Abstract
Background
The aim of the present study was to explore the association between the pretreatment globulin albumin ratio (GAR) and the survival of advanced non-small cell lung cancer (NSCLC) patients.
Methods
Patients hospitalized between January 2007 and December 2010 were enrolled and eliminated according to the inclusion and exclusion criteria. GAR was defined as the absolute globulin value divided by the absolute albumin value. Chi-squared test was performed to compare clinical characteristics in different groups. Kaplan-Meier and Cox regression model were used to determine independent prognostic factors. A P value of ≤0.05 was considered to be statistically significant.
Results
Total 316 patients were finally enrolled. The median progression free survival (PFS) and overall survival (OS) were 210.0 and 430.0 days, respectively. The statistical analyses indicated that pretreatment GAR >0.58 [hazard ratio (HR) =1.52, 95% confidence interval (95% CI): 1.12-2.08, P=0.008 for PFS, HR =1.65, 95% CI: 1.20-2.26, P=0.002 for OS], and pretreatment albumin ≤35 g/L (HR =2.09, 95% CI: 1.20-3.65, P=0.003 for PFS, HR =1.92, 95% CI: 1.10-3.36, P=0.022 for OS) were independent prognostic factors for both PFS and OS.
Conclusions
Our study first established a connection between pretreatment GAR and advanced NSCLC patients, suggesting that GAR was an independent prognostic factor and could be the biomarker for prognosis.
Keywords: Globulin albumin ratio (GAR), prognostic factor, non-small cell lung cancer (NSCLC)
Introduction
Lung cancer is still the leading cause of cancer death in the world, and non-small cell lung cancer (NSCLC) accounts for approximately 85% of lung cancer (1). The majority of NSCLC patients are diagnosed at advanced stage, which is thought to be one important reason for the short survival of lung cancer. To prolong the survival of advanced NSCLC patients, sensitive and specific factors for classifying cancer risk and predicting survival are always desired in clinic to help guiding treatment, and several prognostic factors have been identified in previous studies (2,3). However, some factors have limitations in clinical application because of their tissue-specific expression and high cost of testing, making the efficiency and accuracy of the existing factors need to be improved. There is still need for a promising predictive factor that can be simply detected and closely linked to survival for advanced NSCLC patients.
Serum albumin is generally applied to assess the nutritional status and severity of disease and also used to evaluate the progression and prognosis of some disease, such as operable colorectal cancer, and hepatic disease (4). Previous studies observed that serum albumin is a prognostic factor for various cancers including lung cancer (5-8). Low albumin predicates a poor survival of cancer patients, and high level of albumin is associated with a better survival (8). However, as an index of serum biochemistry, albumin level can be interfered by many factors, which limit its application and credibility in clinic (9).
Another biochemistry index, globulin is demonstrated in research to be interfered by the body status and several disease. One type of globulin, sex hormone-binding globulin (SHBG), is suggested to be associated with the poor survival of hormone related cancer (10-13). Previous studies also demonstrated that hormone was involved in NSCLC. Estrogen receptor β, a hormone receptor, is one of the factors which involved in promoting the development of NSCLC (14,15). Thus it can be seen that globulin may be one prognostic factor for NSCLC patients.
Here we gave a hypothesis that since globulin and albumin are both serum chemistry indexes, taken these two together, globulin albumin ratio (GAR) could reduce the influence to least and may be an effective prognostic factor for advanced NSCLC patients. The aim of the present study was to investigate the association between the pretreatment GAR and the response to treatment, and survival of patients with advanced NSCLC.
Patients and methods
Patients
Patients first hospitalized in the department of respiratory medicine signed a written informed consent which demonstrated that the results of examinations in hospital may be used in respective studies in future. The informed consent and research proposal of this respective study was approved by chairman of the ethics committee of Jinling Hospital (Nanjing, China). Patients hospitalized between January 2007 and December 2010 consecutively enrolled into the present retrospective study. The inclusion criteria are: (I) patients were hospitalized for the primary diagnosis, therapy-naïve; (II) patients were histologically diagnosed primary NSCLC; (III) patients were staged according to the Tumor-Node-Metastasis (TNM) criteria (AJCC criteria 2009) and in stage IIIB and IV, including those in stage IIIA but not able to surgery or not accept the operation; (IV) if took chemotherapy, patients had at least two cycles of first-line platinum-based combination chemotherapy and a response evaluation after treatment; (V) all clinical data was available. Patients were excluded if they had clinical evidence of inflammation in nearly one month, immunity disease, hematology disease or end-stage liver disease.
Clinical and laboratory data collection
Clinical characteristics including gender, age, smoke status, histology, differentiation, TNM stage, metastasis organ, metastasis number, metastasis symptom, and the Eastern Cooperative Oncology Group Performance Status Scale (ECOG PS) were recorded for all patients. First-line platinum-based chemotherapy was consisted of platinum with third-generation chemotherapy agent and therapy response evaluation by whole body tumor scanning was taken after two cycles of treatment. The Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 was applied for the evaluation of response. The response was assessed by radiologist and treating physician, and reviewed by the investigator Yanwen Yao. The response of treatment was also collected as a clinical characteristic. During the data collection, all the investigators were set blinded to the GAR value of the patients.
Follow-up time was defined as the interval time from diagnosis to 31 May 2012. Progression free survival (PFS) was defined as the time from diagnosis until disease progressed or death of any cause. If the patients were dead during the follow-up time, overall survival (OS) was defined as the interval between the date of diagnosis and the date of death. Otherwise, OS time was defined as the interval time between the date of diagnosis and 31 May 2012.
For all study subjects, the value of albumin and globulin testing 1 day before diagnosis were recorded. GAR was defined as the absolute globulin value divided by the absolute albumin value.
Statistical analysis
Data was summarized with the number of subjects and median value, and the optimal cutoff value of pretreatment GAR was estimated by receiver operating characteristics (ROC) curve, as the value at the largest Youden Index. Chi-squared test and RIDIT analysis were performed to compare baseline clinical characteristics in different groups. Mann-Whitney U test or Kruskal-Wallis H test was used to compare categorical end-points and two-sample t-test was used to compare continuous variables after data transformation.
Univariate analysis was performed to determine the significance of variables using logistic regression model for response rate and Cox regression model was performed for PFS and OS. Survival curve was estimated by Kaplan-Meier analysis and the log-rank test was utilized to examine the significance of the differences of survival distributions between groups. Subsequently, the variables with P≤0.05 enter into multivariate analysis. Cox proportional hazards regression model was used to determine the independent prognostic factor. Generally, a P value of ≤0.05 was considered to be statistically significant for all analyses. All statistical analyses were performed using the Statistical Package for the Social Sciences software program version 18.0 (SPSS Inc., Chicago, IL, USA).
Result
Baseline patient characteristics
According to the inclusion criteria, total 420 NSCLC patients at stage III and IV entered in the present study. Final 316 patients were finally enrolled into the study by further referring to the exclusion criteria, excluding seven patients with clinical evidence of anemia and three patients with hepatic disease before diagnosis, 52 patients who had took single agent chemotherapy or targeted therapy, 42 patients did not take at least two cycles of therapy or have a response evaluation.
Baseline characteristics are presented in Table 1. In all patients, the mean age was 61.8 years, 217 were males (68.7%), 135 patients (42.7%) were never smokers and 242 patients had ECOG PS score ≤1. The number of patients in stage III and IV was 81 and 235, respectively. In 235 patients with metastasis, the most common metastatic site was bone [109], followed by intrapulmonary metastasis including pleura [80], brain [58], liver [13], adrenal gland [13] and other sites [10]. Forty two patients had two or more metastatic sites including 19 patients had both bone and brain metastases. A total of 105 patients ever had symptom caused by metastasis, such as pain, dizziness and vomit.
Table 1. Clinical characteristics of all 316 advanced NSCLC patients.
| Characteristic | Data |
|---|---|
| No. of patients | 316 |
| Age (mean ± sd, years) | 61.8±11.3 |
| Gender (female/male) | 99/217 |
| Smoke status (never/ever) | 135/181 |
| Histology (non-squamous/squamous) | 206/110 |
| Differentiation (well and moderate/poor) | 72/244 |
| TNM stage (III/IV) | 81/235 |
| Tumor stage (T1/T2/T3/T4) | 41/107/33/135 |
| Node stage (N0/N1/N2/N3) | 44/30/168/74 |
| Metastasis stage (none/regional/distant) | 81/80/155 |
| Metastasis organ (none/bone/brain/intra-lung/liver/adrenal gland/others) | 81/109/58/80/13/13/10 |
| Metastasis number (none/single/multiple) | 81/187/48 |
| Metastasis symptom (never/ever) | 211/105 |
| ECOG PS ≤1/>1 | 242/74 |
| Chemotherapy (CR + PR + SD/PD) | 117/36 |
TNM stage, Tumor-Node-Metastasis stage; ECOG PS, the Eastern Cooperative Oncology Group Performance Status Scale; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NSCLC, non-small cell lung cancer.
In all study subjects, 153 patients received platinum-based chemotherapy and took a clinical response evaluation. Chemotherapy was chosen according to the tumor histology and patient’s intention. Among these patients, 107 had docetaxel and platinum combined therapy, 17 had pemetrexed and platinum combination, and 29 had gemcitabine and platinum combination. One (0.7%) patients got complete response (CR), 37 (24.7%) patients had partial response (PR), 79 (51.6%) patients had stable disease (SD) and 36 (23.5%) patients had progressive disease (PD), as shown in Table 1.
A total of 107 (40.2%) patients survived till 31 May 2012. The median PFS of these survived patients was 276.0 days (mean ± sd, 330.76±20.3) and the median OS was 516 days (mean ± sd, 590.7±21.9). The median PFS of all 316 patients was 180 days (mean ± sd, 228.25±11.2) and the median OS was 376.5 days (mean ± sd, 408±14.9).
Separate globulin and albumin analysis
Separate globulin and albumin was respectively analyzed. The cut-off value for globulin and albumin were chosen according to the normal range of these two indexes in serum biochemistry test and were 35 and 27 g/L, respectively.
Patients with pretreatment globulin <27 g/L had a higher prevalence of young patients (age <65 years) (P=0.001), histology of non-squamous (P=0.003), poor differentiation (P=0.021), metastasis stage (P=0.035), while patients with pretreatment albumin >35 g/L had more ever smokers (P=0.014).
GAR analysis
The best cut-off value of GAR was chosen at 0.58 according to the ROC curve (Figure 1). The area under curve (AUC) of GAR was 0.600 [95% confidence interval (95% CI): 0.536-0.664, P=0.003]. Patients who had an elevated pretreatment GAR (>0.58) were identified as high GAR group and 181 patients (57.3%) were in this group. The left 135 patients (42.7%) were identified as low GAR group.
Figure 1.
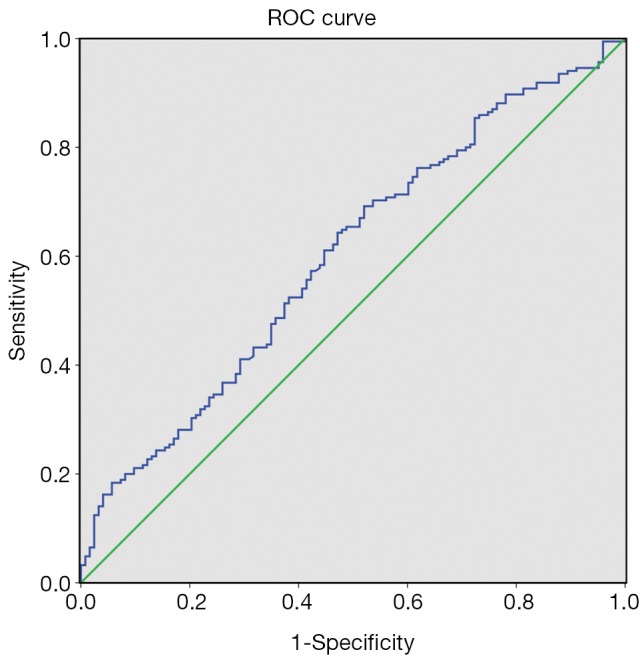
Receiver operating characteristics (ROC) curve of pretreatment globulin albumin ratio.
The distribution of clinical characteristics in GAR subgroup is shown in Table 2. Patients with pretreatment GAR >0.58 had a higher prevalence of high age (P=0.004), histology of squamous carcinoma (P=0.000) and poor differentiation (P=0.025). GAR had no significant difference in PS, TNM stage and reaction of chemotherapy.
Table 2. Distribution of clinical characteristics stratified by pretreatment GAR.
| Characteristic | GAR ≤0.58 | GAR >0.58 | P value |
|---|---|---|---|
| Patients | 135 | 181 | |
| Age (years) | |||
| Mean ± sd (range) | 60.2±10.7 | 63.0±11.6 | |
| <65/≥65 | 90/45 | 91/90 | 0.004 |
| Gender (female/male) | 44/91 | 55/126 | 0.676 |
| Smoke status (never/ever) | 66/69 | 69/112 | 0.056 |
| Histology (non-squamous/squamous) | 103/32 | 103/78 | 0 |
| Differentiation (well and moderate/poor) | 39/96 | 33/148 | 0.025 |
| TNM stage (III/IV) | 36/99 | 45/136 | 0.716 |
| Tumor stage (T1/T2/T3/T4) | 22/46/9/58 | 19/61/24/77 | 0.156 |
| Node stage (N0/N1/N2/N3) | 23/13/70/29 | 21/17/98/45 | 0.552 |
| Metastasis stage [none (M0)/regional (M1a)/distant (M1b)] | 36/36/63 | 45/44/92 | 0.763 |
| Metastasis organ (none/bone/brain/intra-lung/others) | 36/48/23/26/15 | 45/61/35/54/21 | 0.359 |
| Metastasis number (none/single/multiple) | 36/84/15 | 45/103/33 | 0.218 |
| Metastasis symptom (never/ever) | 94/41 | 117/64 | 0.352 |
| ECOG PS (≤1/>1) | 108/27 | 134/47 | 0.215 |
| Chemotherapy | 77 | 76 | |
| CR + PR + SD/PD | 58/19 | 59/17 | 0.737 |
TNM stage, Tumor-Node-Metastasis stage; ECOG PS, the Eastern Cooperative Oncology Group Performance Status Scale; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; GAR, globulin albumin ratio.
Median PFS in patients with pretreatment GAR ≤0.58 was 360.0 days (mean ± sd, 569.0±55.6) compared with 180.0 days (mean ± sd, 310.4±28.1) in patients with GAR >0.58 (P=0.001). Median OS in GAR ≤0.58 group and GAR >0.58 group was 619.0 days (mean ± sd, 851.8±68.9) and 343.0 days (mean ± sd, 468.6±31.2), respectively (P=0.000). The Kaplan-Meier curves of PFS and OS stratified by pretreatment GAR are respectively shown in Figures 2,3.
Figure 2.
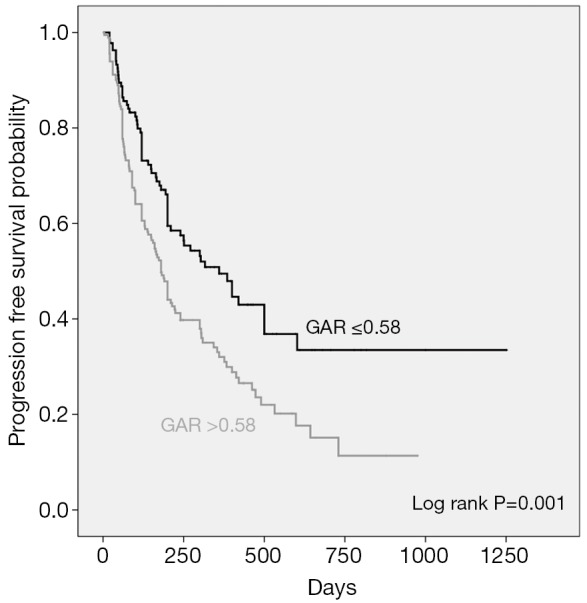
Kaplan-Meier curves showing progression free survival (PFS), stratified by the pretreatment globulin albumin ratio.
Figure 3.
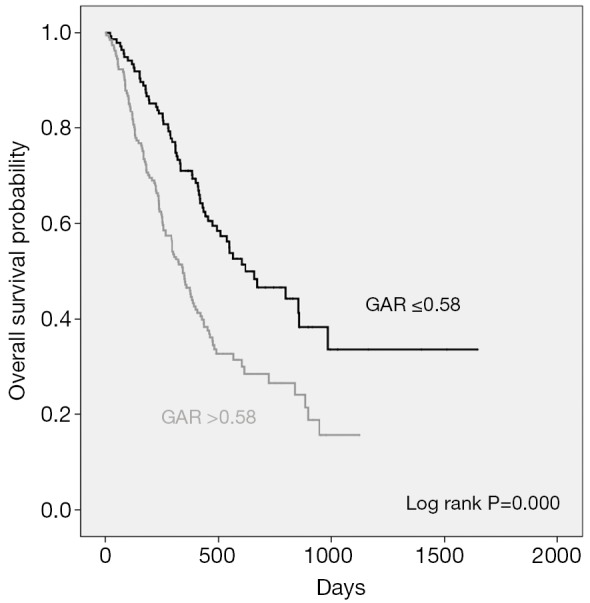
Kaplan-Meier curves showing overall survival (OS), stratified by the pretreatment globulin albumin ratio.
Univariate response rate and survival analysis
Poor differentiation [odd ratio (OR) =3.137, 95% CI: 1.029-9.565, P=0.044] and multiple metastasis sites (OR =5.278, 95% CI: 1.586-17.564, P=0.007) were associated with poor response to first-line platinum-based combination chemotherapy (PD versus CR, PR, SD) at first evaluation. As shown in Table 3.
Table 3. Univariate analysis of clinicopathological factors, serum biochemical index and response rate, PFS and OS in 316 patients.
| Parameter | Response rate |
PFS |
OS |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |||||||||||||||
| Age | |||||||||||||||||||||||
| <65 | 1 | 1 | 1 | ||||||||||||||||||||
| ≥65 | 0.649 | 0.285-1.476 | 0.302 | 1.263 | 0.948-1.681 | 0.11 | 1.553 | 1.165-2.070 | 0.003 | ||||||||||||||
| Gender | |||||||||||||||||||||||
| Female | 1 | 1 | 1 | ||||||||||||||||||||
| Male | 1.119 | 0.498-2.513 | 0.786 | 1.408 | 1.018-1.947 | 0.039 | 1.566 | 1.131-2.169 | 0.007 | ||||||||||||||
| Smoke status | |||||||||||||||||||||||
| Never | 1 | 1 | 1 | ||||||||||||||||||||
| Ever | 0.924 | 0.434-1.967 | 0.838 | 1.317 | 0.979-1.770 | 0.069 | 1.603 | 1.189-2.161 | 0.002 | ||||||||||||||
| Histology | |||||||||||||||||||||||
| Non-squa | 1 | 1 | 1 | ||||||||||||||||||||
| Squamous | 0.547 | 0.219-1.366 | 0.197 | 1.135 | 0.846-1.524 | 0.398 | 1.313 | 0.978-1.763 | 0.07 | ||||||||||||||
| Differentiation | |||||||||||||||||||||||
| Non-poor | 1 | 1 | 1 | ||||||||||||||||||||
| Poor | 3.137 | 1.029-9.565 | 0.044 | 1.296 | 0.908-1.850 | 0.153 | 1.232 | 0.863-1.759 | 0.251 | ||||||||||||||
| TNM stage | |||||||||||||||||||||||
| III | 1 | 1 | 1 | ||||||||||||||||||||
| IV | 2.296 | 0.879-5.997 | 0.09 | 1.718 | 1.207-2.446 | 0.003 | 1.63 | 1.145-2.321 | 0.007 | ||||||||||||||
| Tumor stage | |||||||||||||||||||||||
| T1 | 1 | 1 | 1 | ||||||||||||||||||||
| T2 | 1 | 0.269-3.724 | 1 | 0.902 | 0.547-1.489 | 0.688 | 1.057 | 0.641-1.743 | 0.828 | ||||||||||||||
| T3 | 4 | 0.733-21.838 | 0.109 | 2.128 | 1.202-3.767 | 0.01 | 2.957 | 1.666-5.248 | 0 | ||||||||||||||
| T4 | 1.792 | 0.543-5.919 | 0.338 | 1.313 | 0.813-2.121 | 0.265 | 1.351 | 0.836-2.181 | 0.219 | ||||||||||||||
| Node stage | |||||||||||||||||||||||
| N0 | 1 | 1 | 1 | ||||||||||||||||||||
| N1 | 2 | 0.334-11.969 | 0.448 | 1.953 | 1.072-3.557 | 0.029 | 2.143 | 1.174-3.909 | 0.013 | ||||||||||||||
| N2 | 1.406 | 0.366-5.399 | 0.619 | 1.495 | 0.924-2.417 | 0.101 | 1.626 | 1.005-2.631 | 0.048 | ||||||||||||||
| N3 | 3.111 | 0.781-12.384 | 0.108 | 1.89 | 1.116-3.200 | 0.018 | 1.764 | 1.042-2.986 | 0.035 | ||||||||||||||
| Metastasis stage | |||||||||||||||||||||||
| M0 | 1 | 1 | 1 | ||||||||||||||||||||
| M1a | 1.705 | 0.514-5.656 | 0.384 | 1.198 | 0.773-1.858 | 0.419 | 1.195 | 0.770-1.855 | 0.426 | ||||||||||||||
| M1b | 2.58 | 0.955-6.969 | 0.061 | 2.061 | 1.428-2.976 | 0 | 1.887 | 1.308-2.721 | 0.001 | ||||||||||||||
| Metastasis number | |||||||||||||||||||||||
| None | 1 | 1 | 1 | ||||||||||||||||||||
| Single | 1.77 | 0.651-4.810 | 0.263 | 1.557 | 1.082-2.240 | 0.017 | 1.502 | 1.044-2.161 | 0.028 | ||||||||||||||
| Multiple | 5.278 | 1.586-17.564 | 0.007 | 2.648 | 1.669-4.201 | 0 | 2.283 | 1.443-3.612 | 0 | ||||||||||||||
| Metastasis symptom | |||||||||||||||||||||||
| Never | 1 | 1 | 1 | ||||||||||||||||||||
| Ever | 0.692 | 0.243-1.975 | 0.492 | 1.157 | 0.783-1.711 | 0.464 | 1.209 | 0.817-1.787 | 0.342 | ||||||||||||||
| ECOG PS | |||||||||||||||||||||||
| ≤1 | 1 | 1 | 1 | ||||||||||||||||||||
| >1 | 1.16 | 0.468-2.876 | 0.748 | 1.657 | 1.216-2.257 | 0.001 | 1.654 | 1.213-2.256 | 0.001 | ||||||||||||||
| Albumin | |||||||||||||||||||||||
| >35 | 1 | 1 | 1 | ||||||||||||||||||||
| ≤35 | 0.141 | 0.012-1.604 | 0.114 | 2.458 | 1.442-4.192 | 0.001 | 2.75 | 1.614-4.684 | 0 | ||||||||||||||
| Globulin | |||||||||||||||||||||||
| ≤27 | 1 | 1 | 1 | ||||||||||||||||||||
| >27 | 0.65 | 0.279-1.517 | 0.319 | 1.266 | 0.948-1.691 | 0.109 | 1.471 | 1.101-1.965 | 0.009 | ||||||||||||||
| GAR | |||||||||||||||||||||||
| ≤0.58 | 1 | 1 | 1 | ||||||||||||||||||||
| >0.58 | 0.814 | 0.382-1.735 | 0.594 | 1.664 | 1.233-2.247 | 0.001 | 1.959 | 1.449-2.649 | 0 | ||||||||||||||
OR, odd ratio; HR, hazard ratio; CI, confidence interval; TNM stage, Tumor-Node-Metastasis stage; ECOG PS, the Eastern Cooperative Oncology Group Performance Status Scale; GAR, globulin albumin ratio; PFS, progression-free survival; OS, overall survival.
Result of univariate survival analysis demonstrated that GAR was a prognostic predictor. A high pretreatment GAR >0.58 was associated with worse PFS [hazard ratio (HR) =1.664, 95% CI: 1.233-2.247, P=0.001]. Other PFS prognostic variables were male (HR =1.408, P=0.039), TNM stage IV (HR =1.718, P=0.003), distant metastasis stage M1b (HR =2.061, P=0.000), single metastasis site (HR =1.557, P=0.017), multiple metastasis sites (HR =2.648, P=0.000), ECOG PS >1 (HR =1.657, P=0.001), and low albumin ≤35 g/L (HR =2.458, P=0.001), shown in Table 3.
Pretreatment GAR >0.58 (HR =1.959, P=0.000), age <65 years (HR =1.553, P=0.003), male (HR =1.566, P=0.007), ever smokers (HR =1.603, P=0.002), TNM stage IV (HR =1.630, P=0.007), single metastasis site (HR =1.502, P=0.028), multiple metastasis sites (HR =2.283, P=0.000), ECOG PS >1 (HR =1.654, P=0.001), albumin ≤35 g/L (HR =2.750, P=0.000) and globulin >27 g/L (HR =1.471, P=0.009) was associated with OS (Table 3).
Multivariate response rate and survival analysis
The significant factors in univariate survival analysis were enrolled into a multivariate Cox proportional regression for the test of independent factors. The statistical analysis data indicated that pretreatment GAR >0.58 (HR =1.524, P=0.008), pretreatment albumin ≤35 g/L (HR =2.093, P=0.003), ECOG PS >1 (HR =1.607, P=0.003), TNM stage IV (HR =3.235, P=0.000), distant metastasis (HR =1.600, P=0.018), and male (HR =1.439, P=0.032) were independent prognostic factors for PFS. Pretreatment GAR >0.58 (HR =1.651, P=0.002) was also associated with OS independently.
Other independent factors were pretreatment albumin ≤35 g/L (HR =1.922, P=0.022), ECOG PS >1 (HR =1.614, P=0.003), TNM stage IV (HR =3.371, P=0.000), distant metastasis (HR =1.515, P=0.031), age ≥65 years (HR =1.555, P=0.005) and ever smokers (HR =1.651, P=0.002) (Table 4).
Table 4. Result of multivariate analysis regarding PFS and OS in 316 advanced NSCLC patients.
| Variable | PFS |
OS |
|||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| GAR | |||||||
| ≤0.58 | 1 | 1 | |||||
| >0.58 | 1.52 | 1.12-2.08 | 0.008 | 1.65 | 1.20-2.26 | 0.002 | |
| Albumin | |||||||
| >35 | 1 | 1 | |||||
| ≤35 | 2.09 | 1.20-3.65 | 0.003 | 1.92 | 1.10-3.36 | 0.022 | |
| ECOG PS | |||||||
| ≤1 | 1 | 1 | |||||
| >1 | 1.61 | 1.18-2.19 | 0.003 | 1.61 | 1.18-2. 20 | 0.003 | |
| Metastasis stage | |||||||
| M0 | 1 | 1 | |||||
| M1b | 1.6 | 1.06-2.42 | 0.018 | 1.51 | 1.04-2.21 | 0.031 | |
| TNM stage | |||||||
| III | 1 | 1 | |||||
| IV | 3.23 | 1.99-5.24 | 0 | 3.37 | 2.05-5.55 | 0 | |
| Gender | |||||||
| Female | 1 | – | |||||
| Male | 1.44 | 1.03-2.01 | 0.032 | – | – | – | |
| Age | |||||||
| <65 | – | 1 | |||||
| ≥65 | – | – | – | 1.55 | 1.14-2.12 | 0.005 | |
| Smoke status | |||||||
| Never | – | 1 | |||||
| Ever | – | – | – | 1.65 | 1.20-2.26 | 0.002 | |
HR, hazard ratio; CI, confidence interval; ECOG PS, the Eastern Cooperative Oncology Group Performance Status Scale; GAR, globulin albumin ratio; PFS, progression free survival; OS, overall survival; NSCLC, non-small cell lung cancer; TNM stage, Tumor-Node-Metastasis stage.
Sensitivity and specificity analysis
According to ROC curve shown in Figure 4, AUCs of GAR and albumin were 0.600 (95% CI: 0.536-0.664, P=0.003) and 0.397 (95% CI: 0.333-0.460, P=0.002), respectively. The sensitivity and specificity of GAR at 0.58 were 62.2% and 53.7%, while those of albumin at 35 were 89.7% and 4.1%.
Figure 4.
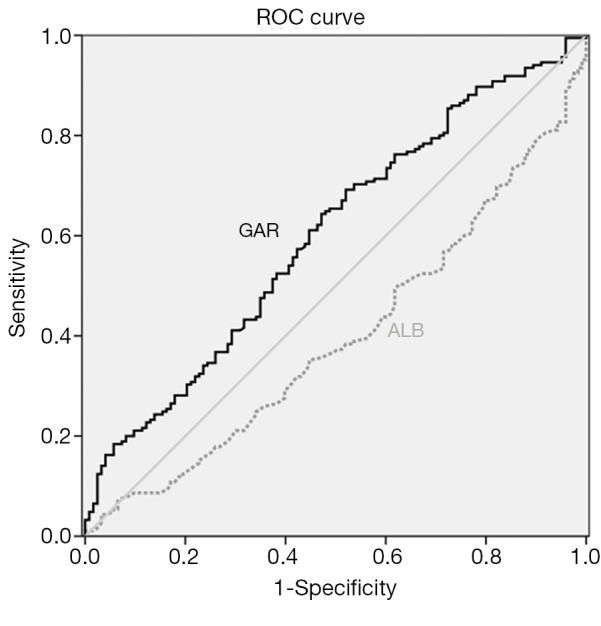
Comparison of receiver operating characteristics (ROC) curve of pretreatment globulin albumin ratio and albumin level.
Discussion
In our study, pretreatment GAR was demonstrated to be associated with PFS and OS for advanced NSCLC patients for the first time. Patients with pretreatment GAR >0.58 had worse PFS and OS. In multivariate survival analysis, after adjusting to age, gender, smoke status, TNM stage and ECOG PS, GAR remained to be an independent factor associated with PFS and OS. Besides GAR, albumin was also proven to be an independent prognostic factor for worse survival in the present study.
Albumin, which is produced by liver, helps to maintain intravascular oncotic pressure and acts as a free radical scavenger (8). The association between albumin and cancer was reported in a large investigation that serum albumin and body mass index were significantly lower in cancer than in non-cancer subjects (16). And in patients with advanced or terminal cancer, it was found that low baseline serum albumin level (<3.7 g/dL) predicted shorter survival (17). Previous studies successively proved that low albumin level was associated with malignant disease and was related to poor prognosis in many cancers, such as breast cancer, ovarian cancer, bladder cancer and lung cancer (7,9,18-21). Our observation also observed an independent association between low pretreatment serum albumin level and poor survival in advanced NSCLC patients.
The role of serum albumin in predicting prognosis may be due to the metabolic which is a reflection of both malignancy of cancer and status of body (9). However, albumin may also be related to metabolic changes caused by many factors, such as stress, illness, hepatic insufficiency, and depletion of visceral protein mass or synthesizing ability (22,23). The volatility of albumin limits the application in clinic.
To avoiding the limitation of albumin, our present study gives a hypothesis that taking albumin and globulin together, the GAR would be a predicting factor associated with prognosis of NSCLC. The result of our study supported this hypothesis and GAR was proved to be a strong factor than albumin in predicting survival for advanced NSCLC patients. The reason for this may be that globulin was not only another protein produced by liver, but also associated with cancer survival. Several studies suggested that globulin especially SHBG was a biomarker for cancer risk in breast cancer and prostate cancer patients (10,11,13,24). Löfgren et al. suggested that SHBG was associated with estrogen receptor which was also related to initiation and development of NSCLC (25-27).
Our present study was a single-institution retrospective study with limited number of included patients. However, this study focused on the advanced NSCLC patients, and more than 300 patients were finally enrolled in this study. Compared with other existing factors, GAR also has some advantages: it can be simply obtained rather than other invasive operation, low costing and efficiency. The association between GAR and worse prognosis of NSCLC patients is confirmed in our study, proving the accuracy of GAR as a biomarker in clinic.
Taken together, our study first established a connection between pretreatment GAR and advanced NSCLC patients, suggesting that GAR was an independent prognostic factor and could be the biomarker for prognosis. The clinical utility of GAR still needs to be confirmed with prospective analysis.
Conclusions
In summary, the results provide novel evidence that pretreatment serum GAR serves a useful prognostic predictor for advanced NSCLC patients. Accordingly, GAR could be used in clinic to better define the baseline risk in cancer patients.
Acknowledgments
All work was completed at the Department of Respiratory Medicine, Jinling Hospital, Nanjing University School of Medicine. This work was supported in part by the Natural Science Fund of Jiangsu Province (BK2011658) to Yong Song.
Disclosure: The authors declare no conflict of interest.
References
- 1.Siegel R, Naishadham D, Jemal A.Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30 [DOI] [PubMed] [Google Scholar]
- 2.Yao Y, Gu X, Zhu J, et al. Hormone replacement therapy in females can decrease the risk of lung cancer: a meta-analysis. PLoS One 2013;8:e71236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao Y, Yuan D, Liu H, et al. Pretreatment neutrophil to lymphocyte ratio is associated with response to therapy and prognosis of advanced non-small cell lung cancer patients treated with first-line platinum-based chemotherapy. Cancer Immunol Immunother 2013;62:471-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujii T, Sutoh T, Morita H, et al. Serum albumin is superior to prealbumin for predicting short-term recurrence in patients with operable colorectal cancer. Nutr Cancer 2012;64:1169-73 [DOI] [PubMed] [Google Scholar]
- 5.Kim HK, Kim S, Sung HK, et al. Comparison between preoperative versus intraoperative injection of technetium-99 m neomannosyl human serum albumin for sentinel lymph node identification in early stage lung cancer. Ann Surg Oncol 2012;19:1343-9 [DOI] [PubMed] [Google Scholar]
- 6.Arrieta O, Michel Ortega RM, Villanueva-Rodríguez G, et al. Association of nutritional status and serum albumin levels with development of toxicity in patients with advanced non-small cell lung cancer treated with paclitaxel-cisplatin chemotherapy: a prospective study. BMC Cancer 2010;10:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Espinosa E, Feliu J, Zamora P, et al. Serum albumin and other prognostic factors related to response and survival in patients with advanced non-small cell lung cancer. Lung Cancer 1995;12:67-76 [DOI] [PubMed] [Google Scholar]
- 8.Asher V, Lee J, Bali A.Preoperative serum albumin is an independent prognostic predictor of survival in ovarian cancer. Med Oncol 2012;29:2005-9 [DOI] [PubMed] [Google Scholar]
- 9.Bizzo SM, Meira DD, Lima JM, et al. Serum albumin and vascular endothelial growth factor in epithelial ovarian cancer: looking at adnexal tumor drainage. Arch Gynecol Obstet 2011;283:855-9 [DOI] [PubMed] [Google Scholar]
- 10.Adly L, Hill D, Sherman ME, et al. Serum concentrations of estrogens, sex hormone-binding globulin, and androgens and risk of breast cancer in postmenopausal women. Int J Cancer 2006;119:2402-7 [DOI] [PubMed] [Google Scholar]
- 11.Kristal AR, Schenk JM, Song Y, et al. Serum steroid and sex hormone-binding globulin concentrations and the risk of incident benign prostatic hyperplasia: results from the prostate cancer prevention trial. Am J Epidemiol 2008;168:1416-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naik S L D, Hedau S, Bahadur AK, et al. Sex hormone binding globulin in breast cancer. Indian J Clin Biochem 2008;23:250-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson DJ, Healey CS, Baynes C, et al. Identification of common variants in the SHBG gene affecting sex hormone-binding globulin levels and breast cancer risk in postmenopausal women. Cancer Epidemiol Biomarkers Prev 2008;17:3490-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verma MK, Miki Y, Abe K, et al. Co-expression of estrogen receptor beta and aromatase in Japanese lung cancer patients: gender-dependent clinical outcome. Life Sci 2012;91:800-8 [DOI] [PubMed] [Google Scholar]
- 15.Tang H, Liao Y, Chen G, et al. Estrogen upregulates the IGF-1 signaling pathway in lung cancer through estrogen receptor-β. Med Oncol 2012;29:2640-8 [DOI] [PubMed] [Google Scholar]
- 16.Göransson J, Jonsson S, Lasson A.Pre-operative plasma levels of C-reactive protein, albumin and various plasma protease inhibitors for the pre-operative assessment of operability and recurrence in cancer surgery. Eur J Surg Oncol 1996;22:607-17 [DOI] [PubMed] [Google Scholar]
- 17.Viganó A, Bruera E, Jhangri GS, et al. Clinical survival predictors in patients with advanced cancer. Arch Intern Med 2000;160:861-8 [DOI] [PubMed] [Google Scholar]
- 18.Lambert JW, Ingham M, Gibbs BB, et al. Using preoperative albumin levels as a surrogate marker for outcomes after radical cystectomy for bladder cancer. Urology 2013;81:587-92 [DOI] [PubMed] [Google Scholar]
- 19.Lis CG, Grutsch JF, Vashi PG, et al. Is serum albumin an independent predictor of survival in patients with breast cancer? JPEN J Parenter Enteral Nutr 2003;27:10-5 [DOI] [PubMed] [Google Scholar]
- 20.Ohnoshi T, Hiraki S, Nakata Y, et al. Pretreatment serum albumin concentration and lactic dehydrogenase activity as prognostic factors in patients with small cell lung cancer. Acta Med Okayama 1982;36:487-90 [DOI] [PubMed] [Google Scholar]
- 21.Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J 2010;9:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baumgartner RN, Koehler KM, Romero L, et al. Serum albumin is associated with skeletal muscle in elderly men and women. Am J Clin Nutr 1996;64:552-8 [DOI] [PubMed] [Google Scholar]
- 23.Anton AH. The relation between the binding of sulfonamides to albumin and their antibacterial efficacy. J Pharmacol Exp Ther 1960;129:282-90 [PubMed] [Google Scholar]
- 24.Sawada N, Iwasaki M, Inoue M, et al. Plasma testosterone and sex hormone-binding globulin concentrations and the risk of prostate cancer among Japanese men: a nested case-control study. Cancer Sci 2010;101:2652-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Löfgren L, von Schoultz E, Fernstad R, et al. Are estrogen receptor content in breast cancer and effects of tamoxifen on sex hormone-binding globulin markers for individual estrogen sensitivity? J Steroid Biochem Mol Biol 2006;99:76-9 [DOI] [PubMed] [Google Scholar]
- 26.Wang XY, Wang Y, Liu HC. Tamoxifen lowers the MMP-9/TIMP-1 ratio and inhibits the invasion capacity of ER-positive non-small cell lung cancer cells. Biomed Pharmacother 2011;65:525-8 [DOI] [PubMed] [Google Scholar]
- 27.Giovannini M, Belli C, Villa E, et al. Estrogen receptor (ER) and epidermal growth factor receptor (EGFR) as targets for dual lung cancer therapy: not just a case? J Thorac Oncol 2008;3:684-5 [DOI] [PubMed] [Google Scholar]


