Abstract
Objective
Chimney” techniques used to extend landing zones for endovascular aortic repair(chEVAR) have been increasingly reported; however, concerns about durability and patency remain. The purpose of this analysis was to examine mid-term outcomes of chEVAR.
Methods
All patients at the University of Florida treated with chEVAR were reviewed. Major adverse events(MAEs) were recorded and defined as any chimney stent thrombosis, type 1a endoleak in follow-up, reintervention, 30-day/in-hospital death and/or ≥ 25% decrease in estimated glomerular filtration rate after discharge. Primary end-points included chimney stent patency and freedom from MAE. Secondary end-points included complications and long-term survival.
Results
From 2008–2012, 41 patients[age ± standard deviation(SD); 73±8; male 66%(N=27)] were treated with a total of 76 chimney stents(renal, N=51; superior mesenteric artery, N=16 celiac artery, N=9) for a variety of indications: juxtarenal, 42%(N=17, 1 rupture); suprarenal, 17%(N=7), and thoracoabdominal aneurysm, 17%(N=7); aortic anastomotic pseudoaneurysm, 15%(N=6; 3 ruptures), type 1a endoleak after EVAR, 7%(N=3), and atheromatous disease, 2%(N=1). Two patients had a single target vessel abandoned due to cannulation failure and one had a type 1a endoleak at case completion(technical success = 93%). Intraoperative complications occurred in 7 patients(17%), including graft maldeployment with unplanned mesenteric chimney(N=2) and access vessel injury requiring repair(N=5). Major postoperative complications developed in 20%(N=8). 30-day and in-hospital mortality were 5%(N=2) and 7%(N=3), respectively.
At median follow-up of 18.2(range 1.4–41.5) months, 28 of 33(85%) patients with available postoperative imaging experienced stabilization or reduction of AAA sac diameters. Nine(32%) patients developed endoleak at some point during follow-up [type 1a, 7%(N=3); type 2, 10%(N=4); indeterminate, 7%(N=3)], and one patient underwent open, surgical conversion. The estimated probability of freedom from reintervention(±standard error mean) was 96±4% at both 1 and 3 years. Primary patency of all chimney stents was 88±5% and 85±5% at 1 and 3 years, respectively. Corresponding freedom from MAEs was 83±7% and 57±10% at 1 and 3 years. The 1 and 5-year actuarial estimated survival for all patients was 85±6% and 65±8%, respectively.
Conclusions
These results demonstrate that chEVAR can be completed with a high degree of success; however perioperative complications and MAEs during follow-up, including loss of chimney patency and endoleak may occur at a higher rate than previously reported. Elective use of chEVAR should be performed with caution and comparison to open and/or fenestrated EVAR is needed to determine long-term efficacy of this technique.
Introduction
Approximately 20–30% of patients are unsuitable anatomic candidates for standard endovascular aortic aneurysm repair(EVAR)1, 2. Within this subgroup, 50–60% of cases are ineligible for EVAR due to proximal aortic neck anatomy limitations2, 3. To overcome these challenges, a variety of endovascular procedures have emerged to extend proximal landing zones including custom fenestrated/branched grafts, surgeon-modified devices, as well as “chimney”, “periscope” and “sandwich” EVAR techniques. The chimney technique(chEVAR) was originally described as an adjunctive salvage procedure to treat unintentionally covered branch vessels4. However, multiple reports of short-term success have led to increasing enthusiasm for chEVAR, and these techniques are being used for primary treatment of juxtarenal, as well as suprarenal and thoracoabdominal aortic pathologies5–8.
Despite early success of the chEVAR procedure, many concerns about durability remain. The worldwide reported chEVAR experience is comprised of < 300 patients with < 400 target vessels with a mean follow-up of <11 months7–10. The limited published experience with this procedure restricts ability to determine guidelines for patient or anatomic selection criteria, as well as device choice, implantation technique and surveillance. Furthermore, the lack of prospective data comparing chEVAR to open aortic or fenestrated/branched repair make it difficult to define what role chEVAR should have in contemporary practice. Lastly, few data exist regarding major adverse events during follow-up (e.g. change in renal function, stent thrombosis, reintervention, mortality, etc.) after chEVAR and their clinical consequences.
The purpose of this analysis is to review our experience with chEVAR and report our mid-term outcomes.
Methods
Approval for this study was obtained from the University of Florida College of Medicine Institutional Review Board(#161-2012).
Database, definitions and subjects
A retrospective review of a prospectively maintained endovascular aortic registry was completed to analyze all chEVAR procedures performed at the University of Florida from January 2008 to December 2012. The “chimney” technique was defined as intentional deployment of a stent/stent-graft(s) into visceral aortic branch vessels immediately parallel to an aortic endoprosthesis that covered the target vessel ostia. “Sandwich”5 and “periscope”11 techniques were selectively used and reviewed in this analysis. Brachiocephalic or internal iliac artery chimney stents were excluded unless patients received a visceral aortic branch chimney stent. In these cases, the brachiocephalic or internal iliac artery stent was recorded as a procedural adjunct and not analyzed as a chimney stent. Similarly, patients undergoing fenestrated/branched repair were included only if they simultaneously received a visceral aortic chimney stent.
Comorbidities were defined and severity graded upon Society for Vascular Surgery(SVS) reporting guidelines(high-risk ≥ 8)12(Appendix Table 1). Stent patency, procedure related success, adjuncts, endoleaks and complication severity were defined using SVS reporting standards13. Stent patency was verified by contrast opacification on follow-up computed tomographic angiography(CTA) and/or with duplex ultrasound. Centerline measurements(Aquarius 3D; TeraRecon Inc, San Mateo, CA) were completed on all available pre/postoperative CT scans to discern aneurysm and stent morphology. Aneurysm sac diameters were considered stable if < 5mm of growth was measured on postoperative CT scans. Chimney stent compression(i.e. ‘kinking’) was documented if imaging demonstrated a ≥ 50% reduction in luminal cross-sectional area. Additional anatomic parameters that were measured included maximum aortic diameters and angulation. The “chimney” neck was measured similar to the method of Lee et al.14 to document the seal zone between the chimney stent and the aortic endoprosthesis.
Clinical practice
All patients received preoperative thin-cut(≤ 2mm) CTA(aortic arch to pelvis) and device planning was completed using a three-dimensional workstation(Aquarius 3D). Patient and device selection, as well as implantation technique were left to the surgeon’s discretion. In general, patients were offered chEVAR only if they were deemed high-risk for open surgery15 and had anatomic selection criteria as previously reported from our group7. Postoperative surveillance consisted of CTA at 1-month, 6-months and annually, thereafter. Excluding patients with documented allergy, dual antiplatelet therapy was started postoperatively and consisted of clopidogrel(75mg/day) and aspirin(81mg/day) for at least 1-month, followed by aspirin 81mg/day, indefinitely. Routine documentation of blood pressure and anti-hypertensive regimen occurred with each follow-up clinic visit. Serum creatinine levels were checked daily as an inpatient and obtained concurrently with each follow-up CT. If patients had renal insufficiency(i.e. eGFR<50 mL/min/1.73m2), routine surveillance consisted of chimney stent duplex and non-contrasted CT.
Timing and need for reintervention were left to the operative surgeon’s decision. All Type 1a and III endoleaks were considered significant and warranted therapy unless there were compelling medical or anatomic factors limiting remedial options. Typically, persistent type II endoleak(≥ 6-months after chEVAR) associated with ≥ 5mm of aneurysm diameter increase prompted reintervention. Although no formal protocol for reintervention existed, additional findings that frequently prompted reintervention included chimney stent luminal cross-sectional area reduction ≥ 50% on CT and/or visceral/renal duplex peak systolic velocity ≥ 300cm/s with concurrent eGFR decrease ≥ 25% from the value at discharge.
Chimney EVAR technique
A majority of patients were repaired in a hybrid operating room using a fixed imaging system under general anesthesia. Percutaneous bilateral femoral and left brachial access was completed as previously described for procedures requiring a single chimney stent16. If more than one vessel required chimney stent placement, bilateral percutaneous brachial access or an open axillary conduit was employed. In most cases, a 7 or 8Fr 90-cm sheath was placed over a 0.035 inch Rosen wire(Cook Medical, Inc, Bloomington, Ind) after successful target vessel catheterization. If multiple vessels were cannulated, individual visceral vessel sheath access was achieved prior to aortic endograft deployment. A 20mm overlap between the aortic and chimney stents was planned in a majority of cases. Once the aortic endoprosthesis was deployed (oversized ≥ 20% to outer diameter of seal zone), the chimney stent(s)(iCAST, Atrium Medical, Hudson, NH; Viabahn, W.L. Gore & Associates, Flagstaff, AZ; Zilver, Cook Medical, Inc, Bloomington, Ind) were deployed. Additional stents were placed as needed to ensure that the chimney extended 5–10 mm above the aortic stent.
As a final step, a simultaneous “kissing” balloon technique, with an aortic balloon and branch vessel balloon(s), was used to mold the proximal seal zone. If completion angiography demonstrated a type 1a endoleak, repeat ballooning was performed. In selected cases, a self-expanding stent was placed within the chimney stent and extended into the native vessel to ease the transition between the rigid stent-graft and compliant target vessel to prevent kinking. Finally, selective use of intravascular ultrasound occurred to verify chimney stent expansion and/or relative position to the top of the aortic endoprosthesis.
End-points, definitions and statistics
Primary end-points included chimney stent patency and freedom from major adverse events(MAEs). Secondary end-points included complications and long-term survival. MAEs were defined as a composite end-point that included any chimney stent thrombosis, type 1a endoleak in follow-up, reintervention, 30-day/in-hospital death and/or ≥ 25% decrease in estimated glomerular filtration rate after discharge.
Estimated glomerular filtration rate(eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration(CKD-EPI) formula17. Acute kidney injury(AKI) and acute renal failure(ARF) were based on the RIFLE(Risk, Injury, Failure, Loss and End-stage kidney disease) criteria18. Early changes in renal function were calculated by comparing the preoperative laboratory value to that obtained on date of discharge. Midterm renal function was determined by comparing the discharge value and the most recent available laboratory value.
All analysis was completed using the R statistical software package(Vienna, Austria; V.2.15.0). Differences in renal function were calculated using the non-parametric Kruskal-Wallis and exact Wilcoxon scores tests. Continuous variables were analyzed using a Student t test or Wilcoxon Mann-Whitney test, and categorical variables were compared with a Fisher’s exact test, when indicated. Long-term survival, patency, reintervention, and freedom from MAEs were estimated using Kaplan-Meier curves and differences determined with a log-rank test. A P-value < .05 was considered significant.
Results
Patient cohort
From 2008–2012, 1223 patients were treated with either open(N=376) or endovascular(N=847) surgery for abdominal and thoracoabdominal aortic disease. Standard EVAR for infrarenal aortic aneurysm was completed in 291 patients while 442 patients underwent TEVAR for a variety of indications. During the study interval, 73 patients received fenestrated endograft repair which began being offered in 2010. A total of 41 patients underwent chEVAR which comprised 5% of all endovascular repairs. These patients received 76 chimney stents(renal, N=51; superior mesenteric, N=16; celiac, N=9). The demographics and comorbidities are outlined in Table 1.
Table 1.
Patient characteristics and comorbidities
| Feature | N = 41(%) |
|---|---|
| Age, mean ± SD, years | 73±8 |
| Male | 27 (66%) |
| BMI | 26±7 |
| Comorbidities | No. (%) |
| Hypertension | 36 (88) |
| Dyslipidemia | 31 (78) |
| Coronary artery disease | 26 (63) |
| Smoking | 23 (56) |
| Chronic obstructive pulmonary disease | 21 (51) |
| Congestive heart failure | 13 (32) |
| Diabetes | 7 (17) |
| Renal insufficiencyb | 8 (20) |
| CVOD | 6 (15) |
| PVOD | 3 (7) |
| Composite Total, mean ± SD | 3.5±1.6 |
| SVS Comorbidity score, median | 7 (IQR 6–9) |
SD, standard deviation; BMI, body mass index; CVOD, cerebrovascular occlusive disease; PVOD, peripheral vascular occlusive disease.
Renal insuffiency = estimated glomerular filtration < 50 mL/min/1.73m2; SVS, Society for Vascular Surgery Comorbidity score (≥8 = ‘high-risk’); IQR, interquartile range
Preoperative characteristics
Preoperative aneurysm diameters, indications, and mode of presentation are highlighted in Table 2. Fourteen patients (34%) had a history of prior endovascular or open aortic surgery. Repair was elective in 30(73%) cases while three patients presented with ruptured pathology(juxtarenal aneurysm-1; post-surgical pseudoaneurysm-2). Details of the implanted chimney stents are demonstrated in Table 3. The most frequent configuration consisted of either bilateral renal chimneys(N=13;32%) or a single renal along with one or more mesenteric chimney(s)(N=10; 24%). A variety of aortic endograft and chimney stent combinations were used(Appendix Table 2), with the most prevalent being a Cook Zenith bifurcated endograft with iCAST stents(N=10;24%).
Table 2.
Preoperative anatomy, prior aortic related procedures, and clinical presentation
| Feature | N = 41(%) |
|---|---|
| AAA diameter (cm±SD) | 6.5±1.2 |
| Previous open aneurysm repair | 6 (15) |
| Prior EVAR | 4 (10) |
| Prior TEVAR | 4 (10) |
| Anatomic indication | No. (%) |
| Juxtarenal AAA | 17 (42) |
| Suprarenal AAA | 7 (17) |
| Post-surgical pseudoaneurysm | 6 (15) |
| Type 1a endoleak after EVAR | 3 (7) |
| Thoracic aneurysm | 3 (7) |
| Type IV TAAA | 2 (5) |
| Type II TAAA | 1 (5) |
| Aortic dissection with aneurysm | 1 (2) |
| Atheromatous disease with embolization | 1 (2) |
| Presentation | No. (%) |
| ASA 4 | 37 (90) |
| Urgent/symptomatic | 8 (20) |
| Emergent/rupture | 3 (7) |
AAA, abdominal aortic aneurysm; EVAR, endovascular abdominal aortic aneurysm repair; TEVAR, thoracic endovascular aortic repair; TAAA = Crawford Extent thoracoabdominal aneurysm; ASA, American Society of Anesthesia
Table 3.
Target vessels and chimney stent combinations
| Feature | No. |
|---|---|
| Total number chimney stents implanted | 76 |
| Visceral vessels originally targeted | 75 |
| Unplanned chimney | 3 |
| Technically unable to cannulate | 2 |
| Right renal artery | 26 |
| Left renal artery | 25 |
| Superior mesenteric artery | 16 |
| Celiac artery | 9 |
| Chimney combinations | N = 41 (%) |
| Bilateral renal | 13 (32) |
| Single renal + SMA and/or celiac | 10 (24) |
| Single renal | 7 (17) |
| Single celiac | 5 (12) |
| Bilateral renal + SMA and/or celiac | 4 (10) |
| Single SMA | 2 (5) |
SMA, superior mesenteric artery
Perioperative details
A majority(N=31;78%) of patients had either access vessel and/or intraprocedural adjuncts employed to facilitate repair. Table 4 details the various adjuncts and procedure related variables. Two patients had a single target vessel abandoned due to cannulation failure and one had type 1a endoleak at case completion (technical success = 93%). The type 1a endoleak failed to resolve after repeat balloon angioplasty, but was not detected on 1-month follow-up CTA. Intraoperative complications occurred in 7(17%) cases including: graft maldeployment with unplanned mesenteric or renal chimney(N=2), and access vessel injury requiring repair(N=5). Two unplanned chimney stents were used in elective cases of juxtarenal AAA managed initially with a Cook Zenith bifurcated endograft and bilateral renal chimney iCAST stent-graft configuration. The most common access vessel injury was related to brachial artery thrombosis(N=3) requiring thrombectomy. One patient suffered axillary artery avulsion requiring axillo-brachial bypass. The remaining complication was a femoral pseudoaneurysm after percutaneous access that underwent repair.
Table 4.
Perioperative adjuncts and procedure related variables
| Feature (N = 41) | No. (%) |
|---|---|
| Access vessel adjunct* | |
| Axillary conduit | 9 (22) |
| Open iliac conduit | 5 (12) |
| Endovascular iliac conduit | 1 (2) |
| Iliac angioplasty/stent | 3 (7) |
| Femoral endarterectomy | 3 (7) |
| Iliac occlusion plug | 2 (5) |
| Hypogastric bypass | 1 (2) |
| Brachial-brachial bypass | 1 |
| Femorofemoral bypass | 1 |
| Common femoral interposition graft | 1 |
| Intraprocedural adjunct | |
| Aortic cuff | 9 (22) |
| Simultaneous TEVAR/EVAR | 3 (7) |
| Visceral vessel fenestration | 2 (5) |
| Graft modification (removal stent ring) | 2 (5) |
| Renal artery embolization | 2 (5) |
| Hypogastric embolization | 1 (2) |
| Subclavian artery embolization | 1 |
| Visceral stent (non-chimney) | 1 |
| Brachiocephalic chimney | 1 |
| Diameter reducing wires | 1 |
| Atrial inflow balloon occlusion | 1 |
| Preoperative carotid-subclavian bypass | 1 |
| Infrainguinal vessel embolectomy | 1 |
| Procedure variable | mean ± SD or No.(%) |
| Cerebrospinal fluid drain | 7 (17) |
| Procedure time, min | 269±123 |
| Flouroscopy time, min | 88±56 |
| Contrast exposure, mL | 163±72 |
| Estimated blood loss, mL | 456±376 |
| Packed red blood cell transfusion, units | 1.4±2.4 |
| Primary technical success | 38 (93) |
| Intraprocedural complication | 7 (17) |
| Completion angiogram Type 1a endoleak | 1 (2) |
Multiple adjuncts were used in individual patients
Postoperative outcomes
Major postoperative complications developed in 20%(N=8) of patients. The 30-day and in-hospital mortality were 5%(N=2) and 7%(N=3), respectively. Two patients experienced stroke(5%) and one of these patients died in-hospital due to respiratory failure followed by a fatal malignant arrhythmia on postoperative day 10. The second stroke patient had significant aphasia and a lateralizing motor deficit. This patient ultimately recovered at time of most recent follow-up, however they had a persistent speech impediment. The most common complication after chEVAR was secondary to impaired renal function(exclusive of patients who underwent intentional renal coverage) and was observed in 20%(N=8) of cases. Two(5%) patients had a new postoperative requirement for hemodialysis. These two patients had thoracoabdominal aneurysms that were managed electively with multiple telescoping chimney stents within a “sandwich” aortic stent graft configuration5 to the mesenteric and renal vessels. Postoperatively, one of the subjects developed colonic ischemia and subsequent multi-organ failure that resulted in death on postoperative day 12. The second patient developed pelvic and lower extremity atheroembolization and respiratory failure. The patient survived hospitalization but has remained on hemodialysis. The remaining tabulation of postoperative outcomes is outlined in Table 5.
Table 5.
Perioperative outcomes of chimney EVAR
| Feature, No. (%) | N = 41 |
|---|---|
| Intraoperative death | 0 |
| 30-day death | 2 (5) |
| In-hospital death | 3 (7) |
| Unplanned reoperation | 2 (5) |
| Any complication* | 20 (49) |
| Any major complication | 8 (20) |
| Renal complication (any)Ϯ | 8 (20) |
| eGFR decrease ≥ 25% | 6 (15) |
| New need for hemodialysis | 2 (5) |
| Respiratory failure/pneumonia | 5 (12) |
| Gastrointestinal | 3 (7) |
| Cardiac | 3 (7) |
| Stroke/TIA | 2 (5) |
| Spinal cord ischemia | 1 (2) |
| Disposition | |
| Home | 26 (63) |
| Inpatient facility | 12 (29) |
Multiple complications occurred 9 (21.9%) patients;
Acute kidney injury was defined based on the RIFLE criteria18; TIA, transient ischemic attack
Follow-up and mid-term outcomes
At median follow-up of 18.2(range 1.4–41.5) months, 28 of 33(85%) patients with available postoperative imaging experienced stabilization or reduction of AAA sac diameters. Nine (32%) patients developed endoleak at some point during follow-up [late (>30 day) type 1a, 7%(N=3); type 2, 10%(N=4); indeterminate, 7%(N=3)]. One patient(2%) underwent open, surgical conversion that was initially treated for dissection-related suprarenal aneurysm and ultimately determined to have Marfan syndrome. Additional postoperative anatomical measurements of the chEVAR patients are highlighted in the Appendix Table 3.
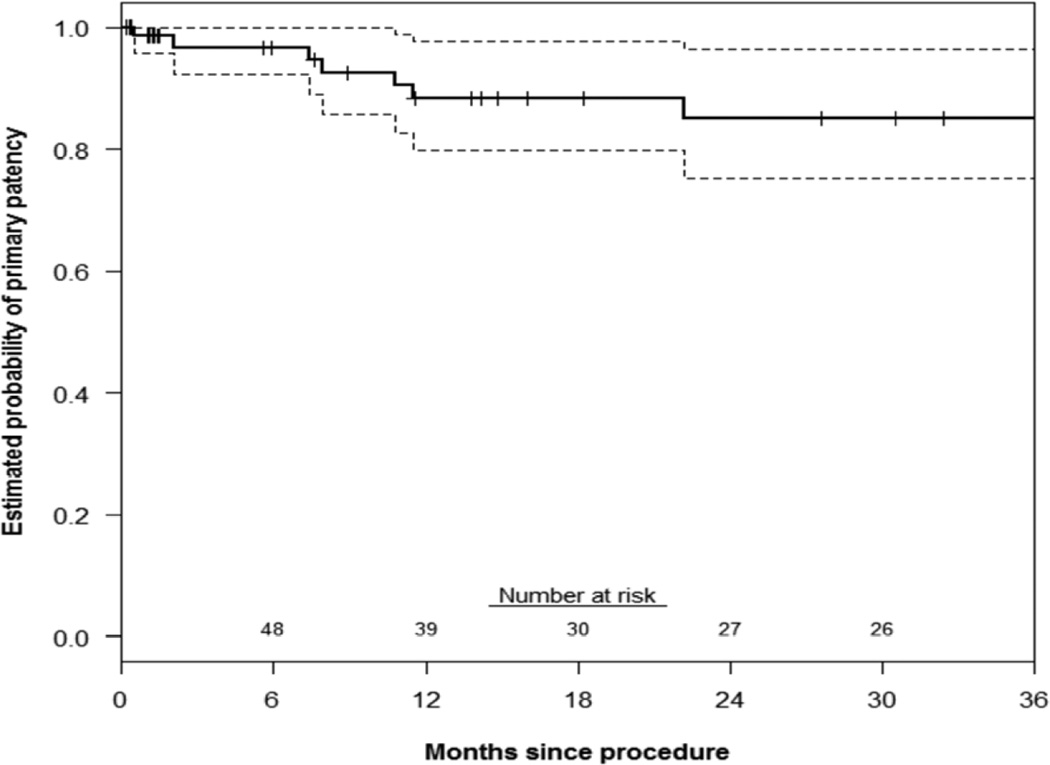
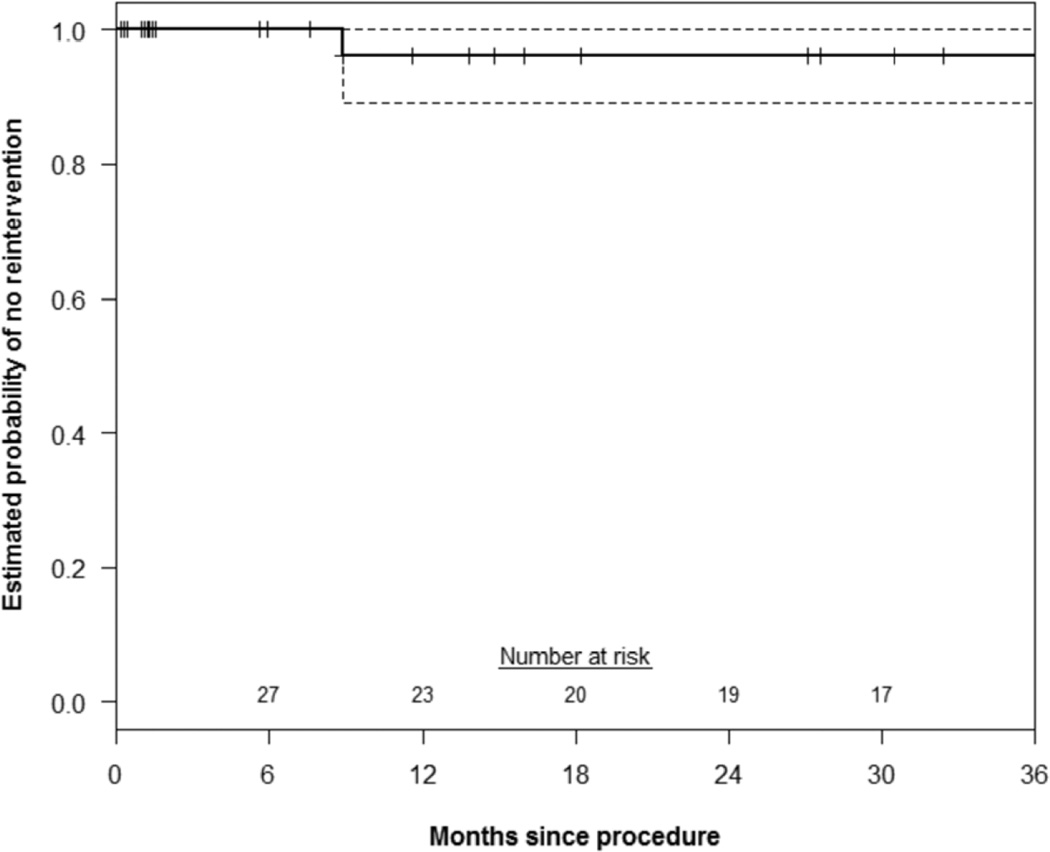
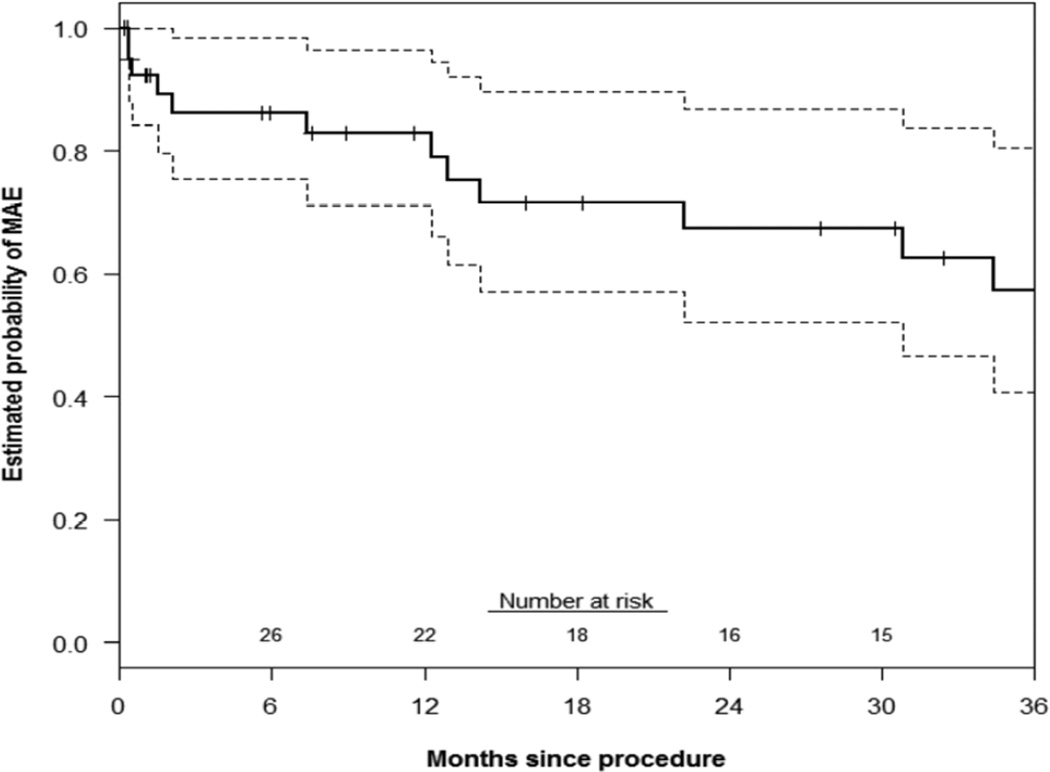
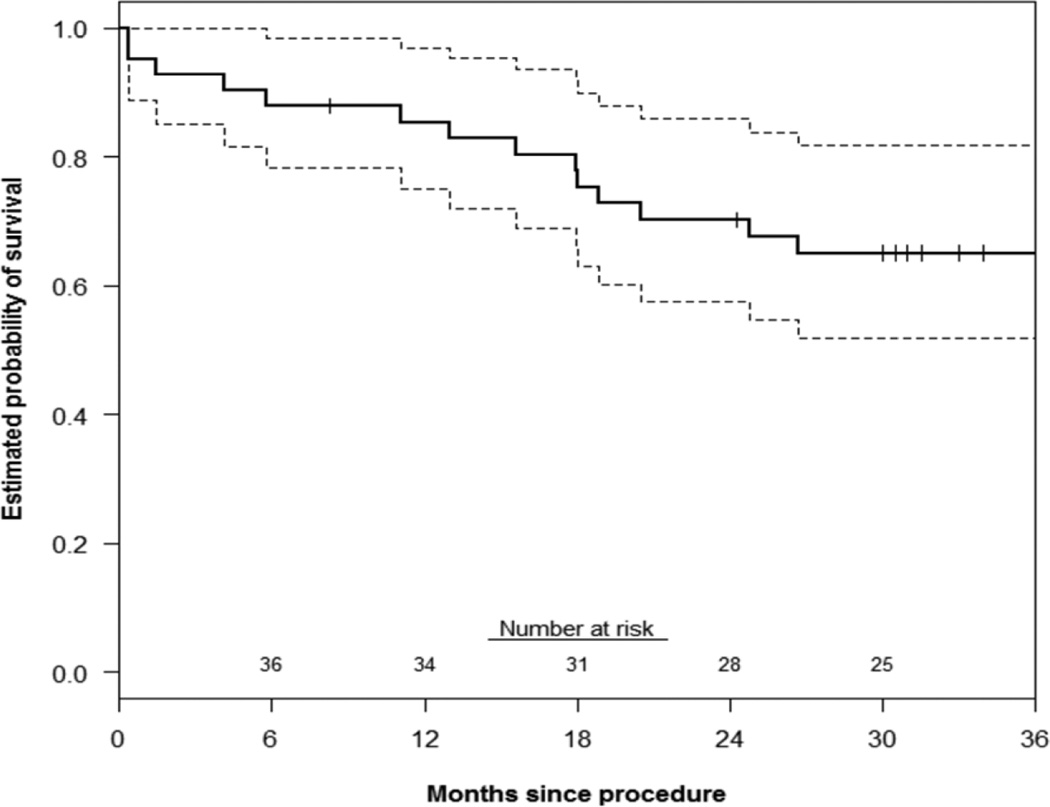
Primary patency of all chimney stents was 88±5% and 85±5% at 1 and 3 years, respectively(Figure 1). There was no significant difference in renal or mesenteric chimney stent patency(log-rank P=.84). The estimated probability of freedom(±standard error mean) from reintervention was 96±4% at both 1 and 3 years(Figure 2). Corresponding freedom from MAE was 83±7% and 57±10% at 1 and 3 years(Figure 3). Details of the MAEs are cataloged in Table 6. Of note, the three patients who experienced ≥ 25% decrease in eGFR all had normal preoperative renal function and no intentional renal vessel coverage. The 1 and 5-year actuarial estimated survival for all patients was 85±6% and 65±8%, respectively (Figure 4).
Figure 1.
This Kaplan-Meier curve demonstrates the estimated probability of primary stent patency up to 3 years (n=76 stents). Overall, 9 stents -- 1 celiac, 6 renal, and 2 SMA – occluded. Seven of those occlusions (9%) occurred within three years. Accounting for patients with less than 3 years of follow-up, the estimated probability of primary patency at 3 years is 85% (95% CI: 75–95%). The standard error of the mean is <10% at all displayed intervals.
Figure 2.
This Kaplan-Meier curve demonstrates the estimated probability of freedom from re-intervention after chimney EVAR up to 3 years. Overall, 3 of 41 patients experienced the need for reintervention. Only 1 of those reinterventions occurred within 3 years. Accounting for patients with less than 3 years of follow-up, the KM estimated probability of freedom from reintervention at 3 years is 96% (95% CI: 88–99%). The standard error of the mean is <10% at all displayed intervals.
Figure 3.
This Kaplan-Meier curve demonstrates the estimated probability of freedom from major adverse events (MAEs) up to three years after ChEVAR. Overall, 13 of 41 patients suffered an MAE, which included either chimney stent thrombosis, type 1a endoleak in follow-up, re-intervention, 30-day/in-hospital death, and/or ≥ 25% decrease in estimated glomerular filtration rate after discharge. Twelve of these MAEs occurred within 3 years. Accounting for patients with less than 3 years of follow-up, the KM estimated probability of freedom from MAE at 3 years is 57% (95% CI: 37–77%). The standard error of the mean is <10% at all displayed intervals.
Table 6.
Description of major adverse events after chimney EVAR
| Patient | Original indication | MAE Description | Time(m) | Outcome |
|---|---|---|---|---|
| 1 | Type 1a endoleak | ≥25% ↓ eGFR | 12.3 | No dialysis |
| 2 | Juxtarenal AAA | ≥25% ↓ eGFR | 12.9 | No dialysis |
| 3 | Suprarenal AAA | 30-death | 0.4 | Death |
| 4 | Juxtarenal AAA | ≥25% ↓ eGFR | 30.8 | No dialysis |
| 5 | Type 1a endoleak | Type 1a endoleak | 34.4 | Pending revision |
| 6 | Type 1a endoleak | SMA chimney thrombosis | 7.5 | Asymptomatic |
| 7 | Juxtarenal AAA | In-hospital death | 1.5 | Death |
| 8 | Juxtarenal AAA | Bilateral renal chimney thrombosis | 40.4 | Dialysis, renal bypass |
| 9 | Juxtarenal AAA | Bilateral renal chimney thrombosis | 2.1, 10.8 | Dialysis |
| 10 | Pseudoaneurysm | Celiac stent thrombosis | 22.2 | Celiac bypass |
| 11 | Dissection with aneurysm | Type 1a endoleak | 14.2 | Conversion |
| 12 | Pseudoaneurysm | L renal chimney thrombosis | 0.5 | No dialysis |
| 13 | Suprarenal AAA | L renal chimney thrombosis | 11.5 | No dialysis |
AAA, abdominal aortic aneurysm; eGFR, estimated glomerular filtration rate was calculated using the CKD-EPI formula17; SMA, superior mesenteric artery
Figure 4.
This Kaplan-Meier curve demonstrates the estimated probability of survival up to 3 years after ChEVAR. At three years, 58% (95% CI: 39–74%) of patients were alive after repair which is consistent with published reports of open aortic repair. The standard error of the mean is <10% at all displayed intervals.
Discussion
The results of this analysis confirm that chEVAR can be applied to a variety of clinical situations with a high-degree of technical success. Surgical conversion is rare and a majority of patients experience aneurysm sac stabilization or regression. However, perioperative complications, including access vessel injury and postoperative renal insufficiency are common. More importantly, with increasing follow-up time, chimney stent thrombosis and/or decline in renal function after discharge may occur at a higher rate than previously reported.
The original description of chEVAR was reported as a ‘bail out’ maneuver when inadvertent renal artery coverage occurred during EVAR4. However, this technique has evolved into a primary treatment strategy for a variety of indications5, 8, 19. Enthusiasm for chEVAR has been further engendered by reports describing high technical success and excellent short-term outcomes6, 9, 14. Additionally, alternative strategies such as fenestrated-branched techniques have a number of potential issues including increased cost, long wait times for customization, and a need for complex endovascular skills to implant and/or modify devices, as well as requirement for industry oversight and training. In contrast, chEVAR is a readily available technique that can be completed using endovascular skills familiar to most practicing vascular surgeons and applied to elective and emergent settings. These potential advantages of chEVAR compared to other methods of juxtarenal or paravisceral aortic disease management are likely primary drivers of the rapid adoption in many centers, and increasing reports in the literature.
To date, descriptions of chEVAR have been overwhelmingly optimistic with multiple groups describing excellent technical success(≥95%) and short-term chimney patency rates(≥90%)8, 19. Further, aneurysm sac stabilization/regression is documented in more than 90% of cases8, 19. The results in the existing literature include a report from our own group, with similar findings in early follow-up7. Despite the generally positive tone of most publications, several series have reported postoperative complication rates ranging from 0–35%8, 20–22. Eight(20%) patients in this study experienced postoperative complications. If intraoperative complications(N=7;17%) are tabulated with the postoperative complications, the major morbidity rate was 24%(N=10; some experienced intra-and postoperative complications). This rate is comparable or even exceeds morbidity of fenestrated/branched or open repair of juxtarenal aneurysm disease23–26. Katsargyris and colleagues documented that postoperative renal injury occurred on average in 18.5%, 9.8%, and 12% of juxtarenal aneurysm cases following open surgery, fenestrated EVAR, and chEVAR, respectively27 which is consistent with our experience.
Notably, two patients(5%) had documented stroke in our series, including one fatal stroke, which represents a risk somewhat unique to chEVAR due to the required manipulation of brachiocephalic vessels to complete the procedure. Other investigators have also reported that the requisite brachial access and arch manipulation during chEVAR increases stroke risk(3.2%) compared to fenestrated(0.3%) or open juxtarenal aneurysm repair(0.1%)(P=.01)27.
Furthermore, type 1a endoleak after chEVAR occurs at a significantly higher rate than fenestrated repair(4.3%vs.10%, P=.002)27. Interestingly, this complication occurred in only three patients(7%) in our experience. This lower rate may in part be explained by our consistent practice of achieving at least 20mm of proximal seal. However, this strategy resulted in ≥ 2 chimneys being required in 66%(N=27) of cases and may have contributed to the observed elevated rate of late chimney stent thrombosis. Although our type 1a endoleak rate was low, the consequences of this problem cannot be overstated. One patient underwent conversion and the other patients were pending reintervention at the time of manuscript preparation. The cause of type 1a endoleak in chEVAR is attributed to the so-called “gutters” around the chimney grafts which reflect the degree of conformational change the aortic endograft undergoes to accommodate the interposition of the chimney stent between the aortic endograft and native aorta. Some have reported less gutter issues depending on chimney device selection(self-expanding vs. balloon expandable stent-graft), however greater patient numbers are needed to determine if there are significant differences28.
The cumulative reported chEVAR experience has mean follow-up of only 10.7±1 months7, 9, 14, 20, 29 and a number of the MAEs in this series occurred beyond that time period. Three patients who had documented stent thrombosis events received a Cook Zenith + Atrium iCAST configuration, however the other 3 cases had different device combinations. Notably, half of these failures occurred beyond 11.5 months, and had no obvious radiographic signs of impending failure on surveillance CT imaging. Interestingly, more than 10 different device combinations were used to treat 9 different pathologies(Appendix Table 2) in our experience. This variability could be criticized as a weakness in our chEVAR strategy; however, a majority of these choices have been reported and operative planning/device selection was predicated upon device and patient specific anatomic constraints7, 8, 20.
The chimney stent can lead to deformation and alteration of branch vessel anatomy, potentially impacting end organ perfusion. This notion is illustrated by the 3 patients in our series who experienced a ≥ 25% eGFR decline after discharge. These patients had normal preoperative renal function and no intentional/unintentional renal abandonment occurred during their procedures. Gradual renal function decline, while a previously reported long-term outcome in open juxtarenal26, 30 and fenestrated endograft31 repair, occurred between 12.3–30.8 months postoperatively for the chEVAR patients in this series.
The relationship of the main aortic device to the chimney stent, and variability in mechanical properties that exist between the available devices underscores the problem with adopting these procedures without rigorous comparative effectiveness data. The device-device and device-aortic interactions are difficult to predict since they depend on numerous factors, including intrinsic device characteristics, stent oversizing32, aortic wall quality, as well as the angulation, calcification and thrombus burden of the aorta and its branches. The outward radial force exerted on the chimney stent by the aortic device, and likelihood of chimney compression and/or target vessel axis deviation is unpredictable, particularly given the various device combinations and dynamic physiologic environment of the aorta. To counteract the extrinsic compression on the chimney stent, some authors recommend placing a second self-expanding stent within a chimney stent-graft; however the effectiveness of this practice is unknown.
Several additional challenges exist when trying to understand optimal patient selection for chEVAR. A variety of ‘high-surgical risk’ definitions have been reported, and most commonly are documented as a clinical impression of the patient’s cardiologist and/or surgeon6, 14. This selection bias is difficult to account for due to study heterogeneity and imprecise comorbidity severity grading. In our group’s experience, we use a combination of the SVS comorbidity scoring system12 and preoperative prediction of 1-year mortality with aneurysm repair33 to facilitate decision-making regarding which patients to offer therapy. Indeed, this bias is reflected in the fact that > 90% of the patients in this series were ASA 4, however long-term survival was consistent with published outcomes for surgical management of abdominal aortic disease30, 34.
In addition to our own chEVAR outcomes, we have had increasing referrals for chEVAR failure leading to open conversion35, further tempering our enthusiasm for these procedures. Our current practice is to avoid elective use of chEVAR, and these techniques are generally reserved for salvage of inadvertent renal coverage during EVAR or for emergent procedures in prohibitively high surgical risk patients who are too unstable to await device modification for surgeon-modified repair15. When chEVAR is used, we adhere to the principles that the proximal landing zone should be radiographically normal aorta, and optimally there should be at least 20mm of overlap between the aortic graft and chimney stent(s). Ideally, the target vessel should have a downward angle to the aortic centerline of flow, and self-expanding stents are routinely used to reinforce the chimney stent-graft as well as to ease transition into the distal vessel if vessel kinking or tortuosity is encountered. Additionally, we have adopted a more aggressive imaging surveillance protocol which includes CTA at 1-month, 3-months and every 6-months thereafter.
This study has several important limitations. The intrinsic limitations of a small sample size and single-center, retrospective nature of the analysis makes Type 2 error very possible. No standardized treatment algorithm was present over the study period and the selection bias for which patients receiving open or fenestrated/branched EVAR vs. chEVAR undoubtedly influenced application of the technique. Use of a composite end-point, particularly inclusion of renal function change after discharge, could be criticized since this is not a routinely reported outcome in most EVAR/TEVAR trials. The decision to add this endpoint was based on the evolving literature surrounding the impact of renal function decline on long-term mortality after endovascular or open aortic repair36, 37. The lack of standardized utilization of postoperative duplex ultrasound imaging may have led to under appreciation of in-stent stenosis which may have been linked to chimney thrombosis. However, currently there are neither universally accepted validated duplex criteria for renal/mesenteric stent surveillance nor a threshold that warrants prophylactic reintervention without any pre-existing temporal change in renal function. No case controls of open or fenestrated EVAR patients were presented to provide insight into the outcomes of these procedures in our practice. Because our selection bias evolved during the study period, especially after 2010 when fenestrated repair became the preferred approach, we felt the patient populations were so different that it would be difficult to draw meaningful conclusions from this type of analysis.
Another potential limitation is that we included chimney, periscope (N = 2) and sandwich stent-grafts (N = 4) for analysis. Two adverse events occurred in the periscope/sandwich patients and the decision to include these patients was based on the concept that blood flow is primarily a pressure driven phenomenon. The direction of the stented visceral vessel would not be expected to differentially impact the flow dynamics through the stent. Instead, the hemodynamic differences between the various ‘chimney’ techniques would be related to inertial components of the blood which could lead to flow perturbations through the bent portion of the ‘chimney’ stent. In general, inertial components are much less significant than the hemodynamic elements of flow and therefore primarily contribute to second order hemodynamic effects, the impacts of which are beyond the scope of this manuscript. However, frictional forces between the chimney/periscope and sandwich techniques may be dissimilar, potentially leading to differential risk of stent deformation.
In conclusion, this study demonstrated that chEVAR is a technique that can be completed with a high degree of technical success and acceptable perioperative mortality, however; morbidity is significant. At mid-term follow-up, an increasing rate of MAEs, including stent thrombosis and reintervention was observed. Due to the rate of perioperative complications, a lack of high-quality comparative effectiveness studies, and the collectively short-term follow-up available in the literature, we feel that chEVAR should not be used routinely in the elective setting, but reserved for patients who are prohibitively high-risk for an open operation, and/or who are not candidates for other forms of treatment such as fenestrated/branched grafts.
Appendix
Appendix Table 1.
Comorbidity abbreviations and definitions
| Comorbidity (abbreviation) | Definition |
|---|---|
| Hypertension (HTN) | chart history, any antihypertensive drug |
| Coronary artery disease (CAD) | chart history, angina, coronary artery bypass, percutaneous coronary angioplasty |
| Chronic obstructive pulmonary disease (COPD) | chart history, smoking history >20 pack/years, abnormal pulmonary function tests, medication |
| Smoking | current, prior if > 10 pack years |
| Diabetes (DM) | oral hypoglycemics, insulin |
| Congestive heart failure (CHF) | New York Heart Association Class II or greater |
| Chronic renal insufficiency | creatinine ≥1.5 mg/dL, estimated glomerular filtration rate <50 mL/min/1.73m2 or dialysis dependence13 |
| Dyslipidemia | chart history or medication |
| Cerebrovascular occlusive disease (CVOD) | transient ischemic attack, stroke, carotid endarterectomy, angioplasty |
| Peripheral vascular occlusive disease (PVOD) | ankle brachial index < 0.9, prior peripheral arterial open or endovascular intervention |
Appendix Table 2.
Anatomic indications and strategy for aortic endograft-chimney stent combinations
| Juxtarenal aneurysm | N = 17 |
| Cook Zenith + iCast | 5 |
| Cook Zenith + Zilver | 3 |
| Cook Zenith + cuff + iCast | 3 |
| Endologix + cuff + iCast | 2 |
| Cook Zenith + cuff + Viabahn/iCast | 1 |
| Gore EVAR + Viabahn | 1 |
| Cook Zenith + Talent cuff + Zilver | 1 |
| Endologix + TX2 + iCast/Zilver | 1 |
| Suprarenal aneurysm | N = 7 |
| Cook Zenith + iCast | 3 |
| Cook Zenith + Renu cuff + iCast | 1 |
| Cook Zenith + TX2 + iCast | 1 |
| Cook Zenith + TX2 + iCast/Viabahn | 1 |
| Cook Zenith + cuff + iCast/Viabahn | 1 |
| Post-surgical pseudoaneurysm | N = 6 |
| Cook Zenith + iCast | 2 |
| Talent + TX2 + iCast | 1 |
| Cook Zenith + TX2 + iCast | 1 |
| TAG + TX2 + Zilver | 1 |
| Renu + Zilver | 1 |
| Type 1a endoleak after EVAR | N = 3 |
| TX2 + iCast | 2 |
| Cook Zenith AUI + Zilver | 1 |
| Thoracic aneurysm | N = 3 |
| TX2 + Zilver | 2 |
| Medtronic Captiva + Viabahn | 1 |
| Type IV TAAA | N = 2 |
| Zenith + TX2 + Viabahn | |
| Type II TAAA | N = 1 |
| Zenith + TX2 + iCast/Viabahn | |
| Aortic dissection with aneurysm | N = 1 |
| Zenith AUI + TX2 + iCast | |
| Atheromatous disease | N = 1 |
| Gore EVAR + TAG + Zilver |
Appendix Table 3.
Postoperative anatomical measurements after chEVAR
| Feature (N = 76 chimney stents in 41 patients) | No.(%) |
|---|---|
| Available postoperative CTA | 33 (81%) |
| Preoperative aortic aneurysm diameter (±SD) | 65±12mm |
| Average postoperative change in maximal aneurysm diameter | −6.0mm |
| Aortic diameter at the celiac artery | 26.3mm |
| Aortic diameter at the SMA | 26.8mm |
| Aortic diameter at the lowest renal | 30.8mm |
| Achieved “Chimney” seal zone | 25.5mm |
| Stent compression ≥ 50% on postoperative CTA | 25 (33%) |
| Any chimney stent thrombosis | 8 (11%) |
| Type 1a endoleak on follow-up CTA | 3 (7%) |
Scientific Session I - Thursday, January 16, 2014
Discussant: Timothy M. Sullivan, MD (Minneapolis, MN)
Critical Analysis of the Mid-Term Results After Chimney EVAR: Cause for Concern
Paper Presented by: Dr. Salvatore Scali (Gainesville, FL) Salvatore.scali@surgery.ufl.edu
Discussion
The concept of chEVAR is one that made little sense to me from its inception, given the Euclidean realities of placing two cylinders within another, creating ‘gutters’ which thrombose, creating a ‘pseudo-seal’ at the proximal aneurysm neck. We seem to have conveniently forgotten past lessons, namely that this semi-liquid thrombus is able to transmit pressure to the aneurysm sac. Based on careful examination of your results, you have appropriately recommended chEVAR solely for urgent situations; this careful analysis and introspection remains an important hallmark of our specialty.
Schiro, in Annals of Vascular Surgery reports a high incidence of sac enlargement and rupture, even in patients whose aneurysms were initially excluded. Several Type I endoleaks developed late. Given these data, should patients having chEVAR be followed more aggressively? When discovered, how should ‘gutter’ endoleaks be treated?
You report a decline in kidney function in 15% of patients, presumably in part related to partial chimney collapse. Stent placement within the chimney may alleviate this issue, but a perfectly cylindrical chimney theoretically creates larger gutters. What are your thoughts regarding the proper balance?
‘Gutter’ endoleaks also occur in patients treated with snorkels to the internal iliac arteries in patients with aortoiliac aneurysms, but seem to be more benign. Can you speculate on this apparent difference?
Response
Thank you, Dr. Sullivan. To your first question regarding the surveillance protocol, I think one of the most disconcerting things that we found in this analysis was that as we reviewed all available postoperative imaging, even for patients that subsequently went on to experience stent thrombosis, there weren’t always clues on the postoperative CT scans that demonstrated architectural changes to the chimney stent that may tip you off to the potential of impending failure. So with respect to the question, should these patients have more rigorous surveillance? The short answer is that I am not certain that our data would strongly support that recommendation since several failures occurred without clinical or radiographic signs, however due to our concerns about the relatively unpredictable nature of the chimney to aortic endograft and native aorta interaction, we would recommend imaging at 1, 3, and every 6-months, thereafter for the first 24 months and then probably tailor the intervals based on impressions of stent architecture and aneurysm sac involution.
The bigger issue though is the second part of your first question regarding how to manage a gutter leak, resulting in a persistent type Ia endoleak with sac enlargement. Some authors recommend that you extend the repair proximally and get more overlap, however this can be more challenging than it sounds and may require additional chimney lengthening and/or visceral stenting. Notably, we had a relatively low incidence of type Ia endoleak in our series compared to what is reported in the literature which is probably related to the 25 millimeter average seal that was achieved with the repair which may have consequently led to elevated rates of chimney stent failure due to the increased overlap. With that being said, the impact of this complication cannot be overstated as one patient underwent conversion. We have a second patient who is likely to ultimately undergo conversion so barring the ability to extend more proximally, which often involves putting more chimney stents into the renal or mesenteric vessels, I do not think that there is a straightforward solution.
To your second question about the drop in GFR, again, to my previous point, there weren’t always clues on postoperative imaging that the patients were developing stent deformation. However, even in vessels thought to have optimal anatomy for chimney placement, we have seen deformation without clinical consequences such as a change in GFR, stent thrombosis or abdominal pain. I would like to bring your attention to one example. Many authors embrace the notion that a down going vessel is more advantageous for a chimney stent procedure. The SMA is classically a down going vessel however these slides are an example of a suprarenal aortic aneurysm that was repaired with chimney EVAR in our practice. Initial chimney placement resulted in some stent compression without clinical symptoms or duplex velocities suggestive of stenosis. However, over time CT scans demonstrated greater compression and eventual thrombosis, which surprisingly and fortunately was asymptomatic for this patient. Some authors might argue "well you should place additional self-expanding stents to support the stent graft" which highlights one of the challenges with interpreting the chimney literature. Specifically, there is no standardized technique. We used combinations of virtually all known aortic endografts and stent/stent grafts due to the complexity of the disease patterns we treated and no single device combination seemed to be more frequently associated with failures. I think it is very difficult to reliably predict the biomechanical properties of the interface between the different aortic endografts and the various permutations of chimney stents or stent grafts that are reported. For example, two of the patients in this series that experienced bilateral renal chimney stent thrombosis had stent grafts (1 patient-had Fluencys and a Zenith graft, 1 patient- had Viabahns and a Gore graft) with internally supported self-expanding Zilver stents and they still ended up with thrombosis.
Chimney stent failure is not simply explained by architecture and stent deformation. In an effort to try and understand the differences in hemodynamic perturbations that occur in stented visceral vessels after chimney and fenestrated EVAR, one of my colleagues, Scott Berceli has some preliminary data that demonstrates that there is tremendous variation in shear stress that occurs in the visceral vessels after chimney stent placement. These studies may provide mechanistic insights as to why it is that some of these stents go on to fail even without architectural abnormalities on CT or alterations in duplex velocities.
With respect to your third question about why is it that an internal iliac chimney with a Type 1b leak may have a more benign course compared to a type 1a leak. I am unaware of any compelling clinical data that could argue for relative ‘safety’ of one leak compared to the other. Conceptually, from a purely flow dynamic and pressure principle argument, it should not make a difference where the type I endoleak originates. However, I am aware that there are some suggestions in the literature that support the notion that type 1b internal iliac chimney leaks may be more benign. One possible explanation is that there are different biomechanical properties of the iliac limbs compared to the hoop strength that you encounter with an aortic endograft leading to better conformability between the iliac stents. Also the length of overlap is often different. It is possible that the longer internal iliac chimney stent gutter has a smaller cross-sectional diameter leading to relatively greater flow resistance and pressure drop compared to a shorter proximal aortic gutter with larger diameters. Also, depending on how the internal iliac chimney is constructed, the ‘type 1b leak’ may really be behaving like a type 2 leak depending on the configuration of the proximal aortic stent and distal external iliac seal zone, especially if you have constructed an ‘internal’ chimney.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 38th Annual Meeting for the Southern Association of Vascular Surgery Thursday, January 16th, 2014 Palm Beach, Florida
References
- 1.Leurs LJ, Kievit J, Dagnelie PC, Nelemans PJ, Buth J. Influence of infrarenal neck length on outcome of endovascular abdominal aortic aneurysm repair. J Endovasc Ther. 2006;13:640–648. doi: 10.1583/06-1882.1. [DOI] [PubMed] [Google Scholar]
- 2.Aburahma AF, Campbell JE, Mousa AY, Hass SM, Stone PA, Jain A, et al. Clinical outcomes for hostile versus favorable aortic neck anatomy in endovascular aortic aneurysm repair using modular devices. J Vasc Surg. 2011;54:13–21. doi: 10.1016/j.jvs.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Linsen MA, Jongkind V, Nio D, Hoksbergen AW, Wisselink W. Pararenal aortic aneurysm repair using fenestrated endografts. J Vasc Surg. 2012;56:238–246. doi: 10.1016/j.jvs.2011.10.092. [DOI] [PubMed] [Google Scholar]
- 4.Greenberg RK, Clair D, Srivastava S, Bhandari G, Turc A, Hampton J, et al. Should patients with challenging anatomy be offered endovascular aneurysm repair? J Vasc Surg. 2003;38:990–996. doi: 10.1016/s0741-5214(03)00896-6. [DOI] [PubMed] [Google Scholar]
- 5.Kolvenbach RR, Yoshida R, Pinter L, Zhu Y, Lin F. Urgent endovascular treatment of thoraco-abdominal aneurysms using a sandwich technique and chimney grafts--a technical description. Eur J Vasc Endovasc Surg. 2011;41:54–60. doi: 10.1016/j.ejvs.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Donas KP, Pecoraro F, Torsello G, Lachat M, Austermann M, Mayer D, et al. Use of covered chimney stents for pararenal aortic pathologies is safe and feasible with excellent patency and low incidence of endoleaks. J Vasc Surg. 2012;55:659–665. doi: 10.1016/j.jvs.2011.09.052. [DOI] [PubMed] [Google Scholar]
- 7.Bruen KJ, Feezor RJ, Daniels MJ, Beck AW, Lee WA. Endovascular chimney technique versus open repair of juxtarenal and suprarenal aneurysms. J Vasc Surg. 2011;53:895–904. doi: 10.1016/j.jvs.2010.10.068. discussion 904-895. [DOI] [PubMed] [Google Scholar]
- 8.Moulakakis KG, Mylonas SN, Avgerinos E, Papapetrou A, Kakisis JD, Brountzos EN, et al. The chimney graft technique for preserving visceral vessels during endovascular treatment of aortic pathologies. J Vasc Surg. 2012;55:1497–1503. doi: 10.1016/j.jvs.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Donas KP, Pecoraro F, Bisdas T, Lachat M, Torsello G, Rancic Z, et al. Ct angiography at 24 months demonstrates durability of evar with the use of chimney grafts for pararenal aortic pathologies. J Endovasc Ther. 2013;20:1–6. doi: 10.1583/12-4029.1. [DOI] [PubMed] [Google Scholar]
- 10.Schlosser FJ, Muhs BE. Commentary: Midterm results of endovascular aortic repair with chimney stent-grafts. J Endovasc Ther. 2013;20:7–12. doi: 10.1583/12-4029C.1. [DOI] [PubMed] [Google Scholar]
- 11.Wilson A, Zhou S, Bachoo P, Tambyraja AL. Systematic review of chimney and periscope grafts for endovascular aneurysm repair. Br J Surg. 2013;100:1557–1564. doi: 10.1002/bjs.9274. [DOI] [PubMed] [Google Scholar]
- 12.Chaikof EL, Fillinger MF, Matsumura JS, Rutherford RB, White GH, Blankensteijn JD, et al. Identifying and grading factors that modify the outcome of endovascular aortic aneurysm repair. J Vasc Surg. 2002;35:1061–1066. doi: 10.1067/mva.2002.123991. [DOI] [PubMed] [Google Scholar]
- 13.Chaikof EL, Blankensteijn JD, Harris PL, White GH, Zarins CK, Bernhard VM, et al. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg. 2002;35:1048–1060. doi: 10.1067/mva.2002.123763. [DOI] [PubMed] [Google Scholar]
- 14.Lee JT, Greenberg JI, Dalman RL. Early experience with the snorkel technique for juxtarenal aneurysms. J Vasc Surg. 2012;55:935–946. doi: 10.1016/j.jvs.2011.11.041. discussion 945-936. [DOI] [PubMed] [Google Scholar]
- 15.Scali ST, Waterman A, Feezor RJ, Martin TD, Hess PJ, Jr, Huber TS, et al. Treatment of acute visceral aortic pathology with fenestrated/branched endovascular repair in high-surgicalrisk patients. J Vasc Surg. 2013;58:56–65. doi: 10.1016/j.jvs.2012.12.043. e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee WA, Brown MP, Nelson PR, Huber TS. Total percutaneous access for endovascular aortic aneurysm repair ("preclose" technique) J Vasc Surg. 2007;45:1095–1101. doi: 10.1016/j.jvs.2007.01.050. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Venkataraman R, Kellum JA. Defining acute renal failure: The rifle criteria. J Intensive Care Med. 2007;22:187–193. doi: 10.1177/0885066607299510. [DOI] [PubMed] [Google Scholar]
- 19.Tolenaar JL, van Keulen JW, Trimarchi S, Muhs BE, Moll FL, van Herwaarden JA. The chimney graft, a systematic review. Ann Vasc Surg. 2012;26:1030–1038. doi: 10.1016/j.avsg.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 20.Coscas R, Kobeiter H, Desgranges P, Becquemin JP. Technical aspects, current indications, and results of chimney grafts for juxtarenal aortic aneurysms. J Vasc Surg. 2011;53:1520–1527. doi: 10.1016/j.jvs.2011.01.067. [DOI] [PubMed] [Google Scholar]
- 21.Ohrlander T, Sonesson B, Ivancev K, Resch T, Dias N, Malina M. The chimney graft: A technique for preserving or rescuing aortic branch vessels in stent-graft sealing zones. J Endovasc Ther. 2008;15:427–432. doi: 10.1583/07-2315.1. [DOI] [PubMed] [Google Scholar]
- 22.Suominen V, Pimenoff G, Salenius J. Fenestrated and chimney endografts for juxtarenal aneurysms: Early and midterm results. Scand J Surg. 2013;102:182–188. doi: 10.1177/1457496913490464. [DOI] [PubMed] [Google Scholar]
- 23.Early results of fenestrated endovascular repair of juxtarenal aortic aneurysms in the united kingdom. Circulation. 2012;125:2707–2715. doi: 10.1161/CIRCULATIONAHA.111.070334. [DOI] [PubMed] [Google Scholar]
- 24.Greenberg RK, Sternbergh WC, 3rd, Makaroun M, Ohki T, Chuter T, Bharadwaj P, et al. Intermediate results of a united states multicenter trial of fenestrated endograft repair for juxtarenal abdominal aortic aneurysms. J Vasc Surg. 2009;50:730–737. doi: 10.1016/j.jvs.2009.05.051. e731. [DOI] [PubMed] [Google Scholar]
- 25.Ockert S, Schumacher H, Bockler D, Malcherek K, Hansmann J, Allenberg J. Comparative early and midterm results of open juxtarenal and infrarenal aneurysm repair. Langenbecks Arch Surg. 2007;392:725–730. doi: 10.1007/s00423-006-0141-6. [DOI] [PubMed] [Google Scholar]
- 26.Tsai S, Conrad MF, Patel VI, Kwolek CJ, LaMuraglia GM, Brewster DC, et al. Durability of open repair of juxtarenal abdominal aortic aneurysms. J Vasc Surg. 2012;56:2–7. doi: 10.1016/j.jvs.2011.12.085. [DOI] [PubMed] [Google Scholar]
- 27.Katsargyris A, Oikonomou K, Klonaris C, Topel I, Verhoeven EL. Comparison of outcomes with open, fenestrated, and chimney graft repair of juxtarenal aneurysms: Are we ready for a paradigm shift? J Endovasc Ther. 2013;20:159–169. doi: 10.1583/1545-1550-20.2.159. [DOI] [PubMed] [Google Scholar]
- 28.Donas KP, Bisdas T, Torsello G, Austermann M. Technical considerations and performance of bridging stent-grafts for iliac side branched devices based on a pooled analysis of single-center experiences. J Endovasc Ther. 2012;19:667–671. doi: 10.1583/JEVT-12-3917MR-R.1. [DOI] [PubMed] [Google Scholar]
- 29.Larzon T, Eliasson K, Gruber G. Top-fenestrating technique in stentgrafting of aortic diseases with mid-term follow-up. J Cardiovasc Surg (Torino) 2008;49:317–322. [PubMed] [Google Scholar]
- 30.Chiesa R, Tshomba Y, Mascia D, Rinaldi E, Logaldo D, Civilini E. Open repair for juxtarenal aortic aneurysms. J Cardiovasc Surg (Torino) 2013;54:35–45. [PubMed] [Google Scholar]
- 31.Kristmundsson T, Sonesson B, Dias N, Tornqvist P, Malina M, Resch T. Late outcomes after fenestrated endovascular repair for juxtarenal aortic aneurysm. J Vasc Surg. 2013 doi: 10.1016/j.jvs.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 32.Corbett TJ, Molony DS, Callanan A, McGloughlin TM. The effect of vessel material properties and pulsatile wall motion on the fixation of a proximal stent of an endovascular graft. Med Eng Phys. 2011;33:106–111. doi: 10.1016/j.medengphy.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 33.Beck AW, Goodney PP, Nolan BW, Likosky DS, Eldrup-Jorgensen J, Cronenwett JL. Predicting 1-year mortality after elective abdominal aortic aneurysm repair. J Vasc Surg. 2009;49:838–843. doi: 10.1016/j.jvs.2008.10.067. discussion 843-834. [DOI] [PubMed] [Google Scholar]
- 34.Jongkind V, Yeung KK, Akkersdijk GJ, Heidsieck D, Reitsma JB, Tangelder GJ, et al. Juxtarenal aortic aneurysm repair. J Vasc Surg. 2010;52:760–767. doi: 10.1016/j.jvs.2010.01.049. [DOI] [PubMed] [Google Scholar]
- 35.Scali ST, Feezor RJ, Huber TS, Beck AW. Acute bilateral renal artery chimney stent thrombosis after endovascular repair of a juxtarenal aortic aneurysm. J Vasc Surg. 2013 doi: 10.1016/j.jvs.2013.10.037. (article in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mills JL, Sr, Duong ST, Leon LR, Jr, Goshima KR, Ihnat DM, Wendel CS, et al. Comparison of the effects of open and endovascular aortic aneurysm repair on long-term renal function using chronic kidney disease staging based on glomerular filtration rate. J Vasc Surg. 2008;47:1141–1149. doi: 10.1016/j.jvs.2008.01.039. [DOI] [PubMed] [Google Scholar]
- 37.Nathan DP, Brinster CJ, Woo EY, Carpenter JP, Fairman RM, Jackson BM. Predictors of early and late mortality following open extent iv thoracoabdominal aortic aneurysm repair in a large contemporary single-center experience. J Vasc Surg. 2011;53:299–306. doi: 10.1016/j.jvs.2010.08.085. [DOI] [PubMed] [Google Scholar]