Abstract
Objective
To assess the suitability of instrumented gait and balance measures for diagnosis and estimation of disease severity in PD.
Methods
Each subject performed iTUG (instrumented Timed-Up-and-Go) and iSway (instrumented Sway) using the APDM® Mobility Lab. MDS-UPDRS parts II and III, a postural instability and gait disorder (PIGD) score, the mobility subscale of the PDQ-39, and Hoehn & Yahr stage were measured in the PD cohort. Two sets of gait and balance variables were defined by high correlation with diagnosis or disease severity and were evaluated using multiple linear and logistic regressions, ROC analyses, and t-tests.
Results
135 PD subjects and 66 age-matched controls were evaluated in this prospective cohort study. We found that both iTUG and iSway variables differentiated PD subjects from controls (area under the ROC curve was 0.82 and 0.75 respectively) and correlated with all PD severity measures (R2 ranging from 0.18 to 0.61). Objective exam-based scores correlated more strongly with iTUG than iSway. The chosen set of iTUG variables was abnormal in very mild disease. Age and gender influenced gait and balance parameters and were therefore controlled in all analyses.
Interpretation
Our study identified sets of iTUG and iSway variables which correlate with PD severity measures and differentiate PD subjects from controls. These gait and balance measures could potentially serve as markers of PD progression and are under evaluation for this purpose in the ongoing NIH Parkinson Disease Biomarker Program.
Keywords: Parkinson, Mobility Lab, Severity, Diagnosis, Gait, Balance
Introduction
Objective assessment tools of PD severity are needed to accelerate progress in discovering disease modifying therapies. As gait abnormalities are characteristic of PD, assessment of gait could potentially enable estimation of disease severity. The gold standard gait assessment device involves the use of high speed 3D cameras.1–4 These infrared motion capture systems, such as Vicon®, require reflective markers to be placed on the body part to be measured. These systems are expensive to acquire and are limited to evaluation of only a few strides and as such are unsuitable for measuring gait variability which may be an important aspect of gait dysfunction in neurologic disease.5 A more widely used tool for the study of gait in PD is the GaitRite® system6–9 which involves having a patient walk on a special mat embedded with sensors, and which produces similar results to a 3D camera system while requiring less setup time and cost.10 However, such instrumented mat systems are unable to measure aspects of gait that do not involve contact of the foot with the ground, such as arm and trunk movements, which are known to be affected in PD.
Postural instability is another key feature of PD which increases with disease severity.11 Computerized dynamic posturography (CDP) by devices such as the NeuroCom Smart Balance Master® measures the body sway of subjects as they stand on a force plate.12 These systems have been used to measure balance in normal pressure hydrocephalus,13 progressive supranuclear palsy,14 and PD15 but involve non-portable, expensive equipment which make them impractical for routine use in a PD clinic.
The APDM® Mobility Lab16 utilizes inertial sensors attached to the wrists, ankles, chest, and back to quantify postural sway, postural transitions, trunk, and upper and lower limb movements. This system provides detailed information regarding gait and balance. While a few studies with this or a similar system have elucidated differences between early PD subjects and controls,17–20 only a single study with a small number of subjects attempted to correlate quantitative gait parameters with PD severity.21
As part of the National Institutes of Neurological Disorders and Stroke (NINDS) Parkinson Disease Biomarker Program (PDBP), we are undertaking to evaluate the potential of the APDM® Mobility Lab to serve as a marker of disease progression. For this report, we used baseline measurements in a cohort of PD and control subjects to identify gait and balance parameters that distinguish PD subjects from controls and that correlate with disease severity.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
The study protocol was reviewed and approved by the Institutional Review Board of the University of Texas Southwestern Medical Center (UTSWMC). Written informed consent was obtained from all participants. The study was registered on clinicaltrials.gov with registration number NCT01767818.
Subjects
PD patients were recruited from the Clinical Center for Movement Disorders at UTSWMC from December 2012 to January 2014 to participate in a 5-year biomarker discovery project. All PD patients met UK PD Society Brain Bank criteria22 and were either de novo previously untreated with dopaminergic medication with ioflupane iodine-123 injection (DaTscan) confirmation, or were treated with dopaminergic drugs (levodopa or dopamine agonists) and known to be clinically responsive. Patients with motor fluctuations were assessed in the on state. Eligibility was limited to PD subjects in stages 1–4 of the Hoehn and Yahr (H&Y) scale in the on state so that all subjects would be able to participate in gait assessments. Age-matched controls were recruited from PD patient spouses, faculty, and staff. Each subject performed the instrumented Timed-Up-and-Go (iTUG) and the instrumented Sway (iSway) tests using the APDM® Mobility Lab. Clinical severity of PD was measured using MDS-UPDRS parts II and III, the mobility subscale of the Parkinson’s Disease Questionnaire (PDQ-39)23, and the H&Y scale. The postural instability gait disorder subscore (PIGD) was calculated by summing scores for MDS-UPDRS 3.9 (arising from chair), 3.10 (gait), 3.11 (freezing of gait), 3.12 (postural stability), and 3.13 (posture). These PD severity scales were not administered to the control subjects. There were no missing data.
Experimental protocol
Six movement sensors called Opals® consisting of 3-axis accelerometer, gyroscope and magnetometer (Mobility Lab, APDM Inc., Portland, OR) were attached to each subject: one on each ankle and wrist, the lower back, and the upper chest. For the iTUG, the subjects stood up, walked 6 meters, turned 180 degrees, walked back to the chair, and sat down. This test is useful in examining key aspects of gait such as stride velocity, cadence, arm swing, and trunk movement during turns, standing, and sitting. For the iSway, the subjects stood still with their hands across their chests and their feet positioned a set distance apart for recording parameters such as mean sway area, path length, jerk, and sway distance in the mediolateral and anteroposterior directions. For both the iTUG and iSway, the test was performed three times with the median values being reported and analyzed.
Statistics
iTUG and iSway each yield 101 and 47 measurements of which 86 and 46 represent unique variables, respectively. Given this large number of variables compared to the number of subjects (201), minimizing the number of variables examined was considered in depth. Thus, we avoided any form of stepwise selection to minimize the extent we capitalized upon chance.
Our first step was to reduce the number of variables and select only the 10 most pertinent ones from each test for further analysis. We chose one set of 10 iTUG and one set of 10 iSway variables that correlated highly with the diagnosis (i.e. presence vs. absence of the disease) and another set of 10 iTUG and 10 iSway variables that correlated with disease severity as measured by MDS-UPDRS part III score, given, of course, that disease was present.
We first calculated Pearson correlation coefficients (r) for each iTUG and iSway variable with both diagnosis and disease severity, and ordered them in descending order by the absolute value of r. Some of the best variables also correlated strongly with each other so if the correlation coefficient was 0.95 or greater between the two variables we kept only one (e.g. “Gait: Stride Length L [Mean]” and “Gait: Stride Length [Mean]” had an r of 0.99 so we kept only the latter, non-lateralized variable. Some cases did not involve lateralized variables but were otherwise highly correlated, e.g. “High frequency power (AP)” and “Low frequency power (AP)” had an r of 0.99 with one another, and we kept only the former based on its slightly higher criterion-related correlation).
We also performed a second variable selection procedure as a check on this first procedure. We randomly chose half the subjects (half PD and half controls) and assigned each variable a rank score based upon (a) its correlation with diagnosis and (b) its correlation with disease severity as defined by its correlation with the MDS-UPDRS part III score. The groups were resampled 10 times and the average rank score was calculated for each iTUG/Diagnosis, iTUG/Severity, iSway/Diagnosis, and iSway/Severity measure. Thus, variables with the lowest average rank scores were those that consistently correlated the best with either the diagnosis or disease severity. Using this procedure we again chose the 10 variables in each of the four groups of variables. Variables chosen using the entire cohort were virtually identical to those chosen using the halves with at most 2 variables differing in each set. There were 2 iTUG and 5 iSway variables that appeared in both the diagnosis set and disease severity set. Variable correlation coefficients and related measures are shown in the Supplemental Table.
Once the variable sets were defined, their psychometric properties were determined and two types of analyses were performed: (a) between-groups analyses, which compared PD patients and controls using the iTUG and iSway diagnosis variables, and (b) within-group analyses, which examined differences within PD patients using the iTUG and iSway severity variables. Age and gender were controlled in all analyses, so the significance of the various test statistics to be reported were tested as increments above the values obtained using age and gender alone.
The between-groups analyses began with comparing the means and standard deviations of the two groups on the iTUG and iSway measures. We also performed logistic regression to examine differences on the iTUG and iSway variables as a function of group membership. These analyses also included ROC analyses of the group differences. We also compared patients with clinically normal gait (MDS-UPDRS 3.10 score of 0) and patients with clinically normal balance (MDS-UPDRS 3.12 score of 0) against controls. An additional comparison was performed to explore the joint influence of gender and diagnosis using the ANOVA type III sum of squares to account for differences in sample sizes. A multivariate ANOVA (MANOVA) was conducted on each of the four sets of predictors (iTUG/Diagnosis, iTUG/Severity, iSway/Diagnosis, and iSway/Severity), with Wilks’ lambda used as an omnibus significance test.
The within-groups analyses used multiple linear regression to examine the relation between the iTUG and iSway measures and the five PD severity measures (MDS-UPDRS part II, MDS-UPDRS part III, PIGD subscore, PDQ-39 mobility subscale, and H&Y stage). We also examined differences between patient types: de-novo (never treated), stable (MDS-UPDRS 4.3 score of 0, meaning no off time), and fluctuator (MDS-UPDRS 4.3 score greater than 0) using ANCOVA. Again, significance was tested as increments over age and gender alone.
Multivariate statistical analyses were performed using SAS v9.3 (SAS Institute Inc., Cary, NC). Randomization and correlation coefficient calculations were performed using Matlab v2014a (Mathworks, Natick, MA).
Results
135 PD patients and 66 controls were included in the study. Demographic and baseline clinical information on the groups is shown (Table 1). Note that there was a male predominance in the PD group and a female predominance in the control group, since many controls were spouses of PD subjects. There was no significant difference in age between PD and control subjects (p = 0.47). Of the PD subjects, 15 were untreated de novo cases with DaTscan confirmation, and the others were on dopaminergic medication with clear evidence for responsiveness. Among the treated subjects, 34 had slight motor fluctuations, 9 had mild, 1 had moderate, and 1 had severe fluctuations (MDS-UPDRS question 4.3). This, together with the average H&Y stage of 2 indicates that our study was comprised chiefly of mildly affected patients.
Table 1.
Demographics and clinical characteristics of subjects (mean ± SD).
| PD (n=135) | Controls (n=66) | ||
|---|---|---|---|
| Age | 64.0 ± 9.9 | 62.9 ± 9.5 | |
| Men | 57% | 41% | |
| MDS-UPDRS | Part I score | 7.3 ± 4.9 | |
| Part II score | 8.8 ± 6.4 | ||
| Part III score | 26.1 ± 12.8 | ||
| Part IV score | 2.3 ± 3.5 | ||
| Hoehn & Yahr | 2.0 ± 0.6 | ||
| PDQ-39 (%) | ADL | 16.0 ± 14.4 | |
| Body Discomfort | 22.1 ± 21.0 | ||
| Cognitive Impairment | 15.6 ± 15.4 | ||
| Communication | 12.8 ± 16.5 | ||
| Emotional | 12.4 ± 14.8 | ||
| Mobility | 13.6 ± 17.5 | ||
| Social Support | 5.2 ± 12.1 | ||
| Stigma | 15.4 ± 18.8 | ||
| On any PD medication | 89% | ||
| On levodopa | 63% |
Psychometric properties
According to the method described, one pair of sets of iTUG and iSway variables was selected for use in the regression analysis of diagnosis and a second pair of sets was selected for the analysis of disease severity. These variable sets are shown (Table 2) along with their mean correlation coefficients (r) over 10 randomization trials. A negative value of r indicates that a measure was less or smaller in PD than control subjects while a positive value indicates the opposite. Variable descriptions, including the units of measure, are shown (Table 3).
Table 2.
iTUG and iSway measures with highest correlation (r) with PD diagnosis and disease severity.
| Diagnosis Set | Disease Severity Set | ||||||
|---|---|---|---|---|---|---|---|
| iTUG | r* | iSway | r* | iTUG | r* | iSway | r* |
| Turn To Sit: Peak Turn Velocity | −0.30 | Path length | 0.18 | Gait: Stride Length [CoV] | 0.46 | Centroidal frequency (AP) | 0.40 |
| Gait: RoM Trunk horizontal [CoV] | −0.26 | High frequency power (AP) | 0.18 | Gait: Stride Length [Mean] | −0.43 | Path length | 0.39 |
| Gait: Stride Length [Mean] | −0.24 | Mean distance (ML) | 0.19 | Turn To Sit: Duration | 0.47 | Jerk (ML) | 0.39 |
| Turn: Number of steps [Mean] | 0.23 | Normalized jerk (AP) | 0.17 | Gait: RoM Knee [Mean] | −0.42 | Mean frequency | 0.40 |
| Gait: Peak Swing Velocity R [CoV] | 0.21 | Path length (AP) | 0.18 | Gait: Stride Velocity [Mean] | −0.40 | Median frequency (AP) | 0.39 |
| Turn: Duration [Mean] | 0.21 | Mean frequency | 0.15 | Gait: RoM Shank L [Mean] | −0.40 | Normalized jerk | 0.38 |
| Turn To Sit: RoM Trunk | −0.23 | Normalized jerk | 0.14 | Gait: Stride Velocity [CoV] | 0.40 | Mean distance (ML) | 0.33 |
| Gait: RoM Knee [Mean] | −0.21 | Range of acceleration | 0.14 | Total Duration | 0.41 | Path length (AP) | 0.30 |
| Gait: RoM Knee R [Mean] | −0.21 | Total sway area | 0.15 | Gait: RoM Shank [CoV] | 0.38 | Centroidal frequency | 0.32 |
| Turn: Peak Velocity [Mean] | −0.20 | Mean velocity (ML) | 0.14 | Gait: RoM Knee L [Mean] | −0.37 | Frequency dispersion (ML) | −0.30 |
CoV=covariance, AP=anteroposterior, ML=mediolateral
average correlation coefficient over 10 randomization trials
Table 3.
Description of select iTUG and iSway variables.
| iTUG | Total Duration (seconds) | Total duration of the ITUG trial, measured from the beginning of the detected sit-to-stand transition to the end of the turn-to-sit transition |
| Gait: Stride Length (%stature) [Mean] | Distance between two consecutive foot falls at the moments of initial contact, normalized for subject height. Mean value over all gait cycles in the trial | |
| Gait: Stride Length (%stature) [CoV] | Distance between two consecutive foot falls at the moments of initial contact, normalized for subject height. Covariance of the measure over all gait cycles in the trial | |
| Gait: Stride Velocity (%stature/s) [Mean] | Walking speed, normalized for subject height. Mean value over all gait cycles in the trial | |
| Gait: Stride Velocity (%stature/s) [CoV] | Walking speed, normalized for subject height. Covariance of the measure over all gait cycles in the trial | |
| Gait: Peak Swing Velocity R (degrees/sec) [CoV] | Peak (95%) angular right shank velocity. Covariance of the measure over all gait cycles in the trial | |
| Gait: RoM Knee (degrees) [Mean] | Angular range of knee motion. Mean value over all gait cycles in the trial | |
| Gait: RoM Knee L (degrees) [Mean] | Angular range of the left knee motion. Mean value over all gait cycles in the trial | |
| Gait: RoM Knee R (degrees) [Mean] | Angular range of the right knee motion. Mean value over all gait cycles in the trial | |
| Gait: RoM Shank (degrees) [CoV] | Angular range of shank motion. Covariance of the measure over all gait cycles in the trial | |
| Gait: RoM Shank L (degrees) [Mean] | Angular range of the left shank motion. Mean value over all gait cycles in the trial | |
| Gait: RoM Trunk horizontal (degrees) [CoV] | Angular range of trunk motion in the horizontal plane. Covariance of the measure over all gait cycles in the trial | |
| Turn: Duration (seconds) [Mean] | Duration of 180 degree turn. Mean value over all gait cycles in the trial | |
| Turn: Number of steps [Mean] | Number of steps in the 180 degree turn. Mean value over all gait cycles in the trial | |
| Turn: Peak Velocity (degrees/sec) [Mean] | Peak (95%) angular trunk velocity while turning. Mean value over all gait cycles in the trial | |
| Turn To Sit: Duration (seconds) | Duration of the turn-to-sit transition | |
| Turn To Sit: Peak Turn Velocity (degrees/sec) | Peak (95%) angular trunk velocity in the during the sit-to-stand transition | |
| Turn To Sit: RoM Trunk (degrees) | Angular range of trunk motion in the sagittal plane during the turn-to-sit transition | |
| iSway | Normalized jerk | Normalized Jerk (normalized to the range of the sway path’s excursion and duration) in the transverse plane |
| Normalized jerk (AP) | Normalized Jerk (normalized to the range of the sway path’s excursion and duration) in the anterior/posterior (forward-backward) direction | |
| Jerk (ML) (m2/s5) | Smoothness of sway from the time derivative of the sway path in the medial/lateral (side-to-side) direction | |
| Mean distance (ML) (m/s2) | Mean distance of the sway path from the center of the path in the medial/lateral (side-to-side) direction | |
| Path length (m/s2) | Total length of the sway path in the transverse plane | |
| Path length (AP) (m/s2) | Total length of the sway path in the anterior/posterior (forward-backward) direction | |
| Range of acceleration (m/s2) | Total range of the sway path in the transverse plane | |
| Mean velocity (ML) (m/s) | Mean velocity of the sway path in the medial/lateral (side-to-side) direction | |
| Total sway area (m2/s5) | Sway area, computed as the area included in the sway path in the transverse plane per unit of time | |
| Mean frequency (Hz) | Mean sway frequency in the transverse plane, calculated from the sway path’s length and duration | |
| Median frequency (AP) (Hz) | Frequency below which 50% of the sway path power in the anterior/posterior (forward-backward) direction is present | |
| Centroidal frequency (Hz) | Frequency of sway from the centroid of the sway path’s power spectrum in the transverse plane | |
| Centroidal frequency (AP) (Hz) | Frequency of sway from the centroid of the sway path’s power spectrum in the anterior/posterior (forward-backward) direction | |
| High frequency power (AP) ((m/s2)2*Hz−1) | Ratio of sway path power in the anterior/posterior (forward-backward) direction that is above 3.5Hz | |
| Frequency dispersion (ML) | Frequency dispersion in the medial/lateral (side-to-side) direction |
Between-Groups results comparing PD to controls
Results of t-tests comparing the selected iTUG and iSway measures in PD versus control subjects are shown (Table 4). As expected, means for iTUG and iSway variables are significantly different between PD and control subjects since only the most discriminating variables were chosen for further analysis. Standard deviations for iTUG variables are comparable for PD and controls, but for iSway measures PD subjects are considerably more variable. Logistic regression models to predict presence or absence of disease achieved high levels of fit both for iTUG and iSway. The area under the ROC curves was 0.82 and 0.75, respectively (Figure 1).
Table 4.
Comparing control subjects with PD, PD normal gait and PD normal balance patients: group means, standard deviations and p values for select clinical measures and iTUG and iSway diagnosis prediction variables.
| Control (N=66) | PD (N=135) | PD normal gait (N=40) | PD normal balance (N=114) | p-val PD vs Con | p-val PD nl gait vs Con | p-val PD nl bal vs Con | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measure | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||
| Age | 62.96 | 9.52 | 64.00 | 9.87 | 63.03 | 10.39 | 63.46 | 9.61 | 0.47 | 0.97 | 0.73 | |
| MDS-UPDRS Part III | 26.08 | 12.80 | 17.08 | 7.08 | 24.40 | 10.94 | ||||||
| MDS-UPDRS Part III PIGD subscore | 2.32 | 2.40 | 0.40 | 0.78 | 1.75 | 1.72 | ||||||
| iTUG | Turn To Sit: Peak Turn Velocity | 178.19 | 36.40 | 150.86 | 38.50 | 167.49 | 43.77 | 153.79 | 37.59 | 0.000 | 0.230 | 0.000 |
| Gait: RoM Trunk horizontal [CoV] | 0.19 | 0.10 | 0.14 | 0.09 | 0.14 | 0.08 | 0.14 | 0.09 | 0.001 | 0.010 | 0.000 | |
| Gait: Stride Length [Mean] | 82.40 | 5.79 | 77.70 | 9.35 | 81.76 | 6.93 | 79.28 | 7.93 | 0.000 | 0.590 | 0.010 | |
| Turn: Number of steps [Mean] | 4.50 | 1.13 | 5.20 | 1.21 | 4.74 | 1.20 | 5.16 | 1.12 | 0.000 | 0.350 | 0.000 | |
| Gait: Peak Swing Velocity R [CoV] | 0.04 | 0.01 | 0.05 | 0.03 | 0.05 | 0.02 | 0.05 | 0.02 | 0.001 | 0.040 | 0.030 | |
| Turn: Duration [Mean] | 2.27 | 0.59 | 2.65 | 0.72 | 2.36 | 0.57 | 2.58 | 0.61 | 0.000 | 0.520 | 0.000 | |
| Turn To Sit: RoM Trunk | 25.90 | 9.95 | 21.80 | 7.27 | 22.06 | 7.28 | 21.83 | 6.88 | 0.004 | 0.040 | 0.000 | |
| Gait: RoM Knee [Mean] | 56.11 | 4.60 | 53.46 | 5.36 | 55.60 | 5.27 | 54.18 | 5.05 | 0.000 | 0.630 | 0.010 | |
| Gait: RoM Knee R [Mean] | 54.18 | 5.71 | 50.91 | 6.65 | 53.15 | 6.27 | 51.66 | 6.22 | 0.000 | 0.350 | 0.010 | |
| Turn: Peak Velocity [Mean] | 160.04 | 31.39 | 142.60 | 33.98 | 156.50 | 37.20 | 144.85 | 32.63 | 0.000 | 0.640 | 0.000 | |
| iSway | Path length | 5.13 | 1.49 | 8.71 | 11.04 | 6.98 | 6.55 | 7.41 | 8.91 | 0.000 | 0.030 | 0.040 |
| High frequency power (AP) | 9.52 | 6.44 | 15.17 | 16.52 | 12.48 | 11.40 | 14.63 | 15.93 | 0.001 | 0.090 | 0.010 | |
| Mean distance (ML) | 0.02 | 0.01 | 0.03 | 0.03 | 0.02 | 0.02 | 0.02 | 0.02 | 0.000 | 0.020 | 0.020 | |
| Normalized jerk (AP) | 1.08 | 0.22 | 1.21 | 0.48 | 1.17 | 0.25 | 1.17 | 0.39 | 0.006 | 0.040 | 0.090 | |
| Path length (AP) | 3.91 | 1.25 | 6.01 | 7.05 | 5.15 | 4.80 | 5.38 | 6.58 | 0.001 | 0.050 | 0.070 | |
| Mean frequency | 0.48 | 0.13 | 0.55 | 0.28 | 0.52 | 0.13 | 0.52 | 0.20 | 0.008 | 0.100 | 0.080 | |
| Normalized jerk | 4.72 | 0.88 | 5.25 | 2.21 | 5.07 | 0.91 | 5.03 | 1.40 | 0.016 | 0.060 | 0.110 | |
| Range of acceleration | 0.37 | 0.13 | 0.48 | 0.39 | 0.43 | 0.30 | 0.44 | 0.32 | 0.005 | 0.160 | 0.120 | |
| Total sway area | 0.00 | 0.00 | 0.01 | 0.04 | 0.01 | 0.02 | 0.01 | 0.04 | 0.006 | 0.080 | 0.160 | |
| Mean velocity (ML) | 0.03 | 0.02 | 0.05 | 0.05 | 0.04 | 0.05 | 0.04 | 0.04 | 0.005 | 0.090 | 0.070 | |
Figure 1.

ROC curves for logistic regression model utilizing iTUG (A) or iSway (B) variables to predict presence of PD diagnosis. The area under the curve is 0.82 for iTUG and 0.75 for iSway.
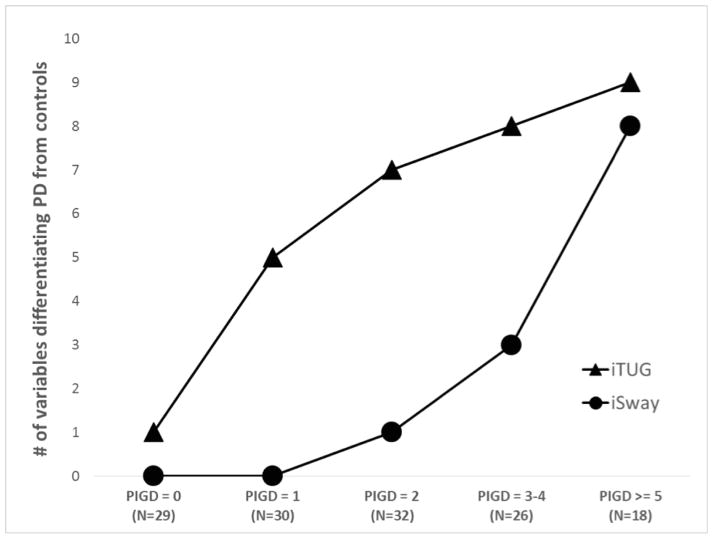
There were 40 patients with clinically normal gait, and only 3 iTUG and 3 iSway variables differentiated them from the controls. Wilks’ lambda evaluating the overall effect of all 10 variables did reach statistical significance for iTUG variables (p = 0.04), but not for iSway (p = 0.2). A much larger subset of patients had clinically normal balance (N=114), and all 10 iTUG variables were able to discriminate them from controls (Wilks’ p < 0.001), but only 3 iSway variables were significant, although together they reached statistical significance (Wilks’ p = 0.01). Stabilograms generated by the ADPM software of a control subject and two PD subjects with clinically normal balance are shown (Figure 2). To explore the question whether iTUG is more sensitive than iSway to presence of disease, we grouped all PD patients into 5 disease severity categories based on their PIGD subscore and compared each group against controls (Figure 3). The number of variables that were able to differentiate patients from controls was higher for iTUG than iSway across all groups, and iTUG variables were abnormal even in very early disease.
Figure 2.
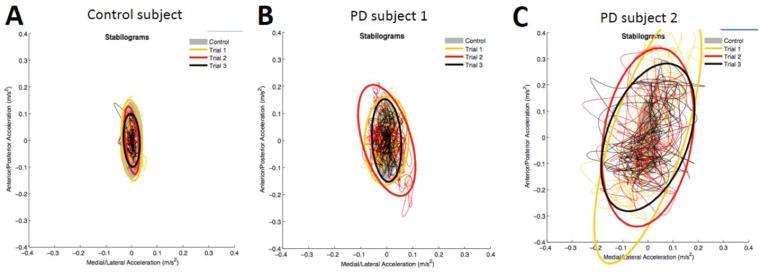
Plots of the sway path in anteroposterior and mediolateral directions in a control subject (A) and two PD subjects with clinically normal balance (B, C).
Figure 3.
More iTUG than iSway measures are abnormal in very mild disease, but both worsen as the disease advances.
As noted above, age and gender correlated significantly with iTUG and iSway variables so these were controlled in all regression analyses. Age was significantly correlated with more than two thirds of variables for both PD and control subjects (data not shown). We explored the influence of gender in more detail using a 2-way ANOVA (Table 5). In the PD cohort men and women had comparable age (p = 0.5) and disease severity (p = 0.4 for MDS-UPDRS part III, p = 0.2 for PIGD subscore). When controlling for gender all iTUG and almost all iSway variables were significantly different between the PD and control groups. When controlling for diagnosis, 3 iTUG and 3 iSway variables were significantly different between the genders, and several more especially among iSway showed a trend toward significance. No interaction terms were found to be significant. Wilks’ lambda was significant for both iTUG and iSway variables, for both gender and diagnosis.
Table 5.
Comparing PD and control subjects by gender using 2-way ANOVA: group means, standard deviations, F statistic and p values for select clinical measures and iTUG and iSway diagnosis prediction variables.
| PD Men (N=77) |
PD Women (N=58) |
Control Men (N=27) |
Control Women (N=39) |
F diagnosis |
p-val diagnosis |
F gender |
p-val gender |
F interaction |
p-val interaction |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measure | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||||||
| Age | 63.51 | 10.27 | 64.65 | 9.37 | 63.40 | 10.59 | 62.65 | 8.83 | 0.50 | 0.480 | 0.020 | 0.900 | 0.400 | 0.527 | |
| MDS-UPDRS part III | 26.86 | 13.02 | 25.05 | 12.54 | 0.417 | ||||||||||
| MDS-UPDRS part III PIGD subscore | 2.08 | 2.02 | 2.64 | 2.80 | 0.180 | ||||||||||
| iTUG | Turn To Sit: Peak Turn Velocity | 143.61 | 35.67 | 160.49 | 40.28 | 167.59 | 32.49 | 185.54 | 37.55 | 15.69 | 0.000 | 11.51 | 0.001 | 0.19 | 0.666 |
| Gait: RoM Trunk horizontal [CoV] | 0.13 | 0.08 | 0.15 | 0.10 | 0.16 | 0.08 | 0.21 | 0.11 | 8.00 | 0.005 | 8.30 | 0.004 | 1.83 | 0.177 | |
| Gait: Stride Length [Mean] | 79.36 | 7.79 | 75.49 | 10.76 | 82.17 | 5.89 | 82.57 | 5.79 | 15.06 | 0.000 | 1.90 | 0.170 | 2.67 | 0.104 | |
| Turn: Number of steps [Mean] | 5.27 | 1.23 | 5.12 | 1.20 | 4.56 | 0.97 | 4.46 | 1.23 | 12.82 | 0.000 | 0.59 | 0.444 | 0.00 | 0.985 | |
| Gait: Peak Swing Velocity R [CoV] | 0.05 | 0.02 | 0.05 | 0.03 | 0.04 | 0.01 | 0.04 | 0.01 | 6.71 | 0.010 | 0.02 | 0.882 | 1.34 | 0.248 | |
| Turn: Duration [Mean] | 2.71 | 0.75 | 2.56 | 0.68 | 2.44 | 0.61 | 2.16 | 0.54 | 9.00 | 0.003 | 5.49 | 0.020 | 0.83 | 0.365 | |
| Turn To Sit: RoM Trunk | 21.72 | 6.38 | 21.91 | 8.38 | 23.32 | 8.26 | 27.68 | 10.70 | 8.12 | 0.005 | 3.48 | 0.064 | 2.95 | 0.088 | |
| Gait: RoM Knee [Mean] | 53.72 | 4.83 | 53.13 | 6.03 | 56.19 | 4.93 | 56.04 | 4.42 | 11.19 | 0.001 | 0.18 | 0.672 | 0.10 | 0.754 | |
| Gait: RoM Knee R [Mean] | 50.43 | 5.56 | 51.54 | 7.87 | 53.01 | 5.51 | 54.99 | 5.78 | 9.79 | 0.002 | 2.20 | 0.139 | 0.14 | 0.714 | |
| Turn: Peak Velocity [Mean] | 139.79 | 34.79 | 146.34 | 32.80 | 154.09 | 30.77 | 164.17 | 31.55 | 8.94 | 0.003 | 3.08 | 0.081 | 0.23 | 0.632 | |
| Wilks’ lambda | 4.80 | <.0001 | 6.75 | <.0001 | 1.37 | 0.189 | |||||||||
| iSway | Path length | 7.10 | 7.98 | 10.84 | 13.91 | 5.29 | 1.49 | 5.02 | 1.49 | 7.72 | 0.006 | 1.590 | 0.209 | 2.12 | 0.147 |
| High frequency power (AP) | 14.95 | 14.72 | 15.46 | 18.77 | 9.11 | 6.81 | 9.81 | 6.24 | 7.13 | 0.008 | 0.080 | 0.780 | 0.00 | 0.965 | |
| Mean distance (ML) | 0.02 | 0.02 | 0.03 | 0.04 | 0.01 | 0.01 | 0.02 | 0.01 | 10.14 | 0.002 | 4.270 | 0.040 | 2.78 | 0.097 | |
| Normalized jerk (AP) | 1.24 | 0.52 | 1.17 | 0.43 | 1.16 | 0.24 | 1.01 | 0.19 | 3.53 | 0.062 | 3.020 | 0.084 | 0.44 | 0.507 | |
| Path length (AP) | 4.71 | 3.19 | 7.72 | 9.90 | 3.98 | 1.33 | 3.86 | 1.20 | 6.94 | 0.009 | 2.740 | 0.099 | 3.20 | 0.075 | |
| Mean frequency | 0.57 | 0.30 | 0.53 | 0.25 | 0.53 | 0.14 | 0.44 | 0.10 | 3.24 | 0.073 | 3.020 | 0.084 | 0.67 | 0.414 | |
| Normalized jerk | 5.45 | 2.63 | 4.99 | 1.46 | 5.09 | 0.94 | 4.47 | 0.74 | 2.39 | 0.124 | 3.580 | 0.060 | 0.08 | 0.778 | |
| Range of acceleration | 0.39 | 0.22 | 0.60 | 0.51 | 0.36 | 0.13 | 0.38 | 0.14 | 6.44 | 0.012 | 5.870 | 0.016 | 3.61 | 0.059 | |
| Total sway area | 0.01 | 0.01 | 0.02 | 0.06 | 0.00 | 0.00 | 0.00 | 0.00 | 4.78 | 0.030 | 2.510 | 0.115 | 2.57 | 0.111 | |
| Mean velocity (ML) | 0.04 | 0.03 | 0.06 | 0.06 | 0.03 | 0.02 | 0.03 | 0.02 | 6.80 | 0.010 | 5.73 | 0.018 | 2.27 | 0.134 | |
| Wilks’ lambda | 1.89 | 0.043 | 2.25 | 0.014 | 0.57 | 0.849 | |||||||||
Within-groups results assessing correlation of measures with severity
The global measure of explained variance (R2), the F statistic (F) and p-values (p) obtained from the multiple least squares regression models predicting five PD severity measures (MDS-UPDRS-II, MDS-UPDRS-III, PIGD subscore, PDQ39-mobility, and H&Y stage) from the iTUG and iSway variables selected for disease severity analysis are shown (Table 6). The predictors related significantly to all five criteria with R2 values ranging from 0.18 to 0.61. Effects were stronger for iTUG than iSway for objective, exam-based scores, but they were comparable for the two subjective, questionnaire-based measures of disability.
Table 6.
Multiple linear regression models relating select clinical measures to iTUG and iSway variables: F statistics, squared correlation coefficients (R2), and p values.
| iTUG Variables for disease severity | iSway Variables for disease severity | |||||
|---|---|---|---|---|---|---|
| Measure | F | R2 | p-val | F | R2 | p-val |
| MDS-UPDRS part II | 2.18 | 0.18 | 0.017 | 2.67 | 0.21 | 0.003 |
| MDS-UPDRS part III | 5.22 | 0.34 | 0.000 | 3.05 | 0.23 | 0.001 |
| MDS-UPDRS part III PIGD subscore | 16.22 | 0.61 | 0.000 | 6.44 | 0.39 | 0.000 |
| PDQ39-mobility | 5.86 | 0.37 | 0.000 | 5.91 | 0.37 | 0.000 |
| Hoehn & Yahr stage | 6.95 | 0.41 | 0.000 | 5.66 | 0.36 | 0.000 |
Since our PD cohort included a wide range of patients, we specifically compared de-novo, stable (no off time), and fluctuator (at least some off time) patients using ANCOVA (Table 7). All treated patients were examined in the medication on state. The three groups were comparable in age (p = 0.9), but fluctuators had significantly worse MDS-UPDRS part III scores (p = 0.005) and PIGD subscores (p=0.002). Two iTUG and 7 iSway variables were significantly worse in fluctuators. When the analysis was repeated controlling for disease severity there were no differences between the groups.
Table 7.
Comparing PD patient types: group means, standard deviations and p values for select clinical measures and iTUG and iSway disease severity variables.
| PD de-novo (N=15) | PD stable (N=69) | PD fluctuator (N=51) | F* | p-val* | F** | p-val** | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Measure | Mean | SD | Mean | SD | Mean | SD | |||||
| Age | 62.73 | 8.14 | 64.19 | 11.06 | 64.12 | 8.70 | 0.14 | 0.871 | 0.35 | 0.704 | |
| MDS-UPDRS part III | 24.20 | 11.41 | 23.14 | 10.22 | 30.61 | 15.06 | 5.51 | 0.005 | |||
| MDS-UPDRS part III PIGD subscore | 2.00 | 1.51 | 1.71 | 1.65 | 3.24 | 3.11 | 6.60 | 0.002 | 1.84 | 0.164 | |
| iTUG | Gait: Stride Length [CoV] | 0.02 | 0.01 | 0.02 | 0.01 | 0.03 | 0.03 | 4.87 | 0.009 | 1.96 | 0.144 |
| Gait: Stride Length [Mean] | 80.62 | 6.90 | 78.28 | 7.67 | 76.06 | 11.62 | 1.66 | 0.193 | 0.64 | 0.530 | |
| Turn To Sit: Duration | 3.98 | 0.66 | 4.17 | 0.77 | 4.36 | 1.12 | 1.21 | 0.301 | 0.47 | 0.628 | |
| Gait: RoM Knee [Mean] | 55.06 | 5.08 | 53.89 | 5.26 | 52.42 | 5.49 | 1.88 | 0.156 | 0.62 | 0.539 | |
| Gait: Stride Velocity [Mean] | 72.32 | 7.03 | 72.34 | 8.51 | 69.79 | 11.81 | 1.07 | 0.345 | 0.02 | 0.977 | |
| Gait: RoM Shank L [Mean] | 75.52 | 6.99 | 73.72 | 9.95 | 71.52 | 10.82 | 1.20 | 0.305 | 0.34 | 0.715 | |
| Gait: Stride Velocity [CoV] | 0.03 | 0.01 | 0.04 | 0.02 | 0.05 | 0.03 | 2.49 | 0.087 | 0.72 | 0.488 | |
| Total Duration | 16.42 | 1.79 | 16.78 | 3.29 | 18.06 | 6.40 | 1.38 | 0.256 | 0.17 | 0.844 | |
| Gait: RoM Shank [CoV] | 0.02 | 0.01 | 0.02 | 0.01 | 0.03 | 0.03 | 3.26 | 0.042 | 1.17 | 0.314 | |
| Gait: RoM Knee L [Mean] | 57.21 | 5.03 | 56.28 | 5.96 | 55.26 | 5.16 | 0.89 | 0.412 | 0.27 | 0.767 | |
| iSway | Centroidal frequency (AP) | 0.54 | 0.21 | 0.57 | 0.18 | 0.68 | 0.31 | 3.57 | 0.031 | 1.41 | 0.249 |
| Path length | 5.37 | 1.78 | 6.58 | 7.80 | 12.57 | 14.77 | 5.42 | 0.006 | 2.97 | 0.055 | |
| Jerk (ML) | 0.00 | 0.00 | 0.01 | 0.04 | 0.04 | 0.13 | 2.77 | 0.067 | 0.97 | 0.383 | |
| Mean frequency | 0.45 | 0.09 | 0.50 | 0.14 | 0.65 | 0.41 | 5.34 | 0.006 | 2.77 | 0.066 | |
| Median frequency (AP) | 0.33 | 0.07 | 0.33 | 0.07 | 0.42 | 0.35 | 2.58 | 0.080 | 0.79 | 0.454 | |
| Normalized jerk | 4.61 | 0.70 | 4.96 | 1.07 | 5.85 | 3.29 | 3.20 | 0.044 | 1.33 | 0.268 | |
| Mean distance (ML) | 0.02 | 0.01 | 0.02 | 0.03 | 0.04 | 0.04 | 4.30 | 0.016 | 2.39 | 0.095 | |
| Path length (AP) | 4.10 | 1.63 | 4.75 | 5.77 | 8.27 | 8.87 | 4.49 | 0.013 | 2.73 | 0.069 | |
| Centroidal frequency | 0.67 | 0.18 | 0.72 | 0.19 | 0.82 | 0.27 | 4.55 | 0.012 | 2.62 | 0.076 | |
| Frequency dispersion (ML) | 0.74 | 0.10 | 0.70 | 0.10 | 0.69 | 0.12 | 1.69 | 0.189 | 1.22 | 0.299 | |
ANOVA (comparing means of the 3 groups)
ANCOVA (comparing means of the 3 groups while controlling for disease severity as measured by MDS-UPDRS3 part III)
Discussion
We present the results of our analysis of quantitative gait (iTUG) and balance (iSway) parameters in a large cohort of early-to-moderate PD patients and controls from which we identified two sets of clinically meaningful iTUG and iSway measures; one that can identify the presence of disease and a second set which can estimate disease severity. iTUG is slightly more sensitive than iSway in identifying disease, and it also correlates better with objective measures of disease severity. There was no difference in gait and balance performance among patients based on treatment status (de novo, non-fluctuator, or fluctuator) when controlling for disease severity. Both iTUG and iSway variables were significantly influenced by age and gender in both PD patients and controls.
The first main finding of our study was that both iTUG and iSway variables are useful in differentiating PD from control subjects. We purposefully chose to limit the number of variables to 10 from each group to show that meaningful clinical conclusions can be drawn from a limited number of predictors. iTUG performed slightly better than iSway with model accuracy exceeding 80%. We further demonstrated that gait performance as measured by iTUG is abnormal compared to control subjects even in very early disease as measured by the PIGD subscore, while iSway measures remain within normal limits for PIGD subscores less than 2. Accordingly, iTUG exhibits slightly higher sensitivity compared to iSway (75% vs 65% for iTUG and iSway when specificity is set to 75%).
The second main finding is that both iTUG and iSway measures are good predictors of PD disease severity as measured by the five clinical outcomes employed. As expected, the PIGD subscore of MDS-UPDRS part III correlated most strongly with the gait and balance parameters. Correlation with MDS-UPDRS part III was somewhat weaker as other features of PD such as limb rigidity, tremor, and speech captured by this score do not influence gait or balance. Interestingly, iTUG correlated better with objective, exam-based measures of disease severity, but iSway was the same or slightly better for subjective, questionnaire-based measures of disease severity (motor activities of daily living). We can only speculate that other clinical characteristics (such as cognitive function, sleep quality or mood symptoms) may affect patients’ perception of motor performance and disproportionately affect balance performance. Given that our selected sets of iTUG and iSway measures correlate with PD severity within a relatively narrow severity range, we anticipate that these sets of measures may be useful as biomarkers of PD progression.
Our analysis highlights two additional important points regarding the design and conduct of gait and balance studies. First, both age and gender significantly influence gait and balance measures. This is true both for PD subjects as well as controls. It is therefore important to account for these characteristics when performing similar studies, and to attempt to recruit cohorts matched for age and gender. This is particularly difficult in regard to gender because PD studies typically enroll more men as the disease is more prevalent in men. In our analysis, we compensated for this by adding age and gender as covariates in regression analyses and used type III sum of square to account for unbalanced groups (as implemented in SAS). We have also found that PD women had somewhat worse balance than PD men despite comparable disease severity scores and medications. Gender alone does not explain this observation since control women had slightly better balance than control men, a finding which has been previously demonstrated in healthy elderly people.24
Second, there was no fundamental difference in gait and balance characteristics of PD patients in regards to treatment status. Our cohort included patients who were untreated, those who had stable responses to treatment and those who experienced wearing off. Patients with wearing off had more advanced disease, but once that was controlled for they were comparable to the rest of the cohort. It is important to note that we examined patients in their morning on state, so we cannot comment if the same conclusion regarding treatment status holds when patients are evaluated in an untreated state. Although previous studies have shown that dopaminergic treatment can impact balance performance,25, 26 we did not observe this in our study when we compared treated and untreated patients as a group (as opposed to measuring change in performance off and on medications in a single subject). This property of being insensitive to treatment status represents a major strength of a putative biomarker since one is more interested in the progress of the underlying disease than in symptoms which can be masked by medication.
Careful variable selection was crucial because the APDM® Mobility Lab generates data on so many variables that differences between PD and control subjects are to be expected by chance. Our major challenge therefore was to reduce the dimensionality of this data in a meaningful way. We verified the robustness of our variable sets by using multiple randomized subsets drawn from our cohort. These variables should therefore be applicable to any cohort of PD patients with mild-to-moderate disease severity (average H&Y score of 2). Both sets of predictors perform reasonably well for both diagnosis prediction and disease severity tracking (data not shown), but by design each was optimized for the specific purpose. Several chosen iTUG variables were “lateralized” (referring to either left or right body part), but given that there were equal numbers of left and right variables we do not believe this was necessarily related to subjects’ handedness or more symptomatic side. Those particular variables may have been selected by chance, and had our correlation coefficient cutoff been less stringent we could have included only non-lateralized variables. A similar finding occurred for iSway variables where we discarded some useful measures just because they were highly intercorrelated and therefore did not contribute any additional useful information (see Supplemental Table for the list of all available variables and their mutual correlation coefficients). So while there is a certain degree of flexibility when choosing clinically pertinent variables, it is clear that some are significantly more informative than others.
Our selected iTUG and iSway variables show many similarities, but also some important differences when compared to prior reports where similar movement sensors (Physilog for iTUG and lumbar MTX Xsens sensor for iSway) were employed for comparison of PD patients and controls.17–19, 21 Of note, these studies used similar cohorts of 12–13 untreated patients, and PD diagnosis was not confirmed by imaging or response to treatment. Both theirs and our study of iTUG identified turning, turn-to-sit and trunk-related parameters as significant when compared to controls. We found that arm swing parameters were only modestly pertinent, while lower extremity measures were more significant. We also found that stride length was very significant, while gait cadence was not. Overall, the biggest difference between ours and prior studies is the much larger number of subjects in the present study, the majority of whom were treated and examined in the medicated state.
Limitations of our study include the unbalanced numbers of subjects and controls, the preponderance of men in the PD group and women in the control group, the lack of longitudinal data to directly evaluate these tools as markers of disease progression, and the lack of sufficient numbers of PD subjects with more advanced disease. We accounted for the gender imbalance by including gender as a covariate in all analyses, and by performing balanced sum of squares analysis. Our results are applicable to early stage PD and cannot be extrapolated to suggest a role in monitoring disease progression at this time. However, we are actively collecting longitudinal clinical, iTUG and iSway data on this cohort (along with new study enrollees) and will analyze longitudinal results in a future project.
In summary, our data suggests that the APDM® Mobility Lab is a useful device for characterizing gait and balance in a cohort of early PD subjects. We demonstrated that our selected sets of iTUG and iSway variables correlate highly with diagnosis and disease severity. Age and gender significantly correlate with gait and balance measures and must be accounted for in these types of studies. The gait and balance measurement system used in this study is relatively inexpensive and easy to use in a clinical setting which makes it attractive for large multicenter studies of gait and balance in PD. Our long term goal is to validate these measures in a longitudinal study of PD subjects and evaluate them as potential biomarkers of disease progression. In addition, our identification of these sets of iTUG and iSway variables may facilitate future studies aimed at identifying better treatment options for gait and balance impairment in PD.
Supplementary Material
Highlights.
We evaluated the APDM® Mobility Lab in Parkinson disease.
We employed a statistical method to reduce the variables of interest.
We found a set of 20 variables that differentiate PD from controls.
Another set of 20 variables correlate with symptom severity.
This device may be useful for objectively tracking disease progression.
Acknowledgments
This study was supported by NINDS grant U01NS082148. We thank Heather Askew, Julia Koch, Beverly Romero, William Thayer, and Ashley Gerald for their help with study coordination, Joan Reisch and Guanghua Xiao for their input into the statistical analysis, Patty Smith for her help with gait lab assessments, Lesli Brown for administrative assistance, Charlene Supnet for editorial assistance, Lars Holmstrom for technical assistance with Mobility Lab, and Dwight German for his contributions to the PDBP project at UT Southwestern.
Footnotes
Author Contributions:
D.C. Dewey: Study design, drafting/revising the manuscript for content, analysis and interpretation of data.
S. Miocinovic: Study design, drafting/revising the manuscript for content, analysis and interpretation of data, and statistical analysis.
I. Bernstein: Study design, drafting/revising the manuscript for content, analysis and interpretation of data, and statistical analysis.
P. Khemani: Study design, drafting/revising the manuscript for content, analysis and interpretation of data, acquisition of data.
R.B. Dewey, III: Study design, analysis and interpretation of data, acquisition of data.
R. Querry: Study design, drafting/revising the manuscript for content, analysis and interpretation of data, acquisition of data.
S. Chitnis: Study design, drafting/revising the manuscript for content, acquisition of data.
R.B. Dewey, Jr.: Study design, drafting/revising the manuscript for content, analysis and interpretation of data, study supervision, obtaining funding, acquisition of data.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Defebvre L, Blatt JL, Blond S, et al. Effect of thalamic stimulation on gait in Parkinson disease. Arch Neurol. 1996;53:898–903. doi: 10.1001/archneur.1996.00550090108016. [DOI] [PubMed] [Google Scholar]
- 2.Fritz B, Rombach S, Godau J, et al. The influence of Nordic Walking training on sit-to-stand transfer in Parkinson patients. Gait Posture. 2011;34:234–238. doi: 10.1016/j.gaitpost.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Johnsen EL, Mogensen PH, Sunde NA, et al. Improved asymmetry of gait in Parkinson’s disease with DBS: gait and postural instability in Parkinson’s disease treated with bilateral deep brain stimulation in the subthalamic nucleus. Mov Disord. 2009;24:590–597. doi: 10.1002/mds.22419. [DOI] [PubMed] [Google Scholar]
- 4.Krystkowiak P, Blatt JL, Bourriez JL, et al. Effects of subthalamic nucleus stimulation and levodopa treatment on gait abnormalities in Parkinson disease. Arch Neurol. 2003;60:80–84. doi: 10.1001/archneur.60.1.80. [DOI] [PubMed] [Google Scholar]
- 5.Hausdorff JM. Gait variability: methods, modeling and meaning. J Neuroeng Rehabil. 2005;2:1–9. doi: 10.1186/1743-0003-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chien SL, Lin SZ, Liang CC, et al. The efficacy of quantitative gait analysis by the GAITRite system in evaluation of parkinsonian bradykinesia. Parkinsonism Relat Disord. 2006;12:438–442. doi: 10.1016/j.parkreldis.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Kegelmeyer DA, Parthasarathy S, Kostyk SK, et al. Assistive devices alter gait patterns in Parkinson disease: Advantages of the four-wheeled walker. Gait Posture. 2013;38:20–24. doi: 10.1016/j.gaitpost.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 8.Lohnes CA, Earhart GM. The impact of attentional, auditory, and combined cues on walking during single and cognitive dual tasks in Parkinson disease. Gait Posture. 2011;33:478–483. doi: 10.1016/j.gaitpost.2010.12.029. [DOI] [PubMed] [Google Scholar]
- 9.Williams AJ, Peterson DS, Earhart GM. Gait coordination in Parkinson disease: Effects of step length and cadence manipulations. Gait Posture. 2013;38:340–344. doi: 10.1016/j.gaitpost.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Webster KE, Wittwer JE, Feller JA. Validity of the GAITRite walkway system for the measurement of averaged and individual step parameters of gait. Gait Posture. 2005;22:317–321. doi: 10.1016/j.gaitpost.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Hsiao IT, Weng YH, Hsieh CJ, et al. Correlation of Parkinson Disease Severity and 18F-DTBZ Positron Emission Tomography. JAMA Neurol. 2014;71:758–766. doi: 10.1001/jamaneurol.2014.290. [DOI] [PubMed] [Google Scholar]
- 12.Pickerill ML, Harter RA. Validity and reliability of limits-of-stability testing: a comparison of 2 postural stability evaluation devices. J Athl Train. 2011;46:600–606. doi: 10.4085/1062-6050-46.6.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lundin F, Ledin T, Wikkelso C, et al. Postural function in idiopathic normal pressure hydrocephalus before and after shunt surgery: A controlled study using computerized dynamic posturography (EquiTest) Clin Neurol Neurosurg. 2013;115:1626–1631. doi: 10.1016/j.clineuro.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Ondo W, Warrior D, Overby A, et al. Computerized posturography analysis of progressive supranuclear palsy: a case-control comparison with Parkinson’s disease and healthy controls. Arch Neurol. 2000;57:1464–1469. doi: 10.1001/archneur.57.10.1464. [DOI] [PubMed] [Google Scholar]
- 15.Nocera JR, Horvat M, Ray CT. Impaired step up/over in persons with Parkinson’s disease. Adapt Phys Activ Q. 2010;27:87–95. doi: 10.1123/apaq.27.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mancini M, King L, Salarian A, et al. Mobility Lab to Assess Balance and Gait with Synchronized Body-worn Sensors. J Bioengineer & Biomedical Sci. 2012;S1:1–5. doi: 10.4172/2155-9538.S1-007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mancini M, Carlson-Kuhta P, Zampieri C, et al. Postural sway as a marker of progression in Parkinson’s disease: a pilot longitudinal study. Gait Posture. 2012;36:471–476. doi: 10.1016/j.gaitpost.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mancini M, Salarian A, Carlson-Kuhta P, et al. ISway: a sensitive, valid and reliable measure of postural control. J Neuroeng Rehabil. 2012;9:59. doi: 10.1186/1743-0003-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salarian A, Horak FB, Zampieri C, et al. iTUG, a sensitive and reliable measure of mobility. IEEE Trans Neural Syst Rehabil Eng. 2010;18:303–310. doi: 10.1109/TNSRE.2010.2047606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salarian A, Zampieri C, Horak FB, et al. Analyzing 180 degrees turns using an inertial system reveals early signs of progression of Parkinson’s disease. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:224–227. doi: 10.1109/IEMBS.2009.5333970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zampieri C, Salarian A, Carlson-Kuhta P, et al. The instrumented timed up and go test: potential outcome measure for disease modifying therapies in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2010;81:171–176. doi: 10.1136/jnnp.2009.173740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes AJ, Daniel SE, Kilford L, et al. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peto V, Jenkinson C, Fitzpatrick R. PDQ-39: a review of the development, validation and application of a Parkinson’s disease quality of life questionnaire and its associated measures. J Neurol. 1998;245 (Suppl 1):S10–14. doi: 10.1007/pl00007730. [DOI] [PubMed] [Google Scholar]
- 24.Basta D, Rossi-Izquierdo M, Soto-Varela A, et al. Mobile posturography: posturographic analysis of daily-life mobility. Otol Neurotol. 2013;34:288–297. doi: 10.1097/MAO.0b013e318277a29b. [DOI] [PubMed] [Google Scholar]
- 25.Beuter A, Hernandez R, Rigal R, et al. Postural sway and effect of levodopa in early Parkinson’s disease. Can J Neurol Sci. 2008;35:65–68. doi: 10.1017/s0317167100007575. [DOI] [PubMed] [Google Scholar]
- 26.Revilla FJ, Larsh TR, Mani A, et al. Effect of dopaminergic medication on postural sway in advanced Parkinson’s disease. Front Neurol. 2013;4:202. doi: 10.3389/fneur.2013.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.