranging from 4%-24% depending on the population studied and symptom status (3–6). Subclinical non-calcified atherosclerotic plaque is more prevalent in males, and those with diabetes, active smoking, a family history of premature CHD, or those presenting with chest pain symptoms (7–9). These data suggest that although the absolute 10-year risk is low for a cardiovascular event among asymptomatic patients with a CAC score of 0, there may be subsets in whom risk is higher and for whom the overall predicted risk may be a misattribution. Data addressing this question in asymptomatic patients with a CAC score of 0 and a positive family history of CHD have not been published to the best of our knowledge. We therefore investigated the CHD and cardiovascular disease (CVD) event rates in a diverse population of individuals with a 0 CAC score at baseline comparing those with versus without a family history of CHD. We hypothesized that after adjustment for standard risk factors, the observed event rates would be higher in the group with a positive family history.
Methods
The Multi-Ethnic Study of Atherosclerosis (MESA) is a longitudinal, population-based study of 6,814 men and women, initially free of clinical cardiovascular disease, aged 45–84 years at baseline, and recruited from 6 field centers: Baltimore, MD; Chicago, IL; Forsyth County, NC; Los Angeles, CA; New York, NY; and St. Paul, MN. Specific racial/ethnic groups enrolled included white, black, Hispanic and Chinese. Approximately 50% of the participants enrolled were female. Details of the MESA recruitment strategy are published elsewhere (10). The baseline visit took place between July 2000 and September 2002. MESA was approved by institutional review boards at each site, and all participants gave written informed consent. The design of the study has been described in detail previously (11).
Information was obtained at the baseline exam in 2000–2002 regarding age, sex, ethnicity, and medical history by questionnaires. At this exam, participants also reported the presence or absence of a family history of CHD. The family history of CHD was obtained by asking participants whether any parent, sibling or child had experienced a heart attack. The age of the family member at the time of their heart attack was not obtained during the baseline exam, and therefore was not available for analysis. Current smoking was defined as having smoked in the last 30 days, whereas former smoker was defined as an individual who is not currently smoking but had smoked ≥ 100 cigarettes in his or her lifetime. Diabetes was defined as a fasting glucose ≥ 126 mg/dL or on hypoglycemic medication. Use of antihypertensive and other medications was based on clinic staff entry of prescribed medications. Resting blood pressure was measured 3 times in the seated position using a Dinamap model Pro 100 automated oscillometric sphygmomanometer (Critikon, Tampa, FL), and the average of the second and third readings was recorded. Hypertension was defined as a systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or use of medications together with a self-reported diagnosis of high blood pressure. Total and high-density lipoprotein cholesterol and triglyceride levels were measured from blood samples obtained after a 12-hour fast. Low-density lipoprotein cholesterol was calculated with the Friedewald equation (12). The Framingham Risk Score for men and women was calculated on the basis age, total cholesterol, high-density lipoprotein cholesterol, current smoking status, high blood pressure, and the use of anti-hypertensive medications (13).
CAC was assessed by chest computed tomography using either a cardiac-gated electronbeam computed tomography scanner (Chicago, Los Angeles, and New York field centers) or a multi-detector computed tomography scanner system (Baltimore, Forsyth County, and St. Paul field centers). Certified technologists scanned all participants twice over phantoms of known physical calcium concentration. A radiologist or cardiologist read all computed tomography scans at a central reading center (Los Angeles Biomedical Research Institute at Harbor-UCLA in Torrence, CA). We used the average Agatston score (14) for the 2 scans in all analyses. Carr et al. (15) have reported the details of the MESA computed tomography scanning and interpretation methods.
At the time of analysis, the cohort had been followed for incident CVD and CHD events for a median of 10 years. At intervals of 9–12 months, a telephone interviewer contacted each participant to inquire about interim hospital admissions, cardiovascular outpatient diagnoses, and deaths. Trained personnel abstracted medical records suggesting possible cardiovascular events. Two physicians independently classified the events and assigned incidence dates. If, after review and adjudication, disagreements persisted, a full mortality and morbidity review committee made the final classification. For the purposes of this study, we used all CVD and CHD events as the endpoint. Specifically, the endpoint included myocardial infarction, CHD death, resuscitated cardiac arrest, angina, stroke, stroke death, or other CVD death for total CVD events and myocardial infarction, CHD death, resuscitated cardiac arrest and angina for total CHD events (11).
There were 3,416 participants with CAC = 0 at baseline. Of these, 183 were missing data for family history, 17 were removed because they lacked follow-up time and an additional 31 participants were removed due to missing covariate information. Values from the baseline exam were used for the covariates. Only the first CVD or CHD event for each participant was considered. The annual incident rates of first CVD and CHD events, for subjects with and without family history, were estimated with Poisson rate models. The family history groups were compared with rate ratios. Robust standard error was used. Kaplan-Meier graphs compared the cumulative probability distributions over time between family history groups. Differences between the curves were tested using non-parametric log rank and Peto-Peto tests. Cox proportional hazards models were used to estimate the effect of family history on time to incident CVD or CHD, adjusted for other covariates. Controlling covariates included age, gender, race, Framingham Risk Score, and use of baseline medications (statins and aspirin). The proportional hazards assumption was tested with Schoenfeld residuals and time interacting covariates. The number needed to treat (NNT) for statin and aspirin was calculated separately using the subset of participants who were not on the medication at exam 1, using a 10-year time interval and relative risk estimates for statins and aspirin from the Cochrane meta-analyses. Since some participants were started on statins or aspirin at a later date, the risk ratios were adjusted to compensate for the fraction of the exposure time on the medications. The 10-year number needed to treat was then rescaled to 5 years. All statistical analyses were performed using STATA 12.0 and SAS 9.3 for Windows.
Results
At the time of analysis a total of 3,185 subjects had a baseline CAC score of 0 and complete data, thus comprising the study cohort for this analysis (mean age 58 years, 37% male). There were 1,185 (37%) subjects with a self-reported family history of CHD in a first degree relative. Table 1 shows the baseline characteristics according to the presence or absence of a family history of CHD. The two groups had a similar median age, comparable frequencies of most CVD risk factors and comparable Framingham Risk Scores. However, the group with a positive family history of CHD had a lower percentage of males and differed in ethnicity (more Whites and less Chinese Americans). Despite no differences in the frequency of dyslipidemia or Framingham Risk Scores, baseline use of statins and aspirin were more common in those with a family history of CHD.
Table 1.
Baseline characteristics according to Family History
| Baseline CAC Score = 0 | |||
|---|---|---|---|
| Variable | FamHx + (n = 1185) |
FamHx - (n = 2000) |
p-value |
| Mean Age (Years) | 58 | 58 | 0.27 |
| Men | 370 (31%) | 799 (40%) | <0.0001 |
| Ethnicity | <0.0001 | ||
| White | 477 (40%) | 584 (29%) | |
| Chinese | 68 (6%) | 303 (15%) | |
| African-American | 373 (31%) | 609 (30%) | |
| Hispanic | 267 (23%) | 504 (25%) | |
| Dyslipidemia* | 336 (28%) | 515 (26%) | 0.11 |
| Smoker | 0.09 | ||
| Never | 637 (54%) | 1142 (57%) | |
| Former | 372 (31%) | 608 (30%) | |
| Current | 176 (15%) | 250 (13%) | |
| Hypertension† | 452 (38%) | 658 (33%) | 0.003 |
| Diabetes Mellitus | 102 (9%) | 182 (9%) | 0.64 |
| Impaired Fasting Glucose | 144 (12%) | 230 (12%) | 0.58 |
| Mean Framingham Risk Score | 6.1% | 6.2% | 0.84 |
| Baseline Statin Use | 139 (12%) | 174 (9%) | 0.005 |
| Baseline Aspirin Use | 256 (22%) | 309 (16%) | 0.000 |
Dyslipidema – based on adult treatment panel (ATP) III definitions of total and/or low-density cholesterol.
Hypertension – systolic blood pressure >140 mmHg, diastolic blood pressure > 90 mmHg, or use of medications together with a self-reported diagnosis of high blood pressure.
FamHx = Family history of coronary heart disease
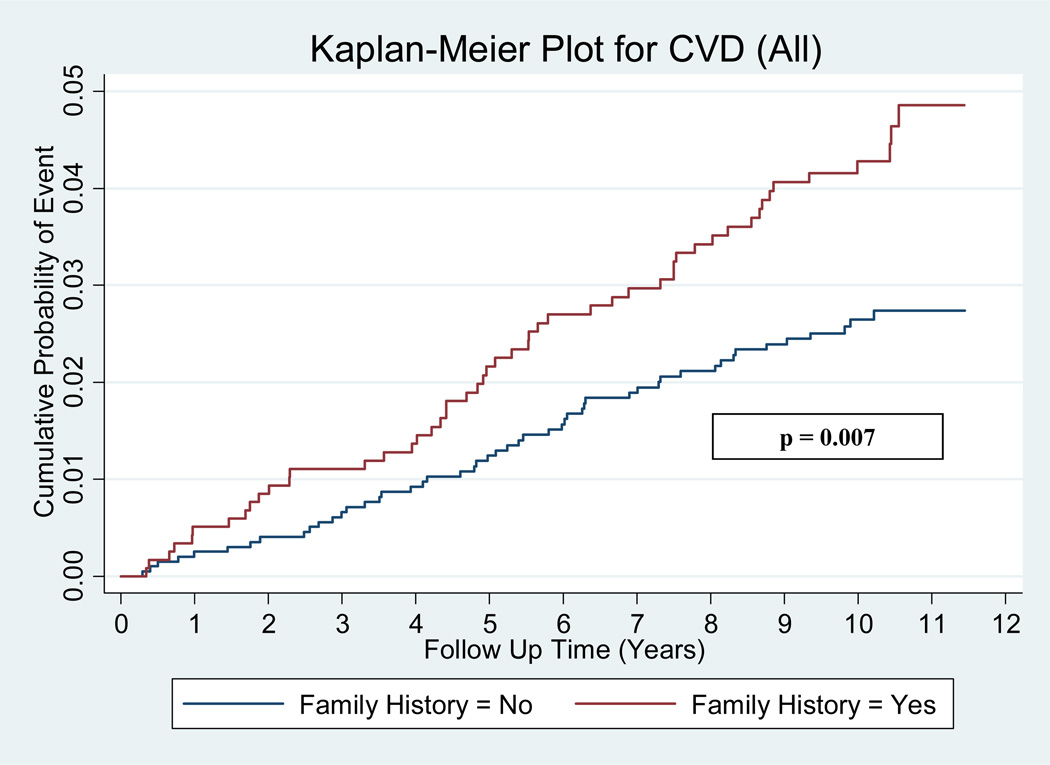
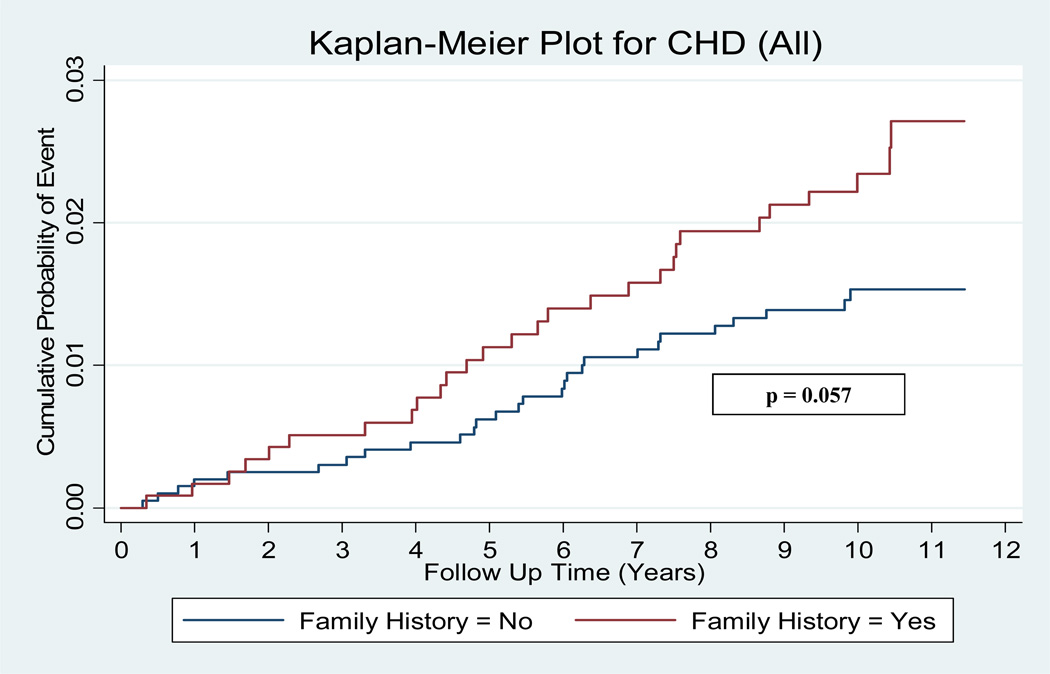
Over the median 10 year follow-up period 101 (3.2%) participants had CVD events and 56 (1.8%) had CHD events. Among individuals with a positive family history for CHD, 51/1185 (4.3%) experienced CVD events (versus 2.5% for negative family history), while 28/1185 (2.4%) had CHD events (versus 1.4% for negative family history). The annual incident event rate in the group with a positive family history for CHD compared to those with negative family history was 0.44% vs. 0.26% for CVD events (rate ratio 1.70, p = 0.007) and 0.24% vs. 0.14% (rate ratio 1.67, p = 0.056) for CHD events. Figures 1 and 2 illustrate the cumulative probabilities for CVD and CHD events among the subjects with a positive family history versus negative family history. There was a statistically significant difference in the cumulative probability for CVD events, while CHD events were marginally non-significant.
Figure 1.
Kaplan-Meier curves for CVD events. Kaplan-Meier curves for all cardiovascular disease (CVD) events according to family history positive versus family history negative individuals with a baseline coronary artery calcium (CAC) score of 0.
Figure 2.
Kaplan-Meier curves for CHD events. Kaplan-Meier curves for all coronary heart disease (CHD) events according to family history positive versus family history negative individuals with a baseline coronary artery calcium (CAC) score of 0.
Following adjustment for age and gender, a family history of CHD was significantly associated with both CVD (hazard ratio 1.73, 1.17-2.56) and CHD (hazard ratio 1.60, 1.08-2.38) events. After additional adjustment for ethnicity, Framingham Risk Score and baseline use of aspirin or statin, a family history of CHD was only significantly associated with CVD events (hazard ratio 1.72, 1.01-2.91).
Of the 3,185 subjects in this analysis, only 3,167 had complete data involving aspirin use. A separate analysis involving this smaller cohort revealed no important differences in outcomes. Due to the limited number of events in the CAC=0 cohort the Framingham Risk Score was chosen as a surrogate covariate. A separate analysis involving the major individual risk factors revealed no important differences (Table 2). A model involving smoking, hypertension, diabetes, diabetes by time interaction, statin and aspirin medication revealed no significant change in the hazard for CVD events (hazard ratio 1.54, 1.04-2.30). Due to the limited number of CHD events, a similar model involving the individual risk factors and medications was not possible. Hypertensive medications were also added as a controlling covariate with no important changes in the hazard ratios for CVD and CHD events. The new cardiovascular risk score was also substituted for the Framingham Risk Score in the fully adjusted model with no important differences in events noted (hazard ratios 1.58, 1.06-2.34 for CVD and 1.54, 0.90-2.62 for CHD). The potential for interaction between the effect of family history and time on the hazards for CVD and CHD events was also investigated with no significant interaction noted (p=0.83 for CVD and p=0.62 for CHD). Gender and ethnic differences were also investigated, with no important differences in either CVD or CHD events for gender. For ethnicity, Caucasians (relative to Chinese-Americans) were found to have a slightly higher hazard for CVD events, which was marginally significant at a p=0.04.
Table 2.
Events for Positive Family History According to Individual Risk Factors (Baseline CAC = 0)
| Risk Factor | CVD Events* | CHD Events* | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Smoking | 1.56 (1.05-2.32) | 0.027 | 1.52 (0.90-2.60) | 0.120 |
| Hypertension | 1.57 (1.05-2.33) | 0.027 | 1.55 (0.91-2.63) | 0.107 |
| Hyperlipidemia | 1.59 (1.07-2.37) | 0.021 | 1.56 (0.92-2.65) | 0.102 |
| Diabetes | 1.61 (1.08-2.39) | 0.019 | 1.57 (0.92-2.67) | 0.098 |
Adjusted for age, gender, ethnicity, baseline medication and individual risk factor. For CVD, diabetes interacts with time. Cardiovascular disease (CVD); confidence interval (CI); coronary artery calcium (CAC); coronary heart disease (CHD); hazard ratio (HR)
Discussion
In a large, ethnically diverse population with a 0 CAC score at baseline, we found that individuals with a family history of CHD had a significantly increased risk of both CVD and CHD events. This relation persisted after adjustment for age, gender, ethnicity, Framingham Risk Score and baseline medication use.
Our study expands on the current body of literature concerning family history of CHD and future CVD events. Prior studies have examined the relation between family history of premature CHD and subclinical atherosclerosis (as measured by coronary artery calcium) and found strong and consistent associations across diverse populations, including those with low Framingham Risk (16), women (17) and those with varying types of family history [first-degree versus second-degree relative, parent versus sibling] (18,19). Even among those with 0 CAC at baseline, a family history of premature CHD was found to be associated with greater incident CAC development (20). In a related study to ours, Budoff and colleagues found elevated CHD events among asymptomatic individuals with a baseline CAC score ranging from 0–10 and a family history of heart attack (21). These findings are consistent with the present analysis but lacked adequate statistical power to be significant. For our study we focused on the family history data available at the baseline MESA exam (history of heart attack in any parent, sibling or child, regardless of age at onset). While we feel this makes our study more generalizable to the population at-large, questions arise as to how our results would differ if we further stratified family history into premature onset versus late-onset. To address this question we performed a sensitivity analysis using family history data from MESA exam 2. Although there was a progressive increase in event rates according to family history status (family history none = 0.26 per 100 person-years, family history late onset = 0.37 per 100 person-years, family history premature = 0.44 per 100 person-years), the absolute differences were small and the overall effect of the group was marginally non-significant (p=0.086). We believe this sensitivity analysis is consistent with our main finding but is statistically underpowered.
Reasons for the elevated CVD and CHD event rates in those with a family history of CHD and 0 baseline CAC are complex and likely multifactorial. A number of prior studies have documented non-calcified plaque of varying extent and severity ranging from 4–24% (3–6). Although different patient populations and study designs were used, it appears that certain individuals are more likely to fall into this category of subclinical CHD with zero calcium, namely those with diabetes, male gender, active smoking, a family history of premature CAD, or those presenting with chest pain symptoms (7–9). Thus, a greater pool of lipid-rich, noncalcified plaque should be considered as a potential contributing factor to the elevated event rates in this population. Other considerations include a shorter “warranty period” for a 0 CAC score (with more accelerated atherosclerosis in those with a family history of CHD), as well as more rapid development and higher acuity risk factors for CHD over time. A less likely but possible explanation is that individuals in this group are experiencing CVD events unrelated to atherosclerosis, including cardioembolic events, vasospasm, or coronary or aortic dissections. Finally, the threshold of CAC detection used in MESA (although well validated) could result in small calcified plaques being undetected. Consistent with this possibility, a nearly 3 fold increase in CHD events has been described among those with CAC scores between 1–10 versus 0 (21).
The clinical implications of our findings need to take into account absolute as well as relative increases in event rates in those with positive family history. The annual CVD and CHD event rates for the entire MESA cohort with a baseline CAC score of 0 was 0.33% and 0.18% respectively. A family history of CHD increased the event rate to 0.44% for CVD events and 0.24% for CHD events. To better understand the clinical significance of our findings, we calculated the 5 year number needed to treat (NNT) with statins, and also with aspirin, using previously published summary estimates for CVD and CHD events (22,23). The number needed to treat with statins for 5 years to prevent 1 CVD event is 197 for family history positive versus 327 for family history negative individuals. For aspirin, the number needed to treat for 5 years to prevent 1 CVD event is 312 for family history positive versus 605 for family history negative individuals. These results were minimally changed when a premature family history of CHD was considered.
Although the recent AHA/ACC guidelines do not consider family history of CHD as part of the 10-year or lifetime risk estimates for CVD, it is endorsed as an important factor to consider for individuals with borderline significant risk estimates (24). We feel our results are clinically important, and provide additional support for this recommendation even in patients with a 0 CAC score.
A family history of coronary heart disease is a potent risk factor
We investigated its significance in those with a CAC score of 0
A 70% increase in cardiovascular events was noted in those with a family history
Family history is a potent risk factor, even among those with CAC scores of 0
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
This research was supported by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-RR-025005 from NCRR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blaha M, Budoff MJ, Shaw LJ, Khosa F, Rumberger JA, Berman D, Callister T, Raggi P, Blumenthal RS, Nasir K. Absence of Coronary Artery Calcification and All-Cause Mortality. JACC: Cardiovasc Imaging. 2009;2:692–700. doi: 10.1016/j.jcmg.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Mosler TP, Tseng PH, Flores FR, Callister TQ, Raggi P, Berman DS. Long-Term Prognosis Associated With Coronary Calcification Observations From a Registry of 25,253 Patients. J Am Coll Cardiol. 2007;49:1860–1870. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 3.Cho I, Chang H-J, Sung JM, Pencina MJ, Lin FY, Dunning AM, Achenbach S, Al-Mallah M, Berman DS, Budoff MJ, Callister TQ, Chow BJW, Delago A, Hadamitzky M, Hausleiter J, Maffei E, Cademartiri F, Kaufmann P, Shaw LJ, Raff GL, Chinnaiyan KM, Villines TC, Cheng V, Nasir K, Gomez M, Min JK. Coronary Computed Tomographic Angiography and Risk of All-Cause Mortality and Nonfatal Myocardial Infarction in Subjects Without Chest Pain Syndrome From the CONFIRM Registry (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) Circulation. 2012;126:304–313. doi: 10.1161/CIRCULATIONAHA.111.081380. [DOI] [PubMed] [Google Scholar]
- 4.Choi E-K, Choi SI, Rivera JJ, Nasir K, Chang S-A, Chun EJ, Kim H-K, Choi D-J, Blumenthal RS, Chang H-J. Coronary Computed Tomography Angiography as a Screening Tool for the Detection of Occult Coronary Artery Disease in Asymptomatic Individuals. J Am Coll Cardiol. 2008;52:357–365. doi: 10.1016/j.jacc.2008.02.086. [DOI] [PubMed] [Google Scholar]
- 5.Gottlieb I, Miller JM, Arbab-Zadeh A, Dewey M, Clouse ME, Sara L, Niinuma H, Bush DE, Paul N, Vavere AL, Texter J, Brinker J, Lima JoAC, Rochitte CE. The Absence of Coronary Calcification Does Not Exclude Obstructive Coronary Artery Disease or the Need for Revascularization in Patients Referred for Conventional Coronary Angiography. J Am Coll Cardiol. 2010;55:627–634. doi: 10.1016/j.jacc.2009.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haberl R, Tittus J, Bohme E, Czernik A, Richartz BM, Buck, Steinbigler P. Multislice spiral computed tomographic angiography of coronary arteries in patients with suspected coronary artery disease: An effective filter before catheter angiography? Am Heart J. 2005;149:1112–1119. doi: 10.1016/j.ahj.2005.02.048. [DOI] [PubMed] [Google Scholar]
- 7.Bamberg F, Dannemann N, Shapiro MD, Seneviratne SK, Ferencik M, Butler J, Koenig W, Nasir K, Cury RC, Tawakol A, Achenbach S, Brady TJ, Hoffmann U. Association Between Cardiovascular Risk Profiles and the Presence and Extent of Different Types of Coronary Atherosclerotic Plaque as Detected by Multidetector Computed Tomography. Arterioscler Thromb Vasc Biol. 2008;28:568–574. doi: 10.1161/ATVBAHA.107.155010. [DOI] [PubMed] [Google Scholar]
- 8.Funabashi N, Asano M, Komuro I. Predictors of non-calcified plaques in the coronary arteries of 242 subjects using multislice computed tomography and logistic regression models. Int J Cardiol. 2007;117:191–197. doi: 10.1016/j.ijcard.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Rivera JJ, Nasir K, Cox PR, Choi E-K, Yoon Y, Cho I, Chun E-J, Choi S-I, Blumenthal RS, Chang H-J. Association of traditional cardiovascular risk factors with coronary plaque subtypes assessed by 64-slice computed tomography angiography in a large cohort of asymptomatic subjects. Atherosclerosis. 2009;206:451–457. doi: 10.1016/j.atherosclerosis.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 10.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacobs DR, Jr, Kronmal R, Liu K, Nelson JC, Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-Ethnic Study of Atherosclerosis: Objectives and Design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 11.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O'Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary Calcium as a Predictor of Coronary Events in Four Racial or Ethnic Groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 12.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, Without Use of the Preparative Ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 13.Expert Panel on the, Evaluation, and Treatment of High Blood Cholesterol. Executive summary of the third report of the national cholesterol education program (ncep) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel iii) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 14.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte JM, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 15.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified Coronary Artery Plaque Measurement with Cardiac CT in Population-based Studies: Standardized Protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) Study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 16.Nasir K, Budoff MJ, Wong ND, Scheuner M, Herrington D, Arnett DK, Szklo M, Greenland P, Blumenthal RS. Family History of Premature Coronary Heart Disease and Coronary Artery Calcification: Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2007;116:619–626. doi: 10.1161/CIRCULATIONAHA.107.688739. [DOI] [PubMed] [Google Scholar]
- 17.Michos ED, Vasamreddy CR, Becker DM, Yanek LR, Moy TF, Fishman EK, Becker LC, Blumenthal RS. Women with a low Framingham risk score and a family history of premature coronary heart disease have a high prevalence of subclinical coronary atherosclerosis. Am Heart J. 2005;150:1276–1281. doi: 10.1016/j.ahj.2005.02.037. [DOI] [PubMed] [Google Scholar]
- 18.Taylor AJ, Bindeman J, Bhattarai S, Feuerstein IM, O'Malley PG. Subclinical Calcified Atherosclerosis in Men and Its Association With Family History of Premature Coronary Heart Disease in First- and Second-Degree Relatives. Prev Cardiol. 2004;7:163–167. doi: 10.1111/j.1520-037x.2004.03639.x. [DOI] [PubMed] [Google Scholar]
- 19.Nasir K, Michos ED, Rumberger JA, Braunstein JB, Post WS, Budoff MJ, Blumenthal RS. Coronary Artery Calcification and Family History of Premature Coronary Heart Disease: Sibling History Is More Strongly Associated Than Parental History. Circulation. 2004;110:2150–2156. doi: 10.1161/01.CIR.0000144464.11080.14. [DOI] [PubMed] [Google Scholar]
- 20.Pandey AK, Blaha MJ, Sharma K, Rivera J, Budoff MJ, Blankstein R, Al-Mallah M, Wong ND, Shaw L, Carr J, O'Leary D, Lima JAC, Szklo M, Blumenthal RS, Nasir K. Family history of coronary heart disease and the incidence and progression of coronary artery calcification: Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2014;232:369–376. doi: 10.1016/j.atherosclerosis.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Budoff MJ, McClelland RL, Nasir K, Greenland P, Kronmal RA, Kondos GT, Shea S, Lima JAC, Blumenthal RS. Cardiovascular events with absent or minimal coronary calcification: The Multi-Ethnic Study of Atherosclerosis (MESA) Am Heart J. 2009;158:554–561. doi: 10.1016/j.ahj.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor F, Ward K, Moore THM, Burke M, Davey Smith G, Casas JP, Ebrahim S. Statins for the primary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews. 2011;1:1–38. doi: 10.1002/14651858.CD004816.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson PPR, Aarabi M, Wallis E. Aspirin for primary prevention of coronary heart disease. Cochrane Database of Systematic Reviews. 2004;1:1–6. doi: 10.1002/14651858.CD004586. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson J, Schwartz JS, Shero ST, Smith SC, Sorlie P, Stone NJ, Wilson PWF. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]