Abstract
Background
Adherence behaviors have not been examined among adolescents undergoing laparoscopic adjustable gastric banding (LAGB). In addition, studies of youth receiving bariatric surgery have not considered the influence of psychopathology on postoperative adherence.
Objective
The purpose of this study was to evaluate predictors and correlates of adherence to post-surgery visits among a sample of adolescents undergoing LAGB.
Setting
Psychiatry Department, University Medical Center, United States.
Methods
Postoperative visits with surgical staff were analyzed over the two years following surgery (n= 101 adolescents). Growth mixture modeling examined trends in adherence.
Results
A three-class solution provided the best fit to the data. The classes from the final model were characterized by class 1 (61.6%) demonstrating high levels of adherence over the 24 months following LAGB, class 2 (28.5%) showing a more gradual decline in adherence, and class 3 (9.9%) with an accelerated decline in adherence. Higher levels of preoperative depressive symptoms and more preoperative episodes of loss of control over eating decreased the likelihood of adherence. Class 3 adolescents had significantly higher estimated 24-month body mass indices than Classes 1 or 2.
Conclusions
Variable patterns of follow-up visit adherence were identified among adolescents receiving LAGB, which were predicted by depressive symptoms and loss of control over eating. The trajectory characterized by a rapid decline in adherence to follow-up visits was also associated with less weight loss.
Keywords: Bariatric Surgery, Adolescents, Adherence, Follow up, Laparoscopic Adjustable Gastric Banding
INTRODUCTION
Variability in weight loss is a significant clinical problem for both adults and adolescents undergoing bariatric procedures. Postoperative excess weight loss can fluctuate widely among adolescents (e.g., 4% 64%1), and for adults receiving laparoscopic adjustable gastric banding (LAGB), the majority evidence weight plateaus or regain starting approximately six months following surgery(2). Adherence, or “the extent to which a person’s behavior coincides with medical or health advice” (p. e4753) is a likely contributor to variable weight loss outcomes(4–8). Adherence behaviors are important to the overall clinical management of individuals receiving bariatric surgery, as missing routine appointments results in suboptimal care, and failure to follow guidelines for diet or vitamin supplementation affects weight loss(6) and risk for malnutrition(9, 10). However, extant research on bariatric surgery(4, 6, 8, 11–16) includes a range of different adherence behaviors (e.g., attendance at postoperative appointments or support groups, following recommendations for diet and physical activity or multivitamin therapy), which limits comparisons across studies. Additional research is needed to better understand modifiable factors affecting adherence.
Several studies have evaluated the influence of psychosocial variables on postoperative adherence behaviors among adults receiving bariatric surgery. Depressive symptoms, negative affect, and eating disorders, relate to postoperative adherence (e.g., self-reported adherence to postoperative diet, postoperative appointment attendance) in some studies(4, 6, 14, 17), but not others(12, 18). Adolescence is a unique developmental stage characterized by notable rates of non adherence and the initiation of self-management of medical care for chronic illnesses(3, 19). Among adolescents receiving bariatric surgery, adherence to postoperative diet is incomplete(9), and attendance at routine visits following gastric bypass declines over time(20). To date, adherence behaviors have not been examined among adolescents undergoing LAGB. The relatively frequent visit schedule required for band adjustments post-LAGB may present a challenge to adherence generally, and for the adolescent population specifically. In addition, although psychological factors are well-documented influences on treatment adherence among adolescents with other chronic medical conditions(21–24), studies of youth have not considered the influence of psychopathology on post-bariatric surgery adherence.
Thus, the purpose of the study was to evaluate clinic appointment attendance in the two years following LAGB in a sample of adolescents, including rates and patterns of adherence to visits(3) within and across participants. The study also aimed to examine predictors and correlates of postoperative visit attendance. We predicted that subgroups demonstrating different attendance patterns would be identified within the sample based on previous research demonstrating heterogeneity among adolescent surgical candidates (e.g., 25). In addition, we hypothesized that like adults receiving bariatric surgery(6, 14) or adolescents with chronic health problems(21–24), psychosocial problems such as elevated depressive symptoms, low levels of quality of life, and eating pathology would predict adherence post-LAGB. Further, as in adults receiving LAGB(7, 8), we hypothesized pre- and post-surgical adherence to clinic visits would be related to weight loss outcomes.
METHODS AND PROCEDURES
Participants
Participants were 101 adolescent candidates enrolled in a university hospital bariatric surgery program between August 2006 and December 2009. All adolescents received LAGB under an FDA approved Investigational Device Exemption. Primary eligibility criteria included: 1) age 14–17 years, 2) BMI > 40 kg/m2 or > 35 kg/m2 with serious comorbid conditions (e.g., Type II diabetes); 3) a 5-year or more history of obesity with failed attempts at diet and medical management for at least one year, and followed in the surgery program for at least six months; 4) for females, appropriate contraception and not planning to become pregnant in the year following surgery, 5) no medical contraindications for surgery, and 6) absence of current self-induced vomiting. Written informed assent and consent, respectively, were provided by adolescents and their parent(s) to receive LAGB and an Institutional Review Board reviewed and approved the protocol.
Procedures
Prior to surgery, adolescents and parent(s) met with the surgical staff for routine visits and completed at least one evaluation with a psychologist or psychiatrist, which included self-report assessments of psychiatric functioning and a clinical interview. Data from these visits with surgery and psychiatry were used as predictors of adherence in the current study. Detailed information about the pre-surgery assessments and clinical interview has been published elsewhere(26).
Predictors of Adherence to Clinic Appointments
Psychiatric Symptoms
Prior to surgery, adolescents completed the following assessments:
Beck Depression Inventory (BDI27): The psychometric properties of the BDI are well-established for measuring depressive symptoms in adolescents(28), and this assessment is commonly administered in studies of adolescents receiving bariatric surgery (e.g., 29, 30). The total score was used, with higher scores reflecting more depressive symptoms.
Pediatric Quality of Life Inventory (PedsQL31): The PedsQL is a 23 item measure of health-related quality of life, with the total score (used in the analyses described below) ranging from 0–100, with higher scores indicating better quality of life. The PedsQL total score is a reliable and valid measure of quality of life that discriminates between clinical and non-clinical populations(32).
Eating Disorders Examination-Questionnaire (EDE Q33) and/or Questionnaire on Eating and Weight Patterns-Revised (QEWP34): Adolescents completed the QEWP or EDE-Q, or both. The EDE-Q is a 38-item measure of eating disorder symptoms with adequate reliability and validity(35), which has been used for overweight youth(36) and adolescents with eating disorders(37). The QEWP is a 28-item self report instrument with appropriate psychometrics (e.g., 38) designed to assess dieting and weight history, and symptoms of binge eating disorder. The QEWP measure of objective binge eating episodes has been used in other research on youth receiving bariatric surgery(29). Only the EDE-Q assesses subjective bulimic episodes, or consuming an amount of food that is not objectively large, but is seen by the individual as large, with a sense of loss of control. This study utilized a composite variable based on objective or subjective bulimic episodes by EDE-Q or objective binge eating episodes from the QEWP to code the presence or absence of ‘loss of control over eating’ episodes, or any eating characterized by a loss of control regardless of the amount consumed(39).
Demographic variables
Age, gender, and race/ethnicity were obtained by clinical interview. Measures of height and weight were obtained during routine visits with the surgery staff, and were used to calculate body mass index (kg/m2). Median household income was obtained from the 2000 US Census Bureau American Factfinder program (http://factfinder.census.gov) using the zip code for the participants’ primary home address. Use of census-based data has been shown to be a valid method of overcoming the absence of information about socioeconomic status in medical records(40).
Distance from Clinic
Similar to other studies(11, 20), participants’ home addresses were obtained from clinical charts, and Google maps determined the travel distance from the adolescent’s home to the medical center campus.
Pre surgery visits with surgical staff
A chart review identified the total number of visits adolescents attended prior to surgery. The total amount of time between the adolescents’ first visit with the surgical staff and LAGB was also calculated.
Outcome Measures
Measure of Clinic Appointment Attendance
By study protocol, routine appointments with surgical staff were scheduled to occur on 17 occasions, including: one week, two weeks, four weeks, six weeks, and twelve weeks post-LAGB and monthly thereafter for the initial 12 months, and then at 15, 18, and 24 months post-surgery. The clinic visit schedule was developed by the team’s pediatric surgeon and pediatric endocrinologist, in consultation with adult bariatric surgeons and the medical center’s diabetes treatment center, to ensure close medical monitoring of potential postoperative risk and diabetes management. Data from these visits were collapsed into 10 time-points to better reflect current clinical practice in our program, including: week two (week 1 or 2), week six (week 4 or 6), month three (month 3 or 4), month six (month 5 or 6), month eight (month 7 or 8), month 10 (month 9, 10, or 11), month 12 (month 12, 13, or 14), month 15 (month 15, 16, 17), month 18 (month 18, 19, or 20), and month 24 (month 22, 23, or 24).
Adherence was coded into four outcomes at each time point: (1) attending the visit within the expected time-frame; (2) attending the visit outside of the expected time-frame; (3) not attending the visit; and (4) not attending the visit and never returning for additional visits. The expected time-frame for attending visits was ± 7 days for weekly visits (week 1, 2, 4, 6, 12) and ± 14 days for monthly visits in the first postoperative year and the 15, 18, and 24 month visits. Adherence to time-frame was first determined for the 17 original expected visits and appointments were coded in only one time-point. After the visits were collapsed, priority was given to any on-time attendance within the 10 time-points.
Other summary variables, including the total number of visits attended with surgical staff over the two postoperative years, the number of visits attended within an expected time-frame, and time to drop-out were also examined. Drop-out was coded as the point at which an adolescent failed to return for any additional visits.
Body Mass Index
Post-LAGB height and weight were measured during routine visits attended with surgical staff.
Statistical Analysis
A series of growth mixture models (GMMs) estimated the trajectory of change in clinic appointment attendance over 24 months following LAGB, and classified similar participants into groups. Growth mixture models assume data that are not available (e.g., 24.5% of BMIs across time points when adolescents did not attend a clinic visit) are missing at random. Tests of baseline differences between participants with and without missing BMIs, and correlations between patterns of missingness and unobserved variables, were not statistically significant, which suggests that the missing at random assumption was appropriate. Growth for attendance was modeled as a single class using linear, quadratic, cubic, and via nonlinear spline. Best fit for the model was evaluated by considering lowest Bayesian Information Criterion (BIC). A statistically significant variance for the random intercept and slope provided evidence of population heterogeneity in patterns of attendance, and a visual inspection of the plotted trajectories for adherence confirmed this variability. Subsequently, GMMs with additional classes were tested (2–5 classes), with the number of classes for the final model selected by Lo Mendel-Rubin chi square tests, which evaluates the relative improvement in model fit for each increased number of classes, and entropy. After final GMMs were estimated, pre-operative covariates hypothesized to relate to post-surgery adherence were entered to examine their predictive value on class membership. We tested covariate influence using the Modal ML method(41) in Mplus using the 3STEP approach described by Asparohov and Muthen (2013; see http://www.statmodel.com/examples/webnotes/webnote15.pdf). The same methods were used to examine differences between groups on BMI at the end of 24-months as a distal outcome.
RESULTS
Demographic Characteristics
The sample was 28 males (27.7%) and 73 females (72.3%), with a mean age of 15.8 ± 1.1 years and average BMI of 47.8 ± 7.2 kg/m2, and 34.7% classified as white (n = 35), 39.6% classified as Hispanic/Latino (n = 40), 20.8% classified as African American (n = 21), and 5.0% classified as of another race (n = 5). Baseline mean total scores on the BDI and PedsQL were 9.0 ± 8.3 and 72.9 ± 15.2, respectively.
Growth Mixture Models
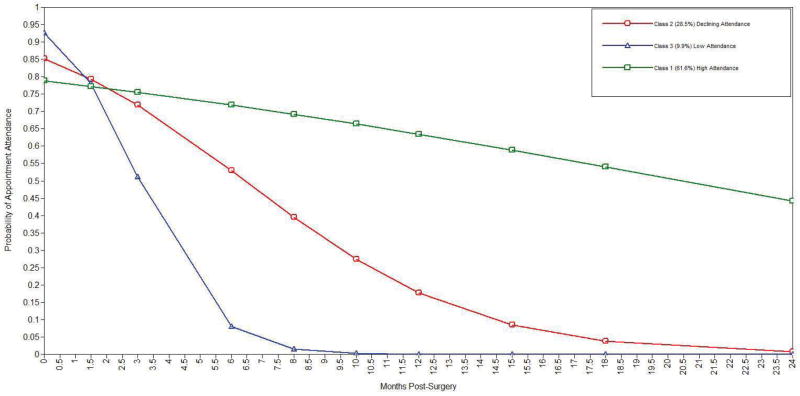
Lo-Mendel-Rubin chi-squares tests indicated a 3-class solution provided a significant improvement over the 2-class model and the best BIC and entropy of the 2–5 class models [3-class parameters=13, loglikelihood= 985.35, BIC=1996.69, entropy=0.887]. The three classes from this model are characterized by class 1 (61.6%) with high levels of adherence over the 2-year follow-up, class 2 (28.5%) showing a more gradual decline in adherence, and class 3 (9.9%) with an accelerated decline in adherence. The rate of change in adherence for class 3 (μslope = 0.86, SE = 0.19, p < 0.001) was greater than class 1 (μslope = −0.07, SE = 0.01, p < 0.001) and class 2 (μslope = −0.285, SE = 0.035, p < .001). The variance estimate for slope was not significant (VAR = 0.001, SE = 0.001, p = 0.21), indicating that the individual variability in adherence over time was accounted for by class membership. Figure 1 summarizes these trajectory changes in probability of dropout over time; probabilities of other types of adherence are available upon request. On average, adolescents attended 9.09 appointments (range of 2–17). Sixty adolescents were seen for a month 24 visit (months 22, 23, or 24), and the median time of drop-out among the 41 other adolescents was the month 10 visit (months 9, 10, or 11).
Figure 1.
Trajectory Change in the Probability of Dropout over the 24 Months following Laparoscopic Adjustable Gastric Banding among 101 Adolescents
Baseline predictors were examined, including: gender, age, race/ethnicity, distance from treatment center, clinically significant symptoms (loss of control over eating, total BDI score, total PedsQL); Table 1 summarizes the effects of the final model of baseline covariates on these trajectories. The covariate effects indicate that change in adherence was significantly predicted by baseline total BDI score. For every 5 unit increase in BDI, the adherence rate decreased by a factor of 0.27. Loss of control over eating was also a significant predictor of class membership; reporting loss of control episodes increased the odds of being in the class characterized by early dropout (Class 3) by 3.51 relative to being classified as maintaining consistent adherence throughout follow up (Class-1). Other psychiatric and demographic variables were not significant predictors of either trajectory or rate of change in adherence over the 24 months of follow-up.
Table 1.
Summary of Baseline Predictors of Adherence Trajectory
| Slope | Class 1 versus Class 3 | Class 2 versus Class 3 | |
|---|---|---|---|
| β(SE) | β(SE) | β(SE) | |
| Body Mass Index | −0.006 (0.004) | 0.122 (0.363) | 0.294 (0.198) |
| Presence of Loss of Control over Eating Episodes | −0.017 (0.018) | 1.27 (0.508)* | −0.609 (0.614) |
| Baseline Pediatric Quality of Life Scale Total Score | 0.003 (0.010) | 1.13 (1.33) | −0.545 (0.467) |
| Gender | −0.049 (0.027) | −6.47 (7.58) | 0.061 (1.14) |
| Median household income by zip code | −0.005 (0.004) | −0.168 (0.510) | 0.412 (0.226) |
| Baseline Beck Depression Inventory Total Score | −0.027 (0.007)** | −1.61 (1.55) | −0.002 (0.262) |
p < 0.05,
p < 0.01
Twenty-four month post-surgery BMI significantly differed by group, with Class 3 having significantly higher estimated BMIs than Class 1 (Mdiff BMI = 4.23, SE = 1.13, p < 0.001) and Class 2 (Mdiff BMI = 2.57, SE = 1.15, p < 0.05). Class 1 did not significantly differ from class 2 (Mdiff BMI = 1.44, SE = 1.19, p < 0.74).
DISCUSSION
Adolescents attended an average of 53% of the expected clinic appointments with surgery staff over the two years following surgery. Modeled trajectories identified three distinct patterns among participants, including: (1) consistently high levels of attendance (Class 1); (2) a gradual decline in attendance (Class 2) and; (3) a more rapid decline in attendance (Class 3). Adolescents characterized by failing to attend visits soon after receiving LAGB had a significantly higher average BMI two years post-surgery in comparison to the other subgroups. Change in adherence over time was significantly predicted by baseline total scores on the Beck Depression Inventory and loss of control eating episodes, with higher levels of depressive symptoms and the presence of loss of control eating associated with early dropout. In adults, postoperative adherence behaviors are affected by elevated depressive symptoms, negative affect, and the combination of mood and eating disorders(4, 6, 14, 17). However, as described previously, no specific associations have been noted between binge eating and adherence outcomes(12). In this study, other factors, including distance from the medical center, gender, and initial BMI were not significant predictors of adherence to clinic appointments. This finding contrasts studies of adults(42), but replicates the one other study of adolescents(20). Unlike prior work with younger(20) and older(18) patients, age was not related to attendance.
The development of interventions for adolescents can be informed by our growth mixture models identifying three homogeneous subgroups of adolescents. In particular, the subsample of adolescents characterized by rapid failure to attend visits also reported increased baseline depressive symptoms and loss of control eating episodes. A focus on reducing pre operative loss of control eating episodes may also be useful to enhance clinical outcomes, as this symptom is an important clinical characteristic among severely obese youth in behavioral or surgical interventions. Loss of control eating appears to decrease the short-term effectiveness of family based treatment(43), and in addition to the effects on clinic appointment attendance noted above, also predicts short term post-LAGB weight loss among adolescents(44).
Similar to programs developed for other chronic illnesses in pediatric populations(45), obese adolescents(46–48), and adults receiving bariatric surgery(49), it might also be possible to capitalize on adolescents’ affinity for novel technology to improve adherence. The development of smartphone or other Internet-based applications for post bariatric surgery monitoring has numerous advantages(49), including facilitating reminders about adherence and collection of self reported information when adolescents are not attending routine visits. Technology also facilitates connecting adolescents and their families across significant distances(3), which could increase support and investment in adherence behaviors. Although some support has been found for the impact of postoperative dietary counseling on adherence in adults(50), it is not yet clear whether intervening pre- or post-surgery would be more effective for increasing attendance at visits for adolescents; however, additional research could determine the optimal window to improve outcomes.
The current study was limited by the reliance on self-report measures, which can be affected by concerns about approval for surgery. Like previous research(20), only information about visit attendance was available for all participants, which is only one component of adherence(3), and it is possible that data on other aspects of postoperative behavior (e.g., adherence to nutritional recommendations) are more relevant in this population. In addition, the original protocol for clinic visits was potentially burdensome, as evidenced by only one adolescent (1.0%) attending all 17 expected appointments, and three of these appointments occurred outside the expected time-frame. The frequency of protocol visits may have resulted in lower rates of overall attendance. As aforementioned, different definitions of adherences are employed across studies (e.g., failure to attend appointment within three weeks of when scheduled(11); inability to follow diet/exercise recommendations, percent attendance at scheduled visits(8); failing to modify eating behavior(12); attendance at > or < 6 visits, number of visits(13), which complicates comparisons between this analysis and extant research on LAGB. The available predictors of adherence did not include variables that may be particularly important for adolescents, such as access to parental health insurance(20), parental barriers to escorting adolescents to visits, or other parental factors (e.g., BMI51). Further, by incorporating an assessment of a broader form of loss of control over eating (the EDE-Q), our measure of this construct was not consistent for all participants. This study also has several important strengths, including sample size, the inclusion of consecutively referred participants, and the duration of follow-up.
As all bariatric interventions carry risk for peri- and postoperative complications(10), surgical teams must weight potential costs and benefits, including risk for non-adherence, before approving an adolescent for surgery. Some distinctive clinical issues must also be considered for adolescents pursuing LAGB. Although LAGB is not approved by the FDA for use among individuals younger than 18, the reversibility, relative safety profile, lower risk of nutritional deficiencies(52), and improved weight loss outcomes in comparison to behavioral therapies(53), are advantages for this age group. However, LAGB also requires adherence to nutritional recommendations to avoid revisional procedures(53) and ongoing follow-up, which was not optimal in our sample. Adolescence presents unique and complex challenges for assessing and improving treatment adherence in bariatric surgery, and it is not yet possible to assess the potential for non-adherence on the basis of existing data. Additional research is needed to determine whether our results generalize to other surgical procedures, and to better understand predictors of the failure to adhere to postoperative treatment that may affect long term weight outcomes.
Acknowledgments
The authors thank B. Timothy Walsh, M.D., Mary Beth Spitznagel, Ph.D., and the members of the Center for Adolescent Bariatric Surgery team.
FINANCIAL SUPPORT: Dr. Sysko is supported, in part, by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK088532-01A1. Tom Hildebrandt was supported, in part, by National Institute on Drug Abuse Grant DA024043-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dillard BE, Gorodner N, Galvani C, et al. Initial experience with the adjustable gastric band in morbidly obese US adolescents and recommendations for further investigation. J Pediatr Gastr Nutr. 2007;45:240–246. doi: 10.1097/MPG.0b013e31805b82fb. [DOI] [PubMed] [Google Scholar]
- 2.Courcoulas AP, Christian NJ, Belle SH, et al. Weight Change and Health Outcomes at 3 Years After Bariatric Surgery Among Individuals With Severe Obesity. JAMA. 2013;310:2416–2425. doi: 10.1001/jama.2013.280928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Modi AC, Pai AL, Hommel KA, et al. Pediatric self-management: a framework for research, practice, and policy. Pediatrics. 2012;129:e473–485. doi: 10.1542/peds.2011-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Júnior WS, do Amaral JL, Nonino-Borges CB. Factors related to weight loss up to 4 years after bariatric surgery. Obes Surg. 2011;21:1724–1730. doi: 10.1007/s11695-011-0420-3. [DOI] [PubMed] [Google Scholar]
- 5.Sarwer DB, Dilks RJ, West-Smith L. Dietary intake and eating behavior after bariatric surgery: threats to weight loss maintenance and strategies for success. Surg Obes Relat Dis. 2011;7:644–651. doi: 10.1016/j.soard.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 6.Sarwer DB, Wadden TA, Moore RH, et al. Preoperative eating behavior, postoperative dietary adherence, and weight loss after gastric bypass surgery. Surg Obes Relat Dis. 2008;4:640–646. doi: 10.1016/j.soard.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El Chaar M, McDeavitt K, Richardson S, Gersin KS, Kuwada TS, Stefanidis D. Does participant compliance with preoperative bariatric office visits affect postoperative excess weight loss? Surg Obes Relat Dis. 2011;7:743–748. doi: 10.1016/j.soard.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 8.Pontiroli AE, Fossati A, Vedani P, et al. Post-surgery adherence to scheduled visits and compliance, more than personality disorders, predict outcome of bariatric restrictive surgery in morbidly obese participants. Obes Surg. 2007;17:1492–1497. doi: 10.1007/s11695-008-9428-8. [DOI] [PubMed] [Google Scholar]
- 9.Sarwer DB, Dilks RJ. Invited commentary: childhood and adolescent obesity: psychological and behavioral issues in weight loss treatment. J Youth Adolesc. 2012;41:98–104. doi: 10.1007/s10964-011-9677-z. [DOI] [PubMed] [Google Scholar]
- 10.Xanthakos SA, Inge TH. Nutritional consequences of bariatric surgery. Curr Opin Clin Nutr Metab Care. 2006;9:489–496. doi: 10.1097/01.mco.0000232913.07355.cf. [DOI] [PubMed] [Google Scholar]
- 11.DeNino WF, Osler T, Evans EG, Forgione PM. Travel distance as factor in follow-up visit compliance in postlaparoscopic adjustable gastric banding population. Surg Obes Relat Dis. 2010;6:597–600. doi: 10.1016/j.soard.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Poole NA, Al Atar A, Kuhanendran D, et al. Compliance with surgical after-care following bariatric surgery for morbid obesity: a retrospective study. Obes Surg. 2005;15:261–265. doi: 10.1381/0960892053268499. [DOI] [PubMed] [Google Scholar]
- 13.Shen R, Dugay G, Rajaram K, Cabrera I, Siegel N, Ren CJ. Impact of participant follow-up on weight loss after bariatric surgery. Obes Surg. 2004;14:514–519. doi: 10.1381/096089204323013523. [DOI] [PubMed] [Google Scholar]
- 14.Toussi R, Fujioka K, Coleman KJ. Pre- and postsurgery behavioral compliance, patient health, and postbariatric surgical weight loss. Obesity. 2009;17:996–1002. doi: 10.1038/oby.2008.628. [DOI] [PubMed] [Google Scholar]
- 15.Modi AC, Zeller MH, Xanthakos SA, Jenkins TM, Inge TH. Adherence to vitamin supplementation following adolescent bariatric surgery. Obesity. 2013;21:E190–195. doi: 10.1002/oby.20031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sawhney P, Modi AC, Jenkins TM, et al. Predictors and outcomes of adolescent bariatric support group attendance. Surg Obes Relat Dis. 2013;9:773–779. doi: 10.1016/j.soard.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorin AA, Raftopoulos I. Effect of mood and eating disorders on the short-term outcome of laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2009;19:1685–1690. doi: 10.1007/s11695-008-9685-6. [DOI] [PubMed] [Google Scholar]
- 18.Wheeler E, Prettyman A, Lenhard MJ, Tran K. Adherence to outpatient program postoperative appointments after bariatric surgery. Surg Obes Relat Dis. 2008;4:515–520. doi: 10.1016/j.soard.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Dew MA, Dabbs AD, Myaskovsky L, et al. Meta-analysis of medical regimen adherence outcomes in pediatric solid organ transplantation. Transplantation. 2009;88:736–746. doi: 10.1097/TP.0b013e3181b2a0e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenkins TM, Xanthakos SA, Zeller MH, Barnett SJ, Inge TH. Distance to clinic and follow-up visit compliance in adolescent gastric bypass cohort. Surg Obes Relat Dis. 2011;7:611–615. doi: 10.1016/j.soard.2011.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fredericks EM, Lopez MJ, Magee JC, Shieck V, Opipari-Arrigan L. Psychological functioning, nonadherence and health outcomes after pediatric liver transplantation. Am J Transplant. 2007;7:1974–1983. doi: 10.1111/j.1600-6143.2007.01878.x. [DOI] [PubMed] [Google Scholar]
- 22.Hilliard ME, Wu YP, Rausch J, Dolan LM, Hood KK. Predictors of deteriorations in diabetes management and control in adolescents with type 1 diabetes. J Adolesc Health. 2013;52:28–34. doi: 10.1016/j.jadohealth.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith BA, Wood BL. Psychological factors affecting disease activity in children and adolescents with cystic fibrosis: medical adherence as a mediator. Curr Opin Pediatr. 2007;19:553–8. doi: 10.1097/MOP.0b013e3282ef480a. [DOI] [PubMed] [Google Scholar]
- 24.Taner Y, Torel-Ergur A, Bahcivan G, Gurdag M. Psychopathology and its effect on treatment compliance in pediatric obesity patients. Turk J Pediatr. 2009;51:466–471. [PubMed] [Google Scholar]
- 25.Sysko R, Zakarin EB, Devlin MJ, Bush J, Walsh BT. A latent class analysis of psychiatric symptoms among 125 adolescents in a bariatric surgery program. Int J Pediatr Obes. 2011;6:289–297. doi: 10.3109/17477166.2010.545411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sysko R, Zandberg LJ, Devlin MJ, Annunziato RA, Zitsman JL, Walsh BT. Mental health evaluations for adolescents prior to bariatric surgery: a review of existing practices and a specific example of assessment procedures. Clinical Obesity. 2013;3:62–72. doi: 10.1111/cob.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 28.Carter CL, Dacey CM. Validity of the Beck Depression Inventory, MMPI, and Rorschach in assessing adolescent depression. J Adolesc. 1996;19:223–231. doi: 10.1006/jado.1996.0021. [DOI] [PubMed] [Google Scholar]
- 29.Kim RJ, Langer JM, Baker AW, Filter DE, Williams NN, Sarwer DB. Psychosocial status in adolescents undergoing bariatric surgery. Obes Surg. 2008;18:27–33. doi: 10.1007/s11695-007-9285-x. [DOI] [PubMed] [Google Scholar]
- 30.Zeller MH, Roehrig HR, Modi AC, Daniels SR, Inge TH. Health-related quality of life and depressive symptoms in adolescents with extreme obesity presenting for bariatric surgery. Pediatrics. 2006;117:1155–1161. doi: 10.1542/peds.2005-1141. [DOI] [PubMed] [Google Scholar]
- 31.Varni JW, Seid M, Rode CA. The PedsQL(™): measurement model for the pediatric quality of life inventory. Med Care. 1999;37:126–139. doi: 10.1097/00005650-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4. 0 generic core scales in healthy and patient populations. Med Care. 2001;39:800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Fairburn CG, Beglin SJ. Assessment of eating disorders: interview or self-report questionnaire. Int J Eating Disord. 1994;16:363–370. [PubMed] [Google Scholar]
- 34.Spitzer RL, Yanovski SZ, Marcus MD. The questionnaire on eating and weight patterns-revised (QEWP-R) New York, NY: New York State Psychiatric Institute; 1993. [Google Scholar]
- 35.Luce KH, Crowther JH, Pole M. Eating Disorder Examination Questionnaire (EDE-Q): norms for undergraduate women. Int J Eat Disord. 2008;41:273–276. doi: 10.1002/eat.20504. [DOI] [PubMed] [Google Scholar]
- 36.Sinton MM, Goldschmidt AB, Aspen V, et al. Psychosocial correlates of shape and weight concerns in overweight pre-adolescents. J Youth Adolesc. 2012;41:67–75. doi: 10.1007/s10964-011-9686-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.le Grange D, Crosby RD, Rathouz PJ, Leventhal BL. A randomized controlled comparison of family-based treatment and supportive psychotherapy for adolescent bulimia nervosa. Arch Gen Psychiatry. 2007;64:1049–56. doi: 10.1001/archpsyc.64.9.1049. [DOI] [PubMed] [Google Scholar]
- 38.Antony MM, Johnson WG, Carr-Nangle RE, Abel JL. Psychopathology correlates of binge eating and binge eating disorder. Compr Psychiatry. 1994;35:386–392. doi: 10.1016/0010-440x(94)90280-1. [DOI] [PubMed] [Google Scholar]
- 39.Tanofsky-Kraff M. Binge eating among children and adolescents. In: Jelalian E, Steele R, editors. Handbook of Child and Adolescent Obesity. New York: Springer Publishers; 2009. [Google Scholar]
- 40.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82:703–710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vermut JK. Latent class modeling with covariates: two improved three-step approaches. Polit Anal. 2010;18:450–9. [Google Scholar]
- 42.Lara MD, Baker MT, Larson CJ, Mathiason MA, Lambert PJ, Kothari SN. Travel distance, age, and sex as factors in follow-up visit compliance in the post-gastric bypass population. Surg Obes Relat Dis. 2005;1:17–21. doi: 10.1016/j.soard.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Wildes JE, Marcus MD, Kalarchian MA, Levine MD, Houck PR, Cheng Y. Self-reported binge eating in severe pediatric obesity: impact on weight change in a randomized controlled trial of family-based treatment. Int J Obes. 2010;34:1143–1148. doi: 10.1038/ijo.2010.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sysko R, Devlin MJ, Hildebrandt TB, Brewer SK, Zitsman JL, Walsh BT. Psychological outcomes and predictors of initial weight loss outcomes among severely obese adolescents receiving laparoscopic adjustable gastric banding. J Clin Psychiatry. 2012;73:1351–7. doi: 10.4088/JCP.12m07690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miloh T, Annunziato R, Arnon R, et al. Improved adherence and outcomes for pediatric liver transplant recipients by using text messaging. Pediatrics. 2009;124:e844–50. doi: 10.1542/peds.2009-0415. [DOI] [PubMed] [Google Scholar]
- 46.Sharifi M, Dryden EM, Horan CM, et al. Leveraging text messaging and mobile technology to support pediatric obesity-related behavior change: a qualitative study using parent focus groups and interviews. J Med Internet Res. 2013;15:e272. doi: 10.2196/jmir.2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woolford SJ, Barr KL, Derry HA, et al. OMG do not say LOL: obese adolescents’ perspectives on the content of text messages to enhance weight loss efforts. Obesity. 2011;19:2382–2387. doi: 10.1038/oby.2011.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woolford SJ, Clark SJ, Strecher VJ, Resnicow K. Tailored mobile phone text messages as an adjunct to obesity treatment for adolescents. J Telemed Telecare. 2010;16:458–461. doi: 10.1258/jtt.2010.100207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas JG, Bond DS, Sarwer DB, Wing RR. Technology for behavioral assessment and intervention in bariatric surgery. Surg Obes Relat Dis. 2011;7:548–557. doi: 10.1016/j.soard.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sarwer DB, Moore RH, Spitzer JC, Wadden TA, Raper SE, Williams NN. A pilot study investigating the efficacy of postoperative dietary counseling to improve outcomes after bariatric surgery. Surg Obes Relat Dis. 2012;8:561–568. doi: 10.1016/j.soard.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 51.Jelalian E, Hart CN, Mehlenbeck RS, et al. Predictors of attrition and weight loss in an adolescent weight control program. Obesity. 2008;16:1318–23. doi: 10.1038/oby.2008.51. [DOI] [PubMed] [Google Scholar]
- 52.Pratt JS, Lenders CM, Dionne EA, et al. Best practice updates for pediatric/adolescent weight loss surgery. Obesity. 2009;17:901–910. doi: 10.1038/oby.2008.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Brien PE, Sawyer SM, Laurie C, et al. Laparoscopic adjustable gastric banding in severely obese adolescents: a randomized trial. JAMA. 2010;303:519–526. doi: 10.1001/jama.2010.81. [DOI] [PubMed] [Google Scholar]