Abstract
Objectives:
The primary objective was to determine the response rate in patients with metastatic pancreatic cancer treated in first line with irinotecan/docetaxel combination (Arm A) or with irinotecan/docetaxel/cetuximab combination (Arm B). Secondary endpoints were progression-free survival (PFS), overall survival (OS), toxicity, and the rate of thromboembolic events with prophylactic enoxaparin sodium.
Patients and Methods:
Patients were eligible who had measurable, metastatic adenocarcinoma of the pancreas, and normal bilirubin. All patients received anticoagulation. Docetaxel (35 mg/m2) and irinotecan (50 mg/m2) were administered once a week for 4 weeks followed by 2 weeks rest (Arm A) alone or with the addition of cetuximab (Arm B). The primary endpoint was response rate.
Results:
A total of 87 eligible patients were enrolled and treated. Grade 3/4 toxicity was observed in 74% of patients in Arm A and 76% in Arm B. The principal grade 3/4 toxicity was diarrhea. Response rates were 4.5% in Arm A and 7% in Arm B. Median PFS and OS were 3.9 and 6.5 months in Arm A and 4.5 and 5.4 months in Arm B.
Conclusions:
Docetaxel/irinotecan combination is associated with considerable toxicity. Objective responses were infrequent and addition of cetuximab in an unselected population was not beneficial, but PFS and OS were comparable with those achieved with other regimens. Docetaxel/irinotecan therapy is active in metastatic pancreatic cancer.
Key Words: pancreatic cancer, docetaxel, irinotecan, cetuximab
Pancreatic cancer is a common cancer in the United States, with a projected 45,220 cases in 2013. It has an extraordinarily high case fatality rate, with 38,460 deaths projected in the same period.1 Standard therapy for advanced disease had been gemcitabine monotherapy.2 The 1-year survival after gemcitabine therapy was reported at 18% for bolus administration and 29% when given by fixed dose rate infusion.3 De novo gemcitabine resistance is likely partially explained by genomic variation in the uptake and metabolism of this agent.4 Thus, alternate treatments have been urgently needed for this disease, and 2 combination regimens—the combination of 5-fluorouracil, leucovorin, oxaliplatin, and irinotecan, known as FOLFIRINOX, and the combination of gemcitabine with nab-paclitaxel, have now been demonstrated to improve survival when compared with gemcitabine alone, albeit median survivals remain under 1 year.5,6 Combinations of gemcitabine with 5-fluorouracil, cisplatin, and oxaliplatin had previously not proven superior to gemcitabine alone.7–9
Among the cytotoxic agents with single-agent activity in pancreatic cancer are docetaxel and irinotecan. Single-agent docetaxel achieves objective antitumor response rates of ≤15%.10,11 Irinotecan has an objective response rate of 9% in pancreatic cancer.12 Preclinical studies show evidence of synergy between taxanes and irinotecan.13–15 The interaction may be schedule dependent, with administration of taxane followed by irinotecan predicted to be optimal. Phase I studies of docetaxel-irinotecan combination demonstrate neutropenia and diarrhea to be the predominant dose-limiting toxicities.16–18 The recommended phase II doses are docetaxel (35 mg/m2) followed by irinotecan (50 mg/m2), when administered weekly for 4 sequential weeks of a 6-week schedule.16 A phase II study of docetaxel/irinotecan combination administered weekly for 4 of 6 weeks in treatment-naive advanced pancreatic cancer reported an objective response rate by WHO criteria of 27%, median survival of 9.4 months, and 1-year survival of 43%.19 The median survival for patients with metastatic disease in that study was 9.0 months. Reni et al20 incorporated mitomycin C with escalating doses of irinotecan and docetaxel on a less continuous schedule and observed no partial responses in 15 patients with up to 2 prior lines of therapy.
The epidermal growth factor receptor (EGFR) is expressed in pancreatic cancer and higher levels of expression are associated with worse survival.21 A phase III trial with the EGFR inhibitor erlotinib demonstrated an improved hazard ratio for survival with adjusted log-rank P-value of 0.038 for addition of erlotinib.22 Cetuximab has also been studied. Although a phase II trial reported median and 1-year survival of 7.1 months and 32%, a subsequent cooperative group phase III trial demonstrated no significant improvement in progression-free survival (PFS) or overall survival (OS).23,24
The current study was conducted to confirm the activity of docetaxel/irinotecan combination, and determine the feasibility of adding cetuximab to this nongemcitabine-containing first-line regimen. Enrollment was confined to patients with metastatic disease, as prior ECOG trials in pancreatic cancer had consistently found different survival in patients with locally advanced and metastatic pancreatic cancer. We added prophylactic low–molecular weight heparin for all patients who were not receiving therapeutic anticoagulation. Pancreatic cancer is strongly associated with venous thromboembolic disease, which predicts for shorter survival.25,26 At the time this study was designed, prophylactic low–molecular weight heparin had not yet been formally evaluated in patients receiving systemic therapy for pancreatic cancer.
MATERIALS AND METHODS
Patient Selection
Patients were eligible who had histologic evidence of pancreatic cancer that was metastatic, and sufficient tumor from needle aspirate or open biopsy to permit immunohistochemical staining for EGFR. Measurable disease, defined as at least 1 primary or metastatic lesion measurable in at least 1 dimension within 4 weeks before randomization, was required. Patients were required to have ECOG performance status of 0 to 1, ability to provide informed consent, no concomitant medical problems that could interfere with the ability to receive therapy, absolute neutrophil count ≥1500 cells/μL, and platelet count >100,000/μL. Estimated creatinine clearance >60 mL/min was required. Patients were eligible who had normal bilirubin, and AST and ALT<2.5× the institutional upper limit of normal (ULN); alkaline phosphatase could be 4× ULN if transaminases were normal. For patients with AST or ALT ≥normal and ≤1.5× ULN, alkaline phosphatase must have been ≤2.5× ULN. Prior systemic chemotherapy was not permitted. Patients could not have neuropathy grade ≥2, a history of congestive heart failure, or uncontrolled arrhythmia. Women who were pregnant or breast feeding were not eligible. The protocol and consent form were approved by the institutional review boards.
Treatment Plan
CT scan of the chest and abdomen were obtained within the 3 weeks before initiating treatment. Laboratory studies, CA19-9, ECG, chest x-ray, and HIV screening were completed within 2 weeks of initiating treatment.
Patients received enoxaparin sodium (Sanofi-Aventis), 40 mg subcutaneously, from start of treatment through completion of protocol therapy as prophylaxis against visceral and deep venous thrombosis, unless they were already receiving therapeutic anticoagulation. Patients received dexamethasone (8 mg) orally 12 hours before and 12 hours after docetaxel to reduce the risk of docetaxel-induced fluid retention. Dexamethasone (10 mg) was also given orally as premedication along with antiemetics. Oral antiemetic therapy was prescribed and patients were instructed on the intensive use of loperamide in the event of diarrhea.27
Treatment Arm A: Combination Chemotherapy
Docetaxel (35 mg/m2) (Sanofi-Aventis) was administered intravenously over 60 minutes. After the completion of the docetaxel infusion, irinotecan (Pfizer) was administered intravenously over 30 minutes at a dose of 50 mg/m2. Chemotherapy was administered once a week (days 1, 8, 15, and 22) for 4 consecutive weeks followed by 2 weeks rest. This constituted a cycle of treatment.
Treatment Arm B: Combination Chemotherapy and Cetuximab
Patients received cetuximab intravenous infusion once a week for 6 weeks. On day 1 of cycle 1, 400 mg/m2 was administered over 120 minutes. Thereafter, 250 mg/m2 was given weekly over 60 minutes. The infusion rate was not to exceed 5 mL/min. Diphenhydramine (50 mg IV) was administered before the initial dose and subsequently at the physician’s discretion. Docetaxel and irinotecan were administered as detailed for arm A.
Treatment was held for ANC<1200/μL, platelet count <75,000/μL, or diarrhea ≥grade 2. Dose modifications were to be made to docetaxel dosing for hypersensitivity, neutropenia, or hepatic toxicity. For patients who retained no fluid but suffered excess dexamethasone toxicity, dexamethasone could be tapered to eliminate the 12-hour prechemotherapy and postchemotherapy doses. Modifications to irinotecan dosing were mandated for neutropenia and diarrhea. If treatment was held in week 4 due to toxicity, therapy was also held week 5 and the subsequent cycle started 1 week early. Toxicities were graded using the Common Toxicity Criteria v. 2.0.
Response Assessment
Response assessment was performed after 2 cycles with CT scanning, multidetector row CT angiography, or MR. RECIST criteria were used to determine response status, and all responses were confirmed after 1 to 2 subsequent cycles.28
Statistical Design
The primary goal of this study was to determine the response rate in patients with metastatic pancreatic cancer treated with irinotecan/docetaxel combination (Arm A) or with irinotecan/docetaxel/cetuximab combination (Arm B). Secondary endpoints were PFS, OS, toxicity, the rate of thromboembolic events when prophylactic enoxaparin sodium is administered, and the proportion of patients with metastatic pancreatic cancer whose tumors overexpress EGFR. A comparison between arms was not formally planned due to limited power.
The overall accrual goal for E8200 was 92 patients (84 eligible patients); 46 patients each (42 eligible patients per arm) were to be randomized equally to the irinotecan/docetaxel and the irinotecan/docetaxel plus cetuximab arms. We expected a 5% hypersensitivity rate in the cetuximab arm, thus an additional 3 patients were added to the accrual goal of both arms in an attempt to ensure at least 40 eligible treated patients on Arm B.
A true response rate of ≥20% in either arm would provide evidence of activity. For both arms, the null hypothesis was that the true response rate is ≤5%. The trial had a 2-stage design in each arm.29 At the initial stage, 22 patients were to be entered on each arm. If at least 2 responses were seen among the first 20 eligible patients of an arm, 24 additional patients were to be entered on that arm. If at least 4 responses were observed among the 42 eligible patients on either arm, then the specified treatment would be considered promising. A toxicity monitoring plan was in place throughout the period of accrual.
This analysis reports on the data as of May 21, 2008. Point estimates and exact 90% confidence intervals (CI) are shown for the primary endpoint of objective response (complete plus partial responses) as well as toxicity severity groupings. Kaplan-Meier estimates were used for OS and PFS.30 OS was defined as time from registration to death from any cause. PFS was defined as the shorter of: (a) the time from registration to progression or (b) the time from registration to death without documentation of progression given that the death occurs within 4 months of the last disease assessment, or registration, whichever is more recent. These cases are censored at the date of last disease assessment without progression, or registration. Data from the 87 eligible and treated patients were used to conduct all analyses in this report with the exception of the analyses related to toxicity, which used data from all 91 treated patients irrespective of eligibility (46 Arm A, 45 Arm B).
RESULTS
Patient Characteristics
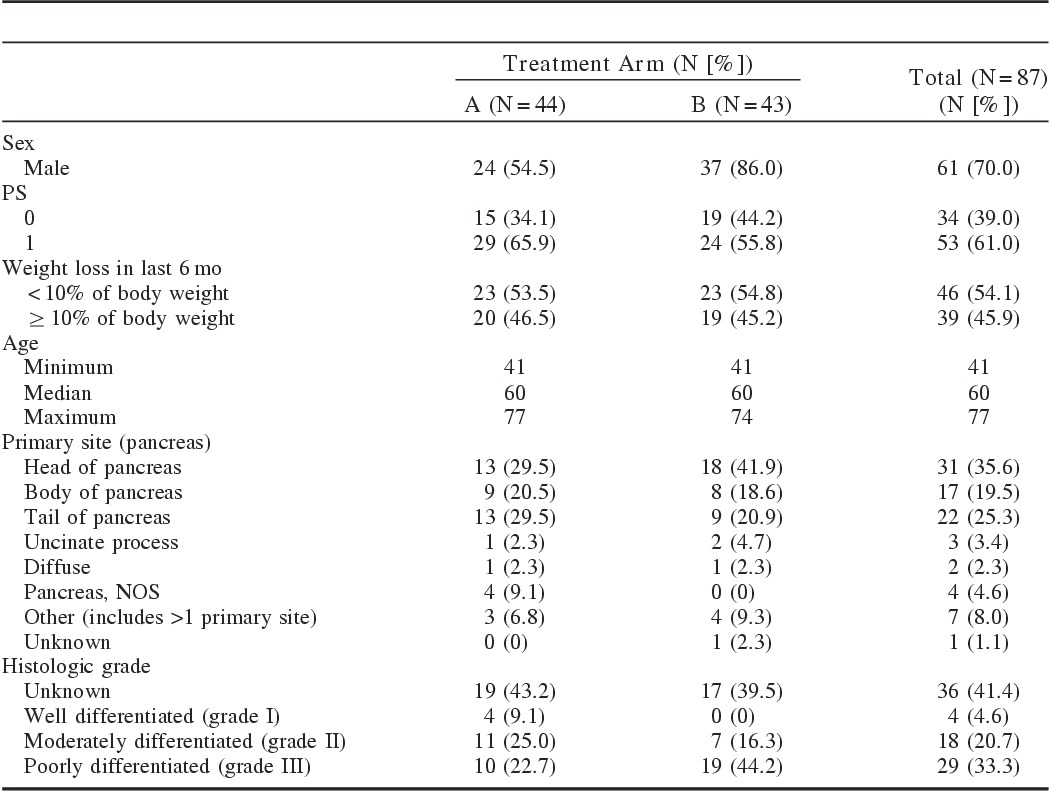
The trial accrued 94 patients between July 2003 and April 2006. Four patients were ineligible yet treated and 3 eligible patients never started assigned therapy. Patient characteristics are shown in Table 1. The median age was 60 years (range, 58 to 77 y). The majority were male (70%). ECOG performance status was 0 for 39% of patients. Weight loss of >10% in the preceding 6 months was reported for 34%.
TABLE 1.
Patient Characteristics
Treatment Information
The median number of cycles was 2 for each arm of the study. The mean number of cycles administered was 2.9 for Arm A and 3.3 for Arm B. The principal reason for treatment discontinuation was disease progression, noted in 54.5% in Arm A and 53.5% in Arm B. An additional 5 patients in Arm A and 6 patients in Arm B discontinued treatment because of clinical progression or symptomatic deterioration.
Toxicity
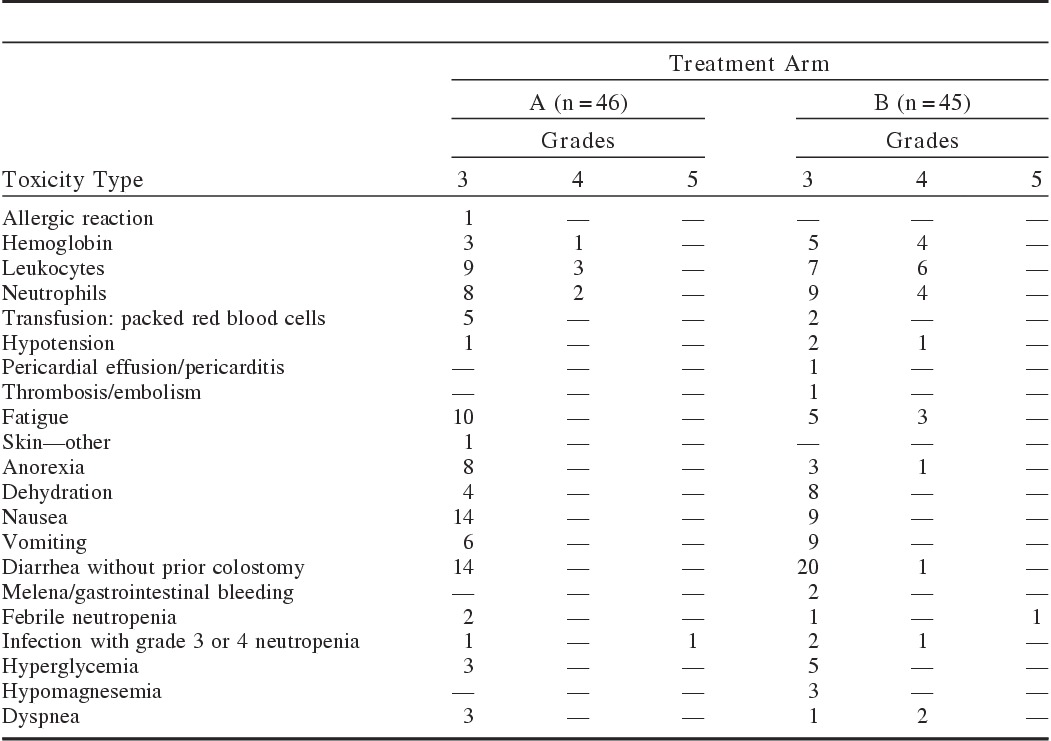
On Arm A, 57% of patients had a worst toxicity grade of 3 and 17% of 4. Toxicity data are presented in Table 2. The most common grade ≥3 toxicities on Arm A were nausea and diarrhea, each 30%. One event of grade 5 toxicity, neutropenic fever after a single day of treatment, was reported in Arm A. In Arm B, 56% had a worst toxicity grade of 3 and 20% had a worst grade of 4. In Arm B, the most common grade 3 or 4 toxicity was diarrhea. There were 2 grade 5 toxicities on Arm B, 1 diarrhea with sepsis and 1 neutropenic fever; these events also occurred in the first cycle. The rate of high-grade toxicities was not statistically different between the arms. The proportion of treated patients who experienced ≥grade 3 diarrhea is 30.4% for Arm A (exact 90% CI [19.4%, 43.4%]), and 46.7% for Arm B (exact 90% CI [33.8%, 59.9%]).
TABLE 2.
Common Grade 3 or 4 Toxicities
Thrombosis/Embolism
The rate of thromboembolic events among the 91 treated patients was 1.10%. There were 77 patients eligible for prophylactic enoxaparin, and of these, 1 reported grade 3 thrombosis/embolism and 1 grade 3 melena, for rates of venous thromboembolism and bleeding of 1.3% each. An exploratory analysis of baseline thrombosis status and outcome was undertaken. Baseline thrombosis was defined by the presence of any of the following at baseline: (a) visceral thrombosis present on CT, (b) splenic vein occlusion present on CT, (c) history of deep vein thrombosis or pulmonary embolism, or (d) indication of thrombosis or embolism on the baseline toxicity form. Complete data were available for 79 of the 87 eligible and treated patients. For the 22 patients with baseline thrombosis, 21 (95%) were known to have died with a median OS of 7.1 months (90% CI [4.5, 9.9]), compared with the 57 patients without baseline thrombosis, of whom 53 were known to have died (93.0%), and for whom the median OS was 6.5 months (90% CI [5.1, 10.7]). Assuming that the 2 treatment arms could be pooled, an exploratory log-rank test of OS between the 2 baseline thrombosis groups had a 2-sided P-value of 0.62.
Response
The RECIST partial response rates were 4.5% (90% CI [1.5%, 18.4%]) in Arm A and 7% (90% CI [2.4%, 19.8%]) in Arm B. One patient among the 44 eligible and treated in Arm A (2.3%) had a confirmed complete response. This patient was alive at last follow-up. A substantial number of patients were not evaluated for response, largely due to clinical deterioration. One patient on each arm began nonprotocol therapy before restaging.
CA19-9
The median CA19-9 level at registration was 1075 U/mL. Patients who had elevated baseline CA19-9 versus normal baseline CA19-9, and those with CA19-9 above the median versus all others, exhibited shorter PFS and OS. Seventy-nine of the 87 (91%) patients had at least 1 follow-up CA19-9 evaluation reported. Overall, 55 of those 79 (70%) had a decline of ≥50% at some follow-up CA19-9.
PFS
The median PFS for the 40 patients with sufficient information in Arm A was 3.9 months (90% CI [2.4, 5.0]). Sufficient information was available for 37 of the 43 eligible and treated patients on Arm B and their median PFS was 4.5 months (90% CI [2.7, 5.6]). The study was not designed to test for differences between the arms.
OS
OS was defined as time from registration to death from any cause. Figure 1 shows the Kaplan-Meier plot for OS for both treatment arms. As of May 21, 2008, 40 of the 44 eligible and treated patients on Arm A were known to have died (90.9%). Median survival for Arm A was 6.5 months (90% CI [5.6, 9.9]). Forty-two of the 43 eligible and treated patients on Arm B are known to have died (97.7%), with a median survival of 5.3 months (90% CI [4.5, 9.4]).
FIGURE 1.
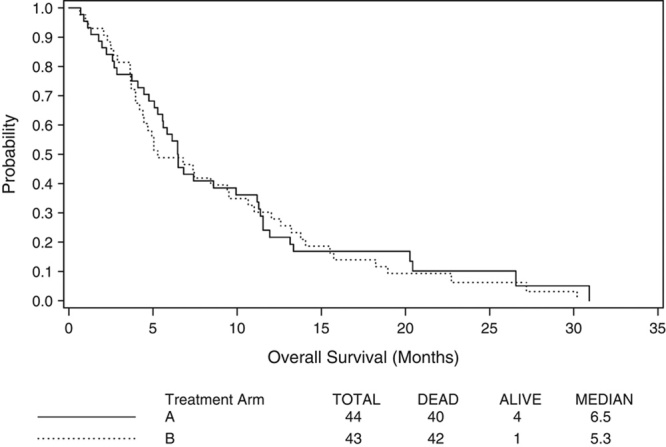
Overall survival in months.
DISCUSSION
The demonstration that FOLFIRINOX combination chemotherapy improves response and survival over gemcitabine represents an important advance in the treatment of metastatic pancreatic cancer.5 More recently, the combination of gemcitabine and nab-paclitaxel has also proven to lead to better OS than gemcitabine monotherapy for this devastating malignancy.6 The 2 regimens have not been compared head-to-head, nor have biomarkers been identified which might guide patient selection for one or the other of these regimens; nonetheless, it seems likely that differences in DNA repair, drug metabolism, or expression of drug targets might contribute to the activity of the elements of these combination regimens in individual patients. Significant differences in the expression of human equilibrative nucleoside transporter-1, ribonucleoside reductase subunit M1, and excision repair cross-complementing gene-1 expression have been documented in pancreatic cancer, and are related to prognosis.31 In this context, our demonstration that docetaxel/irinotecan combination therapy has modest activity in treatment-naive metastatic pancreatic cancer may provide another option in future trials of pharmacogenomically determined therapy in this disease.
The primary endpoint of this study was response rate, and the response rates observed were low. Nonetheless, the rate of CA19-9 decline, time to progression, and survival results of the present study suggest activity. The median PFS of 3.9 and 4.5 months in Arms A and B, respectively, compare favorably to the historical experience with gemcitabine or gemcitabine/erlotinib.2,22 Diarrhea, neutropenic infection, and treatment-related death were observed in each arm of the study and median survivals were not equivalent to those achieved with FOLFIRINOX; thus, the regimen cannot be recommended in unselected patients outside of a clinical trial.
The median OS in the cetuximab arm seemed less favorable than the OS in Arm A. Given the small size of the current study, which was not powered for comparisons between the arms, conclusions regarding the significance of this observation cannot be drawn; possible explanations include differences in use of second-line therapy with gemcitabine or gemcitabine/erlotinib driven by investigator bias or the patients’ toxicity profiles, and chance. The possibility of accelerated disease progression following cetuximab withdrawal cannot be discounted. Such disease acceleration following discontinuation of an EGFR inhibitor has been described in non–small cell lung cancer.32
CONCLUSIONS
Docetaxel and irinotecan combination therapy produces a low objective response rate, and is associated with considerable toxicity. The regimen may add to treatment options for comparison in pharmacogenomically driven trial designs. The addition of cetuximab did not seem to add survival benefit to this regimen in this unselected patient population; addition of cetuximab may also have increased toxicity.
Footnotes
Supported by Grant CA-23318, awarded by the National Cancer Institute, DHHS; Bristol-Myers Squibb; Sanofi; and Pharmacia.
Dr Burtness reports having served as a consultant for Bristol-Myers Squibb, Sanofi-Aventis, Imclone, Lilly and Pharmacia. Dr Berlin reports having served as a consultant for Imclone and Lilly. Dr. Mitchell reports having served as a consultant for Sanofi-Aventis. Dr Benson reports having served as a consultant for Imclone, Lilly, Bristol-Myers Squibb, and Sanofi-Aventis. The remaining authors declare no conflicts of interest.
REFERENCES
- 1.American Cancer Society. Cancer Facts and Figures, 2013. Available at: http://www.cancer.org. Accessed October 15, 2013.
- 2.Burris HA, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. [DOI] [PubMed] [Google Scholar]
- 3.Tempero M, Plunkett W, Ruiz Van Haperen V, et al. Randomized phase II comparison of dose-intense gemcitabine: thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma. J Clin Oncol. 2003;21:3402–3408. [DOI] [PubMed] [Google Scholar]
- 4.Giovannetti E, Mey V, Nannizzi S, et al. Pharmacogenetics of anticancer drug sensitivity in pancreatic cancer. Mol Cancer Ther. 2006;5:1387–1395. [DOI] [PubMed] [Google Scholar]
- 5.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. [DOI] [PubMed] [Google Scholar]
- 6.Von Hoff DD, Ervin TJ, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berlin JD, Catalano P, Thomas JP, et al. Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group trial E2297. J Clin Oncol. 2002;20:3270–3275. [DOI] [PubMed] [Google Scholar]
- 8.Colucci G, Giulani F, Gebbia V, et al. Gemcitabine alone or with cisplatin for the treatment of patients with locally advanced and/or metastatic pancreatic carcinoma: a prospective, randomized phase III study of the Gruppo Oncologia del l’Italia Meridionale. Cancer. 2002;94:902–910. [PubMed] [Google Scholar]
- 9.Louvet C, Labianca R, Hammel P, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23:3509–3516. [DOI] [PubMed] [Google Scholar]
- 10.Lenzi R, Yalcin S, Evans DB, et al. Phase II study of docetaxel in patients with pancreatic cancer previously untreated with cytotoxic chemotherapy. Cancer Invest. 2002;20:464–472. [DOI] [PubMed] [Google Scholar]
- 11.Rougier P, Adenis A, Ducreux M, et al. Phase II study of docetaxel as first line chemotherapy for advanced pancreatic adenocarcinoma. Eur J Cancer. 2000;36:1016–1025. [DOI] [PubMed] [Google Scholar]
- 12.Wagener DJ, Verdonk HE, Dirix LY, et al. Phase II trial of CPT11, irinotecan hydrochloride, in patients with advanced pancreatic cancer, an EORTC early clinical trials group study. Ann Oncol. 1995;6:129–132. [DOI] [PubMed] [Google Scholar]
- 13.Bissery MC, Vrignaud P, Lavelle F. In vivo evaluation of the docetaxel-irinotecan combination. Proc Am Assoc Cancer Res. 1996;37:378.(abstr 2578). [Google Scholar]
- 14.Pei XH, Nakanishi Y, Takayama K, et al. Effect of CPT-11 in combination with other anticancer agents in lung cancer cells. Anticancer Drugs. 1997;8:231–237. [DOI] [PubMed] [Google Scholar]
- 15.Kano Y, Akutsu M, Tsunoda S, et al. In vitro schedule-dependent interaction between paclitaxel and SN-38 (the active metabolite of irinotecan) in human carcinoma cell lines. Cancer Chemother Pharmacol. 1998;42:91–98. [DOI] [PubMed] [Google Scholar]
- 16.Bleickardt E, Argiris A, Rich R, et al. Phase I dose escalation trial of weekly docetaxel plus irinotecan in patients with advanced cancer. Cancer Biol Ther. 2002;1:646–650. [DOI] [PubMed] [Google Scholar]
- 17.Adjei AA, Klein Cheri E, Kastrissios H, et al. Phase I and pharmacokinetic study of irinotecan and docetaxel in patients with advanced solid tumors: preliminary evidence of clinical activity. J Clin Oncol. 2000;18:1116–1123. [DOI] [PubMed] [Google Scholar]
- 18.Couteau C, Risse M-L, Ducreux M, et al. Phase I and pharmacokinetic study of docetaxel and irinotecan in patients with advanced solid tumors. J Clin Oncol. 2000;18:3545–3552. [DOI] [PubMed] [Google Scholar]
- 19.Burtness B, Thomas L, Sipples R, et al. Phase II trial of weekly docetaxel/irinotecan combination in advanced pancreatic cancer. Cancer J. 2007;13:257–262. [DOI] [PubMed] [Google Scholar]
- 20.Reni M, Panucci MG, Passoni P, et al. Salvage chemotherapy with mitomycin, docetaxel and irinotecan (MDI regimen) in metastatic pancreatic adenocarcinoma: a phase I and II trial. Cancer Invest. 2004;22:688–696. [DOI] [PubMed] [Google Scholar]
- 21.Ueda S, Ogata S, Tsuda H, et al. The correlation between cytoplasmic overexpression of epidermal growth factor receptor and tumor aggressiveness: poor prognosis in patients with pancreatic ductal adenocarcinoma. Pancreas. 2004;29:e1–e8. [DOI] [PubMed] [Google Scholar]
- 22.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. [DOI] [PubMed] [Google Scholar]
- 23.Xiong HQ, Rosenberg A, LoBuglio A, et al. Cetuximab, a monoclonal antibody targeting the epidermal growth factor receptor, in combination with gemcitabine for advanced pancreatic cancer: a multicenter phase II Trial. J Clin Oncol. 2004;22:2610–2616. [DOI] [PubMed] [Google Scholar]
- 24.Philip PA, Benedetti J, Corless CL, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group directed intergroup trial S0205. J Clin Oncol. 2010;28:3605–3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zawin M, Camputaro C, Rahman Z, et al. Multirow detector CT detection of subclinical visceral and pulmonary thrombosis in advanced pancreatic cancer is an adverse prognostic indicator. Proc Am Soc Clin Oncol. 2003;22:353.(abstr). [Google Scholar]
- 26.Khorana AA, Fine RL. Pancreatic cancer and thromboembolic disease. Lancet Oncol. 2004;5:655–663. [DOI] [PubMed] [Google Scholar]
- 27.Abigerges D, Armand JP, Chabot GG, et al. Irinotecan (CPT-11) high-dose escalation using intensive high-dose loperamide to control diarrhea. J Natl Cancer Inst. 1994;86:446–449. [DOI] [PubMed] [Google Scholar]
- 28.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. [DOI] [PubMed] [Google Scholar]
- 29.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 31.Fisher SB, Patel SH, Bagci P, et al. An analysis of human equilibrative nucleoside transporter-1, ribonucleoside reductase subunit M1, ribonucleoside reductase subunit M2, and excision repair cross-complementing gene-1 expression in patients with resected pancreas adenocarcinoma: implications for adjuvant treatment. Cancer. 2013;119:445–45332. [DOI] [PubMed] [Google Scholar]
- 32.Riely GJ, Kris MG, Zhao B, et al. Prospective assessment of discontinuation and reinitiation of erlotinib or gefitinib in patients with acquired resistance to erlotinib or gefitinib followed by the addition of everolimus. Clin Cancer Res. 2007;13:5150–5155. [DOI] [PubMed] [Google Scholar]


