Abstract
It has not been reported that cases of alcoholic cardiomyopathy (ACM) combined with acute pulmonary embolism (PE). We hereby present a case of a 48-year-old male with ACM with significant enlargement of the heart and heart failure is described. Then, the patient was seized with acute PE which was confirmed by specific examination and his symptoms.
Keywords: Alcoholic cardiomyopathy (ACM), pulmonary embolism (PE), heart failure
Introduction
Acute pulmonary embolism (PE) is a cardiovascular emergency associated with significant morbidity and a 5-35% mortality for untreated PE (1,2). The classic risk factors for PE are those identified as Virchow’s triad (hypercoagulable state, stasis and injury) (3,4), which include deep vein thrombosis (DVT), major surgery, major trauma, high age, myocardial infarction and chronic heart failure (5). A 48-year-old man presented to Emergency Department with a PE and a history of taking inferior liquor for more than 30 years, 500-750 g per day. The patient was confirmed with alcoholic cardiomyopathy (ACM) by further examinations. We had excluded common risk factors for PE, and here we discuss the relationship between ACM and PE. The following is a detailed report of the case accompanied by a review of previous studies.
Case report
Case one
A 48-year-old male patient presented with chest discomfort for more than one month, accompanied by hemoptysis for ten days. He felt persistent chest discomfort under no obvious predisposing causes at the beginning of March in 2013, mild with no influence on daily life at first but gradually aggravated with abdominal distention, poor appetite and without cough, expectoration and fever. He was diagnosed with ‘coronary heart disease, DM type II, pneumonia and pleural effusion’ by a local hospital on 15th of March, treated for more than 10 days in the hospital with unclear exact details and the symptom alleviated after the treatment. The hemoptysis began with no obvious causes from the middle of April with plentiful sputum, about 50 mL per day, dark red bloody at first and then turning bright and with mucus, accompanied with chest discomfort continually, shortness of breath and dull chest pain mainly located in the bilateral hypochondriac regions and together with poor appetite, upper abdominal distending pain and without fever. The patient was hospitalized in the local Department of Gastroenterology on 25th of April. The results of gastroscopic examination showed chronic superficial gastritis with antral mucosal atrophy, and the examination of cardiac ultrasonography showed left cardiac insufficiency, enlargement of the whole heart, moderate mitral regurgitation, mild to moderate aortic regurgitation, mild tricuspid and pulmonary regurgitation and a severe reduction in ejection fraction (24%). Chest computer tomography (CT) showed the inflammation on bilateral lobes. In the next four days, the patient was given corresponding treatments but with no significant therapeutic efficacy and transferred to our hospital on 30th of April in 2013. In the emergency room, the results of CT angiography (CTA) revealed thrombosis in the branches of bilateral pulmonary arteries, bilateral pneumonia and right pleural thickening (Figure 1). While deep venous ultrasound of lower limbs revealed bilateral femoral venous valve insufficiency and bilateral calf muscular venous thrombosis. Then, the patient was hospitalized in our department as ‘PE and pulmonary infection’.
Figure 1.
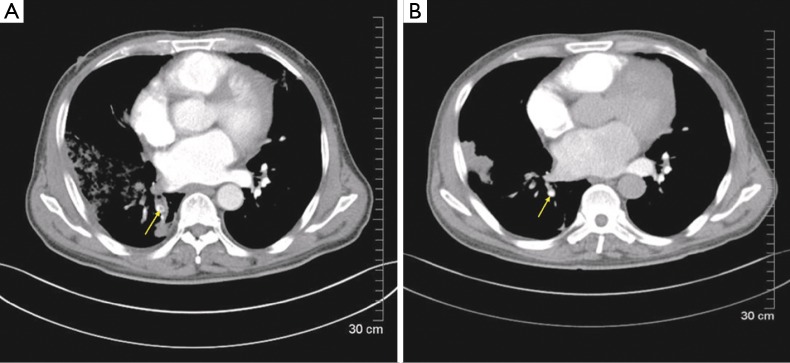
CTA revealed an embolus located in the right inferior pulmonary artery, which was disappeared after the treatment (yellow arrow). CTA, computer tomography angiography.
Past illness: the patient has a history of taking inferior liquor for more than 30 years, 500-750 g per day and smoking for more than 20 years, 10+ cigarettes per day.
Physical examination: the whole skin mucous membrane dyed mild yellow and had no rash or petechia. Right thorax had stronger tactile fremitus and slightly flat percussion sound. Respiratory sound was bilateral rough and moist rales were heard in bilateral lower lung. Systolic cardiac murmurs could be heard on mitral valve area, III/6 and conducted to left axilla.
Laboratory examination: blood routine test: WBC: 15.64×109/L, N: 80.20%; liver function: AST: 172.9 U/L, ALT: 272 U/L, ALP: 166.4 U/L, TBIL: 70.4 µmol/L, DBIL: 45.9 µmol/L, IBIL: 24.5 µmol/L. Coagulation indicators: PT: 14.2 t, FIT: 5.0 g/L, INR: 1.06, APTT: 1.22 t, TT: 17.20 t, D-dimers: 9.55 mg/L, BNP: 2,205 pg/mL, CRP: 148 mg/L. ECG: (I) sinus tachycardia; (II) left atrial and ventricular enlargement; (III) left axis deviation. Blood gas analysis: PO2: 111 mmHg, PCO2: 39 mmHg. Tumor markers: CEA, NSE, CY21-1, CA-199, CA-125(-), HCY(-), sputum cultivation(-), ESR, HLA-B27, RF(-), IgA, IgM, IgG, C3, C4(-).
Heart magnetic resonance imaging (MRI) at 3 T (GE Signa Excite HD scanner) enhancement scanning: cardiac insufficiency (Figure 2, panel A-D). 2D-heart ultrasound: cardiac enlargement, mainly appearing on left atrium and ventricle, mitral, aortic, tricuspid and pulmonary valves incompetence, and the ejection fraction was 40.2% (Figure 3, panel A,B).
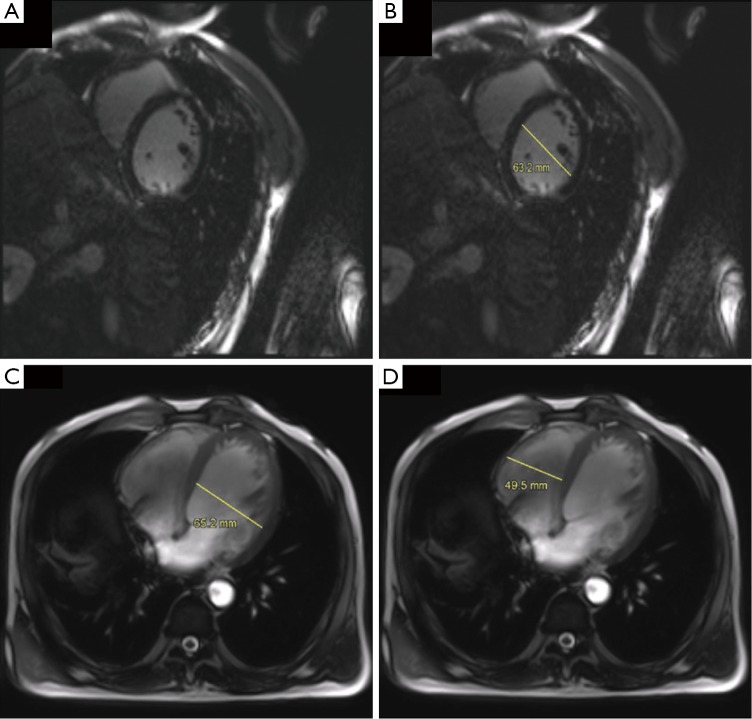
Figure 2.
Heart MR3T showed enlargement of the whole heart. (A,B) On the short axis, the left ventricle’s diameter was 63.2 mm at the end of diastole; (C,D) the diameter of left and right ventricle was 65.2 and 49.5 mm, respectively (through the axial view). MR3T, magnetic resonance imaging at 3 T (GE Signa Excite HD scanner) enhancement scanning.
Figure 3.
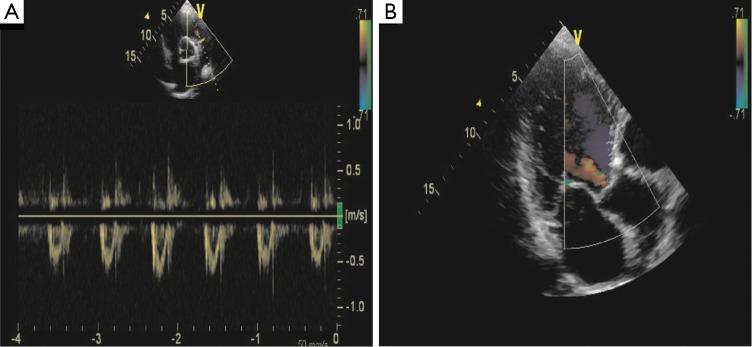
2D-heart ultrasound showed a diffuse hypokinesia of the left ventricle with a mild mitral regurgitation and a moderate reduction fraction (40.2%).
Treatment: the patient received cefoperazone and tazobactam as an anti-infection therapy, tanreqing and ambroxol to reduce phlegm, enoxaparin as an anti-coagulation therapy, alprostadil to drop the pressure of pulmonary artery down, hydrochlorothiazide and spirolactone to improve cardiac function and then treated with warfarin as an anti-coagulation therapy. During the whole process, the patient gave up drinking totally. He wanted to take some alcohol sometime, especially when his condition improved, but the abstinence symptoms did not experience. Gradually, chest discomfort, chest pain and hemoptysis were alleviated and pulmonary rales reduced obviously.
On May 11th, chest CTA showed most thrombosis were absorbed, a little shadow of embolism in the right lower branch of pulmonary artery, multiple patching shadow in bilateral lungs which were considered as the infection and right pleural thickening (Figure 1B).
Liver function: ALT: 149.12 U/L, AST: 66.22 U/L, TBIL: 29.2 µmol/L, DBIL: 17.00 µmol/L, IBIL: 12.2 µmol/L. Obviously, these figures were all reduced, and confirmed that the patient’s liver function was recovered after the treatment and abstinence from alcohol.
Coagulation indicators: PT: 38.6 t, APTT: 20.5 t, FIT: 4.20, INR: 3.38, TT: 15.80, D-diners: 5.42 mg/L, BNP: 2,205 pg/mL, CRP: 74 mg/L. Blood routine test: WBC: 6.50×109/L, N: 54%.
On May 14th, the patient had a favorable turn and discharged with doctor’s advice of giving up smoking and drinking, continuing the oral treatment of warfarin, monitoring blood pressure, INR, 2D-heart ultrasound and chest CTA and follow-up by telephone. On May 21st, the feedback of 2D-heart ultrasound showed left ventricular diastolic insufficiency and EF was 62%.
Discussion
PE is a general term of a series of diseases and clinical syndromes which are cause by various emboli (endogenous and exogenous) blocking the pulmonary arterial system, whose clinical symptoms are various and lack of specificity (6). Limited by the condition of examination and the experience of clinical doctors, many mistaken and missed diagnoses exist. Main reason is DVT and dangerous factors contain trauma, long-term bed rest, varicose veins, venous cannula, pelvic and hip surgery, obesity, DM and contraceptive. In addition, various heart diseases are also the main reasons, especially cardiomyopathy, heart valve diseases and infectious endocarditis (5). The hypercoagulable state caused by malignant tumors can also lead to PE. In this case, we highly suspected that primary heart diseases lead to the embolism because of no dangerous factors related to DVT and negative results of rheumatic, immunological or tumor markers.
ACM is a kind of disease that has typical hemodynamic change, symptoms, signs and imaging findings of dilated cardiomyopathy (DCM) and its symptoms can be released or cured after 4-8 weeks of giving up drinking (7). The incidence of this disease is higher in males than in females, also higher in Europe, the US and Russia than in other regions of the world (7,8). In China, there are sporadic case reports. It mostly occurs on male of 30 to 55 years old with over-drinking history for more than ten years. It has various clinical manifestations, including the main features of cardiac enlargement, cardiac insufficiency and arrhythmia (9). The diagnosis criterion contain long history of excessive drinking, the manifestation of cardiac enlargement, CHF and getting better apparently after giving up drinking for 4-8 weeks. In this case, the patient had a long history of drinking massive liquor and stopped drinking from the onset of this disease. His main initial symptom was whole heart enlargement, which similar with manifestations of the DCM. As a matter of fact, heart MRI-3T and 2D-heart ultrasound help us exclude other non-ischemic cardiomyopathy, such as hypertrophic cardiomyopathy and restrictive cardiomyopathy. His younger brother, 46-year-old, has a similar habit of drinking as well. During this hospitalization, we also performed the examination of 2D-heart ultrasound for him, which showed the existence of cardiac diastolic insufficiency and proved the basis of ACM from another point of view.
We hold the opinion that analyzing the case and making a diagnosis can be based on several points below.
Routine thoughts: bilateral calf muscular venous thrombosis→PE→cardiac insufficiency. It seems well-reasoned. But here is a question. PE caused by thrombosis from lower extremities mostly leads to the increase of pulmonary arterial pressure and then increases afterload of right heart, which induces the enlargement of right atrium, right ventricle, pulmonary and the insufficiency of tricuspid. But in this case, the results of 2D-heart ultrasound and heart 3TMRI both shows the enlargement of whole heart, manifested mostly by the hypertrophy of left atrium and ventricle, multiple valves incompetence and whole cardiac insufficiency, which cannot be explained by emboli from systemic circulation;
Monistic thoughts: long-term over-drinking→ACM→cardiac insufficiency→cardiac hemodynamic disorder→primary thrombosis attached to vessel wall falling off→PE. This analysis seems more reasonable;
During the whole treatment, heavy hemoptysis is one contraindication of thrombolytic therapy. The thrombus dissolved quickly only with anticoagulant therapy and without thrombolytic therapy, which was considered as the result of thrombus autolysis. The cardiac function recovered quickly. So, the reason of this case may be the ACM.
Acknowledgements
Grant support: This work was supported by the National Natural Science Foundation of China (Grant No. 81372035, 81170160), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD, Jx10231081), the Foundation of the Health Department of Jiangsu Province (No. H201301), the Six Talents Peak Project of Jiangsu Province (Grant No. 2013WSN035, DG216D5044), the Open Project Program of State Key Laboratory of Natural Medicines, China Pharmaceutical University (No. SKLNMKF201311, 201408).
Disclosure: The authors declare no conflict of interest.
References
- 1.Bounameaux H, Schneider PA, Reber G, et al. Measurement of plasma D-dimer for diagnosis of deep venous thrombosis. Am J Clin Pathol 1989;91:82-5 [DOI] [PubMed] [Google Scholar]
- 2.Bounameaux H, Slosman D, de Moerloose P, et al. Diagnostic value of plasma D-dimer in suspected pulmonary embolism. Lancet 1988;2:628-9 [DOI] [PubMed] [Google Scholar]
- 3.Esmon CT. Basic mechanisms and pathogenesis of venous thrombosis. Blood Rev 2009;23:225-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.López JA, Chen J. Pathophysiology of venous thrombosis. Thromb Res 2009;123Suppl 4:S30-4 [DOI] [PubMed] [Google Scholar]
- 5.Nielsen JD. The incidence of pulmonary embolism during deep vein thrombosis. Phlebology 2013;28Suppl 1:29-33 [DOI] [PubMed] [Google Scholar]
- 6.Lapner ST, Kearon C. Diagnosis and management of pulmonary embolism. BMJ 2013;346:f757. [DOI] [PubMed] [Google Scholar]
- 7.Skotzko CE, Vrinceanu A, Krueger L, et al. Alcohol use and congestive heart failure: incidence, importance, and approaches to improved history taking. Heart Fail Rev 2009;14:51-5 [DOI] [PubMed] [Google Scholar]
- 8.Piano MR. Alcoholic cardiomyopathy: incidence, clinical characteristics, and pathophysiology. Chest 2002;121:1638-50 [DOI] [PubMed] [Google Scholar]
- 9.Laonigro I, Correale M, Di Biase M, et al. Alcohol abuse and heart failure. Eur J Heart Fail 2009;11:453-62 [DOI] [PubMed] [Google Scholar]