Abstract
Mucopolysaccharidoses (MPS) are caused by deficiency of one of a group of specific lysosomal enzymes, resulting in excessive accumulation of glycosaminoglycans (GAGs). We previously developed GAG assay methods using liquid chromatography tandem mass spectrometry (LC-MS/MS); however, it takes 4–5 min per sample for analysis. For the large numbers of samples in a screening program, a more rapid process is desirable. The automated high-throughput mass spectrometry (HT-MS/MS) system (RapidFire) integrates a solid phase extraction robot to concentrate and desalt samples prior to direction into the MS/MS without chromatographic separation; thereby allowing each sample to be processed within ten seconds (enabling screening of more than one million samples per year). The aim of this study was to develop a higher throughput system to assay heparan sulfate (HS) using HT-MS/MS, and to compare its reproducibility, sensitivity and specificity with conventional LC-MS/MS.
HS levels were measured in blood (plasma and serum) from control subjects and patients with MPS II, III, or IV and in dried blood spots (DBS) from newborn controls and patients with MPS I, II, or III. Results obtained from HT-MS/MS showed 1) that there was a strong correlation of levels of disaccharides derived from HS in blood, between those calculated using conventional LC-MS/MS and HT-MS/MS, 2) that levels of HS in blood were significantly elevated in patients with MPS II and III, but not in MPS IVA, 3) that the level of HS in patients with a severe form of MPS II was higher than that in an attenuated form, 4) that reduction of blood HS level was observed in MPS II patients treated with enzyme replacement therapy or hematopoietic stem cell transplantation, and 5) that levels of HS in newborn DBS were elevated in patients with MPS I, II or III, compared to control newborns.
In conclusion, HT-MS/MS provides much higher throughput than LC-MS/MS-based methods with similar sensitivity and specificity in an HS assay, indicating that HT-MS/MS may be feasible for diagnosis, monitoring, and newborn screening of MPS.
Keywords: automated high-throughput mass spectrometry, mucopolysaccharidoses, heparan sulfate, biomarker, newborn screening
1. Introduction
Mucopolysaccharidoses (MPS) are a group of lysosomal storage diseases caused by deficiency of the lysosomal enzymes required for degradation of glycosaminoglycans (GAGs) such as chondroitin sulfate (CS), dermatan sulfate (DS), heparan sulfate (HS), keratan sulfate (KS), and hyaluronan. There are 11 known enzyme deficiencies, resulting in seven distinct forms of MPS with a collective incidence of more than 1 in 25,000 live births. Accumulation of GAGs causes progressive damage of multiple tissues including brain, lung, heart, liver, kidney, and bone. Most clinical signs and symptoms for MPS patients do not appear immediately after birth; however, the subsequent onset of clinical signs and symptoms progresses with age. Although the symptoms and severity of MPS vary with individual patient and subtype of MPS, the average life span in patients with a severe form is one to two decades if untreated.
Currently, enzyme replacement therapy (ERT), hematopoietic stem cell transplantation (HSCT), substrate reduction therapy (SRT), and gene therapy are clinically in use or are being investigated in clinical trials for some types of MPS patient. Starting these treatments at birth or at a very early stage will provide the most significant impact on the clinical course of the disease [1]. To provide better quality of life for the patients, early diagnosis and treatment are required. Since unique clinical features in most MPS patients are not apparent until they are around two years old and general physicians have little knowledge of MPS, most patients are misdiagnosed or undiagnosed until irreversible damage to the brain and/or bones has started. Thus, a novel, accurate, sensitive, economical and rapid diagnostic method applicable to newborn screening (NBS) for MPS is critical to improving therapeutic efficacy.
Currently, conventional screening methods for MPS are dye-spectrometric methods such as dimethylmethylene blue (DMB) [2–4] and alcian blue [5, 6] which measure total GAGs from urine samples. When urine assays provide a positive result, the definitive diagnosis is determined by measuring the enzyme activities in lymphocytes or fibroblasts. However, these current methods cannot be applied to blood and/or tissue extracts without prior protease, nuclease or hyaluronidase treatment [7]. Total GAG concentration in urine does not reflect the severity of the neurological or skeletal signs and symptoms [4] and substantial overlap of the total urine GAG level between age-matched controls and MPS IV patients is observed [4], resulting in misdiagnosis of many patients. Thin layer chromatography, which is a semi-quantitative method for each GAG, is used to allow preliminary classification of patients among subgroups to reduce the number of enzyme assays necessary for final diagnosis [8, 9]. There are several other methods for measuring specific GAGs in blood, including ELISA [10, 11] and HPLC [12, 13]. However, ELISA kits are not commercially available for all types of GAGs and they cannot measure subtype of HS or KS. HPLC is not suitable for mass screening since the assay is time-consuming with low sensitivity.
Newborn screening (NBS) is recognized as an essential, preventive public health program for early identification of diseases in newborns that can affect their long-term health. A suitable method for NBS must be inexpensive and should be performed using a dried blood spot from a Guthrie card. At present there is no reliable NBS to cover all types of MPSs. We and others have developed new methods to assay CS, DS, HS, and KS simultaneously in blood, urine, and/or dried blood spot (DBS) samples by using liquid chromatography tandem mass spectrometry (LC-MS/MS) [7, 14–23]. The LC-MS/MS method not only shows sensitivity and specificity for detecting all subtypes of MPS, but also monitors therapeutic efficacy in MPS patients and animal models [1, 16, 22, 24, 25]; however, since LC processing is time consuming, the main drawback of this method is throughput. This factor may limit its utility for assaying large numbers of samples.
The automated high-throughput mass spectrometry (HT-MS/MS) system (RapidFire; Agilent Technologies) eliminates the chromatographic process, enabling sample-to-sample cycle times to be reduced to seconds, thereby removing the bottleneck of throughput while maintaining the quality and reliability of standard LC-MS/MS read-outs. The sample is aspirated to a matrix for concentration and desalting, followed by direct injection into MS/MS without chromatographic separation. Each sample is processed within ten seconds, yielding much faster throughput than traditional LC-MS/MS based methods. A single 384 well plate can be read in less than 40 min, indicating that this HT-MS/MS system can analyze over one million samples annually. Moreover, HT-MS/MS has been shown to provide sensitivity and specificity equivalent to a scintillation proximity assay [26], and an LC-MS/MS assay for some applications [19, 27]. The speed and efficiency of HT-MS/MS can allow for experiments that would otherwise be deemed untenable under normal circumstances. The HT-MS/MS system has been validated as suitable for many drug discovery programs [26, 28–34], and ADME (Absorption, Distribution, Metabolism and Excretion) based applications [27]. We recently reported that HT-MS/MS could measure HS levels in control human blood much faster than LC-MS/MS, and showed a strong correlation with levels measured by LC-MS/MS [19].
In this study we have evaluated a higher throughput system to assay HS levels in blood and dried blood spots (DBS) from newborn patients with MPS by using HT-MS/MS, and have compared its reproducibility, sensitivity, and specificity with conventional LC-MS/MS.
2. Materials and Methods
2.1. Materials
2.1.1. Standards and enzymes
To digest polymer HS to disaccharides, the enzyme heparitinase was obtained from Seikagaku Corporation (Tokyo, Japan). Chondrosine was used as an internal standard (IS), and unsaturated disaccharides, [ΔDiHS-0S, 2-acetamido-2-deoxy-4-O-(4-deoxy-a-L-threo-hex-4-enopyranosyluronic acid)-D-glucose; ΔDiHS-NS, 2-deoxy-2-sulfamino-4-O-(4-deoxy-a-L-threo-hex-4-enopyranosyluronic acid)-D-glucose] were used for generation of standard curves. Stock solutions of ΔDiHS-0S (100 μg/ml), ΔDiHS-NS (100 μg/ml), and IS (5 mg/ml) were prepared separately in ddH2O. Standard working solutions of ΔDiHS-0S and ΔDiHS-NS, (15.625, 31.25, 62.5, 125, 250, 500, and 1000 ng/ml), each mixed with IS solution (5 μg/ml), were prepared.
2.1.2. Blood and DBS samples
We collected 95 blood (plasma or serum) samples from MPS patients as follows: 55 MPS II patients (age 2.0–35.0 years, mean 11.4 years; 45 severe, 10 attenuated), 18 MPS III patients (age 4.0–27.0 years, mean 12.5 years; 6 IIIA, 12 IIIB), 22 MPS IV patients (age 3.4–56.0 years, mean 17.2 years; 17 IVA, 5 IVB). We obtained the samples from patients with MPS II who received ERT (16) or HSCT (9), and one patient with MPS IIIB who received HSCT. All patients were diagnosed as having below 5% of normal enzymatic activity. We also collected 133 blood (plasma or serum) samples from normal controls. The age of control samples ranged between 0 and 80 years (mean 5.2 years). In previous LC-MS/MS experiments, we confirmed that specificity and sensitivity of each GAG value were comparable using plasma or serum [7, 14–17, 19]. Diagnosis for MPS patients was confirmed by enzyme assay at each institute providing samples. The clinical severity in patients with MPS II was assessed by presence (severe) or absence (attenuated) of central nervous system (CNS) involvement. Clinical phenotypes in all patients with MPS III and MPS IVA were defined as severe, and all patients with MPS IVB were defined as attenuated in terms of growth compared to MPS IVA patients.
We also collected DBS samples from 22 anonymous control newborns and 12 newborns with MPS (6 MPS I, 1 MPS II, and 5 MPS III). These MPS patients had been diagnosed enzymatically and clinically, and DBS samples obtained at birth were provided by the newborn screening center after diagnosis. These control and patient samples were assayed in a blinded manner.
Written informed consent was obtained for each patient prior to initiation of the study, and the study was approved by the institutional review board of each institute providing samples for this study.
2.2 Methods
2.2.1. Sample preparation
Blood specimens and standards were prepared as described previously [18, 19]. First, 10 μl of each serum or plasma sample and 90 μl of 50 mM Tris–hydrochloric acid buffer (pH 7.0) were placed in wells of AcroPrep™ Advance 96-Well Filter Plates that have Ultrafiltration Omega 10K membrane filters (PALL corporation, NY, USA). The filter plates were placed on the receiver and centrifuged at 2,000 g for 15 min to remove free disaccharides. The membrane plates were transferred to a fresh receiver plate. Standards were added to unused wells of the filter plate. Then, 20 μl of IS solution (500 ng/ml), 70 μl of 50 mM Tris–hydrochloric acid buffer (pH 7.0), and 10 μl of heparitinase solution (2 mU per sample) were added to each filter well. The plate was incubated in a water bath at 37 °C for 15 hr and centrifuged at 2,000 g for 15 min. The receiver plate containing disaccharides was stored at −20 °C until analysis by HT-MS/MS or LC-MS/MS. disks of DBS samples (3 mm in diameter) were punched out and incubated in 0.1% bovine serum albumin for 15 min on Omega 10K membrane filters. After centrifuge at 2,000 g for 15 min, the mixture of IS, 50 mM Tris–hydrochloric acid buffer (pH 7.0), and heparitinase solution as described above was added to each filter well, and processed as per the blood samples.
2.2.2. Automated high-throughput mass spectrometry (HT-MS/MS) system (RapidFire)
Samples stored at −20 °C were allowed to thaw at ambient temperature. General operation of the HT-MS/MS has been described previously [35]. In brief, 10 μL of sample was aspirated directly from microtiter plates and loaded onto the RapidFire microscale solid-phase extraction cartridge D (graphitic carbon) to remove salts with HPLC-grade water in a 2.5-sec wash cycle. Analytes were then coeluted into the mass spectrometer in a 3.5-s elution cycle using 25% acetonitrile, 25% acetone containing 5 mM ammonium acetate. ΔDiHS-0S, ΔDiHS-NS and IS were measured using a Sciex API 4000 QQQ mass spectrometer (Applied Biosystems, Concord, Ontario, Canada) in negative ESI mode following multiple-reaction monitoring (MRM) transitions at 378.2/174.3, 416.0/137.7 and 354.3/193.1, respectively. Sample data were integrated using the RapidFire Integrator software (Agilent Technologies, Inc: Santa Clara, CA) resulting in individual AUC (area under the curve) values for each of the three analytes in each sample. Each sample was prepared and analyzed in duplicate on separate days. HS levels were measured and averaged.
2.2.3. LC-MS/MS
The chromatographic system consisted of the 1260 Infinity LC (Agilent Technologies, Palo Alto, CA, USA) and a Hypercarb column (2.0 mm i.d. 50 mm, 5 μm, Thermo Electron, USA). The 6460 Triple Quad mass spectrometer (Agilent Technologies) was operated in the negative ion detection mode. The procedure was described previously [18]. The MRM of each disaccharide was the same as used for HT-MS/MS analysis.
2.2.4. Method validation
Intra-day precision evaluated as coefficient of variation (CV) was determined by replicate analyses (n = 5) of three different control blood samples. Inter-day precision was determined by replicate analyses (n = 5) of three different control blood samples on three separate days.
The selectivity of the assay was investigated by processing and analyzing five independent samples by the method described above without enzymatic digestion. Calibration curves were constructed by plotting the peak area ratio of the analytes to IS against the concentration of the analytes. Each calibration curve consisted of seven calibration points. HS values obtained by HT-MS/MS were compared with those by LC-MS/MS.
2.2.5. Data analysis
The data were analyzed by ANOVA and Tukey’s test. Analysis was performed using SPSS for Windows (version 22.0, IBM, Chicago, IL, USA).
3. Results
3.1. MRM of HT-MS/MS
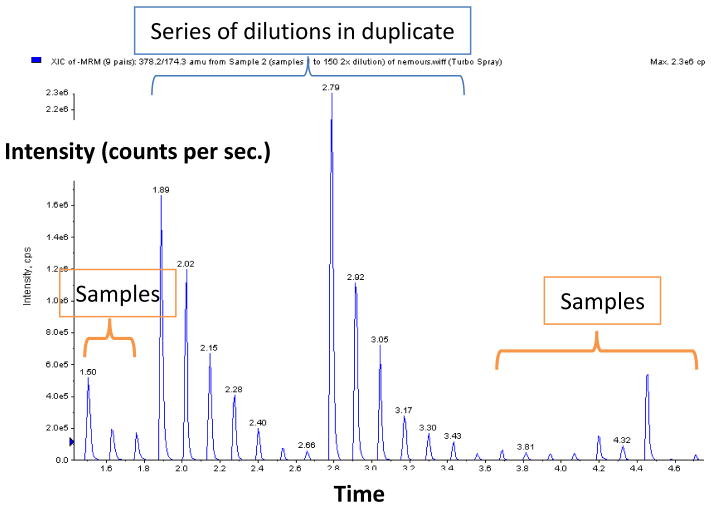
Typical MRM of HT-MS/MS for ΔDiHS-0S standards with serial dilutions and samples is shown in Fig. 1. Similar MRMs were obtained for IS and ΔDiHS-NS. The HT-MS/MS system allowed us to measure 8 – 9 samples per min.
Figure 1.
MRMs of HT-MS/MS. Multiple injections of ΔDiHS-0S (Q1; 378.2, Q3; 174.3) with a series of dilutions (15.625, 31.25, 62.5, 125, 250, 500, and 1000 ng/ml) in duplicate show seven gradient peaks per set of dilutions (surrounding samples also shown).
3.2. Precision
Results of intra- and inter-assay precision for ΔDiHS-0S and ΔDiHS-NS in control blood samples are as follows. The intra-assay precision values/coefficient of variation (CV) determined from the analysis of ΔDiHS-0S and ΔDiHS-NS for control blood samples are less than 14.7 and 14.9%, respectively. The inter-assay precision values/CVs for these disaccharides in control blood samples are less than 13.9 and 13.7%, respectively. These results demonstrate the reproducibility and precision of the method.
3.3. Correlation between HT-MS/MS and LC-MS/MS
All blood samples were also measured with the LC-MS/MS system that separates the disaccharides with a chromatographic system. There were strong correlations between HT-MS/MS and LC-MS/MS in measurements of both ΔDiHS-0S (r2 = 0.9409) and ΔDiHS-NS (r2 = 0.889), and regression coefficients for ΔDiHS-0S and ΔDiHS-NS were 1.2 and 1.0, respectively (Fig. 2)
Figure 2.
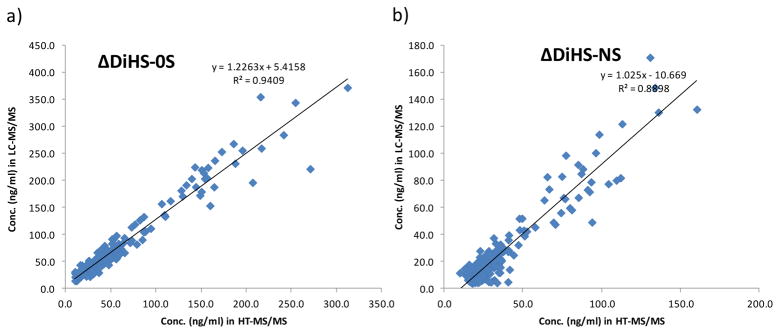
Correlation in HS concentration between LC-MS/MS and HT-MS/MS.
a) ΔDiHS-0S; b) ΔDiHS-NS. Coefficient of determination is r2 = 0.9409 and r2 = 0.889 for ΔDiHS-0S and b) ΔDiHS-NS, respectively; showing a strong correlation in ΔDiHS-0S and ΔDiHS-NS between LC-MS/MS and HT-MS/MS in human blood samples (n = 228).
3.4. Measurements of GAGs in blood
3.4.1. Control
The levels of ΔDiHS-0S and ΔDiHS-NS were the highest in newborns and decreased until three years of age. After three years of age, the levels of these two HS remained stable (Fig. 3).
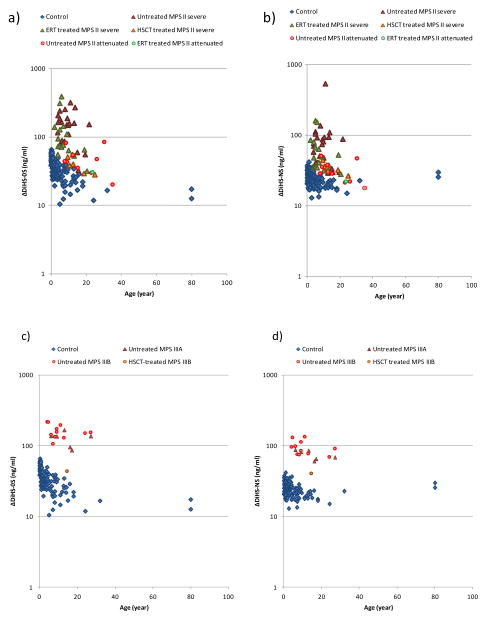
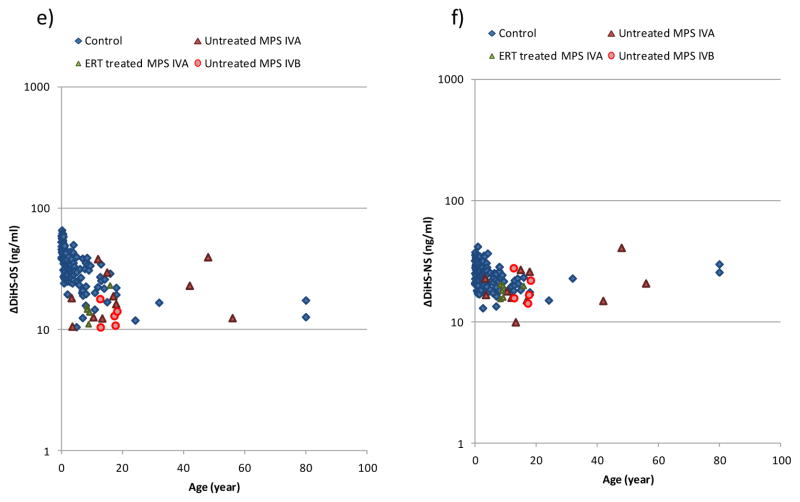
Figure 3.
HS levels in serum of patients with MPS and control subjects. a) ΔDiHS-0S in patients with MPS II, b) ΔDiHS-NS in patients with MPS II, c) ΔDiHS-0S in patients with MPS III, d) ΔDiHS-NS in patients with MPS III, e) ΔDiHS-0S in patients with MPS IV, and f) ΔDiHS-NS in patients with MPS IV.
Results of all specimens from patients and control subjects were plotted on a semilogarithmic scale with respect to age (years).
3.4.2. Mucopolysaccharidosis type II (MPS II)
Levels of blood ΔDiHS-0S and ΔDiHS-NS in untreated patients with a severe form of MPS II (n = 24) were significantly higher, compared with those in control groups (Table 1, Fig. 3). The increase in untreated patients with an attenuated form of MPS II (n = 9) did not reach statistical significance with the small sample size. The level of ΔDiHS-0S in untreated patients with a severe form of MPS II was higher than that in patients with an attenuated form. The level of ΔDiHS-NS was also elevated in the untreated patients with the severe form of MPS II compared to those with the attenuated form (Table 1).
Table 1.
Levels of ΔDiHS-0S and ΔDiHS-NS in patients with MPS control subjects over 3 years of age.
| ΔDiHS-0S (ng/ml) | ΔDiHS-NS (ng/ml) | |
|---|---|---|
| Control | 27.5 ± 8.7 | 22.7 ± 4.8 |
| Untreated MPS II (severe) | 144.5 ± 77.9a | 89.4 ± 100.1a |
| ERT-treated MPS II (severe) | 116.1 ± 101.0a | 61.0 ± 41.4 |
| HSCT-treated MPS II (severe) | 40.3 ± 11.1b,c | 34.1 ± 5.9 |
| Untreated MPS II (attenuated) | 49.2 ± 21.9b | 32.2 ± 11.5 |
| ERT-treated MPS II (attenuated) | 30.5 | 22 |
Number of each group is as follows; control (n = 59), untreated MPS II (severe; n = 24), ERT-treated MPS II (severe; n = 15), HSCT-treated MPS II (severe; n = 6), untreated MPS II (attenuated; n = 9), and ERT-treated MPS II (attenuated; n = 1).
significantly different from the control at p < 0.05,
significantly different from the untreated MPS II severe at p < 0.05,
significantly different from the ERT-treated MPS II severe at p < 0.05
The levels of ΔDiHS-0S in blood from HSCT-treated patients with a severe form of MPS II 8.9 years (average) after treatment were significantly reduced, compared with untreated patients (Table 1). Levels of ΔDiHS-0S in blood from these HSCT treated patients were also significantly lower than in blood from ERT-treated patients (Table 1). Seven patients with the severe form of MPS II provided blood specimens before and an average of two years after starting ERT. Levels of ΔDiHS-0S and ΔDiHS-NS were significantly decreased after ERT treatment (Fig. 4).
Figure 4.
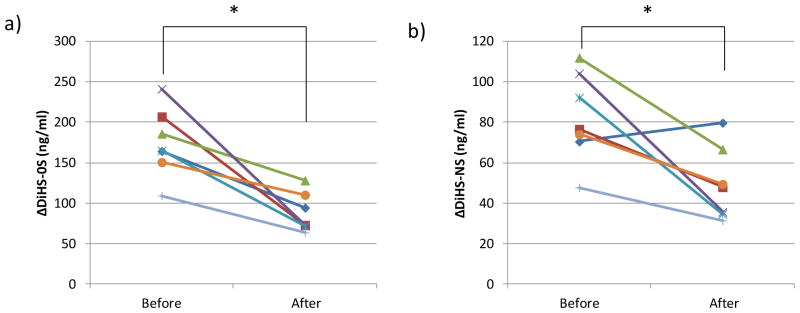
HS levels in serum of seven patients with MPS II who underwent ERT for more than two years. a) ΔDiHS-0S; b) ΔDiHS-NS. Each line describes an individual patient. *: shows significant difference between pre-treatment and post-treatment.
3.4.3. Mucopolysaccharidosis type III (MPS III)
Untreated patients with MPS IIIA and MPS IIIB (n = 6 and n = 11, respectively) had significant elevation of levels of ΔDiHS-0S and ΔDiHS-NS from blood compared with those in control groups (Table 2; Fig. 3). A 15 year-old MPS IIIB female, who had undergone successful HSCT at 1 year and 10 months, had blood levels of ΔDiHS-0S and ΔDiHS-NS in the normal range (Table 2). This patient could speak with limited words, recognize her parents, and walk by herself at the age of 15 years. Thus, her cognitive and physical function was much better, compared with that in untreated age-matched MPS IIIB patients, indicating that the biochemical correction by HSCT resulted in an improved clinical outcome.
Table 2.
Levels of ΔDiHS-0S and ΔDiHS-NS in patients with MPS III and control subjects over 3 years of age.
| ΔDiHS-0S (ng/ml) | ΔDiHS-NS (ng/ml) | |
|---|---|---|
| Control | 27.5 ± 8.7 | 22.7 ± 4.8 |
| Untreated MPS IIIA | 126.6 ± 30.0a | 75.0 ± 11.5 |
| Untreated MPS IIIB | 161.3 ± 35.6a | 95.0 ± 22.3a |
| HSCT treated MPS IIIB | 43.6 | 40.5 |
Number of each group is as follows, Control (n = 59), untreated MPS IIIA (n = 6), untreated MPS IIIB (n = 11), and HSCT-treated MPS IIIB (n = 1)
significantly different from the control at p < 0.05
3.4.4. Mucopolysaccharidosis type IV (MPS IV)
Blood HS level in untreated and ERT-treated patients with MPS IVA (n = 11 and n = 6, respectively) showed the same levels as normal controls. Untreated patients with MPS IVB also had the same range of HS compared with normal controls (Table 3).
Table 3.
Levels of ΔDiHS-0S and ΔDiHS-NS in patients with MPS IV and control subjects over 3 years of age.
| ADiHS-0S (ng/ml) | ΔDiHS-NS (ng/ml) | |
|---|---|---|
| Control | 27.5 ± 8.7 | 22.7 ± 4.8 |
| Untreated MPS IVA severe | 20.9 ± 10.3 | 20.6 ± 8.3 |
| ERT treated MPS IVA severe | 15.2 ± 4.0 | 18.2 ± 2.1 |
| Untreated MPS IVB attenuated | 13.1 ± 3.0 | 19.2 ± 5.5 |
Number of each group is as follows, Control (n = 59), untreated MPS IVA (n = 11), ERT-treated MPS IVA (n = 6), and untreated MPS IVB (n = 5)
3.5. Measurements of GAGs in newborn DBS
The levels of ΔDiHS-0S and ΔDiHS-NS extracted from DBS of newborns later diagnosed with MPS I and MPS III were significantly elevated compared to control newborn samples. The levels of ΔDiHS-0S and ΔDiHS-NS from DBS of a newborn later diagnosed with MPS II were 3 and 1.5 times higher than in DBS from control newborns (Table 4). For all three forms of MPS, ΔDiHS-0S distinguished patients from controls better than ΔDiHS-NS.
Table 4.
Levels of ΔDiHS-0S and ΔDiHS-NS in newborn DBS from control and patients with MPS.
| (ng/spot) | ΔDiHS-0S | ΔDiHS-NS |
|---|---|---|
| Control | 0.10 ± 0.02 | 0.11 ± 0.05 |
| MPS I | 0.49 ± 0.13 * | 0.29 ± 0.11 * |
| MPS II | 0.33 | 0.14 |
| MPS III | 0.50 ± 0.10 * | 0.30 ± 0.13 * |
Number of each group is as follows, Control (n = 22), MPS I (n = 6), MPS II (n = 1), and MPS III (n = 5)
significantly different from the control at p < 0.05.
4. Discussion
We have demonstrated 1) that the RapidFire platform is capable of detecting disaccharides of ΔDiHS-0S and ΔDiHS-NS in blood with the same reproducibility, sensitivity, and specificity as described for the LC-MS/MS method [7, 14–17, 19–22], suggesting that chromatographic separation of disaccharides is not essential for detection and identification of ΔDiHS-0S and ΔDiHS-NS, 2) that HS assays by HT-MS/MS can distinguish untreated patients with MPS II or III from healthy controls, showing the potential use of this technology as a diagnostic platform, 3) that patient groups treated by ERT or HSCT had reduced HS levels, suggesting that HS measured by HT-MS/MS can be used as a biomarker for monitoring therapeutic effect, 4) that the run time of six to eight seconds per sample provides 10–100 fold higher throughput when compared to traditional LC-MS/MS, indicating that HT-MS/MS may be useful for mass screening applications for MPS, and 5) that levels of HS in newborn DBS were elevated in all MPS I, II and III patients, suggesting that HT-MS/MS could be used in a NBS to identify these patients.
In MPS, the undegraded GAGs are stored in lysosomes, secreted into the circulation and excreted into the urine. In this study, blood HS was digested to disaccharides by heparitinase using the same method as described for the LC-MS/MS method [7, 14, 15]. Two subclasses of HS (ΔDiHS-0S and ΔDiHS-NS) were detected by HT-MS/MS simultaneously. The levels of ΔDiHS-0S and ΔDiHS-NS in blood samples from both control and patient subjects, determined by HT-MS/MS, correlated strongly with those determined by LC-MS/MS, both in this and previous studies [15, 17]. The calculated values of ΔDiHS-0S and ΔDiHS-NS using both methods were similar, indicating that LC-MS/MS and HT-MS/MS are comparable in sensitivity and specificity.
Overall, the levels of ΔDiHS-0S and ΔDiHS-NS obtained in blood of normal controls and MPS patients by HT-MS/MS was equivalent to those obtained by LC-MS/MS, indicating that the use of the HT-MS/MS platform did not affect the specificity and sensitivity of the assay. The HT-MS/MS system does not only acquire data much faster than traditional LC/MS/MS systems, but also provides data of a similar quality without using a time-consuming separation process.
MPS II results from a deficiency of iduronate-2-sulfatase, leading to accumulation of HS and DS. In this study ΔDiHS-0S and ΔDiHS-NS levels increased in untreated patients with a severe form of MPS II compared with those in the control groups, as described previously using the LC-MS/MS method [7, 15]. There was a significant increase in the level of ΔDiHS-0S in patients with a severe form of this MPS compared to those with an attenuated form, and the level of ΔDiHS-0S in the ERT- or HSCT-treated group was significantly lower compared with that in the untreated group. Furthermore, the level of ΔDiHS-0S was significantly lower in blood from patients treated by HSCT than in blood from patients treated by ERT. Levels of ΔDiHS-NS were also lower in blood from the HSCT treated patients but the difference was not statistically significant. Consistent with our results, Tanaka et al reported that HSCT given during early stages of MPS II provides effectiveness toward brain and heart disorders with decrease of urinary GAGs level and that patients treated by ERT at a similar stage showed less reduction of urinary GAGs [36]. For ERT, the timing of blood draw after a round of therapy is likely to affect blood levels of GAGs, so more detailed studies are needed to determine whether HSCT or ERT is better at reducing GAGs. In future studies it will be of great interest to explore whether differences in ΔDiHS-0S and/or ΔDiHS-NS levels are associated with clinical severity and/or improvement of CNS involvement.
MPS IIIA and MPS IIIB result from deficiencies of enzymes heparan N-sulphatase and α-N-acetylglucosaminidase, respectively, leading to accumulation of HS. The levels of ΔDiHS-0S and ΔDiHS-NS in untreated patients with MPS IIIA or MPS IIIB were significantly raised compared with those in normal controls, as described previously using LC-MS/MS [7, 15]. The frequency of MPS IIIB is higher in a regional district in Japan, based upon the founder effect [37]. It is noteworthy that one Japanese MPS IIIB patient who received successful HSCT when under two years of age had ΔDiHS-0S and ΔDiHS-NS levels in the normal range. After over 13 years follow-up, the level of neurocognition in this patient is far better than in age-matched untreated patients, although neurological involvement still remains obvious. The finding that the primary storage material, HS, is within the normal range is consistent with the less severe clinical course in this patient, showing that both ΔDiHS-0S and ΔDiHS-NS should be good biomarkers for monitoring the clinical course and therapeutic efficacy in MPS III.
MPS IVA and MPS IVB are caused by the deficiency of N-acetylgalactosamine-6-sulfate sulfatase (GALNS) and β-galactosidase (β-Gal), respectively, and consequently KS but not HS should accumulate in blood. In this study we found that HS levels in patients with MPS IV were the same as the control group, consistent with the predicted result and previous reports [15, 17]. In the United States, nearly 4.2 million neonates undergo NBS annually [38]. There are no specific incidence studies of MPS in the USA, but we estimate that approximately 200 newborns are affected with MPS annually in USA [39–45](20–26). In this study, we show that the levels of ΔDiHS-0S and ΔDiHS-NS from DBS of newborn MPS I, II and III patients are higher than newborn control. De Ruijter et al also reported that the LC-MS/MS method could show elevation of ΔDiHS-0S in the patients with these forms of MPS using newborn DBS samples [20]. Our results lead the possibility of applying HT-MS/MS to NBS for MPS, although further studies with more samples are required. The fact that HT-MS/MS can process the same samples with throughputs up to 10–100 folds faster than conventional LC-MS/MS makes this platform a more appropriate system, due to time-saving and potential cost reductions for NBS.
HS can be elevated in blood due to release from the endothelium during ischemia/reperfusion injury and severe inflammation [46, 47]. It can also be elevated in blood after virus- infection and septic shock [48, 49]. Thus, HT-MS/MS could be a novel high throughput system to identify biomarkers for not only MPS but also for these more common disorders. This manuscript has focused on assay of HS, but CS, DS and KS assays could also be developed for HT-MS/MS to screen for other forms of MPS and other diseases.
In conclusion, we have demonstrated a unique HT-MS/MS method for measurement of HS that can distinguish MPS patients from controls with a similar reproducibility, sensitivity and specificity as established by LC-MS/MS methods, and that the method can be applied to analysis of newborn DBS. The HT-MS/MS should provide both a rapid throughput and economic advantages for monitoring, diagnosis, and NBS for MPS and has the potential of being applied to other disorders.
Highlight.
Automated high-throughput mass spectrometry (HT-MS/MS) is comparable with LC-MS/MS in sensitivity and specificity.
Levels of HS in blood are significantly elevated in patients with MPS II and III.
Levels of HS in patients with a severe form of MPS II are higher than that with an attenuated form.
Reduction of blood HS level is seen in MPS II patients treated with ERT or HSCT.
Levels of ΔDiHS-0S and ΔDiHS-NS in DBS from newborn MPS I, II and III patients are elevated compared to newborn controls.
Acknowledgments
This work was supported by grants from the Austrian MPS Society, International Morquio Organization (Carol Ann Foundation) and Delaware Bioscience Center for Advanced Technology. This work was also supported by Japanese MPS Family Society. R.W.M. and S.T. were supported in part by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of NIH under grant number P20GM103464. The content of the article has not been influenced by the sponsors. F.K. was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico from Brazil (CNPq). Editorial assistance to the manuscript was provided by Michelle Stofa at Nemours/Alfred I. duPont Hospital for Children. We thank Michael Prinsen and Mary Campbell (Center for World Health & Medicine, Saint Louis University) for helpful early discussions about the RapidFire technology.
Footnotes
Conflict of Interest:
All the authors have contributed to this “Original Article” and have no conflict of interest with any other party.
Tsutomu Shimada, Joan Kelly, William A LaMarr, Naomi van Vlies, Eriko Yasuda, Pravin Patel, Robert W. Mason, William Mackenzie, Francyne Kubaski, Roberto Giugliani, Yasutsugu Chinen, Seiji Yamaguchi, Yasuyuki Suzuki, Kenji E. Orii, Toshiyuki Fukao, Tadao Orii, and Shunji Tomatsu declare that they have no conflict of interests.
Compliance with Ethics
Informed Consent:
Informed consent was obtained from the patients and/or their guardians through the physicians who recruited the patients with MPS at each local institute, and approved by the local Institutional Review Board (IRB).
Contributions to the Project:
Tsutomu Shimada contributed to the concept of the project, the planning, execution of experiments (LC-MS/MS), data analysis, and reporting of the work described in the article.
Joan Kelly contributed to the concept of the project, the planning, execution of experiments (HT-MS/MS), data analysis, and reporting of the work described in the article.
William A LaMarr contributed to the concept of the project, the planning, execution of experiments (HT-MS/MS), data analysis, and reporting of the work described in the article.
Naomi van Vlies contributed to collecting samples, data analysis, and reporting of the work described in the article.
Eriko Yasuda contributed to collecting samples, data analysis, and reporting of the work described in the article.
Robert W. Mason contributed to the planning, execution of LC-MS/MS, data analysis, and reporting of the work described in the article.
William G. Mackenzie contributed to collecting samples, data analysis, and reporting of the work described in the article.
Francyne Kubaski contributed to collecting samples, data analysis, and reporting of the work described in the article.
Roberto Giugliani contributed to collecting samples and reporting of the work described in the article.
Yasutsugu Chinen contributed to collecting samples and reporting of the work described in the article.
Seiji Yamaguchi contributed to collecting samples and reporting of the work described in the article.
Yasuyuki Suzuki contributed to collecting samples and reporting of the work described in the article.
Tadao Orii contributed to collecting samples and reporting of the work described in the article.
Toshiyuki Fukao contributed to collecting samples and reporting of the work described in the article.
Kenji E. Orii contributed to collecting samples and reporting of the work described in the article.
Shunji Tomatsu is a Principal Investigator and is responsible for the entire project. He has contributed to the concept of the project, planning, analysis of data, and reporting of the work described in the article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tomatsu S, Montaño AM, Dung VC, et al. Enhancement of drug delivery: enzyme-replacement therapy for murine Morquio A syndrome. Mol Ther. 2010;18:1094–1102. doi: 10.1038/mt.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Act. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 3.de Jong JG, Wevers RA, Liebrand-van Sambeek R. Measuring urinary glycosaminoglycans in the presence of protein: an improved screening procedure for mucopolysaccharidoses based on dimethylmethylene blue. Clin Chem. 1992;38:803–807. [PubMed] [Google Scholar]
- 4.Whitley CB, Spielmann RC, Herro G, Teragawa SS. Urinary glycosaminoglycan excretion quantified by an automated semimicro method in specimens conveniently transported from around the globe. Mol Genet Metab. 2002;75:56–64. doi: 10.1006/mgme.2001.3271. [DOI] [PubMed] [Google Scholar]
- 5.Whiteman P. The quantitative determination of glycosaminoglycans in urine with Alcian Blue 8GX. Biochem J. 1973;131:351–357. doi: 10.1042/bj1310351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burlingame RW, Thomas GH, Stevens RL, Schmid K, Moser HW. Direct quantitation of glycosaminoglycans in 2 mL of urine from patients with mucopolysaccharidoses. Clin Chem. 1981;27:124–128. [PubMed] [Google Scholar]
- 7.Oguma T, Tomatsu S, Montaño AM, Okazaki O. Analytical method for the determination of disaccharides derived from keratan, heparan, and dermatan sulfates in human serum and plasma by high-performance liquid chromatography/turbo ionspray ionization tandem mass spectrometry. Anal Biochem. 2007;368:79–86. doi: 10.1016/j.ab.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 8.Cappelletti R, Del Rosso M, Chiarugi VP. A new electrophoretic method for the complete separation of all known animal glycosaminoglycans in a monodimensional run. Anal Biochem. 1979;99:311–315. doi: 10.1016/s0003-2697(79)80012-3. [DOI] [PubMed] [Google Scholar]
- 9.Hopwood JJ, Harrison JR. High-resolution electrophoresis of urinary glycosaminoglycans: an improved screening test for the mucopolysaccharidoses. Anal Biochem. 1982;119:120–127. doi: 10.1016/0003-2697(82)90674-1. [DOI] [PubMed] [Google Scholar]
- 10.Tomatsu S, Okamura K, Maeda H, et al. Keratan sulphate levels in mucopolysaccharidoses and mucolipidoses. J Inherit Metab Dis. 2005;28:187–202. doi: 10.1007/s10545-005-5673-3. [DOI] [PubMed] [Google Scholar]
- 11.Tomatsu S, Gutierrez MA, Ishimaru T, et al. Heparan sulfate levels in mucopolysaccharidoses and mucolipidoses. J Inherit Metab Dis. 2005;28:743–757. doi: 10.1007/s10545-005-0069-y. [DOI] [PubMed] [Google Scholar]
- 12.Kinoshita A, Sugahara K. Microanalysis of glycosaminoglycan-derived oligosaccharides labeled with a fluorophore 2-aminobenzamide by high-performance liquid chromatography: application to disaccharide composition analysis and exosequencing of oligosaccharides. Anal Biochem. 1999;269:367–378. doi: 10.1006/abio.1999.4027. [DOI] [PubMed] [Google Scholar]
- 13.Yamada H, Miyauchi S, Morita M, et al. Content and sulfation pattern of keratan sulfate in hip osteoarthritis using high performance liquid chromatography. J Rheumatol. 2000;27:1721–1724. [PubMed] [Google Scholar]
- 14.Oguma T, Tomatsu S, Okazaki O. Analytical method for determination of disaccharides derived from keratan sulfates in human serum and plasma by high-performance liquid chromatography/turbo-ionspray ionization tandem mass spectrometry. Biomed Chromatogr. 2007;21:356–362. doi: 10.1002/bmc.760. [DOI] [PubMed] [Google Scholar]
- 15.Tomatsu S, Montaño AM, Oguma T, et al. Validation of disaccharide compositions derived from dermatan sulfate and heparan sulfate in mucopolysaccharidoses and mucolipidoses II and III by tandem mass spectrometry. Mol Genet Metab. 2010;99:124–131. doi: 10.1016/j.ymgme.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Tomatsu S, Montaño AM, Oguma T, et al. Dermatan sulfate and heparan sulfate as a biomarker for mucopolysaccharidosis I. J Inherit Metab Dis. 2010;33:141–150. doi: 10.1007/s10545-009-9036-3. [DOI] [PubMed] [Google Scholar]
- 17.Tomatsu S, Montaño AM, Oguma T, et al. Validation of keratan sulfate level in mucopolysaccharidosis type IVA by liquid chromatography-tandem mass spectrometry. J Inherit Metab Dis. 2010;33(Suppl 3):S35–42. doi: 10.1007/s10545-009-9013-x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 18.Tomatsu S, Fujii T, Fukushi M, et al. Newborn screening and diagnosis of mucopolysaccharidoses. Mol Genet Metab. 2013;110:42–53. doi: 10.1016/j.ymgme.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomatsu S, Shimada T, Mason RW, et al. Assay for glycosaminoglycans by tandem mass spectrometry and its applications. J Anal Bioanal Tech. 2014;S2:006. doi: 10.4172/2155-9872.S2-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Ruijter J, de Ru MH, Wagemans T, et al. Heparan sulfate and dermatan sulfate derived disaccharides are sensitive markers for newborn screening for mucopolysaccharidoses types I, II and III. Mol Genet Metab. 2012;107:705–710. doi: 10.1016/j.ymgme.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 21.de Ruijter J, Ijlst L, Kulik W, et al. Heparan sulfate derived disaccharides in plasma and total urinary excretion of glycosaminoglycans correlate with disease severity in Sanfilippo disease. J Inherit Metab Dis. 2013;36:271–279. doi: 10.1007/s10545-012-9535-5. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 22.de Ru MH, van der Tol L, van Vlies N, et al. Plasma and urinary levels of dermatan sulfate and heparan sulfate derived disaccharides after long-term enzyme replacement therapy (ERT) in MPS I: correlation with the timing of ERT and with total urinary excretion of glycosaminoglycans. J Inherit Metab Dis. 2013;36:247–255. doi: 10.1007/s10545-012-9538-2. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Shimada T, Tomatsu S, Yasuda E, et al. Chondroitin 6-sulfate as a novel biomarker for mucopolysaccharidosis IVA and VII. J Inherit Metab Dis. 2014 doi: 10.1007/8904_2014_311. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montaño AM, Oikawa H, Tomatsu S, et al. Acidic amino acid tag enhances response to enzyme replacement in mucopolysaccharidosis type VII mice. Mol Genet Metab. 2008;94:178–189. doi: 10.1016/j.ymgme.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Tomatsu S, Montaño AM, Ohashi A, et al. Enzyme replacement therapy in a murine model of Morquio A syndrome. Hum Mol Genet. 2008;17:815–824. doi: 10.1093/hmg/ddm353. [DOI] [PubMed] [Google Scholar]
- 26.Quercia AK, LaMarr WA, Myung J, Ozbal CC, Landro JA, Lumb KJ. High-throughput screening by mass spectrometry: comparison with the scintillation proximity assay with a focused-file screen of AKT1/PKB alpha. J Biomol Screen. 2007;12:473–480. doi: 10.1177/1087057107300647. [DOI] [PubMed] [Google Scholar]
- 27.Wu X, Wang J, Tan L, et al. In vitro ADME profiling using high-throughput rapidfire mass spectrometry: cytochrome p450 inhibition and metabolic stability assays. J Biomol Screen. 2012;17:761–772. doi: 10.1177/1087057112441013. [DOI] [PubMed] [Google Scholar]
- 28.Leveridge MV, Bardera AI, LaMarr W, et al. Lead discovery for microsomal prostaglandin E synthase using a combination of high-throughput fluorescent-based assays and RapidFire mass spectrometry. J Biomol Screen. 2012;17:641–650. doi: 10.1177/1087057111435700. [DOI] [PubMed] [Google Scholar]
- 29.Hutchinson SE, Leveridge MV, Heathcote ML, et al. Enabling lead discovery for histone lysine demethylases by high-throughput RapidFire mass spectrometry. J Biomol Screen. 2012;17:39–48. doi: 10.1177/1087057111416660. [DOI] [PubMed] [Google Scholar]
- 30.Plant M, Dineen T, Cheng A, Long AM, Chen H, Morgenstern KA. Screening for lysine-specific demethylase-1 inhibitors using a label-free high-throughput mass spectrometry assay. Anal Biochem. 2011;419:217–227. doi: 10.1016/j.ab.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Atkinson KA, Beretta EE, Brown JA, et al. N-benzylimidazole carboxamides as potent, orally active stearoylCoA desaturase-1 inhibitors. Bioorg Med Chem Lett. 2011;21:1621–1625. doi: 10.1016/j.bmcl.2011.01.113. [DOI] [PubMed] [Google Scholar]
- 32.Highkin MK, Yates MP, Nemirovskiy OV, et al. High-throughput screening assay for sphingosine kinase inhibitors in whole blood using RapidFire® mass spectrometry. J Biomol Screen. 2011;16:272–277. doi: 10.1177/1087057110391656. [DOI] [PubMed] [Google Scholar]
- 33.Langsdorf EF, Malikzay A, Lamarr WA, et al. Screening for antibacterial inhibitors of the UDP-3-O-(R-3-hydroxymyristoyl)-N-acetylglucosamine deacetylase (LpxC) using a high-throughput mass spectrometry assay. J Biomol Screen. 2010;15:52–61. doi: 10.1177/1087057109355319. [DOI] [PubMed] [Google Scholar]
- 34.Holt TG, Choi BK, Geoghagen NS, et al. Label-free high-throughput screening via mass spectrometry: a single cystathionine quantitative method for multiple applications. Assay Drug Dev Technol. 2009;7:495–506. doi: 10.1089/adt.2009.0200. [DOI] [PubMed] [Google Scholar]
- 35.Jonas M, LaMarr WA, Ozbal C. Mass spectrometry in high-throughput screening: a case study on acetyl-coenzyme a carboxylase using RapidFire--mass spectrometry (RF-MS) Comb Chem High Throughput Screen. 2009;12:752–759. doi: 10.2174/138620709789104924. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka A, Okuyama T, Suzuki Y, et al. Long-term efficacy of hematopoietic stem cell transplantation on brain involvement in patients with mucopolysaccharidosis type II: a nationwide survey in Japan. Mol Genet Metab. 2012;107:513–520. doi: 10.1016/j.ymgme.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Chinen Y, Tohma T, Izumikawa Y, Uehara H, Ohta T. Sanfilippo type B syndrome: five patients with an R565P homozygous mutation in the alpha-N-acetylglucosaminidase gene from the Okinawa islands in Japan. J Hum Genet. 2005;50:357–9. doi: 10.1007/s10038-005-0258-4. [DOI] [PubMed] [Google Scholar]
- 38.McCabe LL, McCabe ER. Expanded newborn screening: implications for genomic medicine. Annu Rev Med. 2008;59:163–175. doi: 10.1146/annurev.med.59.110106.132016. [DOI] [PubMed] [Google Scholar]
- 39.Applegarth DA, Toone JR, Lowry RB. Incidence of inborn errors of metabolism in British Columbia, 1969–1996. Pediatrics. 2000;105:e10. doi: 10.1542/peds.105.1.e10. [DOI] [PubMed] [Google Scholar]
- 40.Baehner F, Schmiedeskamp C, Krummenauer F, Miebach E, Bajbouj M, Whybra C, Kohlschütter A, Kampmann C, Beck M. Cumulative incidence rates of the mucopolysaccharidoses in Germany. J Inherit Metab Dis. 2005;28:1011–1017. doi: 10.1007/s10545-005-0112-z. [DOI] [PubMed] [Google Scholar]
- 41.Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. JAMA. 1999;281:249–254. doi: 10.1001/jama.281.3.249. [DOI] [PubMed] [Google Scholar]
- 42.Nelson J. Incidence of the mucopolysaccharidoses in Northern Ireland. Hum Genet. 1997;101:355–358. doi: 10.1007/s004390050641. [DOI] [PubMed] [Google Scholar]
- 43.Nelson J, Crowhurst J, Carey B, Greed L. Incidence of the mucopolysaccharidoses in Western Australia. Am J Med Genet A. 2003;123A:310–313. doi: 10.1002/ajmg.a.20314. [DOI] [PubMed] [Google Scholar]
- 44.Poorthuis BJ, Wevers RA, Kleijer WJ, Groener JE, de Jong JG, van Weely S, Niezen-Koning KE, van Diggelen OP. The frequency of lysosomal storage diseases in The Netherlands. Hum Genet. 1999;105:151–156. doi: 10.1007/s004399900075. [DOI] [PubMed] [Google Scholar]
- 45.Lin HY, Lin SP, Chuang CK, Niu DM, Chen MR, Tsai FJ, Chao MC, Chiu PC, Lin SJ, Tsai LP, Hwu WL, Lin JL. Incidence of the mucopolysaccharidoses in Taiwan, 1984–2004. Am J Med Genet A. 2009;149A:960–964. doi: 10.1002/ajmg.a.32781. [DOI] [PubMed] [Google Scholar]
- 46.Mulivor AW, Lipowsky HH. Inflammation- and ischemia-induced shedding of venular glycocalyx. Am J Physiol Heart Circ Physiol. 2004;286:H1672–1680. doi: 10.1152/ajpheart.00832.2003. [DOI] [PubMed] [Google Scholar]
- 47.Rehm M, Bruegger D, Christ F, et al. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation. 2007;116:1896–1906. doi: 10.1161/CIRCULATIONAHA.106.684852. [DOI] [PubMed] [Google Scholar]
- 48.Nelson A, Berkestedt I, Bodelsson M. Circulating glycosaminoglycan species in septic shock. Acta Anaesthesiol Scand. 2014;58:36–43. doi: 10.1111/aas.12223. [DOI] [PubMed] [Google Scholar]
- 49.Guven FM, Aydin H, Kaya A, et al. The role of heparan sulphate in pathogenesis of Crimean-Congo hemorrhagic fever disease. J Vector Borne Dis. 2013;50:133–136. [PubMed] [Google Scholar]