Abstract
T cells are charged with surveying tissues for evidence of their cognate foreign antigens. Subsequently, they must navigate to effector sites, which they enter through the process of trans-endothelial migration (TEM). During interstitial migration, T cells migrate according to one of two modes that are distinguished by the strength and sequence of adhesions and the requirement for Myosin-IIA. In contrast during TEM, T cells require Myosin-IIA for the final process of pushing their nucleus through the endothelium. A generalized model emerges with dual roles for Myosin-IIA: This motor protein acts like a tensioning or expansion spring, transmitting force across the cell cortex to sites of surface contact and also optimizes the frictional coupling with substrata by modulating the surface area of the contact. The phosphorylation and deactivation of this motor following TCR engagement can allow T cells to rapidly alter the degree to which they adhere to surfaces and to switch to a mode of interaction with surfaces that is more conducive to forming a synapse with an antigen-presenting cell.
Introduction
T cells spend the vast majority of their lifespan in a motile phase during which they scan surfaces of the host seeking to engage their surface-bound T cell receptors (TCRs) [1,2]. Each TCR recognizes a combination of an antigenic peptide fragment bound into a host Major Histocompatibility Complex (MHC) molecule, but most of the host MHC molecules do not or never will bear the correct peptide antigen for TCRs to generate a signal in the T cell. At the cellular level, T cells must therefore engage with surfaces in a way that is both sensitive but also typically transient. If engagements are too short, their TCRs may not engage or find the few MHC molecules loaded with their designated peptide (hereafter ‘pMHC’). If engagements are too long, the T cell will not make the circuit of the host at a rate consistent with efficient immune protection. Clearly, a degree of surface contact is required in order to make membrane-membrane juxtapositions. However were the T cell to contact and interact with its environment with all of its surfaces at once, it would experience significant frictional drag, thus slowing its motility capabilities.
A T cell's motile scanning pattern within lymph nodes has been described as a random walk [3] and in other tissues may also have occasional larger jumps reminiscent of Levy flights [4]. To this extent, we typically assume that T cell migration patterns have been optimized to ‘search’ for antigens in the form of pMHC complexes. T cell motility, which can peak at nearly 25um/min, slows considerably upon the recognition of cognate antigens. Ultimately T cells appear to ‘stop’ and form a conjugate with the pMHC-bearing antigen-presenting cell (APC). At this point, the junction or ‘synapse’ appears to be the dominant T cell behavior and T cells secrete cytokines and signal back-and-forth with APCs and sometimes other T cells for many hours. The synapse profits from more durable and profound membrane contact with the partner APC.
In addition to this nomadic scanning, T cells must squeeze their way into and out of tissues. This allows T cells to exit the blood and reach either new lymph nodes to survey or inflamed tissues, and then to enter the lymphatics to eventually re-enter the blood circulation [5]. T cell exit and entry into vasculature requires the T cell to change the mechanics of its approach to interacting with surfaces since, rather than seeking to glide along an interstitium, or forming an adhesive synapse, the primary reason to contact a surface is now to penetrate it.
Comparing all these requirements, one can see that a T cell needs to utilize many or all of the tools available for eukaryotic cell migration. While other cell types may undergo epithelial-to-mesenchymal (EMT) [6] changes in their motility once in a lifetime, for example as a means for tumor cells to extravasate, T cells need to make these changes every few hours as they move from lymph nodes (or tissues) to the blood and back again, and especially when they recognize pMHC complexes. T cells must also be able to go nearly everywhere in the body, entering and traversing tissues with distinct adhesive environments. In this review, we will outline our understanding of the multiple ‘modes’ by which T cells approach the various surfaces of the different environments they encounter. We will particularly emphasize the role that Myosin-IIA plays as an adjustable force-generating tension system in facilitating many of these diverse motility modes.
Multiple Modes of T cell Motility
Recent data from various groups has highlighted that amoeboid cells such as leukocytes are not restricted to adhesion-dependent motility as described in the ‘haptokinetic’ model defined for fibroblast-like cells. The haptokinetic model proposes that cells bind integrins at the front of the cell and release them at the back, effectively ‘treadmilling’. This requires a certain level of adhesion to convert actin-myosin cytoskeletal protrusion and contraction into forward movement, with maximal migration rates achieved at intermediate adhesion levels [7,8]. However, in a key paper, Sixt and colleagues have shown that dendritic cells in 3-dimensional environments, both in vitro and in vivo, can migrate in the absence of any integrin-based adhesion to the substrate [9]. This is most prominent in 3-dimensional contexts where they can squeeze through fixed lattices. In follow-on work, dendritic cells were further shown to be able to adapt to varying levels of substrate adhesion by modulating their actin polymerization rate [10]. When integrin adhesion is available as a clutch for the cell's actin cytoskeleton, actin polymerization is directly coupled to forward protrusion of the leading edge. Alternatively, in the absence of an integrin clutch mechanism to the underlying substrate, the rate of actin polymerization is increased to compensate for actin filament slippage, allowing dendritic cells to maintain their ability to crawl in response to chemotactic signals over varying substrates [10]. While these data do not exclude that other non-integrin trans-membrane adhesion molecules can function as a clutch mechanism to the substrate, motility in the absence of force-coupling can be explained through actin polymerization-based perpendicular pushing against non-adhesive surfaces [11].
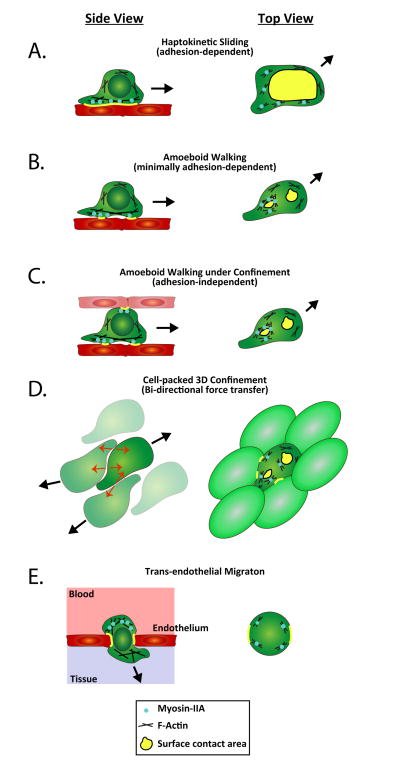
As we will discuss, T cells can use a haptokinetic motility mode with a large contact area with the substrate [12-14]. However, Woolf et al. have shown that LFA-1 adhesion has only a minor contribution to motility in the lymph nodes [15], and studies of T cell motility in 3-dimensional collagen matrices showed that integrin inhibition did not affect motility [16]. This suggested that, like their leukocyte cousins, dendritic cells, T cells can also move independently of integrins. Using TIRF-based imaging approaches, we have shown that T cells can in fact rapidly switch between adhesion-dependent motility and an ‘amoeboid’ motility mode that is not strictly dependent on specific sources of adhesion [13]. In addition, we found that these two T cell motility modes have strikingly different patterns and sequences of adhesions to the substrate, which are formed with different mechanics (Figure 1). The first adhesion- dependent mode closely resembles the motility model proposed for fibroblast-like adherent cells (‘haptokinetic sliding’) and is promoted by a high density of integrin ligand availability (Figure 1A). The second ‘amoeboid walking’ motility mode is characterized by a unique pattern of discontinuous sequential surface contact areas with the substrate and is most prominently utilized in lower adhesion environments (Figure 1B). During T cell amoeboid motility, the contact areas with the substrate are small and short lived, with new contacts formed at the front of the cell and old ones extinguished at the back. This allows T cells to ‘jump’ from one area of substrate contact to another instead of constantly tracking along a single predetermined surface, using a continuously adhesive substrate [13]. Recently, using Fourier traction force microscopy, a similar motility mode generating 2-3 contact areas that simultaneously transmit force to the substrate was reported for other amoeboid cells, including Dictyostelium and neutrophils [17]. This finding suggests that amoeboid motility using multiple small and transient surface contacts is a general phenomenon in amoeboid cells. We also found that the haptokinetic motility mode is slower and that the maximized surface contact areas can provide T cells with a more complete scan of their environment. In contrast, the amoeboid motility mode is faster and maintains only limited surface contact and is thus potentially better suited to rapidly follow chemotactic gradients. As we will discuss, one possibility is that the extensive adhesions of the haptokinetic motility mode generate considerable frictional drag whereas the smaller, quickly extinguishing contacts of the amoeboid motility mode reduce this.
Figure 1.
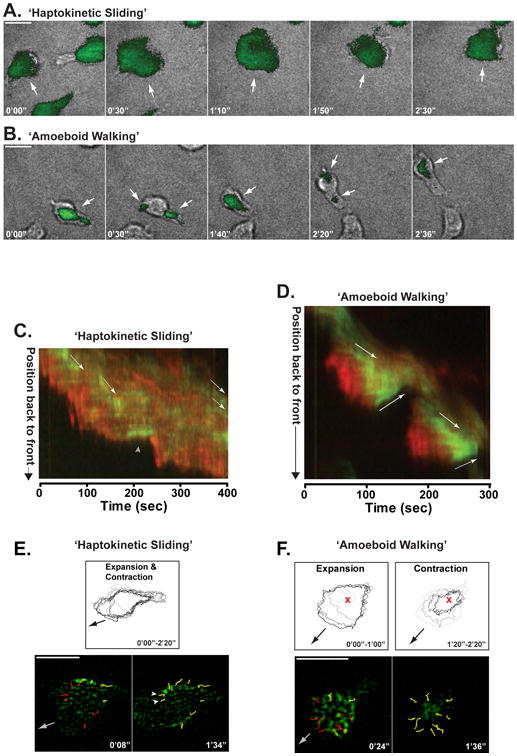
Primary T cells exhibit dual-crawling modes. A. & B.) T cells were labeled with 0.25 M CFSE, plated overnight in chamberslides coated with casein or ICAM-1 and then imaged by TIRF microscopy at 2 s intervals for 5 min. Individual time-points, from left to right, chosen from live imaging time-courses are shown. Brightfield images are overlayed with a TIRF image of the CFSE fluorescence. White arrows highlight separate adhesion areas. Scale bars = 10μm. A.) Representative ‘haptokinetic sliding’ primary T cell crawling on ICAM-1. B.) Representative ‘amoeboid walking’ primary T cell crawling on a casein blocked surface. C. & D.) Kymograph renditions of Myosin-IIA (green) and actin (red) distribution along the path of migration of a representative ‘haptokinetic sliding’ T cell (in C) and a representative ‘amoeboid walking’ T cell (in D). The overlay of the fluorescent intensities of MyosinIIA-GFP (green) and actin-mCherry (red) is shown for each point along a line following the cell path during the time-course. The movement of Myosin-IIA clusters is highlighted by arrows running parallel to the clusters. The gray arrowhead in C indicates a point at which the cell made a turn. E. & F.) Myosin-IIA cluster coalescence in the adhesion areas of crawling T cells. Scale bars = 10μm. E.) Top panel, the outlines of an adhesion area from a ‘haptokinetic sliding’ T cell are shown. The thresholded outlines of an adhesion area were traced at 20 s time intervals, color-coded from gray to black and overlayed to show the contraction and expansion of the adhesion area. The black arrow indicates the general direction of cell migration. E.) Bottom panels, tracking of selected Myosin-IIA clusters in the adhesion area of a ‘haptokinetic sliding’ cell. White arrowheads highlight Myosin-IIA clusters that move against the general direction of crawling of the cell. The complete paths of the clusters are overlayed in red, the tracks transition to yellow as each cluster moves along its path. The gray arrow shows the general direction of cell migration. F.) Top panels, adhesion area expansion and contraction in an ‘amoeboid walking’ T cell. The thresholded outlines of a adhesion area were traced at 20 s time intervals, color-coded from gray to black and overlayed to show the contraction and expansion of the adhesion area. The expansion and contraction phases (color-coded from gray to black) are shown separately to highlight the distinct phases of the adhesion area behavior during ‘amoeboid walking’. The red x is shown as a reference point; the black arrow indicates the general direction of cell migration. F.) Bottom panels, tracking of selected Myosin-IIA clusters in the adhesion area of an ‘amoeboid walking’ cell. The complete paths of the clusters are overlayed in red, the tracks transition to yellow as each cluster moves along its path. The gray arrow shows the general direction of cell migration.
We extended this finding and supported the idea of frictional drag through studies where we varied the degree to which T cells were confined into tight regions while migrating. Experimentally, this was achieved through microfabrication of channels of varying sizes, which can mimic different amounts of unoccupied interstitial space and stiffness within physiological tissues. In small channels (4μm), T cells were squeezed and became extremely elongated and were effectively forced to contact the substrate (channel walls) all along their cell body. As channels got wider, they were able to spread laterally and had more latitude to control both the number and extent of their adhesions. Overall, we found that, in addition to integrin ligand availability, T cell motility modes can also be regulated by the degree of environmental confinement (or potentially the density of cells in vivo) [14]. Therefore, when migrating in 3-dimensional environments that are not extremely tightly packed, T cells can adapt to the degree of adhesiveness and confinement of their environment by using the amoeboid motility mode to achieve efficient migration. A motility ‘optimum’ is reached when T cells can exert force on multiple surrounding surfaces while limiting their adhesion to the substrate; this optimum allows T cells to optimize force- coupling and minimize frictional drag. T cells showed a bell curve distribution in their migration speed relative to the confinement level within 3-dimensional environments [14]. The bell-shape of this curve was still present when integrins coated the microchannel walls, indicating that the ability to optimize was independent of whether there was a specific source of adhesion. In contrast, we found that this bell-shaped optimum was lost when cells lacked Myosin-IIA and, as discussed below, thus lacked the ability to control their cortical tension.
Myosin-IIA Regulates T cell Motility Modes
Myosin-IIA is the only class II myosin expressed in mouse T cells. It is a filamentous motor that is well-established for its role in haptokinetic migration [18,19]. In haptokinetic migration, it functions behind the leading edge lamellopodia, in adhesive contacts within the lamella, and appears to help coordinate retrograde flow [20,21]. We found that T cells are able to migrate, albeit at a significantly slower velocity, even when they lack Myosin-IIA activity. TIRF imaging showed that in the absence of this motor protein they exclusively use the hapokinetic ‘sliding’ mode with continuous propagation of a single adhesion which expands and contracts relatively isometrically (Figure 1C, E) [13,14]. In the absence of Myosin-IIA, T cells also exhibit uropod retraction defects when migrating under conditions of high adhesiveness to the substrate, consistent with a requirement for this motor to ‘pull’ up the uropod at the trailing edge of the surface contact [22,23]. Although we have not undertaken tether assays to directly measure cortical tension, Myosin-IIA appears to regulate tension in organisms from yeast to mammals. We found using TIRF imaging that Myosin-IIA generally restrains adhesiveness and limits the amount of T cell surface that is spread on substrates [13,14]. In this context, Myosin-IIA is critical in promoting fast integrin-independent amoeboid T cell motility mode both in 2- and 3-dimensional environments. We propose that within the small adhesions formed during amoeboid motility, the role of Myosin-IIA is to regulate T cell interaction with the substrate by both mediating cyclical rear-mediated compressions that eliminate existing adhesions and by licensing subsequent adhesions at the front of the cell [13,14] (Figure 1D, F). These properties of Myosin-IIA are likely conserved, at least to a degree, since this motor protein has a role in controlling spreading and motility modes in various cell types including fibroblasts [24] and fish keratocytes [25]. A cartoon model of the motile behaviors of T cells is shown in Figure 2.
Figure 2.
Diverse Modes of T cell Motility. T cell motility modes are shown from side and top perspectives and in multiple tissue contexts. A.) A side view of the haptokinetic mode shows a cell with a single dominant contact along its bottom edge. A top view shows how the single adhesive structure with Myosin-IIA dominantly localized at the rear of this contact can help detach just this edge of the adhesion. B.) In amoeboid ‘walking’ motility, multiple points of contact are made in sequence. Myosin- IIA is localized at the periphery of the rear-most adhesions to dissemble them entirely. C.) A top view of an identical cell in which multiple contacts are made with surfaces on opposite sides of the cell, as in 3D tissue environments. D.) A specific instance of C. in which the opposing surfaces are also motile lymphocytes; force transfer can occur between multiple cells moving in opposite directions without a need for a given cell to contact a fixed structural cell. E.) Transendothelial migration in which adhesion around the rim of the pore serves to anchor the T cell and allow it to compress the nucleus through to the other side.
Myosin-IIA as a Critical Force Transfer Mechanism for Interstital and Trans-endothelial Migration
T cells traverse very different tissue environments with varying levels of adhesion and confinement. To exit the blood circulation, T cells extravasate by squeezing through a restrictive barrier formed by the endothelial cell vascular wall, in a process called trans-endothelial migration (TEM). During this step force generation by the actin-myosin cytoskeleton is required for extravasation. Actin polymerization is mainly needed for the transmigrating cell to extend protrusions through the endothelial cell barrier [26]. Myosin-IIA-mediated contractility is important to allow T cells to complete trans-endothelial migration, through its activity at the rear of the cell, force generated by this motor protein pushes the cell's cytoplasm and squeezes the rigid nucleus through openings in the endothelial cell barrier [27,28]. Indeed nuclear positioning and deformation have been proposed to play an important role during migration under 3-dimensional confinement of various cell types [9,29]. In addition to propelling the nucleus, force generation during TEM can also help de-adhesion during migration. However, the exact mechanisms of how mechanical force generated by the T cell cytoskeleton enables TEM are still unclear.
Surface Spreading controlled by Myosin-IIA and effects on 3D migration
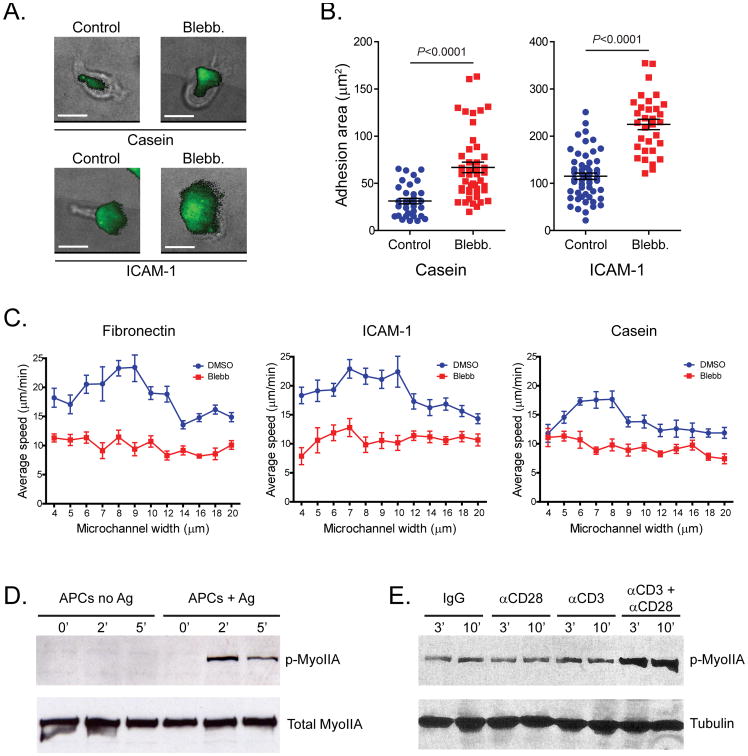
Unsurprisingly, we found that T cells lacking Myosin-IIA are very inefficient during interstitial migration in lymph nodes. The loss of cortical tension appears to license them to have greater contact areas with substrates (Figure 3A-B) and to adhere more effectively to one another [14], likely contributing a higher degree of friction to their movements in T cell-rich zones. Notably, Myosin-IIA null T cells generally ‘spread’ more onto surfaces independently of whether the substrate presents a strong adhesive ligand (e.g. ICAM-1) or not (e.g. Casein) (Figure 3B). Myosin-IIA null T cell speed is overall slower and relatively constant across confinement levels; however, T cells can reach an optimum when they can use Myosin- IIA to control their spreading (Figure 3C) [14].
Figure 3.
A.) Deregulated surface adhesion of T cells lacking myosin IIA. Adhesion areas of control and blebbistatin-treated CFSE-labeled activated CD8+ T cells plated on glass coated with casein or ICAM-1, assessed by TIRF microscopy (green) and brightfield microscopy. Scale bars, 10 μm. B.) Quantification of the adhesion areas of control and blebbistatin-treated activated CD8+ T cells on casein (left) or ICAM-1 (right). Means ±SEM are shown C.) T cell migration efficiency in confined environments is controlled by myosin IIA. Speed of dimethyl sulfoxide (DMSO)- or blebbistatin (Blebb)-treated activated CD8+ T cells injected into microchannels coated with fibronectin, ICAM-1, or casein. Data is presented as a function of the microchannel width. Means ±SEM are shown D.) Myosin-IIA is phosphorylated at threonine residues in response to TCR recognition. DO11.10 T cell blasts were mixed 2:1 with APCs pulsed with antigen, or unpulsed control APCs, and incubated at 37 °C for the indicated times (in minutes). Cell lysates were then analyzed by immunoblotting with a phosphorylation state–specific pThr1939-Myosin- IIA antibody and subsequently stripped and reblotted with a Myosin-IIA antibody. E.) Synergistic effect of CD28 co-stimulation on Myosin-IIA phosphorylation under sub-optimal CD3 stimulation conditions. Naïve CD4+ T cells were stimulated for the indicated times (in minutes) with control IgG, CD3, CD28 or a combination of CD3+CD28 antibodies. Cell lysates were then analyzed by immunoblotting with a phosphorylation state–specific pThr1939-Myosin-IIA antibody. Tubulin proteins levels are shown as a loading control.
How do T cells regulate this surface contact to efficiently detect antigen? One answer is that when TCRs recognize their antigens on the surface of an APC, a calcium signal results in phosphorylation of Myosin- IIA on residues of the heavy chain (Figure 3D) [30]. Furthermore, co-stimulation of CD4+ T cells by the CD28 co-receptor under sub-optimal TCR triggering conditions results in a synergistic increase in the levels of phosphorylated Myosin-IIA heavy chain (Figure 3E, unpublished R.S.F., J.J. and M.F.K.). The phosphorylation of these residues is significant as they are in the coil-coil domain and phosphorylation appears to disrupt Myosin-IIA ability to form higher order oligomers [31]. It is also highly likely that debundling as a result of this transient calcium rise, may allow Myosin-IIA filaments to later rebundle, for example in such a way as to provide compression as opposed to tension. Such a model has yet to be established but at least during the peak of the calcium-mediated TCR signal, it is highly likely that T cells experience decreased cortical tension that would facilitate spreading.
A Generalized Role for Myosin-IIA in Coupling Force Generation from Adhesion to Adhesion and for Modulating Cell Shape to Enhance or Minimize Adhesion Areas
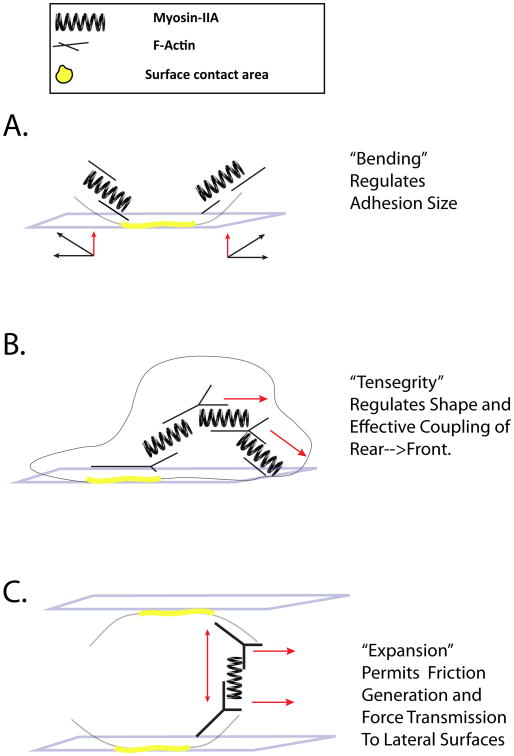
How are these observations of motility and Myosin-IIA loss related and how does this relate to the diversity of motility modes for T cells? We consider a general model in which multiple Myosin-IIA bundles can act as a series of springs within the cell: springs that globally link and locally bend the actin cortex (Figure 4). Under this model, Myosin-IIA can be considered to have multiple types of roles in optimizing interstitial migration as well as TEM. In the case of haptokinetic or amoeboid motility, the tension at the edge of the adhesion area provides a local means of bending the adhered membrane region away from the contact, thus eliminating the adhesion and releasing the uropod (Figure 4A). These membrane contacts would be bent from all sides in an amoeboid walking mode, but mainly from the rear during haptokinetic motility. This model is supported by the radially extinguishing of adhesion areas (Figure 1F) as well as by the presence of inward moving Myosin-IIA clusters during amoeboid motility (Figure 1F). As a possible mechanism of propulsion, a series of Myosin-IIA ‘springs’, emanating from an adhesion area can be modeled to transfer force through linked actin filaments to generate subsequent adhesion areas (Figure 4B). The force coming from what might be considered a ‘tensegrity’ structure provides a mechanism for force transfer from the adhesion in the rear of the cell towards the substrate in front; it also provides a lattice that can force the membrane downward to generate a new adhesion area. We note that very recent work from Lippincott-Schwartz and colleagues, examining myosin II and actin arrays in large adherent cells, supports a role for Myosin II in structurally linking distant regions of the cells [32]. Finally, in the case of amoeboid walking under confinement, it is likely that Myosin-IIA allows multiple adhesion areas on opposing surfaces to be physically coupled by coordinated actin-matrix expansion between two parts of the cell. This coupling in turn allows the cell to use cytoskeletal expansion as a basis for what is effectively torque to generate forward motion from multiple lateral contacts (Figure 4C and discussed by Piel and colleagues in detail [11]). In the case of TEM, this cytoskeletal expansion may allow the T cell cortex to couple to adhesions within the opening between endothelial cells. A separate compression force around the T cell rear may then exert force on the nucleus, squeezing it to extrude through to the other side of the endothelial cell barrier.
Figure 4.
Modeling Myosin- IIA as a spring as a means to explain diverse effects upon T cell motility. T cells are shown similarly to Figure 2. However, here actin filament orientations are proposed to account for the phenotypes observed in Myosin-IIA null T cells. A.) Membrane bending and regulation of the size of an adhesive contact. Myosin-IIA filaments oriented parallel to the membrane and near the edges of the adhesion promote membrane bending thereby regulating adhesive contacts. B.) Propulsion by force transmission through tensegrity structures. Tensegrity structures are generated by multiple Myosin-IIA filaments transducing force between multiple stretches of branched actin filaments. A much larger lattice can be envisioned but only a small one is illustrated for clarity. C.) Friction generation by cytoskeletal expansion. A specific example of forces generated via Myosin-IIA outward force between two stretches of actin filaments oriented perpendicular to two distinct contacts. Through outward force, friction is generated at each surface and the resulting internal actin structure can transmit force to the surfaces in order to generate forward movement.
Conclusions
T cells require many motility modes because the immune system needs to gain access to different kinds of tissues that have a variety of spatial organizations and levels of adhesiveness. This has given rise to a T cell that is able to utilize various force-transducing mechanisms to push and pull its way through these differing environments. It is clear that Myosin-IIA represents a key player for adapting cell motility to diverse tissues. In the event of antigen-encounter, this motor is at least transiently deactivated as the T cell briefly pauses to receive signals and deliver stimuli to its environment. It is likely that other players, notably class I myosins and actin polymerizing and bundling proteins will similarly modulate motility and thereby contribute to immune function.
Acknowledgments
This work was supported by NIH grant R01 AI052116 (MFK), and JDRF (RSF), and in part by the Multiple Sclerosis Society, the Dana Foundation and JDRF (JJ). The authors thank Steve Chmura, Chris Bennett, Priya Pandurangi, Caitlin Sorensen, and Miriam Estin Matthews for contributions to data and concepts.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sumen C, Mempel TR, Mazo IB, von Andrian UH. Intravital microscopy: visualizing immunity in context. Immunity. 2004;21:315–329. doi: 10.1016/j.immuni.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Cahalan MD, Parker I. Choreography of cell motility and interaction dynamics imaged by two- photon microscopy in lymphoid organs. Annu Rev Immunol. 2008;26:585–626. doi: 10.1146/annurev.immunol.24.021605.090620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296:1869–1873. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- 4.Harris TH, Banigan EJ, Christian DA, Konradt C, Tait Wojno ED, Norose K, Wilson EH, John B, Weninger W, Luster AD, et al. Generalized Levy walks and the role of chemokines in migration of effector CD8+ T cells. Nature. 2012;486:545–548. doi: 10.1038/nature11098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masopust D, Schenkel JM. The integration of T cell migration, differentiation and function. Nat Rev Immunol. 2013;13:309–320. doi: 10.1038/nri3442. [DOI] [PubMed] [Google Scholar]
- 6.Greenburg G, Hay ED. Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. J Cell Biol. 1982;95:333–339. doi: 10.1083/jcb.95.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiMilla PA, Barbee K, Lauffenburger DA. Mathematical model for the effects of adhesion and mechanics on cell migration speed. Biophys J. 1991;60:15–37. doi: 10.1016/S0006-3495(91)82027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- 9**.Lammermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Soldner R, Hirsch K, Keller M, Forster R, Critchley DR, Fassler R, et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453:51–55. doi: 10.1038/nature06887. This is a seminal paper describing integrin-independent motility in leukocytes. [DOI] [PubMed] [Google Scholar]
- 10.Renkawitz J, Schumann K, Weber M, Lammermann T, Pflicke H, Piel M, Polleux J, Spatz JP, Sixt M. Adaptive force transmission in amoeboid cell migration. Nat Cell Biol. 2009;11:1438–1443. doi: 10.1038/ncb1992. [DOI] [PubMed] [Google Scholar]
- 11*.Hawkins RJ, Piel M, Faure-Andre G, Lennon-Dumenil AM, Joanny JF, Prost J, Voituriez R. Pushing off the walls: a mechanism of cell motility in confinement. Phys Rev Lett. 2009;102:058103. doi: 10.1103/PhysRevLett.102.058103. This work provides a physical framework for considering force transmission to lateral surfaces in the absence of high affinity molecular adhesions. [DOI] [PubMed] [Google Scholar]
- 12.Smith A, Carrasco YR, Stanley P, Kieffer N, Batista FD, Hogg N. A talin-dependent LFA-1 focal zone is formed by rapidly migrating T lymphocytes. J Cell Biol. 2005;170:141–151. doi: 10.1083/jcb.200412032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobelli J, Bennett FC, Pandurangi P, Tooley AJ, Krummel MF. Myosin-IIA and ICAM-1 regulate the interchange between two distinct modes of T cell migration. J Immunol. 2009;182:2041–2050. doi: 10.4049/jimmunol.0803267. [DOI] [PubMed] [Google Scholar]
- 14*.Jacobelli J, Friedman RS, Conti MA, Lennon-Dumenil AM, Piel M, Sorensen CM, Adelstein RS, Krummel MF. Confinement-optimized three-dimensional T cell amoeboid motility is modulated via myosin IIA-regulated adhesions. Nat Immunol. 2010;11:953–961. doi: 10.1038/ni.1936. This work established a 3D environment-dependent regulation of T cell motility modes by Myosin-IIA. It further demonstrated the distinction between a ‘walking’ (amoeboid) and sliding (haptokinetic) mode, first described in reference 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woolf E, Grigorova I, Sagiv A, Grabovsky V, Feigelson SW, Shulman Z, Hartmann T, Sixt M, Cyster JG, Alon R. Lymph node chemokines promote sustained T lymphocyte motility without triggering stable integrin adhesiveness in the absence of shear forces. Nat Immunol. 2007;8:1076–1085. doi: 10.1038/ni1499. [DOI] [PubMed] [Google Scholar]
- 16.Friedl P, Entschladen F, Conrad C, Niggemann B, Zanker KS. CD4+ T lymphocytes migrating in three- dimensional collagen lattices lack focal adhesions and utilize beta1 integrin-independent strategies for polarization, interaction with collagen fibers and locomotion. Eur J Immunol. 1998;28:2331–2343. doi: 10.1002/(SICI)1521-4141(199808)28:08<2331::AID-IMMU2331>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 17*.Bastounis E, Meili R, Alvarez-Gonzalez B, Francois J, del Alamo JC, Firtel RA, Lasheras JC. Both contractile axial and lateral traction force dynamics drive amoeboid cell motility. J Cell Biol. 2014;204:1045–1061. doi: 10.1083/jcb.201307106. This work confirms the use of multiple adhesive contacts as a means for motility in amoeboid cells beyond T cells, notably dictyostelium and neutrophils. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol. 2009;10:778–790. doi: 10.1038/nrm2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conti MA, Adelstein RS. Nonmuscle myosin II moves in new directions. J Cell Sci. 2008;121:11–18. doi: 10.1242/jcs.007112. [DOI] [PubMed] [Google Scholar]
- 20.Ponti A, Machacek M, Gupton SL, Waterman-Storer CM, Danuser G. Two distinct actin networks drive the protrusion of migrating cells. Science. 2004;305:1782–1786. doi: 10.1126/science.1100533. [DOI] [PubMed] [Google Scholar]
- 21.Giannone G, Dubin-Thaler BJ, Dobereiner HG, Kieffer N, Bresnick AR, Sheetz MP. Periodic lamellipodial contractions correlate with rearward actin waves. Cell. 2004;116:431–443. doi: 10.1016/s0092-8674(04)00058-3. [DOI] [PubMed] [Google Scholar]
- 22.Smith A, Bracke M, Leitinger B, Porter JC, Hogg N. LFA-1-induced T cell migration on ICAM-1 involves regulation of MLCK-mediated attachment and ROCK-dependent detachment. J Cell Sci. 2003;116:3123–3133. doi: 10.1242/jcs.00606. [DOI] [PubMed] [Google Scholar]
- 23.Morin NA, Oakes PW, Hyun YM, Lee D, Chin YE, King MR, Springer TA, Shimaoka M, Tang JX, Reichner JS, et al. Nonmuscle myosin heavy chain IIA mediates integrin LFA-1 de-adhesion during T lymphocyte migration. The Journal of experimental medicine. 2008;205:195–205. doi: 10.1084/jem.20071543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai Y, Biais N, Giannone G, Tanase M, Jiang G, Hofman JM, Wiggins CH, Silberzan P, Buguin A, Ladoux B, et al. Nonmuscle myosin IIA-dependent force inhibits cell spreading and drives F-actin flow. Biophys J. 2006;91:3907–3920. doi: 10.1529/biophysj.106.084806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnhart EL, Lee KC, Keren K, Mogilner A, Theriot JA. An adhesion-dependent switch between mechanisms that determine motile cell shape. PLoS Biol. 2011;9:e1001059. doi: 10.1371/journal.pbio.1001059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nourshargh S, Hordijk PL, Sixt M. Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat Rev Mol Cell Biol. 2010;11:366–378. doi: 10.1038/nrm2889. [DOI] [PubMed] [Google Scholar]
- 27.Jacobelli J, Estin Matthews M, Chen S, Krummel MF. Activated T Cell Trans-Endothelial Migration Relies on Myosin-IIA Contractility for Squeezing the Cell Nucleus through Endothelial Cell Barriers. PloS one. 2013;8:e75151. doi: 10.1371/journal.pone.0075151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soriano SF, Hons M, Schumann K, Kumar V, Dennier TJ, Lyck R, Sixt M, Stein JV. In vivo analysis of uropod function during physiological T cell trafficking. J Immunol. 2011;187:2356–2364. doi: 10.4049/jimmunol.1100935. [DOI] [PubMed] [Google Scholar]
- 29.Friedl P, Wolf K, Lammerding J. Nuclear mechanics during cell migration. Curr Opin Cell Biol. 2011;23:55–64. doi: 10.1016/j.ceb.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobelli J, Chmura SA, Buxton DB, Davis MM, Krummel MF. A single class II myosin modulates T cell motility and stopping, but not synapse formation. Nat Immunol. 2004;5:531–538. doi: 10.1038/ni1065. [DOI] [PubMed] [Google Scholar]
- 31.Dulyaninova NG, Malashkevich VN, Almo SC, Bresnick AR. Regulation of Myosin-IIA Assembly and Mts1 Binding by Heavy Chain Phosphorylation. Biochemistry. 2005;44:6867–6876. doi: 10.1021/bi0500776. [DOI] [PubMed] [Google Scholar]
- 32*.Burnette DT, Shao L, Ott C, Pasapera AM, Fischer RS, Baird MA, Der Loughian C, Delanoe-Ayari H, Paszek MJ, Davidson MW, et al. A contractile and counterbalancing adhesion system controls the 3D shape of crawling cells. J Cell Biol. 2014 doi: 10.1083/jcb.201311104. This very recent work in large adherent cells provides striking images of actin-myosin interactions across different regions of the cell cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]