Abstract
Purpose
To determine risk factors associated with development of a branch retinal vein occlusion (BRVO) among a large group of managed-care plan beneficiaries in the United States (US).
Design
Retrospective longitudinal cohort study.
Participants
All beneficiaries age ≥55 years continuously enrolled for ≥2 years in a managed care network from 2001-2009 who had ≥2 visits to an eye-care provider.
Methods
Multivariable Cox regression analyses were performed to identify socio-demographic factors, ocular and non-ocular conditions that were associated with incident BRVO.
Main Outcome Measure
Hazard of incident BRVO with 95% confidence intervals (CI).
Results
Of the 492,488 enrollees who met the inclusion criteria, 2,283 (0.5%) developed an incident BRVO. After adjustment for confounding factors, blacks (adjusted hazard ratio (aHR)=1.43, CI 1.19-1.73, p=0.0001) had a 43% increased hazard of developing BRVO relative to non-Hispanic whites. Enrollees with hypertension (HTN) alone (aHR=1.78, CI 1.36-2.32, p<0.0001) or HTN along with other metabolic syndrome components (diabetes mellitus (DM) and hyperlipidemia) (aHR=1.44, CI 1.12-1.84, p=0.005) had an increased hazard of developing a BRVO compared to those with none of these conditions. Disease severity was also important; enrollees with end-organ damage caused by HTN had a 107% increased hazard of developing BRVO compared to enrollees without HTN (aHR=2.07, CI 1.75-2.45, p<0.0001). Although there was no association between DM without end-organ damage and BRVO (aHR=0.92, CI 0.81-1.04, p=0.2), individuals with end-organ damage from DM had a 36% increased hazard of developing BRVO (aHR=1.36, CI 1.18-1.57, p<0.0001) compared to those without DM. Although cerebrovascular disease was associated with an increased hazard of developing BRVO (aHR=1.34, CI 1.19-1.52, p<0.0001), other diseases of the vascular system (deep venous thrombosis/pulmonary embolism, peripheral vascular disease, hypercoagulable state, myocardial infarction) or anticoagulant use did not increase the risk of BRVO (p>0.10 for all comparisons).
Conclusions
Both HTN and end-organ damage from DM contribute to arteriosclerosis, atherosclerosis and endothelial dysfunction which appear to be major risk factors for BRVO. Ophthalmologists should emphasize to patients and their primary physicians the importance of effectively managing systemic medical conditions that are associated with BRVO.
Over 16 million people worldwide are affected by branch retinal vein occlusions (BRVO).1 Given that older age is a known risk factor for BRVO, with increasing longevity of the population, the prevalence of venous occlusive disease is likely to rise in the coming decades. BRVOs are associated with significant ocular morbidity; over one in ten patients who suffer from BRVO are left with ≤20/200 best corrected visual acuity in the affected eye.2 Studies reveal that patients who develop BRVO report a decrease in quality of life proportionate to the vision loss in the affected eye, even when good visual acuity is maintained in the uninvolved eye.2 Furthermore, personal and societal costs associated with BRVO are estimated to be much higher than costs for age-matched controls.3
The concept of Virchow’s triad teaches that the following three factors predispose to thrombosis: endothelial damage, abnormal blood flow, and hypercoagulability. In some studies,4-15 but not others,6,7,9,12,13,15,16 systemic disease that raises the risk for endothelial damage or abnormal blood flow—including the components of Virchow’s triad: hypertension (HTN), dyslipidemia, diabetes mellitus (DM), and heart disease—has been associated with retinal vascular occlusion. There has also been debate about whether hypercoagulability predisposes patients to developing retinal vein occlusions,6,17-9 and little work has been done to determine whether a hypercoagulable state (HCS) may affect the risk of BRVO differently from that of central retinal vein occlusion (CRVO).
Previously we studied risk factors associated with CRVO and found that HTN, cerebrovascular disease (CVA), HCS, black race, and end-organ damage from HTN or DM all increased the risk of CRVO.19 We postulated that risk factors for BRVO may differ some from risk factors associated with CRVO because of the different anatomic relationships among retinal vessels. For example, occlusion of a branch retinal vein may be more likely to be secondary to compression from the adjacent artery at an arteriovenous crossing point resulting from hardening of the artery caused by endothelial damage and plaque accumulation. By comparison, the central retinal artery and vein lie next to each other, and so may be more susceptible to occlusion from a condition such as HCS.6 In the present study we sought to identify independent predictors of incident BRVO after adjusting for socio-demographic factors, ocular and medical comorbidities and to determine whether the factors associated with BRVO are similar or dissimilar to those we had previously found to be associated with CRVO.
Methods
Data Source
The Clinformatics DataMart database (OptumInsight, Eden Prairie, MN) contains detailed records of all beneficiaries who had some form of eye care in a large managed care network with members throughout the 48 continental United States. The dataset contains all individuals with ≥1 International Classification of Diseases, Ninth Revision-Clinical Modification (ICD-9-CM) codes20 for eye-related diagnoses (360-379.9), ≥1 Current Procedural Terminology (CPT) codes21 for any eye-related visits, diagnostic, or therapeutic procedures (65091-68899 or 92002-92499), or any other claim submitted by an ophthalmologist or optometrist from January 1, 2001 through December 31, 2009. For each enrollee, we had access to all medical claims for ocular and non-ocular conditions and socio-demographic information including age, sex, race, education level and household net worth. Additionally, the database has records of all outpatient prescriptions since enrollees in the medical plan were also fully enrolled in the pharmacy plan. This database has been used in the past to study patients with several ocular diseases.19,22-24
Since all the data were de-identified to the researchers, the University of Michigan Institutional Review Board determined that this study was exempt from requiring its approval.
Participants and Sample Selection
All beneficiaries who were ≥55 years of age and were continuously enrolled in the medical plan for ≥2 years were included. Prior work has demonstrated that BRVO is uncommon among individuals <55 years old,1 so enrollees who were <55 years old were excluded (Figure 1). Since the outcome of interest was a new diagnosis of BRVO, any individual who had a record of BRVO during their first two years in the plan were omitted from the analysis to exclude non-incident cases. Since BRVO can be asymptomatic, to be even more certain that enrollees did not have the outcome of interest (BRVO) during the 2-year look-back period, we also required ≥1 visits to an eye care provider (ophthalmologist or optometrist) during this time frame so that eye care providers would have had an opportunity to identify enrollees with this condition. We also excluded individuals if they did not have at least one additional eye visit during the follow up period so that an eye care provider would have an opportunity to assess for BRVO during this time period. Beneficiaries not continuously enrolled in the plan were also excluded as they could have received a BRVO diagnosis during their time outside of the plan.
Figure 1.
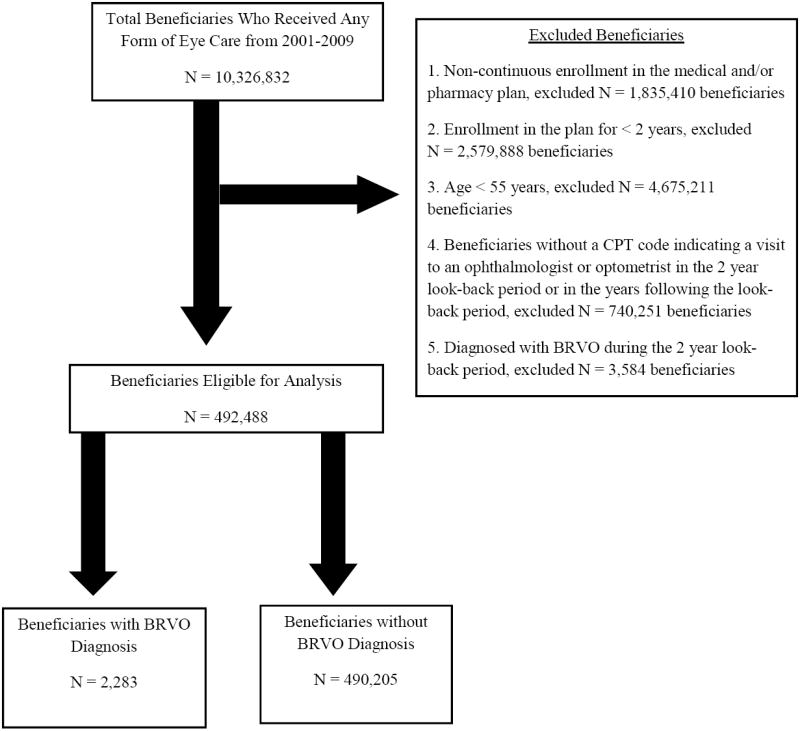
Selection of Beneficiaries for Analysis
CPT, Current Procedural Terminology; BRVO, Branch Retinal Vein Occlusion
Analyses
Statistical analyses were performed using SAS software, version 9.3 (SAS Institute, Cary, NC). Participant characteristics were summarized for the entire sample using means and standard deviations for continuous variables and frequencies and percentages for categorical variables. Our outcome of interest was a new diagnosis of BRVO, which was identified if a beneficiary received an ICD-9-CM diagnosis code for BRVO (362.36) after the index date (which we defined as 2 years after entry into the plan). Cox regression with delayed entry was used to estimate the hazard of being newly diagnosed with a BRVO. All beneficiaries were followed in the model from the index date until they were either diagnosed with BRVO or were censored. Censoring occurred at the date of the enrollee’s last eye examination, as this was conceivably their last opportunity to receive a diagnosis of BRVO by an eye care provider. Multivariable Cox regression models were adjusted for socio-demographic factors, and ocular and systemic comorbidities. All ocular and systemic disease covariates were identified by the ICD-9-CM codes listed in Table 1 (available at http://aaojournal.org).
Independent predictors in the regression model included markers for the three components of Virchow’s triad (Figure 2). The first component is Endothelial Damage, for which we included the following systemic diseases: DM, dyslipidemia, myocardial infarction (MI), congestive heart failure (CHF), CVA, HTN, and peripheral arterial disease (PAD).25-28 The second component of Virchow’s triad is Abnormal Blood Flow, for which we included atherosclerosis secondary to HTN, DM, MI, CHF, CVA, PAD and migraine as a marker of vasospasm.26,28 The third component of Virchow’s triad is Hypercoagulable State, for which we included the following systemic diseases: HCS, cancer, deep vein thrombosis/pulmonary embolism (DVT/PE), and use of oral anticoagulants which may be a surrogate for previous HCS. The use of oral anticoagulants was obtained from beneficiaries’ outpatient pharmacy records. Over-the-counter medication use such as aspirin was unavailable. The oral anticoagulants included in the analysis are listed in Figure 2 and we characterized an enrollee as a user of such medications if she had ≥1 prescription for any such medications during her time in the plan. Because many people have multiple components of metabolic syndrome—DM, HTN, and dyslipidemia— and the presence of multiple metabolic syndrome components could potentially act in an additive or multiplicative way to increase risk of venous occlusive disease as has been seen with other ocular conditions,22 we analyzed these factors both individually and in different combinations with one another.
Figure 2.
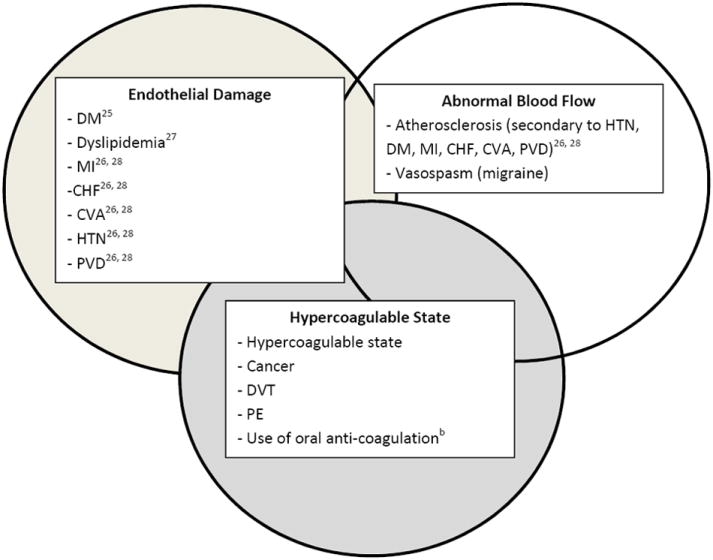
Virchow’s Triad: Medical co-morbidities that may increase the risk of BRVO
aDM = diabetes mellitus; MI = myocardial infarction; CHF = congestive heart failure; CVA = cerebrovascular attack; HTN = hypertension; PVD = peripheral vascular disease; DVT = deep vein thrombosis; PE = pulmonary embolism; BRVO = branch retinal vein occlusion.
bUse of oral anti-coagulation was obtained from pharmacy records and included the following medications: Marevin, Jantoven, Coumadin, Warfarin, Heparin, Clopidogrel, Ticlopidine, Plavix, Miradon, Dicumarol, Enoxaparin, Acenocoumarol, Anisinsione, Sintrom
In the model, glaucoma status, CVA, MI, DVT/PE, and use of anticoagulants were treated as time-dependent covariates since we wanted to be certain the exposure occurred before the outcome of interest. For each of these predictors, at every day after the index date, we assessed if the person had been diagnosed with these time dependent covariates and updated the value of the predictor in the model accordingly. Thus, we captured the time of exposure to these medications or conditions from the index date to the date of the outcome or censoring. Other covariates evaluated, such as DM, HTN, PAD, dyslipidemia, CHF, migraine and HCS, were not treated as time-dependent covariates given the chronic, longstanding nature of these diseases and challenges with identifying an exact date of onset of these conditions. Other model covariates included selected chronic systemic diseases (obstructive sleep apnea, dementia, depression), ocular comorbidities (cataract, pseudophakia / aphakia, macular degeneration, primary open-angle glaucoma (OAG), exfoliation syndrome glaucoma, and ocular hypertension (OHTN)), socio-demographic factors (age, sex, race/ethnicity, household net worth, education, and region of the country of home residence) and the Charlson Comorbidity Index, a measure of overall health.29 OAG, OHTN, and exfoliation syndrome were included in the models because these conditions have previously been shown to be associated with BRVO.12,13,30 We also tested selected interactions in the model.
To explore the impact of the severity of DM and HTN on the risk of developing BRVO, a second Cox regression model was run, controlling for all of the same covariates as in the initial model, except that DM and HTN in the original model were replaced by “uncomplicated” and “complicated” DM and HTN in the second model. We defined “complicated” HTN or DM as those who had evidence of end-organ damage (e.g. nephropathy, neuropathy) from the condition and “uncomplicated” cases as those with no record of end-organ damage from these specific conditions as was done previously.19
Finally, to address potential concerns about miscoding known to exist in claims data, a sensitivity analysis was performed to determine whether the association between the predictor variables and BRVO was affected if we changed our definition of BRVO from requiring a single ICD-9-CM code to identify enrollees with BRVO to requiring an initial and ≥1 confirmatory BRVO diagnosis at a subsequent clinic visit. For all analyses, p<0.05 was considered statistically significant.
Results
Of the 492,488 enrollees who met our inclusion criteria, 2,283 (0.5%) were newly diagnosed with BRVO during the follow-up period (Figure 1). Our study population included 392,718 (79.7%) whites, 23,952 (4.9%) blacks, 16,830 (3.4%) Latinos, 7,949 (1.6%) Asian-Americans, and 3,302 (0.7%) who identified as some “other” race. Race was missing for 47,737 (9.7%) beneficiaries. The mean follow-up from the index date for beneficiaries in our study who developed BRVO was 2.0 ± 1.6 years, which was slightly longer than the mean follow-up for beneficiaries who did not develop BRVO, 1.7 ± 1.5 years (p<0.001). Beneficiaries who did not experience BRVO were younger (65.6 ± 8.1 years) compared to those who developed a BRVO (69.2 ± 8.5 years, p<0.001) (Table 2).
Table 2.
Demographic Characteristics
| Covariate | No BRVO, n, (%) | Incident BRVO, n, (%) | P Value |
|---|---|---|---|
| Age, mean years ±SD, [range] | 65.6±8.1 [55-86.9] | 69.2±8.5 [55-86] | |
| Sex | 0.14 | ||
| Male | 204,594 (41.7) | 1,295 (43.3) | |
| Female | 285,611 (58.3) | 988 (56.7) | |
| Race | 0.02 | ||
| White | 390,914 (79.8) | 1,804 (79.0) | |
| Black | 23,812 (4.9) | 140 (6.1) | |
| Latino | 16,749 (3.4) | 81 (3.6) | |
| Asian -American | 7,902 (1.6) | 47 (2.1) | |
| Other/Missing | 50,828 (10.4) | 211 (9.2) | |
| Education | 0.06 | ||
| Less than High School | 5,173 (1.1) | 27 (1.2) | |
| High School | 166,310 (33.9) | 796 (34.9) | |
| Some College | 181,865 (37.1) | 883 (38.7) | |
| College Diploma | 113,456 (23.1) | 491 (21.5) | |
| Advanced Degree | 1,268 (0.3) | 3 (0.1) | |
| Household Net Worth | <0.0001 | ||
| <$ 25,000 | 23,814 (4.9) | 144 (6.2) | |
| $ 25-75,000 | 22,261 (4.5) | 130 (5.7) | |
| $ 75-150,000 | 48,633 (9.9) | 250 (11.0) | |
| $ 150-500,000 | 206,857 (42.2) | 965 (42.3) | |
| >$ 500,000 | 152,222 (31.1) | 652 (28.6) | |
| Systemic Risk Factors | |||
| Arteriosclerosis | |||
| CVA | 79,676 (16.3) | 712 (31.2) | <0.0001 |
| MI | 39,539 (8.1) | 293 (12.8) | <0.0001 |
| CHF | 72,562 (14.8) | 566 (24.8) | <0.0001 |
| Metabolic Syndrome | <0.0001 | ||
| Only HTN | 41,911 (8.6) | 244 (10.7) | |
| Only DM | 2,699 (0.6) | 10 (0.4) | |
| Only CHOL | 61,121 (12.5) | 136 (6.0) | |
| DM+HTN | 11,617 (2.4) | 71 (3.1) | |
| CHOL+HTN (no DM) | 178,916 (36.5) | 898 (39.3) | |
| DM+CHOL (noHTN) | 9,738 (2.0) | 34 (1.5) | |
| DM+CHOL+HTN | 141,284 (28.8) | 805 (35.3) | |
| Complicateda DM | 74,342 (15.2) | 535 (23.4) | |
| Complicateda HTN | 77,300 (15.8) | 692 (30.3) | |
| Endothelial Damage | |||
| Peripheral Vascular Disease | 94,506 (19.3) | 716 (31.4) | <0.0001 |
| Hypercoagulable State | |||
| DVT/PE | 12,820 (2.6) | 86 (3.8) | 0.0006 |
| Hypercoagulable state | 1,772 (0.4) | 15 (0.7) | 0.02 |
| Oral anti-coagulation | 91,653 (18.7) | 647 (28.3) | <0.0001 |
| Cancer | 93,402 (19.1) | 546 (23.9) | <0.0001 |
| Vasospasm | |||
| Migraine | 27,541 (5.6) | 127 (5.6) | 0.91 |
| Ocular Risk Factors | <0.0001 | ||
| Ocular hypertension | 18,499 (3.8) | 61 (2.7) | |
| Open-angle glaucoma | 70,824 (14.5) | 461 (20.2) | |
| Exfoliation Syndrome | 5,640 (1.2) | 41 (1.8) |
Abbreviations: BRVO, branch retinal vein occlusion; SD, standard deviation; CVA, cerebrovascular accident; MI, myocardial infarction; CHF, congestive heart failure; HTN, hypertension; DM, diabetes mellitus; CHOL, dyslipidemia; DVT/PE, deep vein thrombosis/pulmonary embolism
“Complicated” refers to disease including end-organ damage caused by DM or HTN
Socio-demographic Factors
Compared to whites, blacks had a 43% increased hazard of receiving a BRVO diagnosis (adjusted hazard ratio (aHR) = 1.43, [95% CI 1.19-1.73], p=0.0001) and Asian-Americans had a 39% increased hazard of receiving a BRVO diagnosis (aHR =1.39, [95% CI 1.02-1.89], p=0.03), while Latinos had no significant increased hazard (aHR = 1.08, [95% CI 0.85-1.37], p=0.54) (Table 3). There was no significant association between education and the diagnosis of incident BRVO, (p>0.05 for all comparisons). While there was also no association between different levels of household net worth and BRVO, there was a trend to increasing household net worth being associated with a decreased hazard of incident BRVO (p for trend = 0.02) (Table 3).
Table 3.
Risk Factors for Developing Incident BRVO
| Covariates |
Unadjusted Hazard Ratio [95% Confidence Interval], P Value |
Adjusted Hazard Ratioa [95% Confidence Interval],P Value |
|---|---|---|
| Raceb | ||
| Black | 1.59 [1.34-1.89], <0.0001 | 1.43 [1.19-1.73], 0.0001 |
| Latino | 1.18 [0.94-1.48], 0.15 | 1.08 [0.85-1.37], 0.54 |
| Asian-American | 1.43 [1.07-1.91], 0.02 | 1.39 [1.02-1.89], 0.03 |
| Educationc | ||
| High School Diploma | 0.84 [0.57-1.23], 0.36 | 1.00 [0.66-1.51], 0.99 |
| Some College | 0.77 [0.53-1.14], 0.19 | 0.96 [0.63-1.46], 0.85 |
| College Degree | 0.70 [0.47-1.03], 0.07 | 0.97 [0.63-1.49], 0.90 |
| Advanced Degree | 0.38 [0.12-1.25], 0.11 | 0.42 [0.10-1.79], 0.24 |
| Household Net Worthd | ||
| $ 25-75,000 | 1.00 [0.79-1.27], 0.99 | 1.04 [0.82-1.33], 0.75 |
| $ 75-150,000 | 0.90 [0.73-1.10], 0.30 | 0.89 [0.72-1.11], 0.30 |
| $ 150-500,000 | 0.80 [0.67-0.95], 0.01 | 0.86 [0.72-1.04], 0.12 |
| >$ 500,000 | 0.73 [0.61-0.88], 0.0008 | 0.82 [0.66-1.01], 0.06 |
| Sexe | ||
| Female | 0.96 [0.89-1.05], 0.39 | 1.00 [0.91-1.09], 0.92 |
| Systemic and Ocular Risk Factorsf | ||
| Charlson Comorbidity Index | 1.05 [1.04-1.07], 0.006 | 1.04 [1.01-1.06], 0.002 |
| Cerebrovascular Accident | 1.56 [1.41-1.73], <0.0001 | 1.34 [1.18-1.51] <0.0001 |
| Myocardial Infarction | 1.20 [1.04-1.39], 0.01 | 1.00 [0.85-1.18], 0.98 |
| Congestive Heart Failure | 1.31 [1.19-1.44], <0.0001 | 1.07 [0.94-1.21], 0.31 |
| Only HTN | 1.99 [1.56-2.56], <0.0001 | 1.78 [1.36-2.32], <0.0001 |
| Only DM | 1.63 [0.85-3.14], 0.14 | 1.49 [0.74-2.97], 0.26 |
| Only CHOL | 0.98 [0.75-1.29], 0.90 | 0.95 [0.71-1.27], 0.71 |
| DM + HTN | 2.10 [1.53-2.88], <0.0001 | 1.63 [1.16-2.31], 0.006 |
| CHOL + HTN | 1.60 [1.28-2.00], <0.0001 | 1.36 [1.07-1.73], 0.01 |
| DM + CHOL | 1.55 [1.04-2.31], 0.03 | 1.37 [0.90-2.10], 0.15 |
| DM + CHOL + HTN | 1.88 [1.50-2.35], <0.0001 | 1.44 [1.12-1.84], 0.005 |
| Peripheral Vascular Disease | 1.25 [1.14-1.37], <0.0001 | 1.02 [0.91-1.13], 0.80 |
| DVT/PE | 1.15 [0.85-1.55], 0.36 | 0.98 [0.71-1.35], 0.91 |
| Hypercoagulable State | 1.57 [0.94-2.61], 0.08 | 1.43 [0.84-2.44], 0.19 |
| Oral Anticoagulation | 1.29 [1.17-1.43], <0.0001 | 1.11 [0.98-1.25], 0.10 |
| Cancer | 1.01 [1.04-1.07], <0.0001 | 0.88 [0.77-1.00], 0.06 |
| Migraine | 1.00 [0.84-1.20], 1.0 | 0.96 [0.79-1.17], 0.66 |
| Ocular Hypertension | 0.92 [0.68-1.26], 0.61 | 0.69 [0.52-0.91], 0.01 |
| Open-Angle Glaucoma | 1.11 [1.00-1.24], 0.04 | 1.08 [0.97-1.21], 0.17 |
| Exfoliation Syndrome | 0.69 [0.54-0.89], 0.89 | 0.94 [0.67-1.32], 0.72 |
Abbreviations: BRVO, branch retinal vein occlusion; HTN, hypertension; DM, diabetes mellitus; CHOL, dyslipidemia; DVT/PE, deep vein thrombosis/pulmonary embolism
Adjusted for age, race, sex, education, household net worth, region of the country, glaucoma ocular hypertension, exfoliation syndrome, cataract, pseudophakia, macular degeneration, obstructive sleep apnea, dementia, mood changes, myocardial infarction, congestive heart failure, peripheral vascular disease, stroke, cancer, hypercoagulable state, the use of oral anti-coagulation medications, deep vein thrombosis/pulmonary embolism, migraine, metabolic syndrome (includes hypertension, diabetes mellitus, dyslipidemia alone and in combination), and the Charlson Co-Morbidity Index.
Compared to Whites
Compared to < High School Diploma, p for trend = 0.61
Compared to <$25,000 Household Net Worth, p for trend = 0.02
Compared to Male
Components of Virchow’s Triad
Hypercoagulable State
After controlling for potential confounding factors, none of the diseases associated with HCS (cancer, DVT/PE, use of oral anticoagulants or HCS) were found to be significant risk factors for BRVO, (p>0.05 for all comparisons) (Table 3).
Abnormal blood flow / Endothelial damage
Many of the risk factors associated with abnormal blood flow and endothelial damage conferred an elevated BRVO risk. Enrollees with HTN alone (no DM or dyslipidemia) had a 78% increased risk of BRVO (aHR = 1.78 [95% CI 1.36-2.32], p<0.0001) and those who had a CVA had a 34% increased hazard of developing BRVO (aHR = 1.34 [95% CI 1.18-1.51], p<0.0001). By contrast, the following conditions affecting blood flow were not found to be associated with BRVO: MI, CHF, DM, dyslipidemia, or PAD (p>0.05 for all comparisons, Table 3). Migraine can be associated with vasospasm and could potentially contribute to abnormal blood flow in the retina. In the regression model, migraine did not increase the risk of being diagnosed with a BRVO (p=0.66) (Table 3).
In the multivariable regression, we found that HTN appeared to be the most significant risk factor for developing BRVO, as HTN alone or HTN along with any other components of the metabolic syndrome significantly increased the risk of BRVO. For example, in those with HTN and DM (but no dyslipidemia), the hazard of being diagnosed with incident BRVO increased by 63% (aHR = 1.63 [95% CI 1.16-2.31], p=0.006), by 36% for those with HTN and dyslipidemia (but no DM), (aHR = 1.36 [95% CI 1.07-1.73], p=0.01), and by 44% for those with all three components of metabolic syndrome (aHR = 1.44 [95% CI 1.12-1.84], p=0.005) compared to beneficiaries without any metabolic syndrome components. By comparison, in those with DM alone (no hypertension or dyslipidemia), dyslipidemia alone (no HTN or DM), and dyslipidemia + DM (but no HTN), there was no significant increase in the risk of incident BRVO (p>0.05 for all comparisons) (Table 3).
Severity of Hypertension and Diabetes
In order to evaluate whether the severity of the diabetes or hypertension would impact the risk assessment, we ran a second regression model replacing the various components of metabolic syndrome from the original model with the variables “complicated” and “uncomplicated” DM and HTN, which we defined as those with and without end-organ damage from these diseases. We found that persons with “uncomplicated” hypertension had a 36% increased risk of being diagnosed with BRVO (aHR = 1.36 [95% CI 1.17-1.57], p<0.0001), while those with “complicated” hypertension had a 107% increased risk of being diagnosed with incident BRVO (aHR = 2.07 [95% CI 1.75-2.45], p<0.0001) compared to those without HTN. Those with “uncomplicated” diabetes had no significant increased risk of being diagnosed with BRVO (aHR = 0.92 [95% CI 0.81-1.04], p=0.2), while those with “complicated” diabetes had a 36% increased risk of BRVO (aHR = 1.36 [95% CI 1.18-1.57], p<0.0001), relative to beneficiaries without DM.
Glaucoma
In our study cohort, 3.8% (18,560) of beneficiaries had OHTN, 14.5% (71,285) had OAG, and 1.2% (5,681) had exfoliation syndrome (Table 2). Those with OHTN had a 31% decreased hazard of BRVO (aHR=0.69 [95% CI 0.52-0.91], p =0.01), relative to those without OHTN. There was no significant association between OAG or exfoliation syndrome and BRVO risk (p>0.1 for both comparisons) (Table 3).
Interaction Analyses
We analyzed whether there were significant interactions between race and certain covariates as race was a significant risk factor for developing BRVO in the main regression model. We found no significant interaction between education and race (p>0.05) on BRVO risk. Likewise, the interaction between race and metabolic syndrome with risk of BRVO was not statistically significant (p>0.1).
Sensitivity Analysis
To help address potential concerns about miscoding of BRVO in claims data, we ran a sensitivity analysis requiring a confirmatory diagnosis of BRVO at a separate clinic visit to see whether it would impact the study findings. We found that 1,283 patients (56%) received ≥1 confirmatory BRVO diagnosis. Using this revised definition of the outcome of interest, nearly all of the above-mentioned findings remained unchanged, except only that Asian-American race was no longer a statistically significant risk factor for BRVO (aHR=1.17 [95% CI 0.75-1.83], p=0.49).
Discussion
While some previous population-based and case-control studies identified all the components of metabolic syndrome as risk factors for BRVO, including HTN,5, 6,8-11,14,15 DM 6,16 and dyslipidemia,5,10,14 after adjusting for multiple potential confounding factors, including other metabolic syndrome components, in this analysis of nearly 500,000 managed care beneficiaries from across the US, we found that HTN appears to be the main driver of the increased risk for incident BRVO. Moreover, not only did HTN increase the risk of BRVO, those with more severe HTN were at even greater risk. Compared with normotensive patients, the risk of BRVO was 36% higher among those with “uncomplicated” HTN and 107% higher for those with end-organ damage from HTN.
There has been much controversy in the literature as to whether metabolic syndrome components affect the risk of BRVO. Some prior studies did not find an association between DM and BRVO4, 9, 10, 12, 15 or dyslipidemia and BRVO,4, 15 while other studies found an association between these conditions and BRVO.5,6,10,13 In our study, we found no association between dyslipidemia and BRVO. In fact, the presence of dyslipidemia actually attenuated some of the increased risk of BRVO among persons who had multiple components of metabolic syndrome. Whether this reduction in risk is attributable to the condition itself or perhaps to the medications used to treat dyslipidemia, like statins, requires additional study. We also identified that while persons with “uncomplicated” DM had no significant difference in risk of being diagnosed with BRVO, those with end-organ damage from DM had a 36% increased risk of BRVO compared to those without DM. Given our finding that only those with more severe DM seem to be at increased risk of BRVO, this may help explain some of the conflicting findings in the existing literature on the subject as prior studies may have differed from one another in the proportions of patients with more or less severe DM.
The two components of Virchow’s triad that we identified as most important in increasing the risk of BRVO were abnormal blood flow and endothelial damage. HTN contributes to arteriosclerosis. Hardening of the retinal arteries leads to compression of adjacent retinal veins within the adventitial sheath which facilitates venous stasis and predisposes individuals to thrombosis.6 DM contributes more to atherosclerosis than arteriosclerosis, but may also be acting through its role in causing endothelial dysfunction. The worse the glycemic control in a patient with DM, the more reactive oxygen species (ROS) are present in the bloodstream, and ROS are thought to play a major role in the inflammatory component of diabetes-induced atherosclerosis, endothelial damage and, ultimately, cardiovascular disease .31 Thus, it is not surprising that it was only enrollees with “complicated” DM who demonstrated an elevated risk of BRVO in our study. If the retinal veins are compromised by endothelial dysfunction that may further add to the risk of thrombosis and occlusion as the atherosclerotic, thickened arteries compress the veins. We did not find that hypercoagulability, the third component of Virchow’s triad, significantly affected the risk of BRVO.
Previously, we performed a similar analysis of risk factors associated with CRVO and found some interesting similarities and differences between risk factors for CRVO and BRVO (Table 4). 19 The two models had only one important difference, and that is for the CRVO analysis we excluded subjects with a record of prior CRVO (non-incident cases) and in the BRVO analysis here we excluded subjects with a record of prior BRVO (non-incident cases). The rest of the inclusion and exclusion criteria and the covariates included in both models were the same for the two analyses. We learned that HTN and CVA were risk factors for both BRVO and CRVO. In contrast, HCS was only a significant risk factor for CRVO.19 It appears that all of the components of Virchow’s triad, endothelial damage, abnormal blood flow and hypercoagulability, may contribute to CRVO while abnormal blood flow and endothelial damage are the main risk factors impacting BRVO risk in this population.
Table 4.
Comparison of Risk Factors for BRVO and CRVO
| Risk Factor | Adjusted HR for BRVO [95% Confidence Intervals], p-value |
Adjusted HR for CRVO [95% Confidence Intervals], p-value |
|---|---|---|
| Artery Compressing Vein (Arteriosclerosis) | ||
| Cerebrovascular Accident | 1.34 [1.18-1.51], <0.0001 | 1.45 [1.24-1.70], <0.0001 |
| Myocardial Infarction | 1.00 [0.85-1.18], 0.98 | 0.73 [0.57-0.92], 0.01 |
| Congestive Heart Failure | 1.07 [0.94-1.21], 0.31 | 0.98 [0.83-1.15], 0.79 |
| Metabolic Syndrome | ||
| Only HTN | 1.78 [1.36-2.32], <0.0001 | 1.65 [1.13-2.41], 0.01 |
| Only DM | 1.49 [0.74-2.971], 0.26 | 0.95 [0.29-3.08], 0.93 |
| Only CHOL | 0.95 [0.71-1.27], 0.71 | 1.03 [0.68-1.54], 0.90 |
| DM + HTN | 1.63 [1.16-2.31], 0.0006 | 1.83 [1.16-2.90], 0.01 |
| CHOL + HTN | 1.36 [1.07-1.73], 0.01 | 1.45 [1.04-2.04], 0.03 |
| DM + CHOL | 1.37 [0.90-2.10], 0.15 | 1.52 [0.86-2.71], 0.15 |
| DM + CHOL + HTN | 1.44 [1.12-1.84], 0.005 | 1.57 [1.11-2.23], 0.01 |
| Endothelial Damage | ||
| Peripheral Vascular Disease | 1.02 [0.91-1.13], 0.80 | 1.16 [1.00-1.34], 0.04 |
| Hypercoagulable State | ||
| DVT/PE | 0.98 [0.71-1.35], 0.91 | 0.86[0.55-1.34], 0.50 |
| Hypercoagulable State | 1.43 [0.84-2.44], 0.19 | 2.46 [1.41-4.29], 0.002 |
| Oral Anticoagulation | 1.11 [0.98-1.25], 0.10 | 1.04 [0.89-1.22], 0.63 |
| Cancer | 0.88 [0.77-1.00], 0.06 | 0.95 [0.80-1.13], 0.59 |
Abbreviations: BRVO, branch retinal vein occlusion; CRVO, central retinal vein occlusion; HTN, hypertension; DM, diabetes mellitus; CHOL, dyslipidemia; DVT/PE, deep vein thrombosis/pulmonary embolism; HR = hazard ratio
Bold indicates statistically significant at p< 0.05
Models were run with the same covariates as follows: age (as the time axis), race, sex, education, household net worth, region of the country, glaucoma, ocular hypertension, exfoliation syndrome, cataract, pseudophakia, macular degeneration, obstructive sleep apnea, dementia, mood changes, myocardial infarction, congestive heart failure, peripheral vascular disease, stroke, cancer, hypercoagulable state, the use of oral anti-coagulation medications, deep vein thrombosis/pulmonary embolism, migraine, metabolic syndrome (includes hypertension, diabetes mellitus, dyslipidemia alone and in combination), and the Charlson Comorbidity Index. Models were run using the same inclusion/exclusion criteria except that non-incident cases of CRVO were excluded in the CRVO analysis1 and non-incident cases of BRVO were excluded from the BRVO analysis.
In our analyses, blacks were found to be at increased risk of developing incident ophthalmic venous occlusive disease, even after controlling for HTN, other metabolic syndrome components, and socio-demographic factors. These results support earlier work by the International Eye Disease Consortium which showed a higher prevalence of BRVO among blacks compared with whites.1 There are many hypotheses as to why blacks may be at higher risk of end-organ vascular damage, ranging from reduced access to high-quality healthcare, to racism potentially leading to chronic stress, to living in neighborhoods with higher levels of pollution, and to living in unsafe neighborhoods impacting their ability to exercise which can all lead to an increased burden of vascular disease.32, 33 It is interesting that there was also a trend in our data showing that after controlling for race, education and underlying comorbidities, there was a trend towards increased household net worth being associated with a decreased BRVO risk (p for trend =0.02). Living in poverty, for some of the reasons mentioned above, increases the burden of systemic vascular disease and may also increase the risk of ocular vascular disease.
Our study has several limitations. First, the data source for this analysis only contains information captured in billing codes. Thus, we were limited in our ability to access clinical data on each enrollee including information from fundus photographs on the location of the vein occlusion, findings on fluorescein angiography capturing the severity of the occlusion, visual acuity, intraocular pressure, blood pressure, body mass index, and smoking status. Providers contributing data to this dataset have different levels of experience in diagnosing BRVO and some may have misdiagnosed this condition. We also did not evaluate persons <55 years of age so our findings are not generalizable to younger patients, for whom this condition is quite rare. Likewise, all of the persons in our analysis had health insurance, so our results may not be generalizable to the 16% of Americans who lack health insurance.34
The strengths of our study include the ability to study risk factor associated with BRVO in a cohort of nearly 500,000 patients, a sample size which is nearly 10 times larger than existing studies on this topic.1 The large sample size makes it possible to adjust for multiple ocular and systemic comorbidities and socio-demographic factors and study interaction effects between different variables. Another study strength is that the sample has persons from all over the nation and captures the care of a diverse group of patients by an array of different eye care providers.
This study highlights several key factors that increase the risk of developing BRVO including HTN and end-organ damage from DM. Compared to our prior analysis which identified all three components of Virchow’s triad as risk factors for CRVO, in the present study, it appears that only two of the three components, endothelial dysfunction and abnormal blood flow impact the risk of BRVO.
Not only do these results help us understand how BRVO and CRVO are similar and different from one another, they also reinforce the importance of controlling blood pressure and blood sugar in reducing the risk of this sight-threatening condition. Because HTN and DM cause ocular disease in and of themselves as well as placing patients at risk for vascular occlusions, it is important that ophthalmologists work with primary care providers to educate patients about the importance of controlling the components of metabolic syndrome. Many ophthalmologists and optometrists already educate diabetic patients about the importance of optimal blood pressure and blood sugar control, and this likely heightens patients’ sense of the importance of the matter. If a patient is newly diagnosed with a BRVO, this could be used as an opportunity for reviewing a patient’s blood pressure and blood sugar records in order to advise patients that diabetes and hypertension, and especially poorly controlled, “complicated” diabetes and hypertension can cause vision loss by a variety of mechanisms but that patients can mitigate this risk by controlling their underlying disease. It could also be used as an opportunity for the ophthalmologist to communicate to the patient’s primary care physician (PCP) how the patient’s underlying vascular risk factors have put the patient at risk for this devastating ocular disease, the same way in which an ophthalmologist would communicate with the PCP if a patient developed proliferative diabetic retinopathy. The relationship of these epidemic, chronic systemic diseases to ocular vascular occlusion underscores the importance of having all members of the health care team, including specialists and primary care providers, send the same message to all patients about the necessity of excellent control of diabetes and hypertension as well as primary prevention of these diseases through healthy living.
Supplementary Material
Acknowledgments
Grant support: National Eye Institute K23 Mentored Clinician Scientist Award (JDS:1K23EY019511-01); National Eye Institute Michigan Vision Clinician-Scientist Development Program (PANC:K12EY022299); Blue Cross Blue Shield of Michigan Foundation (JDS, PANC), Heed Foundation Fellowship (PANC); Research to Prevent Blindness (JDS, DCM); National Eye Institute Core Grant EY00703.
The funding organizations had no role in the design or conduct of this research.
Footnotes
Stem MS, Talwar N, Comer GM, Stein JD. A longitudinal analysis of risk factors associated with central retinal vein occlusion. Ophthalmology 2013;120:362-70.
Presented, in part, at: Association for Research in Vision and Ophthalmology Annual Meeting, May 11, 2012.
The authors have no conflicting interests regarding the material discussed in this manuscript.
Supplemental materials are provided at the end of the online version of this manuscript. The following should appear online-only: Table 1.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rogers S, McIntosh RL, Cheung N, et al. International Eye Disease Consortium. The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010;117:313–9. doi: 10.1016/j.ophtha.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Awdeh RM, Elsing SH, Deramo VA, et al. Vision-related quality of life in persons with unilateral branch retinal vein occlusion using the 25-item National Eye Institute Visual Function Questionnaire. Br J Ophthalmol. 2010;94:319– 23. doi: 10.1136/bjo.2007.135913. [DOI] [PubMed] [Google Scholar]
- 3.Fekrat S, Shea AM, Hammill BG, et al. Resource use and costs of branch and central retinal vein occlusion in the elderly. Curr Med Res Opin. 2010;26:223–30. doi: 10.1185/03007990903439046. [DOI] [PubMed] [Google Scholar]
- 4.Appiah AP, Trempe CL. Risk factors associated with branch vs. central retinal vein occlusion. Ann Ophthalmol. 1989;21:153–5. 157. [PubMed] [Google Scholar]
- 5.Cheung N, Klein R, Wang JJ, et al. Traditional and novel cardiovascular risk factors for retinal vein occlusion: the Multiethnic Study of Atherosclerosis. Invest Ophthalmol Vis Sci. 2008;49:4297–302. doi: 10.1167/iovs.08-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayreh SS, Zimmerman B, McCarthy MJ, Podhajsky P. Systemic diseases associated with various types of retinal vein occlusion. Am J Ophthalmol. 2001;131:61–77. doi: 10.1016/s0002-9394(00)00709-1. [DOI] [PubMed] [Google Scholar]
- 7.Johnston RL, Brucker AJ, Steinmann W, et al. Risk factors of branch retinal vein occlusion. Arch Ophthalmol. 1985;103:1831–2. doi: 10.1001/archopht.1985.01050120065021. [DOI] [PubMed] [Google Scholar]
- 8.Kawasaki R, Wong TY, Wang JJ, et al. Body mass index and vein occlusion [letter] Ophthalmology. 2008;115:917–8. doi: 10.1016/j.ophtha.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Klein R, Moss SE, Meuer SM, Klein BE. The 15-year cumulative incidence of retinal vein occlusion: the Beaver Dam Eye Study. Arch Ophthalmol. 2008;126:513–8. doi: 10.1001/archopht.126.4.513. [DOI] [PubMed] [Google Scholar]
- 10.Lim LL, Cheung N, Wang JJ, et al. Prevalence and risk factors of retinal vein occlusion in an Asian population. Br J Ophthalmol. 2008;92:1316–9. doi: 10.1136/bjo.2008.140640. [DOI] [PubMed] [Google Scholar]
- 11.Liu W, Xu L, Jonas JB. Vein occlusion in Chinese subjects [letter] Ophthalmology. 2007;114:1795–6. doi: 10.1016/j.ophtha.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell P, Smith W, Chang A. Prevalence and associations of retinal vein occlusion in Australia. The Blue Mountains Eye Study. Arch Ophthalmol. 1996;114:1243–7. doi: 10.1001/archopht.1996.01100140443012. [DOI] [PubMed] [Google Scholar]
- 13.Sperduto RD, Hiller R, Chew E, et al. Risk factors for hemiretinal vein occlusion: comparison with risk factors for central and branch retinal vein occlusion: the Eye Disease Case-Control Study. Ophthalmology. 1998;105:765–71. doi: 10.1016/S0161-6420(98)95012-6. [DOI] [PubMed] [Google Scholar]
- 14.Weger M, Renner W, Steinbrugger I, et al. Role of thrombophilic gene polymorphisms in branch retinal vein occlusion. Ophthalmology. 2005;112:1910–5. doi: 10.1016/j.ophtha.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 15.Wong TY, Larsen EK, Klein R, et al. Cardiovascular risk factors for retinal vein occlusion and arteriolar emboli: the Atherosclerosis Risk in Communities & Cardiovascular Health studies. Ophthalmology. 2005;112:540– 7. doi: 10.1016/j.ophtha.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 16.Klein R, Klein BE, Moss SE, Meuer SM. The epidemiology of retinal vein occlusion: the Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2000;98:133–41. discussion 141-3. [PMC free article] [PubMed] [Google Scholar]
- 17.Janssen MC, den Heijer M, Cruysberg JR, et al. Retinal vein occlusion: a form of venous thrombosis or a complication of atherosclerosis? A meta-analysis of thrombophilic factors. Thromb Haemost. 2005;93:1021–6. doi: 10.1160/TH04-11-0768. [DOI] [PubMed] [Google Scholar]
- 18.Kalayci D, Gurgey A, Guven D, et al. Factor V Leiden and prothrombin 20210 A mutations in patients with central and branch retinal vein occlusion. Acta Ophthalmol Scand. 1999;77:622–4. doi: 10.1034/j.1600-0420.1999.770602.x. [DOI] [PubMed] [Google Scholar]
- 19.Stem MS, Talwar N, Comer GM, Stein JD. A longitudinal analysis of risk factors associated with central retinal vein occlusion. Ophthalmology. 2013;120:362–70. doi: 10.1016/j.ophtha.2012.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Physician International Classification of Diseases (ICD-9-CM), 9th Revision, Clinical Modification. Chicago, IL: American Medical Association; 2006. [Google Scholar]
- 21.Current Procedural Terminology (CPT 2006 Professional Edition. Chicago, IL: American Medical Association; 2006. [Google Scholar]
- 22.Newman-Casey PA, Talwar N, Nan B, et al. The relationship between components of metabolic syndrome and open-angle glaucoma. Ophthalmology. 2011;118:1318–26. doi: 10.1016/j.ophtha.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stein JD, Kim DS, Mundy KM, et al. The association between glaucomatous and other causes of optic neuropathy and sleep apnea. Am J Ophthalmol. 2011;152:989–98. doi: 10.1016/j.ajo.2011.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stein JD, Kim DS, Niziol LM, et al. Differences in rates of glaucoma among Asian Americans and other racial groups, and among various Asian ethnic groups. Ophthalmology. 2011;118:1031–7. doi: 10.1016/j.ophtha.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Avogaro A, Albiero M, Menegazzo L, et al. Endothelial dysfunction in diabetes: the role of reparatory mechanisms. Diabetes Care. 2011;34(suppl):S285–90. doi: 10.2337/dc11-s239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brunner H, Cockcroft JR, Deanfield J, et al. Endothelial function and dysfunction. Part II: Association with cardiovascular risk factors and diseases. A statement by the Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23:233–46. doi: 10.1097/00004872-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109(suppl):III27–32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 28.Vita JA, Hamburg NM. Does endothelial dysfunction contribute to the clinical status of patients with peripheral arterial disease? Can J Cardiol. 2010;26(suppl):45A–50A. doi: 10.1016/s0828-282x(10)71062-x. [DOI] [PubMed] [Google Scholar]
- 29.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 30.Ritch R, Prata TS, de Moraes CG, et al. Association of exfoliation syndrome and central retinal vein occlusion: an ultrastructural analysis. Acta Ophthalmol. 2010;88:91–5. doi: 10.1111/j.1755-3768.2009.01578.x. [DOI] [PubMed] [Google Scholar]
- 31.Dokken BB. The pathophysiology of cardiovascular disease and diabetes: beyond blood pressure and lipids. Diabetes Spectr. 2008;21:160–5. [Google Scholar]
- 32. [Accessed April 21, 2014];Disparities in health care quality among racial and ethnic minority groups: findings from the National Healthcare Quality and Disparities Reports, 2008 [fact sheet] 2009 Sep; Publication no. 09-0092. Available at: http://www.ahrq.gov/research/findings/nhqrdr/nhqrdr08/minority.html.
- 33.Mustafic H, Jabre P, Caussin C, et al. Main air pollutants and myocardial infarction: a systematic review and meta-analysis. JAMA. 2012;307:713–21. doi: 10.1001/jama.2012.126. [DOI] [PubMed] [Google Scholar]
- 34.U.S. Department of Health and Human Services. [Accessed April 21, 2014];Overview of the uninsured in the United States: a summary of the 2010 current population survey. ASPE Issue Brief. 2011 Sep; Available at: http://aspe.hhs.gov/health/reports/2011/cpshealthins2011/ib.shtml.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


