Abstract
Background
Microbleeds, small perivascular collections of hemosiderin manifested radiologically as hypointensities on gradient-echo magnetic resonance imaging (MRI), are important markers of small vessel pathology. Despite their clinical relevance, little is known about their prevalence and demographic correlates, particularly among ethnically diverse older adults. We examined demographic and clinical correlates of regional microbleeds in a multi-ethnic cohort and examined categorization schemes of microbleed distribution and severity.
Methods
Between 2005 and 2007, 769 individuals participated in a MRI study as part of the Washington Heights/Inwood Columbia Aging Project. Approximately four years later, 243 out of 339 participants (mean age=84.50) who returned for a repeat MRI had gradient-echo scans for microbleed assessment and comprised the sample. We examined the association of deep and lobar microbleeds with age, sex, education, vascular factors, cognitive status and markers of small vessel disease.
Results
Sixty-seven of the 243(27%) participants had at least one microbleed. Individuals with microbleeds were more likely to have a history of stroke than individuals without. When categorized as having either no microbleeds, microbleeds in deep regions only, in lobar regions only, and both deep and lobar microbleeds, hypertension, proportion of strokes, and white matter hyperintensity volume (WMH) increased monotonically across the four groups. Number of lobar microbleeds correlated with WMH volume and diastolic blood pressure.
Conclusions
Microbleeds in deep and lobar locations are associated with worse outcomes than microbleeds in either location alone, although presence of lobar microbleeds appears to be more clinically relevant.
Keywords: cerebral microbleeds, community, small-vessel disease, multi-ethnic, vascular risk factors, white matter hyperintensities, stroke
Introduction
Cerebral microbleeds are perivascular collections of hemosiderin that manifest radiologically as focal signal loss on T2*-weighted gradient-echo magnetic resonance imaging (MRI). Together with white matter hyperintensities (WMH) and lacunar infarcts, microbleeds have recently emerged as an important third MRI marker of small vessel pathology. They are frequently seen among healthy older adults and their prevalence increases with age [1–3]. When distributed in deep and infratentorial regions, such as the basal ganglia, thalamus and brainstem, microbleeds typically are the result of hypertensive vasculopathy [3]. When they appear in cortical and subcortical regions of the cerebral lobes (i.e., “lobar” distribution), microbleeds typically reflect hemorrhagic lesions attributable to cerebral amyloid angiopathy (CAA), which refers to the deposition of beta-amyloid in the media and adventitia of small cerebral arterioles [3–5].
Microbleeds are relevant to cognitive aging in three ways. First, community-based studies and studies with patients meeting clinical criteria for CAA [6] have suggested that higher numbers of microbleeds, as a proxy measure of the severity of CAA pathology, are related to poorer cognition [7–9]. Second, consistent findings have implicated markers of cerebrovascular disease in the clinical presentation and pathogenesis of Alzheimer's disease (AD) [10–13]. That the vast majority of patients with AD have evidence of CAA at autopsy [14] provides evidence of a mechanistic link between cerebrovascular disease and the pathogenesis of AD. Finally, with the implementation of novel anti-amyloid treatments for AD comes the clinical risk of developing microbleeds, or so-called “amyloid-related imaging abnormalities” (ARIA-H) [15,16]. Despite the clinical importance of microbleeds, little is known about their prevalence and clinical and demographic correlates, particularly among racially and ethnically diverse older adults who increasingly comprise the aging population.
The purpose of this study was to examine the demographic and clinical correlates of lobar and deep microbleeds in a multi-ethnic, community-based cohort of older adults residing in northern Manhattan. Based on the extant literature [2,3,17–19] we examined age, sex, education, vascular risk factors (hypertension, blood pressure, diabetes history, smoking history), and cognitive status (dementia, mild cognitive impairment). We were also interested in determining whether microbleeds were related to white matter hyperintensities and history of clinical stroke. Furthermore, there is currently no consensus on how best to categorize the severity of microbleeds. It is unclear, for example, whether simply having a microbleed or the regional (deep or lobar) distribution or number of microbleeds is clinically relevant. So, a second goal was to examine various categorization schemes of the distribution and severity of microbleeds as they relate to these demographic and clinical variables.
Methods
Participants
Participants were drawn from the Washington Heights/Inwood Columbia Aging Project (WHICAP), an ongoing prospective community-based longitudinal study of aging and dementia in northern Manhattan, New York, in residents aged 65 and older that began in 1992. The recruitment procedures and sampling strategies have been described in detail in earlier work [20]. Participants received a full medical, neurological, and neuropsychological examination at each of the follow-up visits, approximately every 18 to 24 months. Beginning in 2005, participants who did not meet criteria for dementia at their previous follow-up were invited to participate in a high-resolution MRI study, as previously described [21]. As a result, 769 participants underwent high resolution structural MRI (“baseline MRI”). The individuals who were eligible for MRI but refused participation, were 1 year older, more likely to be women, and more likely to be African American compared to the recipients of a MRI (Brickman et al. 2008). Among the 769 individuals with MRI scanning, 52 met diagnostic criteria for dementia at the clinical visit that was closest to the MRI scan.
Beginning in 2009 we invited active participants who had baseline MRI scans and were not demented at that time to return for a repeat MRI scan. We completed repeat MRI scans on 339 participants approximately 5 years after their baseline scans. Of the 339 participants, we acquired gradient echo (GRE) scans for microbleed assessment on 243 of them. Compared with the 526 participants who did not have GRE scans, those with GRE scans were similar in age (t(766)=1.782, p=0.075), sex distribution (χ2(1)=0.998, p=0.318), and race/ethnicity (χ2(3)=1.836, p=0.607) at baseline. They were also similar in terms of presence of the APOEε4 allele (χ2(1)=0.007, p=0.932), history of heart disease (χ2(1)=0.214, p=0.643), history of diabetes (χ2(1)=1.171, p=0.279), and smoking history (χ2(1)=0.684, p=0.710). Participants without GRE scans were more likely to have a clinical history of stroke (17% vs. 9%; χ2(1)=5.619, p=0.018).
MRI protocol
An optimized, high-resolution three-dimensional T2*-weighted GRE image (TR=45ms, TE=31ms, flip angle= 13, slice thickness= 2mm) was acquired for microbleed visualization and quantification on a Philips Intera 1.5 T MRI scanner (Best, the Netherlands). Fluid attenuated inversion recovery (FLAIR) T2-weighted MRI scans (TR=11,000ms, TE=144ms, 3mm slice thickness) were acquired for white matter hyperintensities (WMH) quantification. T1-weighted images acquired for additional processing (TR=20ms, TE= 2.1ms, 1.3-mm slice thickness).
Microbleeds were rated by visual inspection following criteria put forth by Greenberg and colleagues [5]. These criteria include the following parameters: a dark (black) lesion on T2*-weighted MRI, which is round or ovoid shaped, surrounded at least half way by parenchyma, and accompanied by a “blooming” effect. The microbleed is devoid of signal hyperintensity on accompanying T1- or T2-weighted sequences and is distinct from other mimics, such as iron or calcium deposits, bone, or vessel flow voids. Microbleeds were classified by location, which included “lobar” in frontal, temporal, parietal, and occipital lobes and “deep,” which included basal ganglia, thalamus, and infratentorial regions. The number of microbleeds and location were coded for each subject. A single operator (IBM) performed all the microbleed reads after achieving excellent inter-rater reliability (ICC>0.93) on a training data set that had been evaluated by an expert (SMR).
Because there is no consensus in the extant literature about the best classification scheme for microbleeds among community participants, we considered the distribution in three distinct ways. First, we compared individuals with at least one microbleeds to those with no microbleeds. Next, we categorized subjects into four mutually exclusive groups, which included no microbleeds, 1 or more deep microbleeds (“deep only”), 1 or more lobar microbleeds (“lobar only”), and 1 or more deep microbleed and 1 or more lobar microbleed (“deep and lobar”). Finally, because of the hypothesized link between lobar microbleeds and beta amyloid, we considered lobar microbleeds as a continuous variable to examine “dose response” individual differences.
Total WMH volume was determined following procedures previously described in detail [22,23]. Briefly, FLAIR images were skull-stripped and a threshold and seed-growing algorithm was applied to identify voxels that fell within an a priori-determined distribution of hyperintense signal. Labeled voxels were summed and multiplied by voxel dimensions to yield total WMH volume. Because the distribution of WMH volume is positively skewed, we log transformed the data.
Clinical variables
Vascular risk factors were ascertained by self-report [24] and included diabetes mellitus, hypertension, smoking history, and clinical stroke. History of heart disease included arrhythmias, coronary artery disease, and congestive heart failure. Stroke was defined according to the World Health Organization criteria [25] and supplemented by a medical examination. These variables were defined dichotomously as absent (0) or present (1). We examined diastolic and systolic blood pressure. Based on blood pressure measurements, we defined three hypertension groups according to the World Health Organization criteria [26]: no hypertension, mild hypertension, and severe hypertension. Mild hypertension was defined as having either a systolic blood pressure of 140 to 159 or diastolic pressure of 90 to 99. Severe hypertension was defined as systolic blood pressure of greater than or equal to 160 or diastolic blood pressure of greater than or equal to 100. Furthermore, presence of history of treatment or diagnosis of hypertension was assessed. Participants who used blood pressure-lowering medication were classified as mild hypertensive, regardless of their blood-pressure at the measurement point. APOE genotyping was available for 232 participants and was determined according to the method of Hixson and Vernier [27], with slight modification [28]. In the analysis, we looked at presence of the APOE e4 allele. Body mass index was calculated as weight in kilograms/(height in meters × height in meters).
Diagnoses of dementia and mild cognitive impairment were made following procedures previously described in detail [29]. Finally, number of years of education was ascertained by self report.
Statistical analysis
The first set of analyses compared demographic and clinical characteristics between individuals with no microbleeds and individuals with at least one microbleed. T-tests were used for continuous variables and chi-squared analysis was used for proportional data. The second set of analyses examined demographic and clinical differences among the four mutually exclusive microbleed distribution groups (no microbleeds, “deep only,” “lobar only,” and “deep and lobar”). One-way analysis of variance (ANOVA) was used for continuous measures and chi-squared analysis was used for proportional data. We hypothesized that lobar microbleeds, in particular, would be associated with poorer clinical outcomes and that individuals with both deep and lobar microbleeds would be even more affected. Thus, we tested the linear trend across the four microbleed groups (no microbleeds, deep only, lobar only, and deep and lobar). Omnibus tests were also followed-up by simple 2-group analyses.
Finally, we examined the association between the number of lobar microbleeds and continuous demographic and clinical variables with simple bivariate correlations. We restricted these analyses to lobar microbleeds only because the distribution of lobar microbleeds was relatively evenly distributed from 0 to 18, whereas of the 26 people with deep microbleeds, 25 of them only had 1.
Results
Gradient echo imaging data were available for 243 individuals and 67 (27%) of those individuals had at least one microbleed.
No microbleed versus one or more microbleeds
Differences between individuals with and without microbleeds are displayed in Table 1. Individuals with microbleeds were more likely to have a clinical history of stroke. At a statistical trend level, those with microbleeds also had more severe WMH than those without. This effect was most prominent among Hispanic participants (χ2(1)=7.555, p = 0.006) and was also observed at a trend level among non-Hispanic Whites (χ2(1)=2.168, p = 0.141), but not among African Americans (χ2(1)=0.046, p = 0.831). The two microbleed groups did not differ in the other clinical and demographic variables, including age; sex and ethnicity distribution; years of education; APOE e4 and e2 frequency; frequency of hypertension, diabetes, and smoking; BMI; and frequency of MCI and dementia.
Table 1.
Demographic and clinical differences between individuals with 1 or more microbleed and those with no microbleeds.
| No Microbleeds | Any Microbleeds | Total Sample | Statistic | ||
|---|---|---|---|---|---|
| N | 176 | 67 | 243 | ||
| Age, mean yrs (SD) | 84.27 (5.36) | 85.22 (5.29) | 84.50 (5.34) | t(241)=1.237, p=0.217 | |
| Sex, N women (%) | 125 (71%) | 44 (65.6%) | 169 (69.5%) | X2(1)=0.656, p=0.418 | |
| Mean (SD), education years, not available for 2 subjects | 10.90 (4.849) | 11.36 (5.459) | 11.04 (5.006) | t(239)=0.635, p=0.526 | |
| Ethnicity N(%) | White/non-hisp or other | 52 (29.5%) | 23 (34.3%) | 75 (30.9%) | X2(2)=1.962, p=0.375 |
| Black/non-hisp | 60 (34.1%) | 26 (38.8%) | 86 (35.4%) | ||
| Hispanic | 64 (36.4%) | 18 (26.9%) | 82 (33.7%) | ||
| Hypertension | No | 96 (57%) | 30 (49%) | 128 (51.8%) | X2(2)=3.924, p=0.141 |
| Mild | 47 (28%) | 15 (24.5%) | 62 (25.1%) | ||
| Severe | 25 (14.8%) | 61 (26%) | 17 (18.1 %) | ||
| Mean (SD) diastolic BP | 72.23 (12.48) | 74.00 (11.17) | 72.81 (12.11) | t(227)=0.974, p=0.331 | |
| Mean (SD) systolic BP | 138.56 (21.21) | 141.92 (25.45) | 139.6 (22.53) | t(227)=1.003, p=0.317 | |
| Mean (SD) pulse pressure | 66.33 (18.58) | 67.92 (22.09) | 66.79 (19.63) | t(227)=0.544, p=0.587 | |
| Heart disease | |||||
| Diabetes, N (%) | 35 (19.9%) | 13 (19.4%) | 48 (19.8%) | X2(1)=0.007, p=0.933 | |
| Smoking N (%), not available for 14 subjects | No | 91 (55.2%) | 27 (42.2%) | 118 (51.1%) | X2(2)=3.525, p= 0.172 |
| Past | 54 (32.7%) | 29 (45.3%) | 83 (36.2%) | ||
| Current | 20 (12.1%) | 8 (12.5%) | 28 (12.2%) | ||
| APOE e4, N (%) APOE not available on 11 subjects | 46 (27%) | 14 (22.5%) | 63 (25.5%) | X2(1)=0.475, p=0.491 | |
| APOE e2, N (%) | 29 (17%) | 12 (19.3%) | 42 (17.7%) | X2(1)=0.165, p=0.685 | |
| Mean (SD) BMI | 27.91 (6.28) | 27.26 (5.07) | 27.78 (5.98) | t(241)=0.752, p= 0.453 | |
| Alcohol N (%) | 14 (7.95%) | 4 (5.97%) | 18 (7.41%) | X2(1)=0.279, p=0.598 | |
| Stroke (signs or symptoms) N (%) | 10 (5.7%) | 10 (14.9%) | 20 (8.2%) | X2 (1)=5.490, p=0.019 | |
| Mean (SD), total WMH, not available for 10 subjects | 8.98 (11.60) | 12.12 (14.03) | 9.70 (12.30) | t(231)= 1.729, p=0.085 | |
| Mean (SD) Log total WMH, not available for 10 subjects | 1.53 (1.25) | 1.80 (1.37) | 1.58 (1.30) | t(231)=1.405, p=0.161 | |
| Dementia N (%) | 5 (2.8%) | 0 (0%) | 5 (2.1%) | X2(1)=1.943,p= 0.163 | |
| MCI N (%), not available for 47 subjects | 27 (19%) | 13 (24%) | 40 (20.4%) | X2(1)=0.617, p=0.432 | |
Comparison across mutually exclusive microbleed groups
Table 2 displays the differences between the groups with no microbleeds, strictly lobar, strictly deep, and microbleeds in both deep and lobar locations. Differences emerged in distribution of hypertension, proportion of strokes, and overall WMH burden. The test of linear trends shows that the severity of these three clinical characteristics increased monotonically across the groups, from individuals with no microbleeds to individuals with both deep and lobar microbleeds. Otherwise, the four groups did not differ in the other demographic or clinical characteristics.
Table 2.
Demographic and clinical differences across the four microbleed groups.
| No Microbleeds | Deep Only | Lobar Only | Deep and Lobar | Statistic | P for linear trend | ||
|---|---|---|---|---|---|---|---|
| N | 176 | 14 | 41 | 12 | |||
| Age, mean yrs (SD) | 84.27 (5.36) | 86.27 (4.84) | 84.59 (5.38) | 86.15 (5.620 | F(3,242)=1.002, P=0.393 | 0.437 | |
| Sex, N women (%) | 125 (71%) | 9 (64.3%) | 27 (65.9%) | 8 (66.7%) | X2(3)=0.675, p=0.879 | 0.477 | |
| Mean (SD), education years, not available for 2 subjects | 10.90 (4.85) | 11.79 (4.63) | 11.90 (5.79) | 8.82 (4.83) | F(3)=1.274, p=0.284 | 0.213 | |
| Ethnicity N (%) | White/non-hisp or other | 52 (29.5%) | 6 (42.9%) | 15 (36.6%) | 2 (16.7%) | X2(6)=4.363, p=0.628 | 0.455 |
| Black/non-hisp | 60 (34.1%) | 5 (35.7%) | 15 (36.6%) | 6 (50%) | |||
| Hispanic | 64 (36.4%) | 3 (21.4%) | 11 (26.8%) | 4 (33.3%) | |||
| Hypertension | No | 96 (57.1%) | 7 (58.3%) | 19 (50%) | 4 (36.4%) | X2(6)=8.637, p=0.195 | 0.044 |
| Mild | 47 (28.0%) | 4 (33.3%) | 7 (18.4%) | 4 (36.4%) | |||
| Severe | 25 (14.9%) | 1 (8.3%) | 12 (31.6%) | 3 (27.3%) | |||
| Mean (SD) systolic BP | 138.56 (21.21) | 136.83 (16.74) | 141.95 (27.60) | 147.36 (26.38) | F(3)=0.755, p=0.520 | 0.158 | |
| Mean (SD) diastolic BP | 72.23 (12.48) | 72.25 (6.89) | 74.74 (11.57) | 73.36 (13.95) | F(3)=0.453, p=0.716 | 0.627 | |
| Mean (SD) pulse pressure | 66.33 (18.58) | 64.58 (16.13) | 67.21 (22.45) | 74.00 (26.85) | F(3)=0.584, p=0.626 | 0.188 | |
| Heart disease | |||||||
| Diabetes, N (%) | 35 (19.9%) | 1 (7.1%) | 8 (19.5%) | 4 (33.3%) | X2(3)=2.804, p=0.423 | 0.616 | |
| Smoking N (%), not available for 14 subjects | No | 91 (55.2%) | 6 (50%) | 16 (39.0%) | 5 (45.5%) | X2(6)=8.466, p=0.206 | 0.110 |
| Past | 54 (32.7%) | 6 (50%) | 20 (48.8%) | 3 (27.3%) | |||
| Current | 20 (12.1%) | 0 (0%) | 5 (12.2%) | 3 (27.3%) | |||
| APOE e4, N (%) not available on 11 subjects | 46 (27.1%) | 2 (14.3%) | 11 (28.9%) | 1 (10%) | X2(3)=2.606, p=0.456 | 0.509 | |
| APOE e2, N (%) | 29 (17.1%) | 1 (7.1%) | 8 (21.1%) | 3 (7.3%) | X2(3)=2.454, p=0.484 | 0.368 | |
| Mean (SD) BMI | 27.91 (6.28) | 29.03 (6.29) | 27.01 (5.04) | 26.06 (3.11) | F(3)=0.785, p=0.503 | 0.183 | |
| Alcohol N (%) | 14 (8.0%) | 2 (14.3%) | 2 (4.9%) | 0 (0%) | X2(3)=2.385, p=0.496 | 0.325 | |
| Stroke (signs or symptoms) N (%) | 10 (5.7%) | 0 (0%) | 7 (17.1%) | 3 (25%) | X2(3)=l 1.482, p=0.009 | 0.013 | |
| Mean (SD), total WMH, not available for 10 subjects | 0.67 (4.06) | 0.15 (1.91) | 2.16 (4.49) | 1.88 (5.13) | F(3)=1.912, p=0.128 | 0.066 | |
| Dementia N (%) | 23 (13.1%) | 0 (0%) | 2 (5.3%) | 1 (8.3%) | X2(3)=3.950, p=0.267 | 0.192 | |
| MCI N (%), not available for 47 subjects | 27 (19.0%) | 2 (15.4%) | 7 (21.9%) | 4 (44.4%) | X2(3)=3.615, 0.306 | 0.194 | |
Relationship between number of lobar microbleeds and clinical variables
Increased number of lobar microbleeds was associated with increased total volume of WMH (r(233)=0.164, p=0.012) and diastolic blood pressure (r(229)=0.170, p=0.010). Otherwise, the number of lobar microbleeds was not associated with BMI, age, systolic blood pressure, pulse pressure, or number of years of education.
Discussion
In this community-based, multi-ethnic cohort of older adults, we studied the prevalence of cerebral microbleeds and their demographic correlates. We found that 27% of these community-based participants had at least one microbleed, a prevalence that is consistent with other community-based imaging studies of older adults [30]. Individuals with at least one microbleed were more likely to have a history of clinical stroke than individuals without; this observation was particularly true among Hispanics and non-Hispanic Whites. Otherwise, the two groups were similar in demographic features and clinical correlates. When we examined whether the distribution of microbleeds differentially related to demographic and clinical features, we found that individuals with microbleeds in lobar regions had more severe WMH than those with microbleeds in strictly deep regions and controls.
Interest in microbleeds has increased recently in the extant literature but there is little consensus about how to classify their severity and distribution. A second goal of this study was to examine various categorization schemes as they relate to different demographic and clinical variables. We categorized microbleeds according to their distribution and by their number because lobar versus deeply distributed microbleeds are assumed to point to different phenomena, including CAA and hypertensive vasculopathy, respectively [3–5]. Linear trends across the four microbleed groups (no microbleeds, deep only, lobar only, and deep and lobar) showed that the severity of hypertension, proportion of strokes, and overall WMH burden increased monotonically across the groups, from individuals with no microbleeds to individuals with both deep and lobar microbleeds. Therefore, microbleeds in deep and lobar locations are associated with worse clinical outcomes than microbleeds in either location alone, although presence of lobar microbleeds appears to be more clinically relevant than presence of deep. When considered as a continuous measure, the number of lobar microbleeds was positively associated with diastolic blood pressure and WMH volume. Our observations suggest that microbleeds distributed in lobar regions may be more clinically relevant in a “dose response” fashion.
The underlying pathology of lobar microbleeds is believed to be CAA, which is supported by evidence from autopsy studies [4,31], population-based studies [5,30], and APOE-alleles studies in populations with lobar microbleeds [32,33]. Microbleeds result when cerebral amyloid angiopathy damages the vessel wall and leads to the leaking of blood into adjacent brain tissue. We and others have hypothesized that WMH represent heterogeneous pathology, including, perhaps CAA, thus providing a potential mechanistic link between regionally distributed white matter disease and AD [11,34–37]. Consistent with previous reports [36,38,39], individuals with microbleeds in lobar regions had more severe WMH than those with microbleeds in strictly deep regions and controls.
Trials with anti-amyloid treatments in AD have shown that although the immunization was effective in removing parenchymal amyloid β plaques, there was no change in CAA [40] and even led to more CAA-related micro-hemorrhagic lesions or so-called “ARIA-H” [41,42]. Spontaneous ARIA in AD patients detected at screening or during treatment with a placebo may suggest that ARIA is an expected phenomenon in AD, reflecting amyloid clearance [16,43]. Indeed, microbleeds, the main component of ARIA-H, are reported in up to 32% of patients with AD [44]. Anti-amyloid treatments may therefore only aggrevate and increase the likelihood of ARIA. Cerebral amyloid angiopathy seems to be the common factor of these spontaneous microbleeds and the microbleeds seen after anti-amyloid treatment. Individuals with microbleeds (27% of the sample in the current study), in particular lobar microbleeds indicative of CAA, might be at risk for development of ARIA if ultimately enrolled in anti-amyloid therapeutic trials and/or may be inappropriate for treatment with amyloid therapies because of an elevated risk of adverse treatment outcomes.
We found that individuals with microbleeds were more likely to have a history of clinical stroke and increased diastolic blood pressure than people without. Vascular risk factors, such as high blood pressure, are risk factors for intracerebral hemorrhage and ischemic stroke and microbleeds are known risk factors for larger intracerebral hemorrhages [19,45–49]. Together with the observations about WMH, these observations show that risk factors for and markers of vascular brain health tend to co-occur.
Regardless of the categorization scheme employed, we did not observe expected associations between microbleeds and several demographic or clinical features, including diabetes, smoking history, APOE, ethnicity, cognitive status, and age whereas other studies have [2,3,9,17–19,32,33,50–55]. Several non-mutually exclusive possibilities could contribute to these negative observations. First, it is likely that 1.5T MRI scanners underestimate the severity of microbleeds. Indeed, higher field-strength magnets are much more sensitive for microbleed detection, such as in the recent study that observed that 21% of individuals with atherosclerotic disease had measurable microbleeds when scanned with a 1.5 T MRI compared with 50% when scanned with a 7 T MRI [56]. It is possible that we underestimated both the number of individuals with microbleeds and the number of microbleeds among individuals with detectable microbleeds. In terms of compatability across studies, those that employ higher field strength magnets are likely more sensitive for microbleed detection, thus increasing statistical power, but the specificity of detection should be comparable across field strengths. Second, there were relatively few individuals with microbleeds, thus limiting statistical power to detect associations. In a similar vein, few individuals met criteria for cognitive impairment (AD or MCI) and had the APOE ε4 allele. Third, our cohort is older, has more medical morbities, and is more ethnically/racially diverse than other study samples in which microbleeds have been investigated. This increased heterogeneity in our cohort may have obscured findings that link microbleeds with factors that are also associated with other clinical outcomes. Finally, the number of statistical analyses conducted without correction may have increased Type I statistical error rates.
Our cohort is on average older than cohorts in other similar efforts [3] and more ethnically/racially diverse than most studies of aging. The population of older adults is living longer and comprises much more ethnic and racial diversity than in the past. Future studies should continue to understand the nature and determinants of brain aging among diverse older adults with sufficient numbers of participants to maximize statistical power.
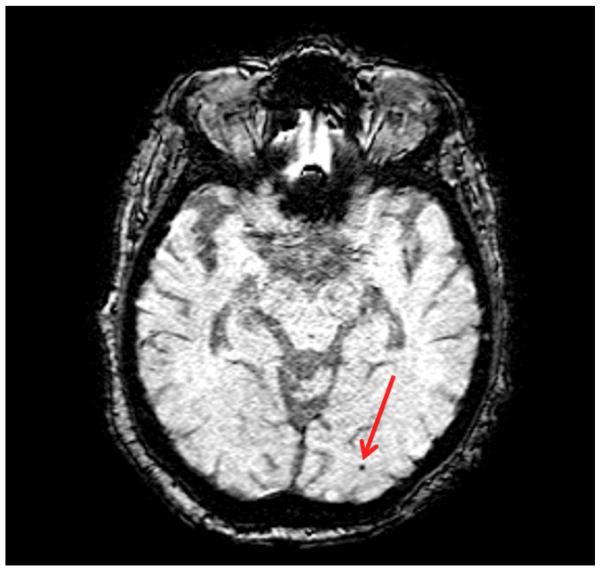
Figure 1.
Example of a microbleed in posterior distribution (red arrow).
Acknowledgements
Sources of funding: This study was sponsored by grants from the National Institutes of Health (AG034189 and AG037212)
References
- 1.Koennecke HC. Cerebral microbleeds on mri: Prevalence, associations, and potential clinical implications. Neurology. 2006;66:165–171. doi: 10.1212/01.wnl.0000194266.55694.1e. [DOI] [PubMed] [Google Scholar]
- 2.Sveinbjornsdottir S, Sigurdsson S, Aspelund T, Kjartansson O, Eiriksdottir G, Valtysdottir B, Lopez OL, van Buchem MA, Jonsson PV, Gudnason V, Launer LJ. Cerebral microbleeds in the population based ages-reykjavik study: Prevalence and location. Journal of neurology, neurosurgery, and psychiatry. 2008;79:1002–1006. doi: 10.1136/jnnp.2007.121913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vernooij MW, van der Lugt A, Ikram MA, Wielopolski PA, Niessen WJ, Hofman A, Krestin GP, Breteler MM. Prevalence and risk factors of cerebral microbleeds: The rotterdam scan study. Neurology. 2008;70:1208–1214. doi: 10.1212/01.wnl.0000307750.41970.d9. [DOI] [PubMed] [Google Scholar]
- 4.Fazekas F, Kleinert R, Roob G, Kleinert G, Kapeller P, Schmidt R, Hartung HP. Histopathologic analysis of foci of signal loss on gradient-echo t2*-weighted mr images in patients with spontaneous intracerebral hemorrhage: Evidence of microangiopathy-related microbleeds. AJNR American journal of neuroradiology. 1999;20:637–642. [PMC free article] [PubMed] [Google Scholar]
- 5.Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, Launer LJ, Van Buchem MA, Breteler MM, Microbleed Study G Cerebral microbleeds: A guide to detection and interpretation. Lancet neurology. 2009;8:165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: Validation of the boston criteria. Neurology. 2001;56:537–539. doi: 10.1212/wnl.56.4.537. [DOI] [PubMed] [Google Scholar]
- 7.Smith EE, Gurol ME, Eng JA, Engel CR, Nguyen TN, Rosand J, Greenberg SM. White matter lesions, cognition, and recurrent hemorrhage in lobar intracerebral hemorrhage. Neurology. 2004;63:1606–1612. doi: 10.1212/01.wnl.0000142966.22886.20. [DOI] [PubMed] [Google Scholar]
- 8.Arvanitakis Z, Leurgans SE, Wang Z, Wilson RS, Bennett DA, Schneider JA. Cerebral amyloid angiopathy pathology and cognitive domains in older persons. Ann Neurol. 2011;69:320–327. doi: 10.1002/ana.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poels MM, Ikram MA, van der Lugt A, Hofman A, Niessen WJ, Krestin GP, Breteler MM, Vernooij MW. Cerebral microbleeds are associated with worse cognitive function: The rotterdam scan study. Neurology. 2012;78:326–333. doi: 10.1212/WNL.0b013e3182452928. [DOI] [PubMed] [Google Scholar]
- 10.Zlokovic BV. Neurovascular pathways to neurodegeneration in alzheimer's disease and other disorders. Nature reviews Neuroscience. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brickman AM, Muraskin J, Zimmerman ME. Structural neuroimaging in altheimer's disease: Do white matter hyperintensities matter? Dialogues Clin Neurosci. 2009;11:181–190. doi: 10.31887/DCNS.2009.11.2/ambrickman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richard E, Moll van Charante EP, van Gool WA. Vascular risk factors as treatment target to prevent cognitive decline. Journal of Alzheimer's disease : JAD. 2012;32:733–740. doi: 10.3233/JAD-2012-120772. [DOI] [PubMed] [Google Scholar]
- 13.de la Torre JC. Cerebral hemodynamics and vascular risk factors: Setting the stage for alzheimer's disease. Journal of Alzheimer's disease : JAD. 2012;32:553–567. doi: 10.3233/JAD-2012-120793. [DOI] [PubMed] [Google Scholar]
- 14.Jellinger KA. Alzheimer disease and cerebrovascular pathology: An update. Journal of neural transmission. 2002;109:813–836. doi: 10.1007/s007020200068. [DOI] [PubMed] [Google Scholar]
- 15.Scheltens P, Goos JD. Dementia in 2011: Microbleeds in dementia--singing a different aria. Nature reviews Neurology. 2012;8:68–70. doi: 10.1038/nrneurol.2011.222. [DOI] [PubMed] [Google Scholar]
- 16.Sperling RA, Jack CR, Jr., Black SE, Frosch MP, Greenberg SM, Hyman BT, Scheltens P, Carrillo MC, Thies W, Bednar MM, Black RS, Brashear HR, Grundman M, Siemers ER, Feldman HH, Schindler RJ. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: Recommendations from the alzheimer's association research roundtable workgroup. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2011;7:367–385. doi: 10.1016/j.jalz.2011.05.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeerakathil T, Wolf PA, Beiser A, Hald JK, Au R, Kase CS, Massaro JM, DeCarli C. Cerebral microbleeds: Prevalence and associations with cardiovascular risk factors in the framingham study. Stroke; a journal of cerebral circulation. 2004;35:1831–1835. doi: 10.1161/01.STR.0000131809.35202.1b. [DOI] [PubMed] [Google Scholar]
- 18.Roob G, Schmidt R, Kapeller P, Lechner A, Hartung HP, Fazekas F. Mri evidence of past cerebral microbleeds in a healthy elderly population. Neurology. 1999;52:991–994. doi: 10.1212/wnl.52.5.991. [DOI] [PubMed] [Google Scholar]
- 19.Tsushima Y, Aoki J, Endo K. Brain microhemorrhages detected on t2*-weighted gradient-echo mr images. AJNR American journal of neuroradiology. 2003;24:88–96. [PMC free article] [PubMed] [Google Scholar]
- 20.Tang MX, Cross P, Andrews H, Jacobs DM, Small S, Bell K, Merchant C, Lantigua R, Costa R, Stern Y, Mayeux R. Incidence of ad in african-americans, caribbean hispanics, and caucasians in northern manhattan. Neurology. 2001;56:49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- 21.Brickman AM, Schupf N, Manly JJ, Luchsinger JA, Andrews H, Tang MX, Reitz C, Small SA, Mayeux R, DeCarli C, Brown TR. Brain morphology in older african americans, caribbean hispanics, and whites from northern manhattan. Arch Neurol. 2008;65:1053–1061. doi: 10.1001/archneur.65.8.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brickman AM, Provenzano FA, Muraskin J, Manly JJ, Blum S, Apa Z, Stern Y, Brown TR, Luchsinger JA, Mayeux R. Regional white matter hyperintensity volume, not hippocampal atrophy, predicts incident alzheimer disease in the community. Arch Neurol. 2012;69:1621–1627. doi: 10.1001/archneurol.2012.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brickman AM, Sneed JR, Provenzano FA, Garcon E, Johnert L, Muraskin J, Yeung LK, Zimmerman ME, Roose SP. Quantitative approaches for assessment of white matter hyperintensities in elderly populations. Psychiatry research. 2011;193:101–106. doi: 10.1016/j.pscychresns.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident alzheimer disease. Neurology. 2005;65:545–551. doi: 10.1212/01.wnl.0000172914.08967.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hatano S. plans for prevention of stroke formulated by who and practice in japan. Nihon rinsho Japanese journal of clinical medicine. 1976;34:131–136. [PubMed] [Google Scholar]
- 26.Whitworth JA, World Health Organization ISoHWG 2003 world health organization (who)/international society of hypertension (ish) statement on management of hypertension. Journal of hypertension. 2003;21:1983–1992. doi: 10.1097/00004872-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Hixson JE, Vernier DT, Powers PK. Detection of ssti restriction site polymorphism in human apoc3 by the polymerase chain reaction. Nucleic acids research. 1991;19:196. doi: 10.1093/nar/19.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayeux R, Ottman R, Maestre G, Ngai C, Tang MX, Ginsberg H, Chun M, Tycko B, Shelanski M. Synergistic effects of traumatic head injury and apolipoprotein-epsilon 4 in patients with alzheimer's disease. Neurology. 1995;45:555–557. doi: 10.1212/wnl.45.3.555. [DOI] [PubMed] [Google Scholar]
- 29.Manly JJ, Tang MX, Schupf N, Stern Y, Vonsattel JP, Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Ann Neurol. 2008;63:494–506. doi: 10.1002/ana.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vernooij MW, Ikram MA, Wielopolski PA, Krestin GP, Breteler MM, van der Lugt A. Cerebral microbleeds: Accelerated 3d t2*-weighted gre mr imaging versus conventional 2d t2*-weighted gre mr imaging for detection. Radiology. 2008;248:272–277. doi: 10.1148/radiol.2481071158. [DOI] [PubMed] [Google Scholar]
- 31.Gilbert JJ, Vinters HV. Cerebral amyloid angiopathy: Incidence and complications in the aging brain. I. Cerebral hemorrhage. Stroke; a journal of cerebral circulation. 1983;14:915–923. doi: 10.1161/01.str.14.6.915. [DOI] [PubMed] [Google Scholar]
- 32.Greenberg SM, Rebeck GW, Vonsattel JP, Gomez-Isla T, Hyman BT. Apolipoprotein e epsilon 4 and cerebral hemorrhage associated with amyloid angiopathy. Ann Neurol. 1995;38:254–259. doi: 10.1002/ana.410380219. [DOI] [PubMed] [Google Scholar]
- 33.Maxwell SS, Jackson CA, Paternoster L, Cordonnier C, Thijs V, Al-Shahi Salman R, Sudlow CL. Genetic associations with brain microbleeds: Systematic review and meta-analyses. Neurology. 2011;77:158–167. doi: 10.1212/WNL.0b013e318224afa3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen YW, Gurol ME, Rosand J, Viswanathan A, Rakich SM, Groover TR, Greenberg SM, Smith EE. Progression of white matter lesions and hemorrhages in cerebral amyloid angiopathy. Neurology. 2006;67:83–87. doi: 10.1212/01.wnl.0000223613.57229.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakata-Kudo Y, Mizuno T, Yamada K, Shiga K, Yoshikawa K, Mori S, Nishimura T, Nakajima K, Nakagawa M. Microbleeds in alzheimer disease are more related to cerebral amyloid angiopathy than cerebrovascular disease. Dementia and geriatric cognitive disorders. 2006;22:8–14. doi: 10.1159/000092958. [DOI] [PubMed] [Google Scholar]
- 36.Pettersen JA, Sathiyamoorthy G, Gao FQ, Szilagyi G, Nadkarni NK, St George-Hyslop P, Rogaeva E, Black SE. Microbleed topography, leukoaraiosis, and cognition in probable alzheimer disease from the sunnybrook dementia study. Archives of neurology. 2008;65:790–795. doi: 10.1001/archneur.65.6.790. [DOI] [PubMed] [Google Scholar]
- 37.Zhu YC, Chabriat H, Godin O, Dufouil C, Rosand J, Greenberg SM, Smith EE, Tzourio C, Viswanathan A. Distribution of white matter hyperintensity in cerebral hemorrhage and healthy aging. Journal of neurology. 2012;259:530–536. doi: 10.1007/s00415-011-6218-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goos JD, Kester MI, Barkhof F, Klein M, Blankenstein MA, Scheltens P, van der Flier WM. Patients with alzheimer disease with multiple microbleeds: Relation with cerebrospinal fluid biomarkers and cognition. Stroke; a journal of cerebral circulation. 2009;40:3455–3460. doi: 10.1161/STROKEAHA.109.558197. [DOI] [PubMed] [Google Scholar]
- 39.Yamada S, Saiki M, Satow T, Fukuda A, Ito M, Minami S, Miyamoto S. Periventricular and deep white matter leukoaraiosis have a closer association with cerebral microbleeds than age. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2012;19:98–104. doi: 10.1111/j.1468-1331.2011.03451.x. [DOI] [PubMed] [Google Scholar]
- 40.Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human alzheimer disease after immunization with amyloid-beta peptide: A case report. Nature medicine. 2003;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- 41.Fisher M, Vasilevko V, Passos GF, Ventura C, Quiring D, Cribbs DH. Therapeutic modulation of cerebral microhemorrhage in a mouse model of cerebral amyloid angiopathy. Stroke; a journal of cerebral circulation. 2011;42:3300–3303. doi: 10.1161/STROKEAHA.111.626655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pfeifer M, Boncristiano S, Bondolfi L, Stalder A, Deller T, Staufenbiel M, Mathews PM, Jucker M. Cerebral hemorrhage after passive anti-abeta immunotherapy. Science. 2002;298:1379. doi: 10.1126/science.1078259. [DOI] [PubMed] [Google Scholar]
- 43.Carlson C, Estergard W, Oh J, Suhy J, Jack CR, Jr., Siemers E, Barakos J. Prevalence of asymptomatic vasogenic edema in pretreatment alzheimer's disease study cohorts from phase 3 trials of semagacestat and solanezumab. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2011;7:396–401. doi: 10.1016/j.jalz.2011.05.2353. [DOI] [PubMed] [Google Scholar]
- 44.Cordonnier C, van der Flier WM. Brain microbleeds and alzheimer's disease: Innocent observation or key player? Brain : a journal of neurology. 2011;134:335–344. doi: 10.1093/brain/awq321. [DOI] [PubMed] [Google Scholar]
- 45.Greenberg SM, Eng JA, Ning M, Smith EE, Rosand J. Hemorrhage burden predicts recurrent intracerebral hemorrhage after lobar hemorrhage. Stroke; a journal of cerebral circulation. 2004;35:1415–1420. doi: 10.1161/01.STR.0000126807.69758.0e. [DOI] [PubMed] [Google Scholar]
- 46.Soo YO, Yang SR, Lam WW, Wong A, Fan YH, Leung HH, Chan AY, Leung C, Leung TW, Wong LK. Risk vs benefit of anti-thrombotic therapy in ischaemic stroke patients with cerebral microbleeds. Journal of neurology. 2008;255:1679–1686. doi: 10.1007/s00415-008-0967-7. [DOI] [PubMed] [Google Scholar]
- 47.Thijs V, Lemmens R, Schoofs C, Gorner A, Van Damme P, Schrooten M, Demaerel P. Microbleeds and the risk of recurrent stroke. Stroke; a journal of cerebral circulation. 2010;41:2005–2009. doi: 10.1161/STROKEAHA.110.588020. [DOI] [PubMed] [Google Scholar]
- 48.Igase M, Tabara Y, Igase K, Nagai T, Ochi N, Kido T, Nakura J, Sadamoto K, Kohara K, Miki T. Asymptomatic cerebral microbleeds seen in healthy subjects have a strong association with asymptomatic lacunar infarction. Circ J. 2009;73:530–533. doi: 10.1253/circj.cj-08-0764. [DOI] [PubMed] [Google Scholar]
- 49.Rosand J. Hypertension and the brain: Stroke is just the tip of the iceberg. Neurology. 2004;63:6–7. doi: 10.1212/01.wnl.0000132843.90333.b2. [DOI] [PubMed] [Google Scholar]
- 50.Cordonnier C, Al-Shahi Salman R, Wardlaw J. Spontaneous brain microbleeds: Systematic review, subgroup analyses and standards for study design and reporting. Brain : a journal of neurology. 2007;130:1988–2003. doi: 10.1093/brain/awl387. [DOI] [PubMed] [Google Scholar]
- 51.Horita Y, Imaizumi T, Niwa J, Yoshikawa J, Miyata K, Makabe T, Moriyama R, Kurokawa K, Mikami M, Nakamura M. analysis of dot-like hemosiderin spots using brain dock system. No shinkei geka Neurological surgery. 2003;31:263–267. [PubMed] [Google Scholar]
- 52.Tsushima Y, Tanizaki Y, Aoki J, Endo K. Mr detection of microhemorrhages in neurologically healthy adults. Neuroradiology. 2002;44:31–36. doi: 10.1007/s002340100649. [DOI] [PubMed] [Google Scholar]
- 53.Qiu C, Cotch MF, Sigurdsson S, Jonsson PV, Jonsdottir MK, Sveinbjrnsdottir S, Eiriksdottir G, Klein R, Harris TB, van Buchem MA, Gudnason V, Launer LJ. Cerebral microbleeds, retinopathy, and dementia: The ages-reykjavik study. Neurology. 2010;75:2221–2228. doi: 10.1212/WNL.0b013e3182020349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takashima Y, Mori T, Hashimoto M, Kinukawa N, Uchino A, Yuzuriha T, Yao H. Clinical correlating factors and cognitive function in community-dwelling healthy subjects with cerebral microbleeds. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2011;20:105–110. doi: 10.1016/j.jstrokecerebrovasdis.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 55.Yakushiji Y, Nishiyama M, Yakushiji S, Hirotsu T, Uchino A, Nakajima J, Eriguchi M, Nanri Y, Hara M, Horikawa E, Kuroda Y. Brain microbleeds and global cognitive function in adults without neurological disorder. Stroke; a journal of cerebral circulation. 2008;39:3323–3328. doi: 10.1161/STROKEAHA.108.516112. [DOI] [PubMed] [Google Scholar]
- 56.Conijn MM, Geerlings MI, Biessels GJ, Takahara T, Witkamp TD, Zwanenburg JJ, Luijten PR, Hendrikse J. Cerebral microbleeds on mr imaging: Comparison between 1.5 and 7t. AJNR American journal of neuroradiology. 2011;32:1043–1049. doi: 10.3174/ajnr.A2450. [DOI] [PMC free article] [PubMed] [Google Scholar]