Abstract
Thoracoscopic mobilization of esophagus and laparoscopic mobilization of stomach with cervical anastomosis is employed widely in minimally invasive esophagectomy (MIE) for esophageal carcinoma. However, it is associated with high incidence of complications, including recurrent laryngeal nerve injury and anastomotic leak. This paper summarizes the key techniques in total laparoscopic and thoracoscopic esophagectomy with intrathoracic anastomosis for MIE in 62 patients of middle or lower esophageal cancer between March 2012 and August 2013. Total laparoscopic and thoracoscopic esophagectomy with intrathoracic anastomosis was performed to treat the middle or lower esophageal cancer. Laparoscopic and thoracoscopic Ivor-Lewis esophagectomy was performed using a circular stapler (Johnson and Johnson) intrathoracically to staple esophagogastric anastomosis and reconstruct the digestive tract. In addition, we performed tension-relieving anastomotic suture and embedded with pedicled omental flap. Compared with the trans-orally inserted anvil (OrVil) approach, the technique reported here is safe, feasible and user-friendly. Total thoracoscopic intrathoracic anastomosis can be performed with a circular stapler (Johnson and Johnson).
Keywords: Thoracoscope, laparoscope, intrathoracic anastomosis, esophageal cancer, minimally invasive esophagectomy (MIE)
Introduction
Currently, minimally invasive esophagectomy (MIE) for esophageal cancer entails laparoscopic and thoracoscopic esophagectomy with cervical anastomosis. Esophagogastric anastomosis is mainly performed by thoracoscopic mobilization of esophagus and laparoscopic mobilization of stomach with cervical anastomosis. Total laparoscopic and thoracoscopic esophagectomy with intrathoracic anastomosis is a major challenge in MIE (1-3), resulting in failure of minimally invasive endoscopic surgery for esophageal cancer (4-6). Cervical anastomosis is still an invasive approach and high incidence of recurrent laryngeal nerve injury and anastomotic leak.
Intrathoracic esophagogastric anastomosis is used for most radical resections of middle or lower esophageal cancer. However, total laparoscopic and thoracoscopic esophagectomy with intrathoracic anastomosis is associated with technical difficulty and complexity. A total of 62 patients with middle or lower esophageal cancer were treated with this approach between March 2012 and August 2013. This paper describes the total laparoscopic and thoracoscopic Ivor-Lewis esophagectomy, focusing on the surgical procedures and the key technical details of thoracoscopic intrathoracic anastomosis using a circular stapler (Johnson and Johnson).
Subjects and methods
Subjects totally 62 patients were enrolled, including 46 males and 16 females, with a mean age of 61±12 years (range, 42-72 years). Progressive dysphagia was the major symptom for 0.5-6 months, with a mean duration of 2.5±2 months. The preoperative barium swallow study was followed by contrast-enhanced computed tomography of the chest, and upper endoscopy and biopsy. The findings revealed a middle or lower esophageal carcinoma occurring as a single tumor in all subjects. The lesions were 2-5 cm in length, with a mean length of 3±1.2 cm. No obvious outward invasion was detected (stage T3 or lower). No significantly enlarged lymph node was detected. CT scan of the head, bone scan and abdominal ultrasonography revealed no distal metastases. Cardiopulmonary evaluation showed no apparent surgical contraindications. Postoperative pathology included squamous carcinoma in 57 cases and adenocarcinoma in 5 cases. Tumors were staged as T1N0M0 in 27 cases, T2N0M0 in 29 cases, T3N0M0 in 5 cases and T3N1M0 in 1 case (UICC esophageal carcinoma pathological stage, 2009).
Anesthesia
All cases underwent intravenous anesthesia and inhalation anesthesia through a double-lumen endotracheal tube. Two-lung ventilation was administered during the abdominal stage, while single left-lung ventilation was performed during the thoracic stage. However, artificial pneumothorax was not employed during the surgery.
Position and incision
Patients were placed in a supine position, and underwent laparoscopy-assisted abdominal surgery with five abdominal ports. The 11-mm incision besides the umbilicus was used as the observation port, and the other four ports were positioned at the incisions in the subcostal region of the left (5-mm port) and right midclavicular lines (5-mm port), and the incisions 3 cm superior to the umbilicus in the left (5-mm port) and right parasternal lines (11-mm port). The surgeon stood on the right side of the patient (Figure 1).
Figure 1.
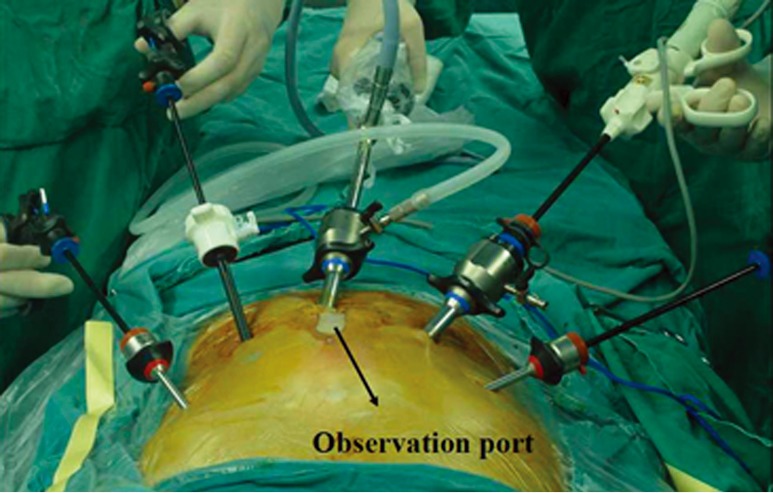
Abdominal incision with five abdominal ports, the surgeon stood on the right side of the patient.
The patient was repositioned to the left lateral decubitus position during the thoracic stage, and was moderately anteverted (4). Thoracoscopy was performed with four thoracic ports. The camera port was located at the 8th intercostal space on the posterior axillary line, the major operation port between the 4th and 6th intercostal space on the anterior axillary line, and the operation port for the assistant at the 7th intercostal space on the scapular line. The incision at the 4th intercostal space on the anterior axillary line was approximately 2.5 cm long, while the other incisions were about 1.5-2 cm in length. The surgeon stood on the abdominal side of the patient (Figure 2).
Figure 2.
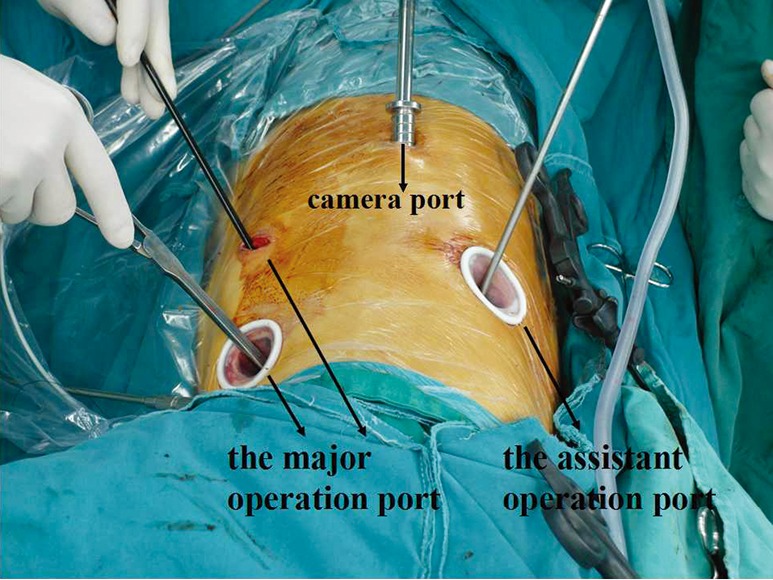
Thoracic incision with four thoracic ports, the surgeon stood on the abdominal side of the patient.
Surgical procedures (Figure S1)
Laparoscopic mobilization of the stomach
The stomach was routinely mobilized, the abdominal lymph node dissected, and the pedicled omental flap was reserved at the site close to the fundus of the stomach. The greater omentum was mobilized towards the margin of the colon to the splenogastric ligament, and then mobilized towards the margin of the stomach. A pedicled omental flap about 10 cm long and 5 cm wide was reserved to embed the anastomosis. A median abdominal incision of about 5 cm in length was cut below xiphoid, and a gastric conduit of about 3.5 cm in diameter was created using a linear stapler (Johnson and Johnson) outside the abdominal cavity. The tip of the gastric conduit was temporarily attached to the surgical specimen with interrupted sutures. A jejunostomy was created about 30 cm distal to the ligament of Treitz.
Thoracoscopic mobilization of thoracic segment of esophagus and tumor
The esophagus was mobilized at the site 4 cm superior to the azygos vein with a cautery hook and ultrasonic scalpel in a bottom-up approach. The azygos vein was ligated using Hem-o-lok clip and divided by ultrasonic scalpel. The pleura surrounding the esophagus at the anastomotic stoma were reserved for subsequent use. Thoracic lymph nodes were systematically dissected, including the left and right recurrent laryngeal nerve chain lymph nodes.
Purse-string suture and insertion of the stapler anvil
Esophageal purse-string suture is the most difficult procedure during the total thoracoscopic intrathoracic anastomosis. The 3-0 Surgipro suture was used for esophageal purse-string suture. The purse-string was hand sewn through the muscular layer of the esophagus about 3 cm superior to the azygos vein, encircling it (five needles). The esophagus was pulled straight downwards during the suturing, altering the position to facilitate the suture. A horizontal incision was then made in the wall of the esophagus, 3 cm distal to the purse-string. First, the oval clamp was inserted through the incision to appropriately expand the purse-string, so as to facilitate the insertion of the stapler anvil, and then, the anvil was inserted into the esophagus through the incision and pushed upward above the purse-string via the working port at the 4th intercostal space on the anterior axillary line. The purse-string was tied using a knot pusher, and the distal esophagus between the purse-string and the incision was then transected. Esophageal mucosal layer was reserved 5 mm longer than the muscular layer to prevent slipping.
Intrathoracic anastomosis
The attached gastric conduit was pulled into the right thoracic cavity via the diaphragmatic hiatus, and the circular stapler was placed through the working port at the 4th intercostal space on the anterior axillary line. The circular stapler was inserted into the gastric conduit in the thoracic cavity, and the side of the gastric conduit was anastomosed to the end of the esophagus by joining the anvil to the stapler. The joining space between the anvil and the stapler was thoroughly checked before the excitation to ensure no involvement of the surrounding tissues. After the anastomosis, the thoracoscope served as an endoscope, which was inserted into the gastric conduit to check the anastomotic stoma, to ensure the integrity of the anastomotic stoma and prevent bleeding. After the examination, a linear stapler (Endo-GIA60-4.1; Johnson and Johnson) was used via the port at the 6th intercostal space on the anterior axillary line to close the stump of the gastric conduit, and the cutting edge was embedded with the seromuscular layer. The position of the cutting margin was over 2 cm from the anastomotic stoma to prevent the occurrence of anastomotic stricture after embedding with the seromuscular layer.
Tension-relieving suture of the anastomotic stoma, embedded with pedicled omental flap
Based on our previous clinical experience, we interrupted suture of the gastric wall below the anastomosis and the pleura surrounding the anastomosis to relieve the tension of the anastomotic stoma. A circular embedding of the anastomosis with pedicled omental flap was performed to effectively prevent the occurrence of anastomotic leak, which was consistent with previous reports (7,8). Interrupted suture was performed at 4, 8 and 12 o’clock position (via the thoracoscopic direction) surrounding the anastomosis. The gastric wall below the anastomosis closely adhered to the reserved pouch-like mediastinal pleura surrounding the esophagus to reduce the tension of the anastomosis. The anastomosis was circularly embedded with the reserved pedicled omental flap, and interrupted suture was performed at the same position to achieve intact and tight embedding of the anastomosis. In the present study, one case (1/62, 1.6%) showed the postoperative anastomotic leak, which was an esophageal leak above the anastomosis. It was probably associated with an accidental injury during the mobilization of the esophagus.
Results
The endoscopic surgeries were successfully performed without conversion to laparotomy or thoracotomy. The mean overall operation time was 260±35 min (range, 250-320 min), the mean thoracoscopic operation time was 155±20 min (range, 110-170 min), and the mean laparoscopic operation time was 115±21 min (range, 90-140 min). The mean estimated blood loss was 210±32 mL (range, 180-270 mL). The mean number of nodes harvested from every patient was 23±5 (range, 11-29). No operative mortality or severe complications occurred, including anastomotic stricture, respiratory failure, trachyphonia or chylothorax. Anastomotic leak was observed in one case (1/62, 1.6%), which was cured with conservative treatment. The patients commenced ambulation 2 days post-operation, with a mild sensation of chest pain. The mean length of hospital stay was 11±2 days (range, 10-14 days; except one case with anastomotic leak). The cases were followed up for 1-17 months, with normal diet. No death or recurrence was observed.
Conclusions
Minimally invasive surgery offers valuable alternatives to enhance traditional open surgery. Total laparoscopic and thoracoscopic esophagectomy with intrathoracic anastomosis overcomes the bottlenecks associated with endoscopic surgery of esophageal cancer, which facilitates the real MIE. It is reported that trans-orally inserted anvil (OrVil) can be used for intrathoracic gastroesophageal anastomosis (9-11). However, OrVil was too expensive to be popular. The transoral insertion of the anvil increases the probability of thoracic infection. Compared with the OrVil approach, the technique reported here is safe, feasible and user-friendly. Total thoracoscopic intrathoracic anastomosis can be performed with a circular stapler (Johnson and Johnson).
Acknowledgements
Disclosure: The authors declare no conflict of interest.
Figure S1.

Laparoscopic and thoracoscopic esophagectomy with intrathoracic anastomosis (12): (I) laparoscopic mobilization of the stomach; (II) thoracoscopic mobilization of thoracic segment of esophagus and tumor; (III) purse-string suture and insertion of the stapler anvil; (IV) intrathoracic anastomosis; (V) tension-relieving suture of the anastomotic stoma, embedded with pedicled omental flap. Available online: http://www.asvide.com/articles/282
References
- 1.Puntambekar SP, Agarwal GA, Joshi SN, et al. Thoracolaparoscopy in the lateral position for esophageal cancer: the experience of a single institution with 112 consecutive patients. Surg Endosc 2010;24:2407-14 [DOI] [PubMed] [Google Scholar]
- 2.Verhage RJ, Hazebroek EJ, Boone J, et al. Minimally invasive surgery compared to open procedures in esophagectomy for cancer: a systematic review of the literature. Minerva Chir 2009;64:135-46 [PubMed] [Google Scholar]
- 3.Nguyen NT, Hinojosa MW, Smith BR, et al. Minimally invasive esophagectomy: lessons learned from 104 operations. Ann Surg 2008;248:1081-91 [DOI] [PubMed] [Google Scholar]
- 4.Levy RM, Trivedi D, Luketich JD. Minimally invasive esophagectomy. Surg Clin North Am 2012;92:1265-85 [DOI] [PubMed] [Google Scholar]
- 5.Gao Y, Wang Y, Chen L, et al. Comparison of open three-field and minimally-invasive esophagectomy for esophageal cancer. Interact Cardiovasc Thorac Surg 2011;12:366-9 [DOI] [PubMed] [Google Scholar]
- 6.Elorza-Orúe JL, Larburu-Etxaniz S, Asensio-Gallego JI, et al. Minimally invasive esophagectomy. Cir Esp 2006;80:151-6 [DOI] [PubMed] [Google Scholar]
- 7.Zhang RQ, Xia WL, Kang NN, et al. Pursestring stapled anastomotic technique for minimally invasive Ivor Lewis esophagectomy. Ann Thorac Surg 2012;94:2133-5 [DOI] [PubMed] [Google Scholar]
- 8.Karaoglanoglu N, Turyilmaz A, Eroglu A.Use of pedicled omentum and endostaplers in esophagogastric anastomosis. Ann Thorac Surg 2007;83:2259-60 [DOI] [PubMed] [Google Scholar]
- 9.Nguyen TN, Hinojosa MW, Smith BR, et al. Thoracoscopic construction of an intrathoracic esophagogastric anastomosis using a circular stapler: transoral placement of the anvil. Ann Thorac Surg 2008;86:989-92 [DOI] [PubMed] [Google Scholar]
- 10.Nguyen NT, Hinojosa MW, Smith BR, et al. Minimally invasive esophagectomy: lessons learned from 104 operations. Ann Surg 2008;248:1081-91 [DOI] [PubMed] [Google Scholar]
- 11.Maas KW, Biere SS, Scheepers JJ, et al. Minimally invasive intrathoracic anastomosis after Ivor Lewis esophagectomy for cancer: a review of transoral or transthoracic use of staplers. Surg Endosc 2012;26:1795-802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ai B, Zhang Z, Liao Y. Laparoscopic and thoracoscopic esophagectomy with intrathoracic anastomosis. Asvide 2014;1:269. Available online: http://www.asvide.com/articles/282 [DOI] [PMC free article] [PubMed]


