Abstract
Objective
Obstructive sleep apnea syndrome (OSAS) is associated with many cardiovascular disorders. Chronic intermittent hypoxia (CIH) is the primary player in OSAS of the many associated factors. This study was in order to investigate the effects of the Adiponectin (Ad) on left ventricular remodeling induced by CIH.
Methods
Forty-five rats were randomly divided into three groups: normal control (NC) group, CIH group and CIH plus Ad supplemented (CIH + Ad) group. After 35 days’ CIH exposure, masson analysis was used to detect the left ventricular fibrosis and western blot was used to measure the protein expression of collagen I, collagen III and TGF-β/smad2/3 pathway. Gene analysis by RT-PCR was used to study the MMP2 and TIMP2.
Results
After CIH exposure, the fibrosis of left ventricular in CIH group was significantly remarkable than that in both NC and CIH + Ad groups (P<0.05), although statistical difference existed between NC and CIH + Ad groups (P<0.05). In addition, the protein expression of collagen I as well as collagen III and the ratio of mRNA levels of MMP2/TIMP2 were the highest in CIH group but the lowest in NC group, with CIH + Ad group in between. There was a significant difference among three groups (all P<0.05). The TGF-β/smad2/3 pathway was activated obviously in CIH group, but less noticeably in CIH + Ad group (P<0.05) with a significant difference in the two groups.
Conclusions
The present study showed that Ad could ameliorate the left ventricular remodeling induced by CIH via inhibition of the expression of TGF-β/smad2/3 pathway.
Keywords: Chronic intermittent hypoxia (CIH), left ventricular remodeling, adiponectin
Introduction
Obstructive sleep apnea syndrome (OSAS), as a common health disorder, is characterized by repeatedly upper airway collapse, resulting in chronic intermittent hypoxia (CIH) within the body. It has been detected OSAS is associated with cardiac dysfunction, which can be improved by positive airway pressure ventilation (CPAP) treatment (1-3). However, the correlation mechanisms between OSAS and associated cardiac damage are still under investigation and more effective treatments are desired for clinical practice since CPAP are not well tolerated by all OSAS patients. Chika Matsumoto et al. reported that intermittent hypoxia could induce left ventricular remodeling (4). Other report showed that oxidative stress, TGF-β and inflammatory cytokines might play important roles in left ventricular remodeling (5). Seong-Man Kim et al. reported that the severity of OSA was correlated with left artricular structural and functional remodeling (6).
Adiponectin (Ad) as a protein derived by the adipose tissue, is abundantly presents in plasma and displayed in three major forms in plasma: trimer, hexamer, and a high-molecular-weight form (7,8). Globular adiponectin (gAd), a proteolytic cleavage product of Ad, also exists in plasma (9). It is reported that Ad has the cardioprotective effect (10) and gAd is significantly more potent in reversing insulin resistance than uncleaved Ad (11). Koichi Fujita et al. reported that Ad protected cardiac from fibrosis induced by Ang II through Activation of PPAR-α (12). However, it remains to be elucidated about the relationship between CIH and left ventricular remodeling as well as the possible intervention role of Ad in vivo. In current study, the CIH model was established to investigate the possible association among them.
Materials and methods
Procedures of this study were approved by the Animal Ethic Committee of Nanjing Medical University.
Animals
Forty-five male Wistar rats (specific pathogen free) 8 weeks of age were purchased from Shanghai Silake Ltd. Inc. The rats were housed in Animal Care Center under the 12:12 hour light-dark cycle and allowed free access to standard chow and tap water, which were randomly divided into three groups with 15 in each group: normal control (NC) group, CIH group, CIH plus Ad supplement (CIH + Ad) group. The method of CIH has been reported previously (13,14). The rats were housed in a cage placed in the chamber of the OxyCycler Oxygen Profile Controller (BioSpherix). The inspired oxygen fraction was changed from ~21% to ~5-6% every 2 min with sustained for 15~20 s. The intermittent hypoxia events persisted 35 days. Rats in NC group were treated with ambient 21% O2 in a separate chamber. Rats in CIH + Ad group were also received the injection of Ad with intravenous at the dosage of 10 µg per time, twice a week for 5 weeks. A similar injection of saline (0.5 mL per time) was carried out in NC group and CIH group. Data were collected at the end of 5th week (day 35).
Tissue processing
After 35 days of experiment, the rats were anesthetized using pentobarbital. The chest was opened for collecting the heart tissue. The heart tissue was quickly isolated and part was stored at –70 °C while part was infused into 4% paraformaldehyde.
Masson analysis
Masson analysis was used to detect the cardiac fibrosis, according to the instruction of manufacturer. After deparaffinization and rehydration, the section incubated in R1 for 1 min, then immersed in R2 for 30 s. The sections were treated with R3 for 8 min. Then R4 was used to incubate the slides for 5 min. The section was analyzed by microscope. To evaluate the fibrosis index of heart tissues, ten random heart fields per tissue section were captured at the 400× magnification.
Quantitative real-time RT-PCR analysis
Total RNA (1 µg) extracted from left ventricular by using the TRIzol reagent (Invitrogen, USA) was reverse transcribed to complementary DNA using Transcriptor First Strand cDNA Synthesis Kit (Roche, Germany). Real-time QPCR was performed by using Power SYBR Green QPCR Master Mix (Applied Biosystems, Foster City, California, USA). The primer for rat MMP2 (Invitrogen, USA): forward (5'-AGGGCACCTCTTACAACAGC-3'); reverse (5'-CCCGGTCATAATCCTCGG TG-3'). The primer for rat TIMP2 (Invitrogen, USA): forward (5'-CAACCCCATCAAGAGGATTC-3'); reverse (5'-CGCAAGAACCATCACTTCTC-3'). The primer for rat β-actin (Invitrogen, USA): forward (5'-CAGGGTGTGA TGGTGGGTATGG-3'); reverse (5'-AGTTGGTGACAATGCCGTGTTC-3'). The cycling parameters were set as follows: 95 °C, 10 minutes and 40 cycles of 95 °C for 15 seconds and 60 °C for 1 minute. A single product obtained was proved by the dissociation curves. The PCR fluorescent signals of all genes were standardized to the β-actin. Comparative and relative quantifications of these gene products normalized to β-actin and the control group were calculated by the 2-△△Ct method.
Western blot analysis
Left ventricular was homogenized using Tissue Protein Extraction Reagent (Thermo scientific, USA) containing 1 mM of PMSF and phosphatase inhibitor cocktail (Roche, Germany). Then the homogenates were centrifuged at 10,000 ×g for 5 minutes and the supernatants were collected. The protein assay kit (Thermo Scientific, Rockford, USA) was used to detect the protein concentration (bicinchoninic acid method). Total left ventricular lysates were used to quantify proteins of TGF-β (Abcam Ltd, USA), smad2/3 (Cell Signaling Technology, USA), collagen I (Abcam Ltd, USA) and collagen III (Abcam Ltd, USA) by western blot. Equal protein amounts (30 µg) of left ventricular lysates were subjected to electrophoresis on 10% sodium dodecyl sulfate PAGE, which transferred to polyvinylidene fluoride membranes (Roche, USA). The 5% bovine serum albumin in TBS with 0.1% Tween-20 at pH 7.6 was used to blot the membranes for 1 h at room temperature, then the membranes were incubated with primary antibodies diluted in 5% bovine serum albumin in TBS with 0.1% Tween-20 at pH 7.6 at 4 °C for one night with gentle shaking, followed by incubation with a peroxidase-labeled secondary antibody diluted in 5% bovine serum albumin in TBS with 0.1% Tween-20 at pH 7.6 for 1 h at 37 °C. The membranes were detected by using enhanced ECL kit (Thermo Scientific, USA) and exposed using the digital imaging system (Molecular Imager® ChemiDocTM XRS + System), which offered sensitive chemiluminescent detection (Bio-Rad Laboratories Inc, Hercules, CA, USA). The intensity of band was normalized to β-actin analyzed using Image Lab 2.0 Software (Bio-Rad Laboratories Inc, USA).
Statistical analysis
Values are presented as means ± SD in three independent experiments. Significant differences between all groups were computed by one-way analysis of variance (ANOVA) using the Student-Newman-Keuls post hoc test for multiple group comparisons. Statistical difference was accepted at P<0.05.
Result
The cardiac fibrosis after CIH
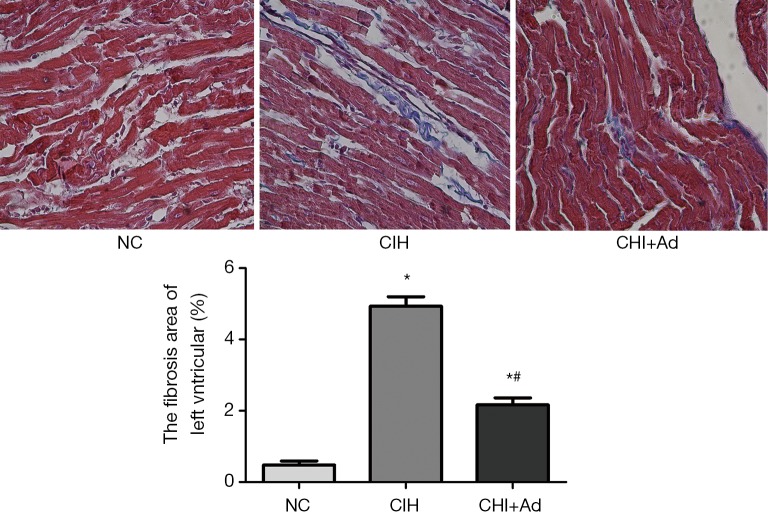
After 35 days’ exposure of CIH, Masson analysis showed the area of cardiac fibrosis of the left ventricular in the CIH group were significantly higher than that in NC and CIH + Ad groups (P<0.05), although there was difference still statistical difference between NC and CIH + Ad groups (P<0.05) (Figure 1). The expression of collagen I and collagen III measured with western blot was the highest in the CIH group but the lowest in the NC group, with the CIH + Ad group in between. There was a significant difference among all the three groups (all P<0.05) (Figure 2).
Figure 1.
The Masson analysis of the left ventricular. The blue represented the fibrosis and the red represented the normal myocardium. *P<0.01 versus NC group; #P<0.05 versus CIH; NC, normal control; CIH, chronic intermittent hypoxia; CIH + Ad, chronic intermittent hypoxia and adiponectin supplement.
Figure 2.
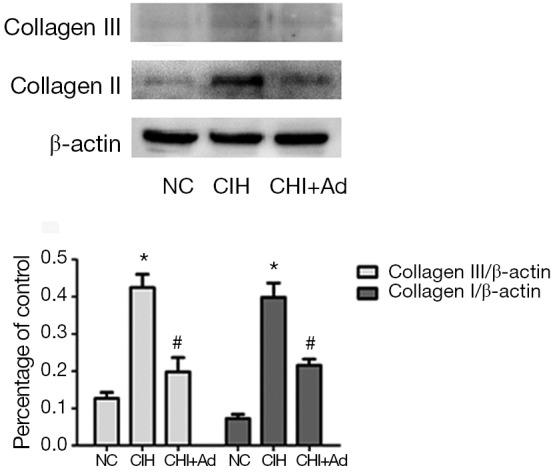
The protein levels of collagen I and collagen III. The protein levels of collagen I and collagen III. Western blot bands of collagen I and collagen III were normalized to β-actin. *P<0.05 versus NC group; #P<0.05 versus CIH group. NC, normal control; CIH, chronic intermittent hypoxia.
Expression of molecules related to CIH-induced cardiac fibrosis
Apart from the finding of the cardiac fibrosis from the myocardium of left ventricle after CIH exposure, a significantly higher ratio of mRNA levels of MMP2/TIMP2 was also detected in CIH group than those in NC group (P<0.05), although there was still a markedly difference between the NC and CIH + Ad groups (P<0.05) (Figure 3).
Figure 3.
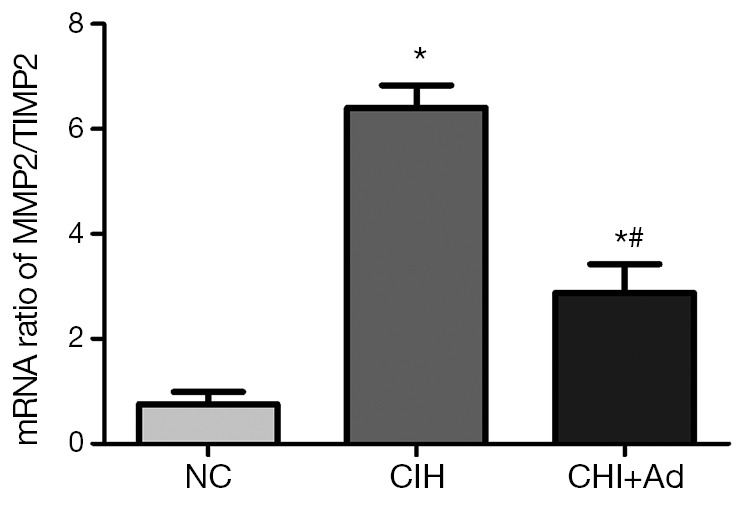
The ratio of mRNA levels of MMP2 and TIMP2. The mRNA expressions of MMP2/TIMP2 in heart of three groups; PCR fluorescent signals for MMP2, TIMP2 were standardized to PCR fluorescent signals obtained from an endogenous reference (β-actin). *P<0.01 versus NC group; #P<0.05 versus CIH. NC, normal control; CIH, chronic intermittent hypoxia.
Since the TGF-β/smad2/3 pathway which is associated with the fibrosis, our further investigate into this pathway revealed that the expression of TGF-β and phosphorylation smad2/3 detected by western blot were significantly enhanced in the CIH group, compared with both NC group and CIH + Ad groups (P<0.05), even though there was a statistical difference between the NC and CIH + Ad groups (P<0.05) (Figure 4).
Figure 4.
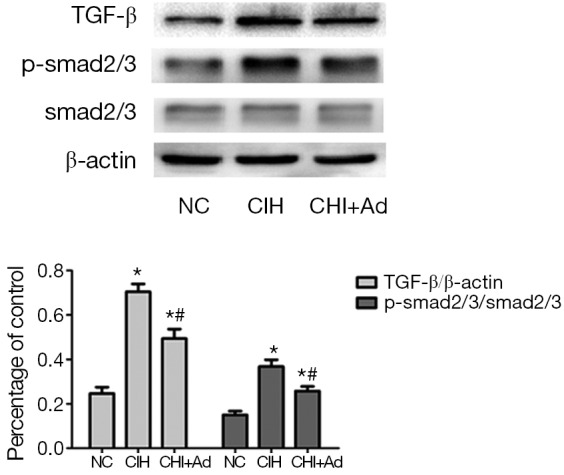
The protein levels of TGF-β/smad2/3 pathway. The protein levels of TGF-β and smad2/3. Western blot bands of TGF-β and smad2/3 were normalized to β-actin. *P<0.05 versus NC group; #P<0.05 versus CIH group. NC, normal control; CIH, chronic intermittent hypoxia.
Discussion
In order to study the effects of Ad on left ventricular remodeling induced by CIH, we established an animal model to mimic CIH. In this study, we found that after 35 days CIH exposure, the CIH could induce the left ventricular remodeling represented by the area of fibrosis and the protein expression of collagen I and collagen III. With Ad supplement, the left ventricular remodeling was ameliorated and the possible mechanism was the inhibition of TGF-β/smad2/3 pathway.
It is well known that OSAS is one of the important risks for several cardiovascular diseases (15). It has been reported that CIH could induce the left ventricular remodeling in mice (4) or rat (14,16,17). Our findings were consisted with them. In the present study, the enhanced fibrosis of left ventricular was also founded in CIH group. Our previous study demonstrated that CIH could induce oxidative stress and myocardium apoptosis (13) and it has been reported that oxidative stress is important for left ventricular remodeling (18). So we speculate that CIH induced the left ventricular remodeling through oxidative stress. However, when Ad was supplemented, we found, the index of the fibrosis was ameliorated. Our findings were consisted with many studies (19). It is well reported that Ad has the effect of cardiac protection (10,20,21). And Koichi Fujita et al. found Ad could protect against cardiac fibrosis induced by angiotensin II (12). Shimano, M’s study showed that Ad-KO mice exhibited greater left ventricular (LV) interstitial fibrosis after the TAC surgery compared with the WT mice (22). So we suggested that Ad could ameliorate the cardiac fibrosis.
MMPs, a family of the proteolytic enzymes for extracellular matrix protein (ECM) degradation, are key players in cardiac matric remolding (23). TIMPs, the tissue inhibitors of metalloproteinases, synthesized proteins which bind to the active MMPs to regulate net proteolytic activity (24). MMPs and TIMPs regulate the matrix degradation which determines the cardiac fibrosis (25). MMP2 is a crucial protein in the process of tissue fibrosis and TIMP2 is the main inhibitory factor of MMP2 in tissue (26). The increased MMP activity and the imbalance between MMP2 and TIMP2 have been implicated in pathological processes (23). Recent study showed that MMP2 activity was responsible for the development of left ventricular hypertrophy in a two kidney, one clip hypertensive rat model (27). In accordance with these reports, we found the elevated ratio of mRNAs levels of the MMP2/TIMP2 were induced by CIH, which represents the imbalance between MMPs and TIMPs. And when the Ad was added, the change of MMP2/TIMP2 was partially improved. This suggested that Ad may protect the myocardium against the fibrosis through regulating the balance between MMPs and TIMPs.
In the present study, we found Ad could improve the left ventricular remodeling induced by CIH and the imbalance between MMPs and TIMPs. TGF-β, as a pleiotropic cytokine, is involved in lots of biological processes such as cell growth and differentiation, embryonic development, fibrosis, cell proliferation and survival and regulation of the inflammatory response (28). The TGF-β signals via the receptors of trans-membrane to activate Smad2/3 phosphorylation, and then the pSmad2/3 complex translocates into the nucleus and induces the pro-fibrotic target genes expression (28-30). It has been reported that the TGF-β signal was an important role in cardiac remodeling (28) and the overexpression of TGF-β could enhance the extracellular matrix protein synthesis (31,32). And in the hypertensive rats induced by one kidney clip, the increased expression of TGF-β, along with temporal changes of MMP2 activity, was associated with simultaneous cardiac remodeling (33). According to these studies, in the present study, we found that the TGF-β pathway was activated by CIH and the supplement of Ad reduced the overexpressions of the TGF-β and Smad2/3.
In conclusion, our results suggested that Ad could protect the myocardium from left ventricular modeling induced by CIH through inhibition of TGF-β/smad2/3 pathway. However, further investigation is still needed to further explore the detailed cardioprotective mechanisms of Ad during CIH.
Acknowledgements
Funding: This work was supported by National Natural Science Foundation of China (No. 81370184 and 81270312).
The authors are grateful to the assistance of Dechao Kong and the PIs of the laboratory of Department of Cardiology.
Disclosure: The authors declare no conflict of interest.
References
- 1.Kasai T, Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J Am Coll Cardiol 2011;57:119-27 [DOI] [PubMed] [Google Scholar]
- 2.Yasuma F, Ogihara A.Long-term treatment of ischemic dilated cardiomyopathy with continuous positive airway pressure. Intern Med 2001;40:1121-7 [DOI] [PubMed] [Google Scholar]
- 3.Grewal RG. Treatment of cardiomyopathy with PAP therapy in a patient with severe obstructive sleep apnea. J Clin Sleep Med 2012;8:581-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsumoto C, Hayashi T, Kitada K, et al. Chymase plays an important role in left ventricular remodeling induced by intermittent hypoxia in mice. Hypertension 2009;54:164-71 [DOI] [PubMed] [Google Scholar]
- 5.Lijnen PJ, Petrov VV, Fagard RH. Induction of cardiac fibrosis by transforming growth factor-beta(1). Mol Genet Metab 2000;71:418-35 [DOI] [PubMed] [Google Scholar]
- 6.Kim SM, Cho KI, Kwon JH, et al. Impact of obstructive sleep apnea on left atrial functional and structural remodeling beyond obesity. J Cardiol 2012;60:475-83 [DOI] [PubMed] [Google Scholar]
- 7.Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 1999;257:79-83 [DOI] [PubMed] [Google Scholar]
- 8.Kishida K, Kando N.Two-stage refinement of query translation in a pivot language approach to cross-lingual information retrieval: An experiment at CLEF 2003. Comparative Evaluation of Multillingual Information Access Systems 2004;3237:253-62 [Google Scholar]
- 9.Fruebis J, Tsao TS, Javorschi S, et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci U S A 2001;98:2005-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ouchi N, Shibata R, Walsh K.Cardioprotection by adiponectin. Trends Cardiovasc Med 2006;16:141-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamauchi T, Kamon J, Waki H, et al. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem 2003;278:2461-8 [DOI] [PubMed] [Google Scholar]
- 12.Fujita K, Maeda N, Sonoda M, et al. Adiponectin protects against angiotensin II-induced cardiac fibrosis through activation of PPAR-alpha. Arterioscler Thromb Vasc Biol 2008;28:863-70 [DOI] [PubMed] [Google Scholar]
- 13.Ding W, Zhang X, Huang H, et al. Adiponectin protects rat myocardium against chronic intermittent hypoxia-induced injury via inhibition of endoplasmic reticulum stress. PLoS One 2014;9:e94545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen L, Einbinder E, Zhang Q, et al. Oxidative stress and left ventricular function with chronic intermittent hypoxia in rats. Am J Respir Crit Care Med 2005;172:915-20 [DOI] [PubMed] [Google Scholar]
- 15.Lattimore JD, Celermajer DS, Wilcox I. Obstructive sleep apnea and cardiovascular disease. J Am Coll Cardiol 2003;41:1429-37 [DOI] [PubMed] [Google Scholar]
- 16.Chen L, Zhang J, Gan TX, et al. Left ventricular dysfunction and associated cellular injury in rats exposed to chronic intermittent hypoxia. J Appl Physiol (1985) 2008;104:218-23 [DOI] [PubMed] [Google Scholar]
- 17.Chen L, Zhang J, Hu X, et al. The Na+/Ca2+ exchanger-1 mediates left ventricular dysfunction in mice with chronic intermittent hypoxia. J Appl Physiol (1985) 2010;109:1675-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inamoto S, Yoshioka T, Yamashita C, et al. Pitavastatin reduces oxidative stress and attenuates intermittent hypoxia-induced left ventricular remodeling in lean mice. Hypertens Res 2010;33:579-86 [DOI] [PubMed] [Google Scholar]
- 19.Amin RH, Mathews ST, Alli A, et al. Endogenously produced adiponectin protects cardiomyocytes from hypertrophy by a PPARgamma-dependent autocrine mechanism. Am J Physiol Heart Circ Physiol 2010;299:H690-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shibata R, Sato K, Pimentel DR, et al. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med 2005;11:1096-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Berendoncks AM, Garnier A, Ventura-Clapier R, et al. Adiponectin: key role and potential target to reverse energy wasting in chronic heart failure. Heart Fail Rev 2013;18:557-66 [DOI] [PubMed] [Google Scholar]
- 22.Shimano M, Ouchi N, Shibata R, et al. Adiponectin deficiency exacerbates cardiac dysfunction following pressure overload through disruption of an AMPK-dependent angiogenic response. J Mol Cell Cardiol 2010;49:210-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mishra PK, Givvimani S, Chavali V, et al. Cardiac matrix: a clue for future therapy. Biochim Biophys Acta 2013;1832:2271-6. [DOI] [PMC free article] [PubMed]
- 24.Spinale FG. Matrix metalloproteinases: regulation and dysregulation in the failing heart. Circ Res 2002;90:520-30 [DOI] [PubMed] [Google Scholar]
- 25.Mizoguchi H, Yamada K.Roles of matrix metalloproteinases and their targets in epileptogenesis and seizures. Clin Psychopharmacol Neurosci 2013;11:45-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshizaki T, Sato H, Furukawa M.Recent advances in the regulation of matrix metalloproteinase 2 activation: from basic research to clinical implication (Review) Oncol Rep 2002;9:607-11 [PubMed] [Google Scholar]
- 27.Rizzi E, Castro MM, Prado CM, et al. Matrix metalloproteinase inhibition improves cardiac dysfunction and remodeling in 2-kidney, 1-clip hypertension. J Card Fail 2010;16:599-608 [DOI] [PubMed] [Google Scholar]
- 28.Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor (TGF)-β signaling in cardiac remodeling. J Mol Cell Cardiol 2011;51:600-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng XH, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol 2005;21:659-93 [DOI] [PubMed] [Google Scholar]
- 30.Samarakoon R, Overstreet JM, Higgins PJ. TGF-β signaling in tissue fibrosis: redox controls, target genes and therapeutic opportunities. Cell Signal 2013;25:264-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eghbali M, Tomek R, Sukhatme VP, et al. Differential effects of transforming growth factor-beta 1 and phorbol myristate acetate on cardiac fibroblasts. Regulation of fibrillar collagen mRNAs and expression of early transcription factors. Circ Res 1991;69:483-90 [DOI] [PubMed] [Google Scholar]
- 32.Rosenkranz S, Flesch M, Amann K, et al. Alterations of beta-adrenergic signaling and cardiac hypertrophy in transgenic mice overexpressing TGF-beta(1). Am J Physiol Heart Circ Physiol 2002;283:H1253-62 [DOI] [PubMed] [Google Scholar]
- 33.Rizzi E, Ceron CS, Guimaraes DA, et al. Temporal changes in cardiac matrix metalloproteinase activity, oxidative stress, and TGF-β in renovascular hypertension-induced cardiac hypertrophy. Exp Mol Pathol 2013;94:1-9 [DOI] [PubMed] [Google Scholar]