Abstract
The traditional view of directional cell migration within a tissue is that it requires a gradient of a soluble attractive chemical that is stable in space and time due to the presence of a source and a sink. However, advances in live imaging technology and the ability to study cell migration in vivo have revealed that endogenous sources and sinks are typically far more varied and complex. Both sources and sinks can be made up of multiple tissues. During long-range migrations, cells tend to divide up their trajectories and follow different source signals in each segment. When a single source signal is used repeatedly in each segment, its expression is dynamically controlled. Source signals can also originate locally from neighboring migrating cells. Sinks are important in some cases but not all, to sculpt a permissive migratory path or allow cells to move from one internediate target to another. Migrating cells themselves can function as sinks to create a gradient out of an initially uniform chemoattractant. These diverse ways of building sources and sinks allow different cell types to navigate distinct trajectories through the same embryo even as the whole embryo undergoes the dramatic changes in cell number, position, arrangement and fate that are the essence of development.
Introduction
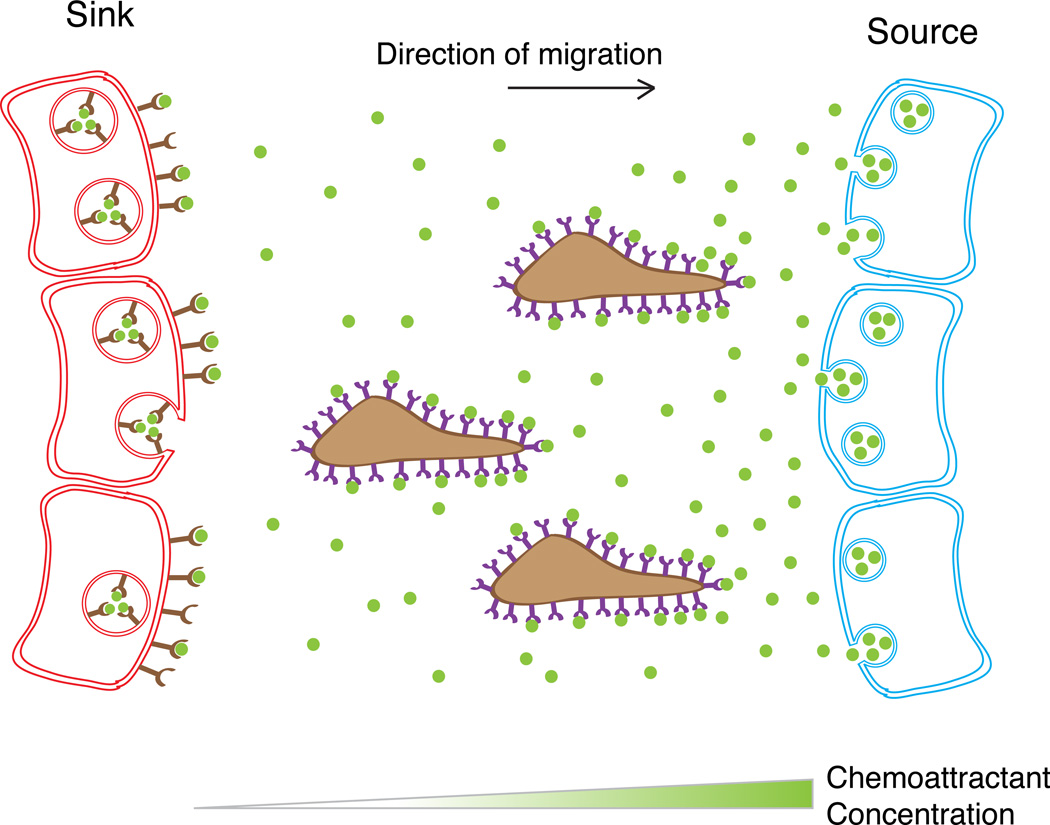
Chemotaxis refers to the directional migration of cells up a gradient of a soluble chemical attractant [1]. Classic examples include migration of Dictyostelium discoideum amoebae toward cAMP [1], fibroblast attraction to growth factors [2,3], chemokine-mediated leukocyte movements [4], and axonal growth cone navigation toward Netrin [5,6]. Establishment of a chemoattractant gradient that is stable in time and space in principle would require a stable source of the molecule and a sink (Figure 1), as proposed for morphogen gradients [7].
Figure 1.
Traditional source and sink model in cell migration. A chemoattractant (green) is secreted from a source tissue and depleted within a sink tissue, thus forming a gradient. Migrating cells polarize in the gradient and migrate to the source.
Using controlled conditions in vitro, such simple gradients can be created, and cells behave as expected, migrating toward the source [2,8]. As live imaging techniques mature, it has become possible to observe directional cell migrations in vivo and to begin to characterize endogenous chemoattractant sources and sinks. These studies reveal that in vivo migrating cells can move through extremely complex and changing environments, traverse through several different types of tissues, carve complex paths, and cover long distances. Cells can travel through three-dimensional interstitial matrices, along individual extracellular matrix fibers, on flat basement membranes, or they can squeeze in between other cells to cross a blood vessel or an epithelium. Due to this complexity, it turns out that sources and sinks are far more diverse and dynamic in vivo than the simple artificial gradients created in vitro. In this review, we use both old and new examples to describe the diverse mechanisms that nature has devised to steer cells along complex trajectories.
Morphogenesis Requires Diverse and Dynamic Sources
In development, it’s not uncommon for migrating cells to travel long distances and make multiple turns. A single stable source of chemoattractant would not be able to direct cells along such complex trajectories. Therefore multiple source signals are often used. This can mean changing where a single chemoattractant is produced over time, or secreting different molecules at different locations and times and altering responsiveness of the migratory cells or growth cones as they move first toward one source and then to another.
One early example can be found in the navigation of vertebrate commissural axons, which travel long distances (several centimeters or 1000 times the diameter of the cell body) to reach their targets. Growth cones, the steering tips of axons, solve this problem by breaking down the trajectory into smaller segments thus necessitating multiple guidance cues and chemoattractant “sources” [5]. The best-studied intermediate target is the central nervous system midline, which secretes chemoattractant factors Netrin-1 and Sonic Hedgehog (Shh) (Figure 2a) [5,9]. Once the axons reach that target, they turn and follow other sources such as Wnt (Figure 2a) [10]. Different sources are thus active at different places and times, breaking a long and complicated journey into smaller steps. At the midline, axons encounter Slit, a secreted protein that causes them to turn on the expression of Roundabout (Robo), which is a negative regulator of Netrin-1 signaling [11,12]. Thus, growth cones can migrate away from the midline after crossing it, even though Netrin-1 is still present.
Figure 2.
Diverse and dynamic sources in different segments of the migration path. (a) Axons respond to different chemoattractants prior to crossing the midline (lef) and after crossing the midline (right) [38]. (b) Bnl (purple) is sequentially expressed at each intermediate target to guide tracheal tip cell migration and branch formation (red). After reaching each intermediate target, bnl expression disappears (dotted circle) and initiates at new site. Arrow: direction of migration.
A different solution is found in Drosophila tracheal system development. A patch of about 80 tracheal cells forms on the surface of each hemisegment of the fly embryo. These cells dive into the embryo and then migrate to form a complex series of interconnected tubules. In contrast to growth cones, which switch from one chemoattractant to another, tracheal cells follow a single guidance signal, the expression of which changes dynamically to allow the cells to switch direction. The branchless locus encodes a fibroblast growth factor (FGF) homolog that is sequentially expressed at intermediate targets along the migration path for each of the tracheal branches. Once the leading tip cell reaches the nearest Bnl source, new expression initiates at another position along the migration path (Figure 2b) [13]. It is not completely clear what serves as a sink to remove Bnl or allows the cells to leave the first Bnl source. However it’s possible that expression of Bnl in each intermediate target is simply transient so that the protein concentration dissipates from the initial source as a new source of Bnl arises.
Cell-cell Interactions Lead to Dynamic Source Signals
Source information can be even more local. In the social amoeba Dictyostelium discoideum, cells relay the cyclic AMP (cAMP) signal from each migrating cell to its immediate neighbor generating a collective behavior called streaming [14]. While Dictyostelium cells migrate individually when nutrients are abundant, upon starvation cells switch to collective behavior, causing them to aggregate and eventually form a multicellular organism. Binding of external cAMP to a receptor known as cAR1 activates adenylyl cyclase, which in turn converts ATP into cAMP. The cAMP is then secreted from the rear of the cell to attract neighboring cells, which then produce more cAMP (Figure 3a), creating a stream. This cAMP relay mechanism can effectively provide collective cell guidance and accelerate cell aggregation [14]. Moreover due to the availability of many mutants defective in specific signaling components, the ease of biochemical analysis in this organism, and high resolution imaging, it has been possible to directly observe and measure the cAMP signal relay and response in living cells [20].
Figure 3.
Sources formed by neighboring cells. (a) A gradient of cAMP (red) produced by a migrating Dictyostelium discoideum amoeba is relayed to neighbors and causes streaming. (b) Later in the aggregation process cAMP production oscillates forming temporal instead of spatial gradients. (c) Wound sites secrete a primary signal such as fMLP peptides (yellow) and induces secretion of the secondary signal LTB4 (orange) causing rapid aggregation of neutrophils (green). (d) Placodal cells (green) function as a source by secreting Sdf1 (orange). NC cells (red) migrate toward placodal cells but upon contact undergo CIL (magenta arrows). Cycles of chemoattraction and contact inhibition produce net directional migration [23].
Later in the process of Dictyostelium aggregation, when there are tens of thousands to millions of cells coming together, the cAMP concentration oscillates, producing waves of extracellular chemoattractant (Figure 3b). Instead of forming a stable spatial gradient, which would soon flatten out due to the low molecular weight and fast diffusion rate of cAMP [15], Dictyostelium produces a temporal gradient of cAMP to guide the migration of its neighbors [16,17]. During the oscillations, the concentration of cAMP increases for a period of time and then decreases (Figure 3b). Cells respond to the increasing concentration by migrating directionally toward the source, and when the concentration decreases they depolarize and migrate non-directionally. This combination of responses leads to a net directional migration. The temporal gradient, relayed by many cells, can mediate robust chemotaxis of many cells over several hundred millimeters [16]. Multiple extracellular and intracellular phosphodiesterases function to degrade the large amount of cAMP produced locally, allowing the system to return to baseline [18,19]. It is possible that temporal oscillation of chemoattractant production is a general phenomenon. However better tools that allow real time measurement of chemoattractant concentrations or responses with high spatial and temporal resolution, such as those used in Dictyostelium studies [20], will be required to detect these types of signals in other living tissues and organisms.
Signal amplification by signal relay does not happen solely in Dictyostelium. During inflammation, neutrophils secrete leukotriene B4 (LTB4) to stimulate the chemotaxis of neighboring neutrophils [21–22]. Neutrophils make up a large proportion of leukocytes in the bloodstream. Upon wound formation, neutrophils follow signals to exit blood vessels and enter the tissue surrounding the wound site. Both in vitro [21] and in vivo [22] cells close to the wound site are able to follow primary signals such as formyl peptides (fMLP) secreted by the wound, and subsequently secrete the secondary chemoattractant LTB4 (Figure 3c). LTB4 secretion is proportional to the fMLP concentration, so that cells closer to the wound secrete more LTB4. A secondary LTB4 gradient thus forms in parallel with that of fMLP and leads to rapid congregation of neutrophils to the wound [21].
Another form of directional migration produced by cell-cell interactions is the elegant chase-and-run mechanism found in Xenopus embryos [23]. Epithelial cells called placodal cells serve as a source of secreted Sdf1, which attracts neural crest cells. Upon contact of the two cell types though, neural crest cells repel the placodal cells by contact inhibition of locomotion (CIL), which is mediated by N-cadherin and PCP signaling. This drives the placodal cells further ahead of the neural crest cells. The placodal cells then initiate another round of Sdf1-mediated attraction (Figure 3d) [23]. This novel mechanism of dynamic attraction and repulsion ensures effective directional migration of both cell types. An open question is how a singular overall polarity is achieved by this interaction. The observation that polarized collective migration of cells can occur when they are explanted and cultured in vitro indicates that the overall polarization is self-generated and does not require external cues from neighboring tissues.
Multiple and Self-generated Sinks Ensure Directional Migration
In theory both a source and a sink are required to create a stable concentration gradient of a diffusible molecule within a confined space. Without a sink, the concentration of chemoattractant (or morphogen) would rapidly rise and soon become uniform. In vitro studies of chemotaxis employ microfluidic devices to generate artificial sources and sinks [24,25]. In vivo, sinks play important roles and help to sculpt complex migration paths through the dynamic environment characteristic of a developing embryo. However natural sinks are diverse and can be made up of a single tissue, multiple tissues, or the migrating cells themselves.
When multiple tissues serve as sinks along the path of migration, they determine the landscape of attractive versus unattractive or repellant regions collectively (Figure 4). For example, during embryonic development in Drosophila, primordial germ cells (PGCs) migrate out of the posterior midgut pocket to reach somatic gonadal precursors (SGP). Multiple tissues (e.g. the central nervous system [CNS] and epidermis) express the lipid phosphate phosphatases wunen (wun) and wunen2 (wun2). Together, they serve as sinks that actively remove phospholipids that are attractive for PGCs. During migration, PGCs avoid wun and wun2-expressing tissues, finding themselves attracted only to regions from which wun and wun2 are absent (Figure 4a) [26]. Multiple wun and wun2-expressing sinks thus sculpt a path of permissive areas.
Figure 4.
Diverse and dynamic sinks. (a) Drosophila PGC cells (green) disperse and migrate our of the posterior midgut pocket (blue) towards somatic gonadal precursors (SGP). They avoid regions where Wun and Wun2 (yellow) phosphatases deplete the chemoattractant [26]. Grey arrow indicates direction of migration. Multiple sinks expressing wun and wun2 are shown in brown. A transverse section of a Drosophila embryo (ventral part) is shown. (b) Migration of cancer epithelial cells (green) from one chamber to another through a connected channel. EGF ligand is shown in blue. (c) Migration of zebrafish lateral line primordium (in red) on a self-generated gradient of Sdf1 (blue). Cxcr7 receptor (brown) is a non-signaling receptor expressed by posterior cells, which serve as a sink. (d) Zebrafish primordial germ cell (green) migration. Red arrow indicates highest point of Sdf1 expression at an intermediate target (orange). Grey arrows indicate direction of migration at two different time points when the source has changed.
Since migrating cells actively take up chemoattractants, they themselves can also serve as chemoattractant sinks. In this case, the same chemoattractant receptor that directs the cells to move can also serve as a sink by locally internalizing the attractant and thus depleting it from the immediate environment [27]. This depletion promotes the initial dispersal of Drosophila primordial germ cells from a tight cluster, which is necessary for them to cross the midgut epithelium, and then this effective repulsion achieved by local depletion of the attractant, stimulates their sorting to both sides of the embryo [28]. Similarly, human cancer cells derived from various epithelial tissues including breast, lung, and pancreas can generate their own gradient by serving as the sink. When migrating through the confined environment of an artificial maze and presented with a uniform concentration of EGF, the majority of cells strikingly take the shortest route through the maze from the entrance to the exit [27]. The reason appears to be that the cancer epithelial cells can locally take up EGF ligands, creating a sink. Molecules then diffuse from the area of highest concentration to the depleted area, forming a gradient (Figure 4b). The cells can thus create and then follow a de novo EGF gradient [27]. An in vivo example comes from chick NC cells. Upon exiting the neural tube, NC cells become subdivided into leading and trailing populations. Leader cells locally deplete VEGF, which is a chemoattractant for NC cells, forming a gradient that the trailing cells follow [29]. These examples of autonomously generated chemical gradients eliminate the need for a pre-existing long-range chemical gradient.
A second example of directional migration in response to a uniformly presented chemoattractant is the movement of the posterior lateral line primordium, which is composed of ~100 cells that migrate collectively from head to tail during zebrafish embryonic development. Along the way, little clusters of cells stop and form major mechanosensory organs along the length of the body [30]. Sdf1 is a secreted molecule present in a stripe along the migratory path. The stripe appears uniform in intensity and cells can follow it in both directions, indicating that Sdf1 is not expressed in a gradient [31]. However a non-signaling receptor, CXCR7 is expressed by cells at the rear of the primordium, potentially creating a local sink, whereas the signaling receptor CXCR4 is expressed throughout the primordium. In a recent study, novel imaging tools allowed visualization of the functional Sdf1 gradient and demonstrated that the concentration of functional Sdf1 is lower in the rear of the tissue than the front, owing to specific expression of the Sdf1-sequestering molecule CXCR7 in the rear (Figure 4c) [32,33]. The lateral line primordium cells can thus migrate directionally even in response to a uniform stripe of Sdf1.
Complex Migration Requires Dynamic Source and Multiple Sinks
Similar to the lateral line primordium, zebrafish primordial germ cells (PGCs) also utilize Sdf1 and CXCR7 for directional migration. However, they follow a more complicated migration path, make multiple turns, and migrate as single cells, so a single stripe of uniform Sdf1 would not be sufficient to guide them. To guide PGCs, multiple intermediate targets along the migration path sequentially express Sdf1 (Figure 4d) [34]. PGCs migrate directionally using a “run and tumble” mechanism analogous to bacterial chemotaxis [35]. When they are moving towards a higher concentration of Sdf1, they move persistently in one direction (i.e. run) for longer periods of time than when they are moving down the concentration gradient. In between the runs, the cells “tumble”, i.e. they temporarily lose polarity and do not move forward until they reassess their surroundings and repolarize [34,36].
This mechanism requires that Sdf1 is produced in limiting amounts and that excess ligand, particularly from the prior segment of the path, is removed. CXCR7 is expressed in multiple somatic tissues along the zebrafish PGC migration path and serves as a sink to shape the gradient of Sdf1 (Figure 4d). It sequesters and internalizes Sdf1, without inducing any downstream signaling [37]. Knocking down CXCR7 causes PGCs to lose polarity and migrate randomly, which can be rescued by reducing sdf1 expression concurrently. The multiple CXCR7 sinks, together with the dynamic sdf1 source expression, ensure that zebrafish PGCs follow the right path and reach their targets at the right time.
Conclusion and Future Directions
Generation of a stable chemoattractant gradient minimally requires a source that produces the chemical and a sink that removes it. However in vivo the landscape is both complex and changing, and this requires that cells employ dynamic and diverse sources and sinks for navigation. Within the same embryo different cells have to find different paths, necessitating a variety of cues, receptors, and overall strategies. In some cases, production of a single chemoattractant can be dynamic enough to guide one type of cell from one intermediate target site to another. Other types of cells see different cues at different points along the way. Within a migrating population, neighboring cells can provide guidance. Sinks are critically important and can be as varied as the sources. These multiple ways of building sources and sinks allow diverse cell types to navigate distinct trajectories through the same embryo even as the whole embryo undergoes the dramatic changes associated with development.
In the future, fluorescent probes with better spatial and temporal resolution will allow visualization of more chemoattractant signals and responses, especially weak or oscillating ones, in living organisms. More advanced imaging techniques will be employed to study the distributions of chemoattractants at single molecule level. The knowledge gleaned from studying diverse sources and sinks in cell migration in vivo may prove useful for tissue engineering and enhanced wound healing, where it will be critically important to steer cells along the correct paths so they can reach the desired final destination as reliably and efficiently as possible.
Acknowledgements
This work was supported by NIH grant R01GM46425 to D.J.M
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Van Haastert PJ, Devreotes PN. Chemotaxis: signalling the way forward. Nat Rev Mol Cell Biol. 2004;5:626–634. doi: 10.1038/nrm1435. [DOI] [PubMed] [Google Scholar]
- 2.Seppa H, Grotendorst G, Seppa S, Schiffmann E, Martin GR. Platelet-Derived Growth-Factor Is Chemotactic for Fibroblasts. Journal of Cell Biology. 1982;92:584–588. doi: 10.1083/jcb.92.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deuel TF, Kawahara RS, Mustoe TA, Pierce GF. Growth-Factors and Wound-Healing - Platelet-Derived Growth-Factor as a Model Cytokine. Annual Review of Medicine. 1991;42:567–584. doi: 10.1146/annurev.me.42.020191.003031. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez-Madrid F, del Pozo MA. Leukocyte polarization in cell migration and immune interactions. EMBO J. 1999;18:501–511. doi: 10.1093/emboj/18.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 6.Ming GL, Wong ST, Henley J, Yuan XB, Song HJ, Spitzer NC, Poo MM. Adaptation in the chemotactic guidance of nerve growth cones. Nature. 2002;417:411–418. doi: 10.1038/nature745. [DOI] [PubMed] [Google Scholar]
- 7.Crick F. Diffusion in embryogenesis. Nature. 1970;225:420–422. doi: 10.1038/225420a0. [DOI] [PubMed] [Google Scholar]
- 8.Tomchik KJ, Devreotes PN. Adenosine 3',5'-monophosphate waves in Dictyostelium discoideum: a demonstration by isotope dilution--fluorography. Science. 1981;212:443–446. doi: 10.1126/science.6259734. [DOI] [PubMed] [Google Scholar]
- 9.Kaprielian Z, Runko E, Imondi R. Axon guidance at the midline choice point. Developmental Dynamics. 2001;221:154–181. doi: 10.1002/dvdy.1143. [DOI] [PubMed] [Google Scholar]
- 10.Lyuksyutova AI, Lu CC, Milanesio N, King LA, Guo NN, Wang YS, Nathans J, Tessier-Lavigne M, Zou YM. Anterior-posterior guidance of commissural Axons by Wnt-frizzled signaling. Science. 2003;302:1984–1988. doi: 10.1126/science.1089610. [DOI] [PubMed] [Google Scholar]
- 11.Stein E, Tessier-Lavigne M. Hierarchical organization of guidance receptors: Silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science. 2001;291:1928–1938. doi: 10.1126/science.1058445. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan A, Kent CB, Charron F, Fournier AE. Switching Responses: Spatial and Temporal Regulators of Axon Guidance. Molecular Neurobiology. 2014;49:1077–1086. doi: 10.1007/s12035-013-8582-8. [DOI] [PubMed] [Google Scholar]
- 13.Sutherland D, Samakovlis C, Krasnow MA. Branchless encodes a Drosophila FGF homolog that controls tracheal cell migration and the pattern of branching. Cell. 1996;87:1091–1101. doi: 10.1016/s0092-8674(00)81803-6. [DOI] [PubMed] [Google Scholar]
- 14.Garcia GL, Parent CA. Signal relay during chemotaxis. J Microsc. 2008;231:529–534. doi: 10.1111/j.1365-2818.2008.02066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shutt DC, Jenkins LM, Carolan EJ, Stapleton J, Daniels KJ, Kennedy RC, Soll DR. T cell syncytia induced by HIV release. T cell chemoattractants: demonstration with a newly developed single cell chemotaxis chamber. J Cell Sci. 1998;111(Pt 1):99–109. doi: 10.1242/jcs.111.1.99. [DOI] [PubMed] [Google Scholar]
- 16.Soll DR, Wessels D, Heid PJ, Zhang H. A contextual framework for characterizing motility and chemotaxis mutants in Dictyostelium discoideum. J Muscle Res Cell Motil. 2002;23:659–672. doi: 10.1023/a:1024459124427. [DOI] [PubMed] [Google Scholar]
- 17.Wessels D, Murray J, Soll DR. Behavior of Dictyostelium Amebas Is Regulated Primarily by the Temporal Dynamic of the Natural Camp Wave. Cell Motility and the Cytoskeleton. 1992;23:145–156. doi: 10.1002/cm.970230207. [DOI] [PubMed] [Google Scholar]
- 18.Saran S, Meima ME, Alvarez-Curto E, Weening KE, Rozen DE, Schaap P. cAMP signaling in Dictyostelium. Complexity of cAMP synthesis, degradation and detection. J Muscle Res Cell Motil. 2002;23:793–802. doi: 10.1023/a:1024483829878. [DOI] [PubMed] [Google Scholar]
- 19.Bader S, Kortholt A, Van Haastert PJ. Seven Dictyostelium discoideum phosphodiesterases degrade three pools of cAMP and cGMP. Biochem J. 2007;402:153–161. doi: 10.1042/BJ20061153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Das S, Rericha EC, Bagorda A, Parent CA. Direct Biochemical Measurements of Signal Relay during Dictyostelium Development. Journal of Biological Chemistry. 2011;286:38649–38658. doi: 10.1074/jbc.M111.284182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Afonso PV, Janka-Junttila M, Lee YJ, McCann CP, Oliver CM, Aamer KA, Losert W, Cicerone MT, Parent CA. LTB4 Is a Signal-Relay Molecule during Neutrophil Chemotaxis. Developmental Cell. 2012;22:1079–1091. doi: 10.1016/j.devcel.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lammermann T, Afonso PV, Angermann BR, Wang JM, Kastenmuller W, Parent CA, Germain RN. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature. 2013;498:371. doi: 10.1038/nature12175. -+. * Showed in vivo that neutrophils use LTB4 to propagate directional signal between cells and migrate to the wound.
- 23. Theveneau E, Steventon B, Scarpa E, Garcia S, Trepat X, Streit A, Mayor R. Chase-and-run between adjacent cell populations promotes directional collective migration. Nature Cell Biology. 2013;15:763. doi: 10.1038/ncb2772. -+. ** Example that cell-cell interaction mediates directional migration of two cell types.
- 24.Torisawa YS, Mosadegh B, Bersano-Begey T, Steele JM, Luker KE, Luker GD, Takayama S. Microfluidic platform for chemotaxis in gradients formed by CXCL12 source-sink cells. Integr Biol (Camb) 2010;2:680–686. doi: 10.1039/c0ib00041h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abhyankar VV, Lokuta MA, Huttenlocher A, Beebe DJ. Characterization of a membrane-based gradient generator for use in cell-signaling studies. Lab on a Chip. 2006;6:389–393. doi: 10.1039/b514133h. [DOI] [PubMed] [Google Scholar]
- 26.Renault AD, Lehmann R. Follow the fatty brick road: lipid signaling in cell migration. Curr Opin Genet Dev. 2006;16:348–354. doi: 10.1016/j.gde.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 27.Scherber C, Aranyosi AJ, Kulemann B, Thayer SP, Toner M, Iliopoulos O, Irimia D. Epithelial cell guidance by self-generated EGF gradients. Integr Biol (Camb) 2012;4:259–269. doi: 10.1039/c2ib00106c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Renault AD, Kunwar PS, Lehmann R. Lipid phosphate phosphatase activity regulates dispersal and bilateral sorting of embryonic germ cells in Drosophila. Development. 2010;137:1815–1823. doi: 10.1242/dev.046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLennan R, Dyson L, Prather KW, Morrison JA, Baker RE, Maini PK, Kulesa PM. Multiscale mechanisms of cell migration during development: theory and experiment. Development. 2012;139:2935–2944. doi: 10.1242/dev.081471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghysen A, Dambly-Chaudiere C. The lateral line microcosmos. Genes Dev. 2007;21:2118–2130. doi: 10.1101/gad.1568407. [DOI] [PubMed] [Google Scholar]
- 31.Haas P, Gilmour D. Chemokine signaling mediates self-organizing tissue migration in the zebrafish lateral line. Dev Cell. 2006;10:673–680. doi: 10.1016/j.devcel.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 32. Dona E, Barry JD, Valentin G, Quirin C, Khmelinskii A, Kunze A, Durdu S, Newton LR, Fernandez-Minan A, Huber W, et al. Directional tissue migration through a self-generated chemokine gradient. Nature. 2013;503:285. doi: 10.1038/nature12635. -+. ** Used fluorescent timer approach to visualize functional Sdf1 gradient generated by migrating tissue.
- 33. Venkiteswaran G, Lewellis SW, Wang J, Reynolds E, Nicholson C, Knaut H. Generation and Dynamics of an Endogenous, Self-Generated Signaling Gradient across a Migrating Tissue. Cell. 2013;155:674–687. doi: 10.1016/j.cell.2013.09.046. ** Another important paper visualizing the functional Sdf1 gradient in zebrafish lateral line primordium
- 34.Doitsidou M, Reichman-Fried M, Stebler J, Koprunner M, Dorries J, Meyer D, Esguerra CV, Leung T, Raz E. Guidance of primordial germ cell migration by the chemokine SDF-1. Cell. 2002;111:647–659. doi: 10.1016/s0092-8674(02)01135-2. [DOI] [PubMed] [Google Scholar]
- 35.Reichman-Fried M, Minina S, Raz E. Autonomous modes of behavior in primordial germ cell migration. Dev Cell. 2004;6:589–596. doi: 10.1016/s1534-5807(04)00074-7. [DOI] [PubMed] [Google Scholar]
- 36.Richardson BE, Lehmann R. Mechanisms guiding primordial germ cell migration: strategies from different organisms. Nat Rev Mol Cell Biol. 2010;11:37–49. doi: 10.1038/nrm2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boldajipour B, Mahabaleshwar H, Kardash E, Reichman-Fried M, Blaser H, Minina S, Wilson D, Xu Q, Raz E. Control of chemokine-guided cell migration by ligand sequestration. Cell. 2008;132:463–473. doi: 10.1016/j.cell.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 38.Charron F, Tessier-Lavigne M. Novel brain wiring functions for classical morphogens: a role as graded positional cues in axon guidance. Development. 2005;132:2251–2262. doi: 10.1242/dev.01830. [DOI] [PubMed] [Google Scholar]