Abstract
Background
Evidence suggests that prolonged operative time adversely affects surgical outcomes. However, whether faster surgeons have better outcomes is unclear, as a surgeon’s speed could reflect skill and efficiency, but may alternatively reflect haste.
Objectives
This study evaluates whether median surgeon operative time is associated with adverse surgical outcomes following laparoscopic Roux-en-Y gastric bypass.
Methods
We performed a retrospective cohort study using statewide clinical registry data from the years 2006 to 2012. Surgeons were ranked by their median operative time and grouped into terciles. Multivariable logistic regression with robust standard errors was used to evaluate the influence of median surgeon operative time on 30-day surgical outcomes, adjusting for patient and surgeon characteristics, trainee involvement, concurrent procedures, and the complex interaction between these variables.
Results
A total of 16,344 patients underwent surgery during the study period. Compared to surgeons in the fastest tercile, slow surgeons required 53 additional minutes to complete a gastric bypass procedure [median (Interquartile range) 139 (133–150) vs. 86 (69–91), p<0.001]. After adjustment for patient characteristics only, slow surgeons had significantly higher adjusted rates of any complication, prolonged length of stay, ED visits or readmissions, and venous thromboembolism (VTE). After further adjustment for surgeon characteristics, resident involvement, and the interaction between these variables, slow surgeons had higher rates of any complication (10.5% vs. 7.1%, p=0.039), prolonged length of stay (14.0% vs. 4.4%, p=0.002), and VTE (0.39% vs. 0.22%, p<0.001).
Conclusions
Median surgeon operative duration is independently associated with adjusted rates of certain adverse outcomes following laparoscopic Roux-en-Y gastric bypass. Improving surgeon efficiency while operating may reduce operative time and improve the safety of bariatric surgery.
Keywords: Outcomes Research, Postoperative Complications, Bariatric Surgery, Laparoscopic Roux-en-Y Gastric Bypass, Quality of Care
INTRODUCTION
A growing body of evidence suggests that prolonged operative duration is associated with adverse surgical outcomes in a wide range of procedures. Many studies have reported a relationship between longer operative durations and surgical site infections (SSI) in general, vascular, orthopedic, and spinal procedures, among others.(1–4) Prolonged operative times have also been associated with increased venous thromboembolic events (VTE) following laparoscopic gastric bypass,(5) and longer hospital stays following hepatic resection and peripheral bypass surgery.(4, 6)
Yet these findings do not necessarily imply that faster surgeons are safer. Many authors assume a causal relationship between operative duration and adverse outcomes, hypothesizing the effect of operative duration on wound infection, for example, may result from prolonged microbial exposure to the incision, or the diminished efficacy of antimicrobial prophylaxis over time.(1, 2, 4) However, studies reporting associations between operative duration and adverse outcomes are inherently confounded by unmeasured patient characteristics associated with the risk of complications. For many procedures, shorter operative durations may simply result from technically straightforward (or easier) operations, which in turn reflect patients of lower risk. In addition, when evaluating the influence of operative duration, operative technique and trainee involvement must also be considered. Though literature on the influence of trainee involvement on clinical outcomes is mixed, numerous studies have reported increases in operative duration secondary to trainee participation.(7–10) And recent studies of laparoscopic gastric bypass suggest that differences in the technique used to construct the gastrojejunostomy can have dramatic effects on postoperative outcomes.(11)
The objective of this study is to better understand the relationship between a surgeon’s median operative duration and postoperative outcomes in patients undergoing complex laparoscopic surgery. To do this, we used data from the statewide Michigan Bariatric Surgery Collaborative (MBSC) to examine risk-adjusted 30-day surgical outcomes of all patients undergoing laparoscopic Roux-en-Y gastric bypass during the period June 2006 to August 2012. We hypothesize that longer median surgeon operative times will be significantly associated with certain adverse outcomes following gastric bypass.
METHODS
Data Sources and Study Population
The MBSC is a payer-funded consortium of hospitals and surgeons who perform bariatric surgery in Michigan. The collaborative collects data on nearly all patients undergoing bariatric surgery in the state each year, and utilizes center-specific outcomes feedback and quarterly collaborative meetings to promote quality improvement and best practices among participating surgeons and hospitals. The specifics of data collection and patient follow-up have been detailed elsewhere.(12–14)
In brief, trained data abstractors review the medical record and collect information on patient demographics and comorbid conditions, perioperative and intraoperative processes, and 30-day outcomes. All variable definitions are standardized. Nurses from the coordinating center of the MBSC audit each hospital annually to ensure data accuracy. For patients who consent to longitudinal follow-up, surveys are administered at baseline and annually after surgery to obtain data on long-term outcomes of bariatric surgery. For this study, data was obtained for all MBSC patients undergoing primary elective laparoscopic Roux-en-Y gastric bypass between June 2006 and August 2012. Thirty-nine surgeons that performed less than 150 procedures (25 procedures per year)(15) during the 6-year study period were excluded to balance sample sizes between terciles, and increase the statistical reliability associated with model estimates.
Median Surgeon Operative Time
In the MBSC database, operative time is defined as minutes from skin incision to skin closure. To address potential bias in the relationship between patient characteristics, procedure difficulty, and individual patient operative times due to unmeasured patient characteristics, in this analysis operative time was defined as the median operative duration of all laparoscopic gastric bypass procedures performed by each surgeon during the study period (median surgeon operative time). Analysis of surgeon-level operative time substantially reduces bias from unmeasured patient characteristics, as it is unlikely that surgeons differ substantially in the proportion of “difficult patients” they operate on. To decrease bias in model estimates,(16) and to account for possible non-linearity in the relationship,(17) surgeons were categorized into 3 equally-sized terciles of median operative time: slow, medium and fast.
Outcome Variables
The primary outcomes of this analysis were postoperative outcomes within 30-days of surgery. Surgical complications included wound infections or dehiscence (requiring antibiotics, opening of the wound, or reoperation), abdominal abscess (requiring percutaneous drainage or reoperation), bowel obstruction (requiring reoperation), anastomotic leak (documented in the medical record and required percutaneous drainage or reoperation), and bleeding (requiring transfusion, reoperation, or splenectomy). Medical complications included pneumonia (requiring treatment with antibiotics) or respiratory failure (requiring 2 or more days of intubation or tracheostomy), myocardial infarction or cardiac arrest, VTE (deep venous thrombosis or pulmonary embolism), and catheter-associated urinary tract infection (UTI). We also examined rates of 30-day mortality, prolonged length of stay (>3 days), emergency department (ED) visits, and 30-day readmissions.
Statistical Analysis
To understand the influence of median surgeon operative time on post-operative outcomes following bariatric surgery, we created two models. The first model used multivariable logistic regression to adjust for patient characteristics only. The full model included median surgeon operative time terciles as the independent variable, and adjusted for patient characteristics, surgeon characteristics (surgeon annual volume and gastrojejunostomy technique), concurrent procedures, and trainee involvement. In addition, we included a three-way interaction between median surgeon operative time, gastrojejunostomy technique, and trainee involvement, to account for modification of the relationship between median surgeon operative time and adverse outcomes by these two factors. In each model, the patient was the unit of analysis. To account for within-surgeon outcome correlation (clustering), we generated robust standard errors for each model.
To adjust for patient characteristics, we created a predictive risk score for serious complications using all available patient covariates. Patient covariates included demographics (age, gender and type of insurance), pre-operative body mass index (BMI), current smoking status, mobility limitations and comorbidities (lung disease, cardiovascular disease, hypertension, hyperlipidemia, gastroesophageal reflux, peptic ulcer disease, cholelithiasis, urinary incontinence, renal failure, diabetes, liver disease, history of VTE, sleep apnea, musculoskeletal disorders, history of hernia repair, and psychological disorders).
In the full model, surgeon annual procedure volume was included as a continuous covariate. Trainee involvement was characterized at the surgeon-level as a dichotomous covariate indicating whether the surgeon works with residents or fellows, using data obtained from an email survey distributed in the summer of 2012. Gastrojejunostomy anastomotic technique, also obtained from survey data, was included as a categorical covariate indicating use of a circular stapler, a linear stapler, or a hand-sewn technique for creation of the gastrojejunostomy.
Baseline characteristics were compared using chi-squared or Kruskal-Wallis test as appropriate. For the full model, risk-adjusted rates were determined by calculating marginal effects at each tercile, and significance was determined by calculating the p-value for the trend of differences in risk-adjusted rates among terciles.(18, 19) For all statistical tests, p-values are two-tailed, and alpha is set at 0.05. All analyses were performed using STATA version 12.1 (StataCorp, College Station, TX). This study was ruled exempt by the University of Michigan Institutional Review Board.
RESULTS
A total of 16,344 patients underwent primary laparoscopic Roux-en-Y gastric bypass by 34 surgeons during the study period. Table 1 details the baseline characteristics of surgeons included in the study cohort, according to tercile of median surgeon operative time. Compared to surgeons in the fastest tercile, surgeons in the slowest tercile required 53 additional minutes to complete a gastric bypass procedure [median (interquartile range) 139 (133–150) vs. 86 (69–91), p<0.001]. A much higher percentage of slow surgeons reported working with residents (90.9% vs. 0%, p<0.001). No significant differences were observed in annual surgeon case volume or choice of gastrojejunostomy technique.
Table 1.
Baseline characteristics of Michigan Bariatric Surgery Collaborative surgeons according to tercile of median surgeon operative time [values are listed as median (interquartile range) or number (percentage)].
| Surgeon Characteristics | Terciles of Median Surgeon Operating Time | p-value | ||
|---|---|---|---|---|
|
| ||||
| Fast | Medium | Slow | ||
|
| ||||
| Surgeons (no.) | 12 | 11 | 11 | |
| Total Cases Performed | 511 (230 – 886) | 309 (252 – 464) | 435 (227 – 636) | 0.764 |
| Median Operating time (min) | 86 (69 – 91) | 107 (102 – 113) | 139 (133 – 150) | < 0.001 |
| Works with Residents | 0 (0) | 6 (54.5) | 10 (90.9) | < 0.001 |
| Volume | ||||
| Annual Case Volume | 97 (46 – 157) | 65 (45 – 134) | 78 (43 – 115) | 0.801 |
| High Volume (>125 cases/year) | 5 (41.7) | 3 (27.3) | 2 (18.2) | 0.577 |
| Gastrojejunostomy Technique | ||||
| Circular | 7 (58.3) | 9 (81.8) | 6 (54.6) | 0.495 |
| Linear | 2 (16.7) | 0 (0) | 1 (9.1) | 0.433 |
| Hand | 1 (8.3) | 0 (0) | 3 (27.3) | 0.231 |
| Mixed | 2 (16.7) | 2 (18.2) | 1 (9.1) | 0.811 |
Baseline patient characteristics according to tercile of median surgeon operative time are shown in Table 2. The mean age of patients undergoing surgery was 45.8 years old, and the mean pre-operative body mass index (BMI) was 47.6 kg/m2. A majority of patients were female and had private insurance, and very few patients were active smokers. Patients undergoing surgery by fast and slow surgeons were generally similar. However, patients undergoing surgery by slow surgeons were more likely to have private insurance, be active smokers, and have cardiovascular disease, gastroesophageal reflux and diabetes. With regard to other patient characteristics, differences were statistically significant, but small and clinically insignificant.
Table 2.
Baseline characteristics of Michigan Bariatric Surgery Collaborative patients according to tercile of median surgeon operative time [BMI: body mass index; NIDDM: Non-insulin Dependent Diabetes Mellitus; VTE: Venous Thromboembolic Events; values are listed as mean ± standard deviation or number (percentage)].
| Patient Characteristics | Terciles of Surgeon Operative Time
|
p-value | ||
|---|---|---|---|---|
| Fast N = 7,235 |
Medium N = 4,264 |
Slow N = 4,845 |
||
|
| ||||
| Demograhics | ||||
| Age | 45.0 ± 11.3 | 47.0 ± 11.6 | 46.0 ± 11.0 | <0.001 |
| Body mass index (pre-op) | 47.5 ± 7.7 | 47.2 ± 7.8 | 48.2 ± 8.0 | <0.001 |
| Male (%) | 1,457 (20.1) | 933 (21.9) | 1,013 (20.9) | 0.083 |
| Private insurance (%) | 4,541 (62.8) | 3,118 (73.1) | 3,366 (69.5) | <0.001 |
| Current smoker (%) | 190 (2.6) | 179 (4.2) | 226 (4.7) | <0.001 |
| Mobility Limitations (%) | 331 (4.6) | 275 (6.4) | 335 (6.9) | <0.001 |
| Comorbidities (%) | ||||
| Musculoskeletal disorder | 6,240 (86.2) | 3,203 (75.1) | 3,714 (76.7) | <0.001 |
| Cardiovascular Disease | 3,743 (51.7) | 2,559 (60.0) | 2,891 (59.7) | <0.001 |
| Hypertension | 3,635 (50.2) | 2,440 (57.2) | 2,817 (58.1) | <0.001 |
| Hyperlipidemia | 3,713 (51.3) | 2,348 (55.1) | 2,631 (54.3) | <0.001 |
| Gastroesophageal reflux | 3,328 (46.0) | 2,174 (51.0) | 2,586 (53.4) | <0.001 |
| Psychological disorder | 3,601 (49.8) | 2,258 (53.0) | 2,284 (47.1) | <0.001 |
| Sleep apnea | 3,163 (43.7) | 2,206 (51.7) | 2,284 (47.1) | <0.001 |
| NIDDM | 2,339 (32.3) | 1,618 (38.0) | 1,766 (36.4) | <0.001 |
| NIDDM requiring insulin | 778 (10.8) | 507 (11.9) | 590 (12.2) | 0.034 |
| Cholelithiasis | 2,034 (28.1) | 1,248 (29.3) | 1,316 (27.2) | 0.083 |
| Lung Disease | 1,990 (27.5) | 1,044 (24.5) | 1,441 (29.7) | <0.001 |
| Urinary incontinence | 1,434 (19.8) | 1,195 (28.0) | 1,223 (25.2) | <0.001 |
| Liver disorder | 266 (3.7) | 227 (5.3) | 244 (5.0) | <0.001 |
| Prior VTE | 248 (3.4) | 172 (4.0) | 248 (5.1) | <0.001 |
| Peptic ulcer disease | 182 (2.5) | 116 (2.7) | 273 (5.6) | <0.001 |
| History of abdominal wall hernia repair | 179 (2.5) | 101 (2.4) | 113 (2.3) | 0.869 |
Table 3 shows unadjusted rates of adverse outcomes during the study period. The most common adverse outcomes following laparoscopic Roux-en-Y gastric bypass include any complication, any surgical complication, ED visit or readmission, and prolonged length of stay. Rates of mortality (0.09%) and anastomotic leak (0.70%) are very low in this cohort. The majority of anastomotic leaks occurred at the gastrojejunal anastomosis (64 of 115, 55.6%). Among patients that experience an anastomotic leak, 94 (81.7%) underwent reoperation and 21 (18.3%) received non-operative management with drainage.
Table 3.
Unadjusted rates of adverse outcomes in Michigan Bariatric Surgery Collaborative patients undergoing laparoscopic Roux-en-Y gastric bypass [values are listed as number (percentage)].
| Adverse Outcome | Unadjusted Rate N=16,344 |
|---|---|
|
| |
| Post-Operative Care | |
| Any Complication | 1,627 (10.0) |
| Serious Complication | 478 (2.9) |
| Any Surgical Complication | 1,352 (8.3) |
| Prolonged Length of Stay | 1,242 (7.6) |
| Reoperation | 353 (2.2) |
| ED Visit or Readmission | 1,634 (10.0) |
| Death | 14 (0.09) |
| Surgical Site | |
| Intraabdominal Abscess | 77 (0.47) |
| Anastomotic Leak | 115 (0.70) |
| Obstruction | 121 (0.74) |
| Wound Complication | 471 (2.9) |
| Bleeding | 432 (2.6) |
| Medical | |
| UTI | 93 (0.75) |
| VTE | 61 (0.37) |
| Cardiac | 19 (0.12) |
| Respiratory | 274 (1.7) |
After adjusting for patient characteristics, there were significantly increased rates of multiple adverse outcomes in patients operated by slow surgeons. Compared to surgeons in the fastest tercile, slow surgeons had significantly higher adjusted rates of any complication (12.9% vs. 8.1%, p=0.022), prolonged length of stay (10.9% vs. 4.6%, p=0.002), ED visits or readmissions (12.2% vs. 7.1%, p=0.014), and VTE (0.69% vs. 0.22%, p=0.005). Adjusted rates of reoperation, mortality, intraabdominal abscess, wound infection, anastomotic leak, obstruction, hemorrhage, cardiac complications, and respiratory complications were not statistically different.
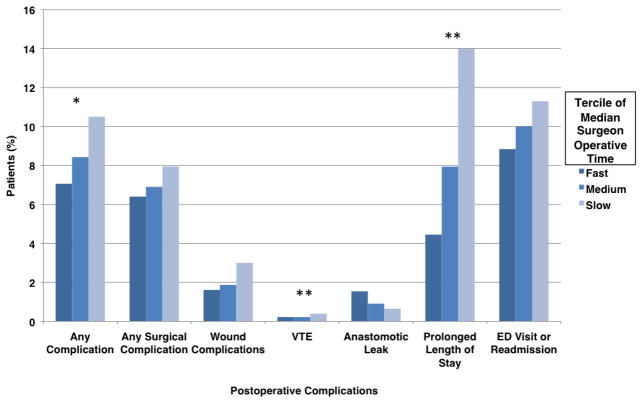
Adjusted rates of 30-day outcomes according to tercile of median surgeon operative time for the full model are shown in Figure 1. After adjusting for patient and surgeon characteristics, concurrent procedures, trainee involvement, and the interaction between median surgeon operative time, trainee involvement, and gastrojejunstomy technique, rates of any complication (10.5% vs. 7.1%, p=0.039), prolonged length of stay (14.0% vs. 4.4%, p=0.002), and VTE (0.39% vs. 0.22%, p<0.001) were significantly increased in slow surgeons. No significant differences were seen in rates of any surgical complication, serious complication, reoperation, mortality, intraabdominal abscess, wound infection, anastomotic leak, obstruction, hemorrhage, cardiac complications, respiratory complications or ED visits or readmissions.
Figure 1.
Rates of 30-day outcomes according to tercile of median surgeon operative time, adjusted for patient and surgeon characteristics, concurrent procedures, trainee involvement, and the interaction between median surgeon operative time, trainee involvement, and gastrojejunstomy technique. (VTE: Venous Thromboembolic Events; ED: Emergency Department; *p-value <0.05; **p-value <0.005).
DISCUSSION
This is the first population-based study to investigate the influence of surgeon median operative time on outcomes following complex laparoscopic surgery. In this analysis, we found that patients undergoing laparoscopic Roux-en-Y gastric bypass by slower surgeons have greater odds of any complication, prolonged length of stay and VTE, even after accounting for patient and surgeon characteristics, and the complex interactions between median surgeon operative time, gastrojejunostomy technique and trainee involvement.
Our findings echo the results of numerous studies that have documented a relationship between operative duration and adverse outcomes at the patient level. Operative duration was included as one of three key components of the validated National Nosocomical Infections Surveillance System risk index.(1, 20, 21) In a study of hospitals participating in the National Surgical Quality Improvement Program (NSQIP), those hospitals considered to be high outliers in rates of SSI performed operations that took significantly longer (128.3 ± 104.3 minutes vs. 102.7 ± 83.9 minutes, p<0.001), when compared to low outlier hospitals.(2) Tan and colleagues specifically investigated the influence of operative duration on post-operative outcomes following femoral-popliteal bypass and found that longer operations were significantly associated with increased infection rates and longer hospital stays following surgery.(4) At the patient level, however, prolonged operative time may simply be a proxy for a more difficult or complex procedure, which would be expected to result in higher rates of postoperative complications.
This is the only study to examine relative operative durations at the surgeon level. These results demonstrate that faster surgeons have lower rates of certain complications, suggesting that, for the most part, faster surgeons may be more efficient that the slower surgeons. This inference is corroborated by the findings of a recent study of bariatric surgery patients that examined the relationship between a surgeon’s technical skill (as rated by surgical colleagues) and adjusted rates of post-operative complications.(22) In that study, surgeons’ operative videos were rated on 5 domains of technical proficiency, one of which was efficiency of motion. Surgeons in the highest quartile of technical skill ratings had significantly lower mean operating room times, and significantly decreased rates of overall complications, surgical site infections, pulmonary complications and mortality, when compared to surgeons in the lowest quartile of skill.
The relationship between median surgeon operative time and anastomotic leak shown in figure 1 is an interesting finding. Though not statistically significant, the direction of influence appears to be reversed: slower surgeons have lower leak rates. It is possible that slower surgeons are more careful during creation of the anastomosis, and this extra attention to detail accounts for the prolonged operative duration. However, given that statistical reliability associated with model estimates of rare events (VTE, anastomotic leak) is decreased,(23, 24) these findings should be interpreted with caution. Nevertheless, published literature corroborates these findings: in the study by Birkmeyer et al. discussed above, no association was observed between surgical skill and anastomotic leak in a similar population,(22) while numerous recently published studies of bariatric surgery have reported significant associations between operative duration and VTE.(5, 10)
This study has several limitations. First, given that this analysis utilizes data for a single operation from a statewide quality improvement collaborative, it may not be generalizable outside the state of Michigan or to other operations. However, we specifically chose laparoscopic gastric bypass because it is a commonly performed complex laparoscopic operation, for which these findings could have significant implications. Second, the MBSC registry does not capture previous abdominal surgery, which likely influences operative duration. However, we included primary elective surgery only and excluded revisions, and we adjusted for history of previous abdominal wall surgery. Moreover, as noted previously, the use of surgeon-level operative time terciles substantially reduces bias due to individual patient characteristics, as it is unlikely that surgeon terciles differ substantially in the proportion of patients with clinically important previous operations.
Third, although the MBSC maintains a robust clinical registry tailored for use with bariatric surgery, we lack granular data on surgeon-specific characteristics such as antibiotic administration, technical skill, and wound closure techniques, and hospital characteristics such as specialty-specific operative teams and enhanced recovery protocols, which may have provided more insight into the mechanisms behind associations reported here. Similarly, although we adjust for the presence of a trainee, we characterize trainee involvement at the surgeon-level and therefore lack granular detail regarding level of training and trainee involvement in individual cases (the proportion of the case the trainee performed and in which steps did the trainee participate). And finally, given the observational nature of the study, there may be unmeasured confounders that bias our results.
These findings may have important implications for clinical quality improvement and surgical training. For institutions and providers seeking to improve the quality of surgical care delivered to patients, this work suggests that strategies that optimize surgeon operative efficiency could improve outcomes. This could be accomplished at an institutional-level by improving coordination of the operative team and limiting unnecessary delays.(25–27) At the surgeon level, improving technical skill and operative efficiency may potentially be addressed by emerging strategies that promote continued skill acquisition and refinement. For example, surgeon coaching or mentoring programs are possible strategies recently proposed to address this issue.(28, 29) Similarly, for programs dedicated to surgical education, given the known associations between trainee involvement, operative duration, and surgical outcomes in laparoscopic operations,(10) these findings further suggest that strategies to enhance the preparation of surgical trainees prior to active participation in the operating room could lead to improved operative efficiency and outcomes.
This work may also have important implications for health policy. Previous literature on surgical quality has concentrated on evaluating and enhancing evidence-based processes of perioperative care. In the present study, we focus on intraoperative care, and show that median surgeon operative time is independently associated with certain adverse outcomes. As the literature highlighting the importance of intraoperative quality continues to grow, future policy efforts should expand their focus to consider novel strategies to evaluate and enhance the quality of care delivered to patients during an operation.
Acknowledgments
Source of Funding: Dr. Reames is supported by a grant from the National Cancer Institute (5T32CA009672-23). This funding source had no involvement in the manuscript herein.
Footnotes
Reprint Requests may be addressed to the corresponding author.
Conflict of Interest: Dr. John Birkmeyer is chief scientific officer and has an equity interest in ArborMetrix Inc, which provides software and analytics for measuring hospital quality and efficiency. The company had no role in this study. All remaining authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leong G, Wilson J, Charlett A. Duration of operation as a risk factor for surgical site infection: comparison of English and US data. J Hosp Infect. 2006;63:255–62. doi: 10.1016/j.jhin.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Campbell DA, Jr, Henderson WG, Englesbe MJ, et al. Surgical site infection prevention: the importance of operative duration and blood transfusion--results of the first American College of Surgeons-National Surgical Quality Improvement Program Best Practices Initiative. J Am Coll Surg. 2008;207:810–20. doi: 10.1016/j.jamcollsurg.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 3.Pull ter Gunne AF, Cohen DB. Incidence, prevalence, and analysis of risk factors for surgical site infection following adult spinal surgery. Spine (Phila Pa 1976) 2009;34:1422–8. doi: 10.1097/BRS.0b013e3181a03013. [DOI] [PubMed] [Google Scholar]
- 4.Tan TW, Kalish JA, Hamburg NM, et al. Shorter duration of femoral-popliteal bypass is associated with decreased surgical site infection and shorter hospital length of stay. J Am Coll Surg. 2012;215:512–8. doi: 10.1016/j.jamcollsurg.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Chan MM, Hamza N, Ammori BJ. Duration of surgery independently influences risk of venous thromboembolism after laparoscopic bariatric surgery. Surg Obes Relat Dis. 2013;9:88–93. doi: 10.1016/j.soard.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 6.Lorenzo CS, Limm WM, Lurie F, Wong LL. Factors affecting outcome in liver resection. HPB (Oxford) 2005;7:226–30. doi: 10.1080/13651820510028864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalton SJ, Ghosh AJ, Zafar N, Riyad K, Dixon AR. Competency in laparoscopic colorectal surgery is achievable with appropriate training but takes time: a comparison of 300 elective resections with anastomosis. Colorectal Dis. 2010;12:1099–104. doi: 10.1111/j.1463-1318.2009.01998.x. [DOI] [PubMed] [Google Scholar]
- 8.Hernandez-Irizarry R, Zendejas B, Ali SM, Lohse CM, Farley DR. Impact of resident participation on laparoscopic inguinal hernia repairs: are residents slowing us down? J Surg Educ. 2012;69:746–52. doi: 10.1016/j.jsurg.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 9.Iordens GI, Klaassen RA, van Lieshout EM, Cleffken BI, van der Harst E. How to train surgical residents to perform laparoscopic Roux-en-Y gastric bypass safely. World J Surg. 2012;36:2003–10. doi: 10.1007/s00268-012-1620-2. [DOI] [PubMed] [Google Scholar]
- 10.Krell RW, Birkmeyer NJ, Reames BN, et al. Effects of resident involvement on complication rates after laparoscopic gastric bypass. J Am Coll Surg. 2013 doi: 10.1016/j.jamcollsurg.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finks JF, Carlin A, Share D, et al. Effect of surgical techniques on clinical outcomes after laparoscopic gastric bypass--results from the Michigan Bariatric Surgery Collaborative. Surg Obes Relat Dis. 2011;7:284–9. doi: 10.1016/j.soard.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Birkmeyer NJO, Share D, Campbell DA, et al. Partnering with payers to improve surgical quality: The Michigan plan. Surgery. 2005;138:815–20. doi: 10.1016/j.surg.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 13.Birkmeyer NJ, Dimick JB, Share D, et al. Hospital complication rates with bariatric surgery in Michigan. JAMA. 2010;304:435–42. doi: 10.1001/jama.2010.1034. [DOI] [PubMed] [Google Scholar]
- 14.Carlin AM, Zeni TM, English WJ, et al. The comparative effectiveness of sleeve gastrectomy, gastric bypass, and adjustable gastric banding procedures for the treatment of morbid obesity. Ann Surg. 2013;257:791–97. doi: 10.1097/SLA.0b013e3182879ded. [DOI] [PubMed] [Google Scholar]
- 15.MBSAQIP Standards. [Accessed August 29th, 2013];Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program Homepage. web site. Available at: http://www.mbsaqip.org/?page_id=54.
- 16.Cochran WG. The effectiveness of adjustment by subclassification in removing bias in observational studies. Biometrics. 1968;24:295–313. [PubMed] [Google Scholar]
- 17.Walter SD, Feinstein AR, Wells CK. Coding ordinal independent variables in multiple regression analyses. Am J Epidemiol. 1987;125:319–23. doi: 10.1093/oxfordjournals.aje.a114532. [DOI] [PubMed] [Google Scholar]
- 18.Ai C, Norton EC. Interaction terms in logit and probit models. Economics Letters. 2003;80:123–29. [Google Scholar]
- 19.Karaca-Mandic P, Norton EC, Dowd B. Interaction terms in nonlinear models. Health Serv Res. 2012;47(1 Pt 1):255–74. doi: 10.1111/j.1475-6773.2011.01314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Culver DH, Horan TC, Gaynes RP, et al. Surgical wound infection rates by wound class, operative procedure, and patient risk index. National Nosocomial Infections Surveillance System. Am J Med. 1991;91:152S–57S. doi: 10.1016/0002-9343(91)90361-z. [DOI] [PubMed] [Google Scholar]
- 21.Gaynes RP, Culver DH, Horan TC, et al. Surgical site infection (SSI) rates in the United States, 1992–1998: the National Nosocomial Infections Surveillance System basic SSI risk index. Clin Infect Dis. 2001;33 (Suppl 2):S69–77. doi: 10.1086/321860. [DOI] [PubMed] [Google Scholar]
- 22.Birkmeyer JD, Finks JF, O’Reilly A, et al. Surgical skill and complication rates after bariatric surgery. N Engl J Med. 2013;369:1434–42. doi: 10.1056/NEJMsa1300625. [DOI] [PubMed] [Google Scholar]
- 23.Dimick JB, Welch HG, Birkmeyer JD. Surgical mortality as an indicator of hospital quality: the problem with small sample size. JAMA. 2004;292:847–51. doi: 10.1001/jama.292.7.847. [DOI] [PubMed] [Google Scholar]
- 24.Krell RW, Hozain A, Kao LS, Dimick JB. Reliability of risk-adjusted outcomes for profiling hospital surgical quality. JAMA Surg. 2014 doi: 10.1001/jamasurg.2013.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee BT, Tobias AM, Yueh JH, et al. Design and impact of an intraoperative pathway: a new operating room model for team-based practice. J Am Coll Surg. 2008;207:865–73. doi: 10.1016/j.jamcollsurg.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 26.Hu YY, Arriaga AF, Roth EM, et al. Protecting patients from an unsafe system: the etiology and recovery of intraoperative deviations in care. Ann Surg. 2012;256:203–10. doi: 10.1097/SLA.0b013e3182602564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russ S, Arora S, Wharton R, et al. Measuring safety and efficiency in the operating room: development and validation of a metric for evaluating task execution in the operating room. J Am Coll Surg. 2013;216:472–81. doi: 10.1016/j.jamcollsurg.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 28.Gawande AA. The New Yorker. 2011. Personal Best; pp. 44–53. [Google Scholar]
- 29.Hu YY, Peyre SE, Arriaga AF, et al. Postgame analysis: using video-based coaching for continuous professional development. J Am Coll Surg. 2012;214:115–24. doi: 10.1016/j.jamcollsurg.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]