Abstract
Objective
The study aimed to construct and manage an acute respiratory distress syndrome (ARDS)/sepsis registry that can be used for data warehousing and clinical research.
Methods
The workflow methodology and software solution of research electronic data capture (REDCap) was used to construct the ARDS/sepsis registry. Clinical data from ARDS and sepsis patients registered to the intensive care unit (ICU) of our hospital formed the registry. These data were converted to the electronic case report form (eCRF) format used in REDCap by trained medical staff. Data validation, quality control, and database management were conducted to ensure data integrity.
Results
The clinical data of 67 patients registered to the ICU between June 2013 and December 2013 were analyzed. Of the 67 patients, 45 (67.2%) were classified as sepsis, 14 (20.9%) as ARDS, and eight (11.9%) as sepsis-associated ARDS. The patients’ information, comprising demographic characteristics, medical history, clinical interventions, daily assessment, clinical outcome, and follow-up data, was properly managed and safely stored in the ARDS/sepsis registry. Data efficiency was guaranteed by performing data collection and data entry twice weekly and every two weeks, respectively.
Conclusions
The ARDS/sepsis database that we constructed and manage with REDCap in the ICU can provide a solid foundation for translational research on the clinical data of interest, and a model for development of other medical registries in the future.
Keywords: Acute respiratory distress syndrome (ARDS)/sepsis registry, research electronic data capture (REDCap), data quality, data management
Introduction
Sepsis is a systemic response to infection that can result in severe complications such as organ dysfunction and septic shock (circulatory failure despite fluid resuscitation) (1). Further, it is the most common etiology of acute respiratory distress syndrome (ARDS) (2). ARDS and sepsis are currently the leading causes of death among critically ill patients, with reported case-fatality rates ranging from 25% to nearly 50% for ARDS, up to 30% for sepsis, 50% for severe sepsis, and 80% for septic shock (3-7).
We have been assisting several large-scale global clinical trials to collect clinical data of our patients with ARDS and sepsis for a long time. However, a special electronic database to manage these precious clinical resources has never been established. Although our in-clinic diagnoses and treatments are in accordance with international guidelines, some excellent clinical findings by senior doctors could not be published owing to lack of clinical evidence. Further, because a large number of ARDS/sepsis patients are registered to the intensive care unit (ICU) every year, it has proved necessary to establish a special ARDS/sepsis registry to collect and manage the data comprising the daily diagnosis and treatment of the patients in the ICU. Our objective is to establish a database that can serve as a convenient and reliable tool in data collection, storage, and management, and to facilitate clinical research aimed at improving diagnosis and treatment, exploring the biomarkers of the diseases, and proposing helpful suggestions for public health decision makers.
We worked with the Applied Health Research Centre (AHRC), a leading academic research organization at St. Michael’s Hospital, Canada, to build and manage our ARDS/sepsis registry. The workflow methodology and software solution of research electronic data capture (REDCap), an NIH-sponsored, HIPAA compliant, free, and secure web-based application designed for data collection and management to support clinical and translational research, was used to construct the registry (8,9). REDCap was originally developed at Vanderbilt University by Paul A. Harris and colleagues in 2004 and is continuously updated and enhanced by the Project REDCap Team at Vanderbilt. Currently, it is being used in more than 111,000 projects by over 143,000 users across the world, including extensive usage by Clinical and Translational Science Awards (CTSAs) and other institutions such as Harvard, Mayo Clinic, and Massachusetts Institute of Technology (MIT) (10). It provides multiple studies and case report forms (CRFs) and very effectively supports data capture for small and medium scale study and is capable of supporting prospective and retrospective studies, as well as multicenter clinical trials. Data collections in microsoft excel and microsoft access can be easily converted to REDCap. The methodology used to construct our ARDS/sepsis registry with REDCap and utilization of the registry for submission, storage, and management of the data of ARDS/sepsis patients for clinical and translational research are presented in this paper. The data presented here are part of a long-term study of ARDS/sepsis patients registered to the ICU.
Methods
REDCap application
REDCap is not an open-source software, but it is available free of charge to institutional partners. It is not difficult to support; however, infrastructural requirements such as a web server that supports PHP, a MySQL database server, and secure sockets layer (SSL) connections need to be satisfied (10). On signing a valid end-user license agreement with Vanderbilt University to become a consortium partner, we obtained access to the software and help resources. It is now hosted on a local server at The First Affiliated Hospital of Guangzhou Medical University.
ARDS/sepsis patients
ARDS/sepsis patients registered to the ICU at The First Affiliated Hospital of Guangzhou Medical University between June and December 2013 were enrolled the project. We define ARDS using the Berlin definition (11), and sepsis in accordance with the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference (1). Patients were eligible for enrollment if they fulfilled the criteria for ARDS or sepsis within 24 hours after ICU admission. Any patient who was pregnant, below 18 years old, had a life expectancy of less than 48 hours, had a history of bone marrow or liver transplantation, had been receiving chemotherapy or radiation therapy within the previous eight weeks, or was positive for the human immuno-deficiency virus was excluded. Clinical data collection began immediately following ICU admission. From each enrolled patient, 5 mL of venous blood was drawn and centrifuged, and the plasma aliquots (200 µL) stored at –80 °C for further analysis.
The study protocol was approved by the Ethics Committee of The First Affiliated Hospital, Guangzhou Medical University. Written informed consent was also obtained from all participants in the study.
Registry description
The research team worked with clinicians to design our discipline-specific, comprehensive registry, which consists of six parts: demographic characteristics, medical history, clinical interventions, daily assessments, clinical outcome, and follow-up. In addition to collecting the clinical data of the patients, we observed the changing state of their illnesses 1, 3, 5, 7, 14, 21, and 28 days after admission. We created a large, prospective, non-interventional database in the registry to collect data describing the management and outcomes of ARDS/sepsis patients in the ICU. All data met accepted clinical standards governing the usage of existing standard terminology concerning ARDS and sepsis. Consistent and comparable formats were also adopted whenever possible. Original clinical data were abstracted from the electronic and written medical records of the patients for 28 days from the day of ICU admission or until discharge, if earlier, and recorded on a CRF. To ensure that the clinical data were collected in the appropriate format necessary for essential analysis, a statistician was assigned to review the database planning process.
Creation process
The ARDS/sepsis registry was designed and implemented on the REDCap platform, which provides (I) an intuitive interface for validated data entry; (II) audit trails to track the history of data entry and revision; (III) procedures for importing data from external sources; (IV) data downloads to Excel, PDF, SAS, SPSS, Stata, and R; and (V) a built-in scheduling calendar, ad hoc reporting tools, and advanced features such as branching logic and calculated fields (8,10).
No formal programming, networking, or database experience was needed to use REDCap because training videos and help resources were available on the REDCap consortium website to familiarize users with REDCap. However, to ensure that the database was information security compliant and aligned with standard operating procedures, an AHRC IT specialist was assigned to assist with project programming. The implementation steps comprised (I) logging in to our hospital network to access REDCap; (II) creating a new project by navigating to the “Create New Project” tab and completing and submitting all required information about the main project settings; (III) creating data collection instruments by defining data variables and their properties; (IV) previewing and testing data entry screens to ensure appropriate dataset yields for planned statistical analyses; (V) configuring study member permissions and user rights; and (VI) moving the project from development to production to ensure data accuracy and integrity after actual collection of clinical data started.
Data entry and quality control
It is important to have a mechanism to reduce the possibility of data entry errors. With REDCap, we restricted data format/type, set ranges for date and numeric fields, and allowed data validation. Data consistency problems such as incorrect data type, values out of range, and outliers for numerical fields can be reported using the data quality module. Further, we applied pre-defined rules that facilitated determination of whether a specific data value might be discrepant, which is very important because our project contains many fields and has many records. Two trained medical staff members worked together on data extraction and the data were cross-checked during the input process. The project director reviewed all the medical records of the first 50 patients to ensure that standard operating procedures, including data collection and entry, were adhered to. Spot checks were also conducted by a statistician every two months. Only five entry mistakes or less per 100 were acceptable, failing which all clinical data underwent a full-scale review or were re-entered and reassessed.
Database management
A project director, a research team, clinicians, an IT specialist, and a statistician worked together to manage the ARDS/sepsis registry. The project director, who was also the project administrator, coordinated the activities of all personnel associated with the database. The database structure was designed based on discussions held with practicing clinicians and the research team. Electronic CRFs were developed by the IT specialist using REDCap and reviewed by the statistician. ICU clinicians identified subjects for the study according to the eligibility and exclusion criteria. Demographic and clinical data capture, entry, and analysis were performed by the research team. The project director and the statistician supervised all data activities to assure quality control (Figure 1).
Figure 1.
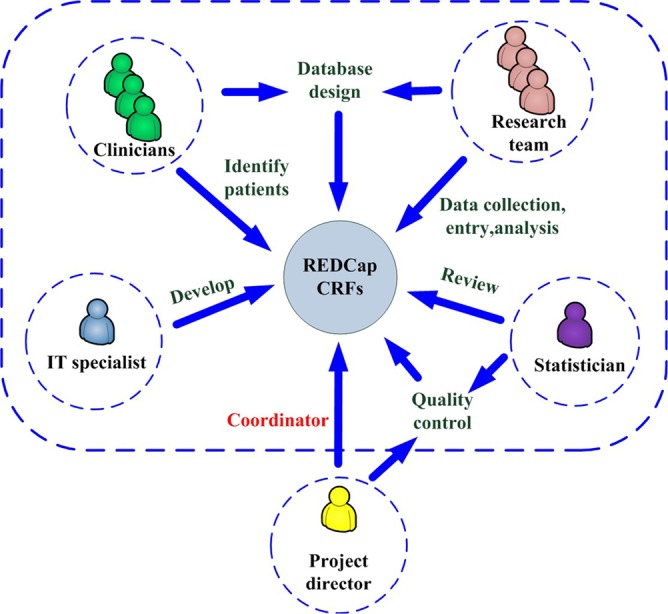
Schematic diagram of the registry management methodology. CRFs, case report forms; REDCap, research electronic data capture.
Results
Inclusion and exclusion of patients
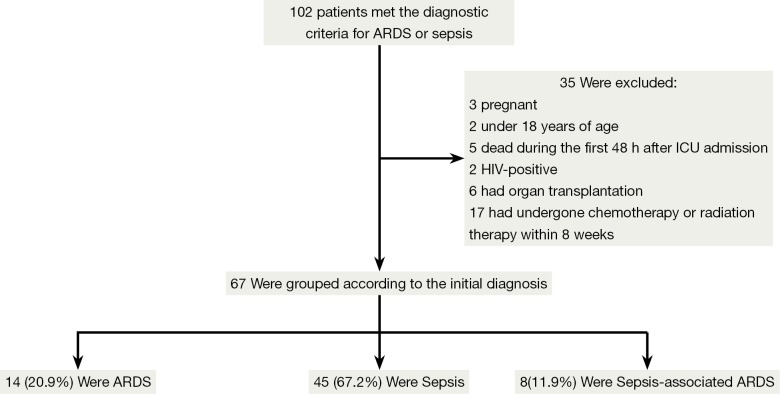
A total of 102 ARDS/sepsis patients registered to the ICU between June and December 2013 were assessed for inclusion in the study. Of this total, 35 were excluded and the remaining 67 eligible patients were enrolled in the registry. Among the eligible patients, 45 (67.2%) were diagnosed with sepsis, 14 (20.9%) with ARDS, and eight (11.9%) with sepsis-associated ARDS at the time of ICU admission (Figure 2).
Figure 2.
Patient inclusion/exclusion flow diagram. ARDS, acute respiratory distress syndrome; ICU, intensive care unit.
Structure of the registry
The registry comprises six categories, demographic characteristics, medical history, clinical interventions, daily assessments, clinical outcome, and follow-up, which are sub-divided into 121 items and 186 variables (Table 1).
Table 1. ARDS/sepsis registry structure.
| Entries | Variables |
|---|---|
| Demographics | Study ID, gender, date of birth, marital status, height, weight, address, smoking, junior physician of ICU, senior physician of ICU, hospital admission date, ICU admission date |
| Medical history | Co-morbidities, surgery history, lung Injury cause |
| Clinical interventions | ARDS/sepsis screening, mechanical ventilation, fluid balance, nutritional support, analgesia, sedation, neuromuscular blockade, transfusion, chest tube, RRT, PICCO, ECMO, IABP, antibiotics, vasopressor, corticosteroids, immunoglobulin |
| Daily assessments | Patient disposition, vitals, lab results, glasgow coma score, SOFA score, APACHE II score, pathogens and sources, chest X-ray, CT scan |
| Clinical outcome | ICU outcome, date of death, cause of death, ventilator on days, ICU expenses, hospitalization expenses |
| Follow-up | 30-, 60-, 90-, 180-, 365-day survival |
ICU, intensive care unit; ARDS, acute respiratory distress syndrome.
Data storage
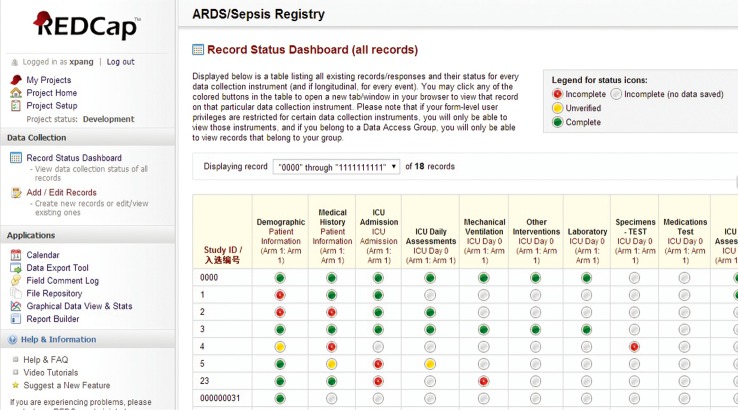
As shown in Figure 3, the record status dashboard of the registry is a table listing all existing records and their status for every data collection instrument. The data collected at the various points in time are categorized accordingly for storage and management. The red, grey, yellow, and green icons signify incomplete, blank, unverified, and complete records, respectively. Clicking any of the colored buttons in the table automatically activates the associated data collection instrument.
Figure 3.
Record status dashboard of the ARDS/sepsis registry. ARDS, acute respiratory distress syndrome; ICU, intensive care unit.
Security of the data
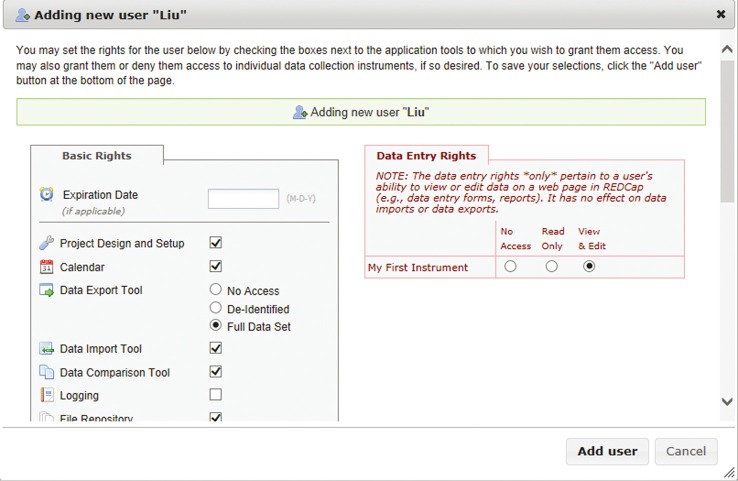
The clinical data maintain full patient confidentiality since they are SSL encrypted and stored on a secure server. Every interaction with the data is logged, creating an audit trail. The project director can also lock the data after all finalization checks have been completed. Researchers can access the database from multiple sites and institutions only with their own unique usernames and passwords. Different levels of data access rights can also be assigned by the project administrator to different users, depending on their roles in the project. These rights include logging, data entry rights, managing survey participants, calendar, data export tool, data import tool, file repository, data quality, project design, and setup (Figure 4).
Figure 4.
User rights that can be granted or restricted.
Enhanced efficiency in data collection to support research
Data were collected and entered into the database twice weekly and every two weeks, respectively, by trained medical staff. It took a trained medical worker approximately 30 minutes per subject to convert clinical data to the eCRF format used in the REDCap platform. The frequent collection ensured timely and efficient acquisition of the clinical data of the patients of interest, significantly reducing the traditional data collection workload and facilitating future clinical research. Moreover, since the data collected can be easily converted to formats used by microsoft excel, microsoft access, SPSS, and SAS, they are convenient to transfer and share.
Discussion
A high quality medical registry can help to monitor and improve the health of ARDS/sepsis patients by facilitating description of the epidemiologic and clinical characteristics of the diseases and evaluation of the effectiveness of interventions. One notable example is the surviving sepsis database of the surviving sepsis campaign (SSC), which contains ten measures of the routine care process and one outcome measure of sepsis patients. It remains the core measurement strategy for the SSC. The SSC was developed in an attempt to increase early recognition and improve outcome in severe sepsis and septic shock patients (12,13). Since proposal of the SSC guidelines in 2004 and their subsequent updating in 2008 and 2012, the survival rate of patients with severe sepsis and septic shock has been significantly improved. Levy et al. found that the hospital mortality decreased significantly from 37% to 30.8% (P=0.001) within 2 years (14). Castellanos-Ortega et al. reported that the hospital mortality in the historical group was significantly higher than in the intervention group (57.3% vs. 37.5%, P=0.001) (15). Miller et al. reported that severe sepsis and septic shock bundle compliances reduced hospital mortality from 21.7% in 2004 to 9.7% in 2010 (16). Significant achievements of ARDS Networks, such as the ALVEOLI Study, and the Fluid and Catheter Treatment Trial (FACTT) study, have also benefitted from comprehensive patient information (17-19).
Although the SSC database is reasonable in design, it is aimed at early recognition and treatment of sepsis patients. Clinical data collection is primarily focused on the characteristics of early condition changes in sepsis patients and the efficacy of initial interventions (like fluid resuscitation, and 3- and 6-hour bundles). Our ARDS/sepsis registry, established using the REDCap platform, facilitates collection and management of the clinical data of patients with ARDS and sepsis in a more comprehensive manner.
The ARDS/sepsis registry collection demographics, diagnoses, interventions, medications, and laboratory data of ARDS/sepsis patients are valuable for clinical research. They can be used to identify patients for clinical research, analyze disease-specific practice patterns, and aid in the development of potential treatment strategies (20,21). In addition, the plasma samples obtained from our patients at ICU admission and stored, frozen at –80 °C, can be analyzed. Combinations of clinical data with biological specimens enhance a better understanding of pathophysiological mechanisms of ARDS and sepsis and help determine biomarkers to improve diagnostic and prognostic accuracy (22).
This ARDS/sepsis registry has several limitations. Although many clinically important variables are included in the database, the data cannot be automatically transferred from the Hospital Information System to REDCap. Therefore, much time and labor are expended collecting and inputting these data into our registry. Although REDCap provides automatic data validation and popup warnings regarding out-of-range values, it does not prevent invalid data from being entered. Consequently, efforts to minimize the possibility of incorrect data being inputted and to control data quality are especially important.
Conclusions
This paper described the infrastructure of our ARDS/sepsis registry and the approach taken to its construction and management with REDCap. Construction of the ARDS/sepsis registry facilitates better translation from clinical practice to scientific research. This methodological study is an effort to fully utilize daily clinical data of ARDS/sepsis patients and to implement a reproducible research system for clinical data integration. Such an effort can be generalized to other medical registries.
Acknowledgements
Source of funding: This study was supported by the National Nature Science Foundation of China (China-Canada Joint Health Research, Grant No. 81361128003) and the Natural Science Foundation of Guangdong Province (Grant No. S2012010008499).
Author contributions: Xiaoqing Pang, Sulong Wu, Yongbo Huang and Pu Mao designed the registry, collected and entered the clinical data. Xiaoqing Liu and Weiqun He designed the registry and identified eligible subjects. Mei Jiang reviewed the registry. Chaoyi Huang developed the eCRFs. Natascha Kozlowski coordinated the activities between Guangzhou and Toronto. Yimin Li and Haibo Zhang supervised all data activities and coordinated the activities of all members.
We sincerely thank the editors for their valuable comments and suggestions.
Disclosure: The authors declare no conflict of interest.
References
- 1.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003;31:1250-6 [DOI] [PubMed] [Google Scholar]
- 2.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med 2005;353:1685-93 [DOI] [PubMed] [Google Scholar]
- 3.Phua J, Badia JR, Adhikari NK, et al. Has mortality from acute respiratory distress syndrome decreased over time?: A systematic review. Am J Respir Crit Care Med 2009;179:220-7 [DOI] [PubMed] [Google Scholar]
- 4.Villar J, Sulemanji D, Kacmarek RM. The acute respiratory distress syndrome: incidence and mortality, has it changed? Curr Opin Crit Care 2014;20:3-9 [DOI] [PubMed] [Google Scholar]
- 5.Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 2003;348:1546-54 [DOI] [PubMed] [Google Scholar]
- 6.Jawad I, Lukšić I, Rafnsson SB. Assessing available information on the burden of sepsis: global estimates of incidence, prevalence and mortality. J Glob Health 2012;2:010404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy MM, Artigas A, Phillips GS, et al. Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect Dis 2012;12:919-24 [DOI] [PubMed] [Google Scholar]
- 8.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franklin JD, Guidry A, Brinkley JF. A partnership approach for Electronic Data Capture in small-scale clinical trials. J Biomed Inform 2011;44Suppl 1:S103-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Research Electronic Data Capture. [Internet]. Cited 2014 May 12. Available online: http://project-redcap.org/
- 11.ARDS Definition Task Force , Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33 [DOI] [PubMed] [Google Scholar]
- 12.Slade E, Tamber PS, Vincent JL. The Surviving Sepsis Campaign: raising awareness to reduce mortality. Crit Care 2003;7:1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dellinger RP, Carlet JM, Masur H, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med 2004;32:858-73 [DOI] [PubMed] [Google Scholar]
- 14.Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med 2010;38:367-74 [DOI] [PubMed] [Google Scholar]
- 15.Castellanos-Ortega A, Suberviola B, García-Astudillo LA, et al. Impact of the Surviving Sepsis Campaign protocols on hospital length of stay and mortality in septic shock patients: results of a three-year follow-up quasi-experimental study. Crit Care Med 2010;38:1036-43 [DOI] [PubMed] [Google Scholar]
- 16.Miller RR, 3rd, Dong L, Nelson NC, et al. Multicenter implementation of a severe sepsis and septic shock treatment bundle. Am J Respir Crit Care Med 2013;188:77-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004;351:327-36 [DOI] [PubMed] [Google Scholar]
- 18.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Wiedemann HP, Wheeler AP, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006;354:2564-75. [DOI] [PubMed]
- 19.Thompson BT, Bernard GR. ARDS Network (NHLBI) studies: successes and challenges in ARDS clinical research. Crit Care Clin 2011;27:459-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prokosch HU, Ganslandt T. Perspectives for medical informatics. Reusing the electronic medical record for clinical research. Methods Inf Med 2009;48:38-44 [PubMed] [Google Scholar]
- 21.Dreyer NA, Garner S. Registries for robust evidence. JAMA 2009;302:790-1 [DOI] [PubMed] [Google Scholar]
- 22.Marshall JC, Reinhart K, International Sepsis Forum Biomarkers of sepsis. Crit Care Med 2009;37:2290-8 [DOI] [PubMed] [Google Scholar]