Abstract
Kinetin (Kn) is a cytokinin growth factor that exerts several anti-aging and antioxidant effects on cells and organs. To investigate the mechanism underlying apoptotic events in aging cells induced by D-galactose (D-gal), we examined the effect of Kn delivered via nuchal subcutaneous injection on D-gal-induced aging and apoptosis in rats. Our results showed that interleukin (IL)-2 levels and mitochondrial membrane potential (ΔΨm) were decreased by Kn in aging rats while IL-6 production and apoptosis increased. In addition, the expression of anti-apoptotic Bcl-2 was low while that of Bax was high in the aging group. After treated with Kn, compared with aging group, there showed obvious difference in Kn group with elevated IL-2, proliferation index, Bcl-2, ΔΨm and decreased IL-6 and Bax in splenic lymphocyte. Based on these results, we concluded that Kn can effectively protect the rat spleen from aging, apoptosis, and atrophy.
Keywords: apoptosis, immunity, kinetin, mitochondrial membrane potential, spleen
Introduction
Kinetin (Kn) is a cytokinin and belongs to a group of gland purine derivatives, and Kn protects against oxidative damage and exerts powerful anti-aging effects. Kn activates superoxide dismutase (SOD) and glutathione peroxidase (GSH-PX), and can serve as a direct free radical antagonist. Additionally, it has shown that Kn exerts significant anti-oxidant and anti-aging effects on skin cells [1,5].
Apoptosis, also known also as programmed cell death, is the result of gene regulation in cells that helps balance the dynamic stability between cell proliferation and death, and apoptosis occurs in aging organs involved in immunity [18]. Mitochondrial membrane potential (ΔΨm) is required to maintain normal mitochondrial function. Low ΔΨm is an important indicator of apoptosis. Decreased ΔΨm leads to reduced ATP synthesis and inhibits mitochondrial oxidative phosphorylation [10]. In addition, a previous study showed that interleukin (IL)-2 and IL-6 influence the aging process, apoptosis, and immunity [11]. In the current study, we measured changes in ΔΨm associated with D-galactose (D-gal)-induced aging and expression of apoptosis-related genes in rat spleen. Results of the current study showed that Kn helped protect spleen lymphocytes from apoptosis and prevented splenic atrophy.
Materials and Methods
Animals
SD (Rattus norregicus) rats (100 rats, 2 months old, average body weight [BW]: 200 ± 20 g, and there was a random distribution of male and female animals in the study) were obtained from the laboratory animal center of Xi'an Jiaotong University (certification of qualification no. 2012-003; China) and housed in a clean environment. The animals were randomly divided into five groups: the control, aging, low Kn dose, middle Kn dose, and high Kn dose groups (20 rats each group).
Reagents and primer design
Kn (K3378) and D-gal were obtained from Sigma (USA). Kn was made into a solution with 0.06 mol/L. Enzyme-linked immunosorbent assay (ELISA) kits specific for IL-2 and IL-6 were obtained from Xinbosheng Biotechnology (China). Lymphocyte separation medium was purchased from Shanghai Huajing Biological Technology (China). A ΔΨm detection kit (JC-1), annexin V-FITC apoptosis detection kit, and cell cycle detection kit were obtained from BiYunTian (China). A total DNA/RNA extraction kit, First Chain UltraSYBR Mixture, and SuperQuickRT cDNA synthesis kit were purchased from CoWin Bioscience (China). The following PCR primers from Mus musculus were used for the experiment.
Bcl-2-F: 5'-GGTTTCATCCAGGATCGAGCA-3'
Bcl-2-R: 5'-CGTCAGCAATCATCCTCTGCA-3'
Bax-F: 5'-TGGACAACATCGCTCTGTGGA-3'
Bax-R: 5'-TCAAACAGAGGTCGCATGCAG-3'
β-actin-F: 5'-ATGGAAGAAGAAATCGCCGC-3'
β-actin-R: 5'-ACACGCAGCTCGTTGTAGAA-3'
Equipment
An A1R laser confocal microscope (Nikon, Japan), BD FACSAria flow cytometer (Becton, Dickinson and Company, USA), and an ELx800 absorbance microplate reader (BioTek Instruments, USA) were used for the study.
Establishment of the aging rat model and treatment
The rats were randomly divided into five groups of 20 animals each and allowed to acclimate for 1 week to reduce stress. The animals were given food and clean water, and the temperature was maintained at 20℃. The control group was continuously injected subcutaneously with normal saline (125 mg/kg; BW) for 45 days. Rats in the other four groups (aging, low Kn, middle Kn, and high Kn groups) were continuously injected subcutaneously with D-gal (125 mg/kg, BW) for 45 days. Starting on experimental day 11, rats in the control group and aging group were given normal saline (125 mg/kg, BW) while rats in other groups were given different doses (5 mg/kg BW, 10 mg/kg BW, and 20 mg/kg BW) of Kn per day until experimental day 45.
Change of the morphology of the spleen lymphocytes was observed continuously to day 45 before the animals were euthanized by injected subcutaneously. The spleen of each rat was collected, weighed, and ground into a 10% tissue homogenate (1 g spleen and 9 g saline, tissue grinders). The homogenate was then centrifuged at 1,500 × g force for 10 min at room temperature (RT). The supernatants were collected and the levels of SOD, GSH-PX, and MDA activity were measured with hydroxylamine, colorimetric, malondialdehyde colorimetric method.
Measurement of IL-2 and IL-6 levels
The serum concentrations of IL-6 and IL-2 were measured on day 45 with a double-antibody sandwich ELISA according to manufacturer's instructions.
Preparation of spleen lymphocyte suspensions
Sterile spleen tissues were recovered from each group of rats on day 45 and ground into a homogenate (in 1 mL PBS buffer) that was centrifuged at 1,000 × g for 20 min at RT. The lymphocytes were removed and cell viability was assessed with trypan blue solution (survival rate >97%; BiYunTian). Last the lymphocytes in suspension (1~10 × 106 cells/mL) were grown in culture medium containing 10% calf serum (Hyclone Laboratories, USA) at 37℃.
Detection of ΔΨm
Some spleen lymphocytes suspension (cell counting, 6 × 106 cells/mL) was carried out according to the manufacturer's instructions (JC-1) using flow cytometry detection and confocal microscope. The results were analyzed using the ratio of polymer (red fluorescence) and monomer (green fluorescence) as changes in the ΔΨm.
Detection of apoptosis
Apoptosis of the lymphocytes was assessed with the annexin V-FITC apoptosis detection kit according to the apoptosis manufacturer's instructions. The results were analyzed and the apoptosis rate was determined by flow cytometry.
Cell cycle analysis
Aliquots of the spleen lymphocyte suspension (~1.17 × 106 cells/mL) were added to 96-well plates (in triplicate for each rat; Corning, USA) with 5 µL of concanavalin A (5 µg/mL; BiYunTian). The cells were washed with PBS and stained with propidium iodide (EMD Millipore, USA). Using flow cytometry, data for Gap0/1 (G0G1), synthesis phase (S), and mitotic phase (G2M) were analyzed and the proliferation index (PI) indicated the spleen lymphocyte proliferation index as follows:
Reverse transcription (RT)-PCR
Total mRNA from the spleen cells was isolated using a Trizol kit (Invitrogen, USA) according to the manufacturer's instructions. cDNA was transcribed from the mRNA using a RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, USA) according to the manufacturer's instructions. PCR was conducted at 94℃ for 5 min followed by 30 cycles of 94℃ for 30 sec, 57℃ for 30 sec, and 72℃ for 1 min. The levels of Bax and Bcl-2 mRNA expression were normalized to that of β-actin (RNA concentration was performed using spectrophotometer; Shimadzu, Japan).
Statistical analyses
Statistical analysis was conducted with SPSS software (ver. 20.0; IBM, USA). A one-way ANOVA was used to perform inter-group comparisons and a post-hoc Duncan's test was used to make multiple comparisons. All data are expressed as the mean ± standard deviation (SD). A p value < 0.05 was considered significant, a p value < 0.01 was considered highly significant, and a p value < 0.0001 was considered extremely significant.
Results
Establishment of the aging rat model
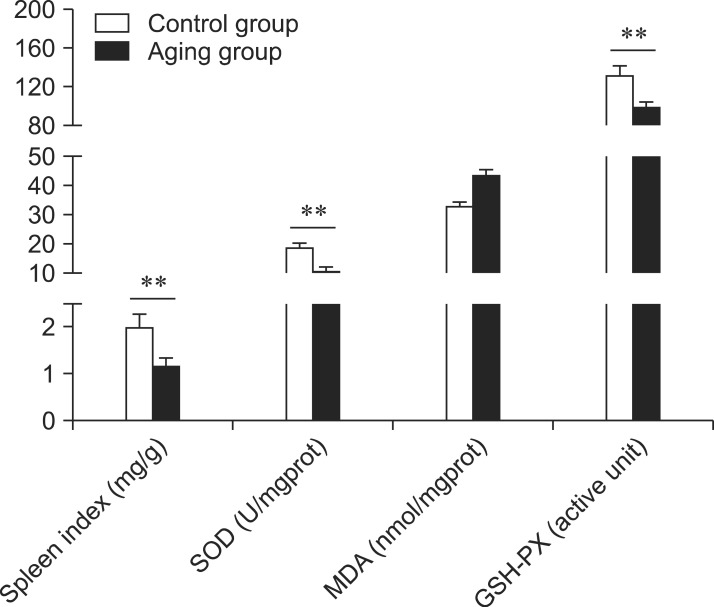
Rats in the aging group showed symptoms of aging characterized by depression, a thick coat, and slow activity. As shown in Fig. 1, these values of spleen index, SOD, GSH-PX, and MDA in the aging group were significantly different (p < 0.01) from those of the control group. These results indicated that the aging rat model was successfully established.
Fig. 1.
Spleen oxidative (spleen index, SOD, MDA, and GSH-PX) indices for the different groups. **p < 0.01.
The change of IL-2 and IL-6 production
As shown in Fig. 2, compared with control group, IL-2 levels were significantly decreased while IL-6 concentrations were significantly increased (p < 0.01) in aging group. After treatment with different doses of Kn, the levels of IL-2 in the spleen was high (p < 0.01) while the IL-6 content was obviously reduced (p < 0.05) in aging group.
Fig. 2.
Difference in IL-2 and IL-6 production in the different groups. After treatment with kinetin (Kn), the expression levels of IL-2 and IL-6 in the control and high dose groups were similar. *p < 0.05 and **p < 0.01; n.s. means no significant difference.
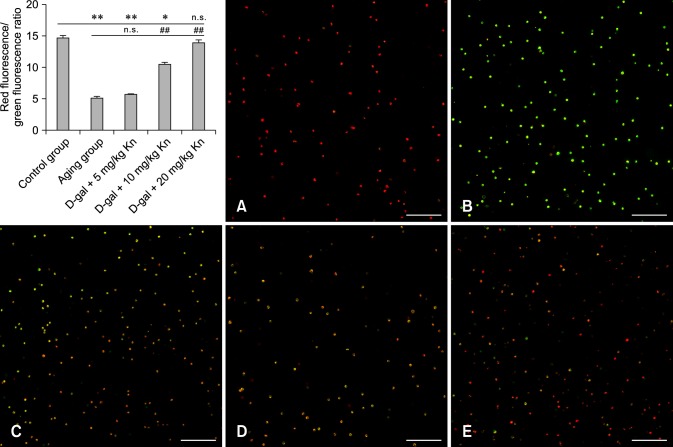
Changes of spleen lymphocyte ΔΨm
The ΔΨm of the control group (indicated by intense red fluorescence) was obviously greater than that in other groups, and the red fluorescent/green fluorescent ratio was 15.66 ± 0.38 as measured by flow cytometry. After treatment with carbonyl cyanide m-chlorophenyl-hydrazone (CCCP, an inhibitor of the mitochondrial electron transport chain; BiYunTian) as a positive control, ΔΨm almost completely disappeared as indicated by staining with JC-1 (green fluorescence). The red fluorescence/green fluorescence ratio for the aging group was significantly lower than that of the control group (p < 0.01). After treatment with Kn, the ΔΨm decreased compared to the control group and increased relative to the aging group (p < 0.01). Additionally, our results also proved that changes in green fluorescence corresponded to alterations in the apoptosis rate (Fig. 3).
Fig. 3.
Spleen lymphocyte mitochondrial membrane potential (ΔΨm) for each group. The red fluorescence/green fluorescence ratios showed that Kn helped restore the ΔΨm in aging cells. ΔΨm (A) of the control group indicated by red fluorescence. (B) After treatment with CCCP, the ΔΨm almost disappeared as indicated by green fluorescence resulting from JC-1 staining. ΔΨm (C) for the aging group, (D) middle Kn dose group, and (E) high Kn dose group. Scale bars = 100 µm.
Changes of apoptosis rates
The lymphocyte apoptosis rate of the aging group increased significantly compared to that of the control group (p < 0.01; Fig. 4). The lymphocyte apoptosis rate of the Kn treatment group (High Kn groups) decreased and the difference between the aging group and control group was extremely significant (p < 0.01; Fig. 4).
Fig. 4.
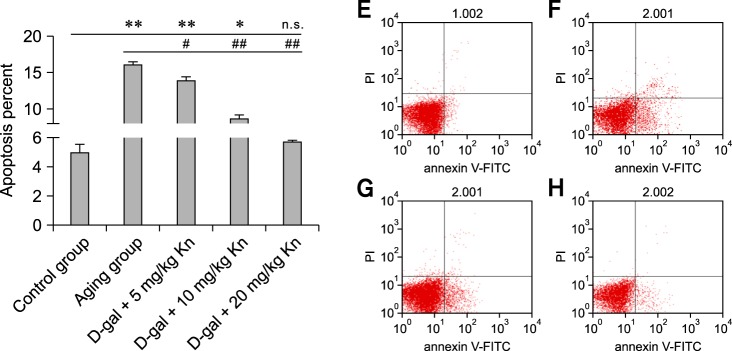
The rates of spleen lymphocyte apoptosis for each group. Spleen lymphocyte apoptosis (E) in the control group; the apoptosis rate was 3.68%. (F) In the aging group, the apoptosis rate was 16.46%. (G) In the middle dose group, the apoptosis rate was 8.32%. (H) In the high dose group, the apoptosis rate was 5.76%. PI: proliferation index.
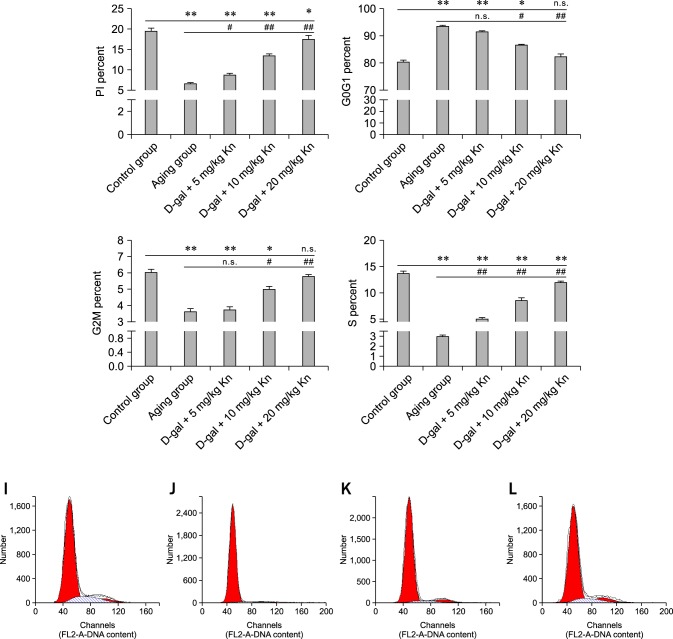
Changes in the cell cycle and PI value
As presented in Fig. 5, the percentage of lymphocytes in the quiescent stage increased for the aging group and PI index was reduced compared to the control group (p < 0.01). After Kn treatment, the number of lymphocytes in the different cell cycle stages showed significant difference (Fig. 5).
Fig. 5.
Changes in the cell cycle and PI values for each group. The percentage of PI, G2M, and S phases in spleen lymphocytes and G0G1 (*p < 0.05; **p < 0.01; n.s., not significant, respectively). Spleen lymphocytes (I) in the control group with a higher percentage of cells in the G2M and S phase. (J) In the aging group, there were reduced proportions of cells in the G2M and S phases. (K) Treatment with the middle Kn dose increased the proportion of cells in the G2M and S phases. (L) The high Kn dose significantly increased the proportion of cells in the G2M and S phases.
Changes in the expression of Bax and Bcl-2 mRNA in rat spleen lymphocytes
From Fig. 6, Bcl-2 mRNA expression in the aging group decreased significantly relative to that in the control animals, and that Bcl-2 mRNA expression was partially restored by Kn treatment but was still significantly reduced compared to the control rats (p < 0.01). Additionally, please consider stating whether or not the dose of Kn affected Bcl-2 expression (e.g., was there a difference between rats given the high dose and ones that received a low dose?). In contrast, the expression of Bax mRNA increased in the aging group compared to the control group (p < 0.01; Fig. 6). However, there was not difference between the high Kn group and control group.
Fig. 6.
Differences in the expression of Bax and Bcl-2 in each group (*p < 0.05 and **p < 0.01; n.s., not significant). The effects of Kn on the expression of Bcl-2 and Bax in spleen lymphocytes. Lane 1, the control group; Lane 2, the aging group; Lane 3, the low dose group (D-gal + 5 mg/kg Kn); Lane 4, the middle dose group (D-gal + 10 mg/kg Kn); Lane 5, the high dose group (D-gal + 20 mg/kg Kn).
Discussion
Animal aging is a spontaneous and inevitable process. Presently, there are many theories about the mechanisms underlying the aging process such as ones involving free radicals, immune decline, and cell apoptosis [20]. Tissues and organs exhibit age-related decreases in functions and atrophy that result in reduced skin tone, brain atrophy, dementia, and lowered immunity [2,7]. Additionally, it has been shown that aging is accompanied by increased apoptosis and decreased immune function [16]. Kn is widely found in plants and animals, and may effectively protect against aging in human fibroblasts [12]. This factor has anti-aging effects, acts synergistically with Vitamin B3 [14], and can affect cell shape as well as the internal cytoskeleton structure [4].
D-gal affects cell metabolism resulting in excessive accumulation of reactive oxygen species with increased free radicals, eventually resulting in aging and a variety of age-related disease [6]. Our current study has shown that SOD and oxidase activity decrease after subcutaneous injection of D-gal according with the previous studies [8,11]. Our results also demonstrated that spleen index, SOD, and GSH-PX decreased in the aging group while MDA activity increased.
IL-2 and IL-6 secreted by lymphocytes play a pivotal role in immune regulation. During the aging process, IL-2 levels decrease while IL-6 production increases [3]. Our results showed that Kn increased the expression of both IL-2 and IL-6. Interestingly, our results proved a well character compared to aging model, and rescue was positively correlated with the Kn dosage. The results clearly demonstrate the effect of Kn on immune regulation and anti-aging activities.
With aging, there is a substantial reduction in ΔΨm and excessive apoptosis as observed by green fluorescence using JC-1 staining [10]. Agreeing to previous results, mitochondrial membrane potential treated with Kn showed lower in control group than aging model group, indicating that Kn had obvious anti-aging effect to confront spleen aging rats and maintained higher ΔΨm. A previous study indicated that apoptosis rates increase with increasing age [19]. Our results demonstrated that Kn prevented apoptosis of the rat spleen lymphocytes in a dose-dependent manner in the aging group, and effectively inhibited D-gal-induced apoptosis.
The aging has been reported for the comprehensive performance and the most notable features of cell cycle is of block in G0G1 phase while cells in S phase and G2M phase reduced PI index [9,15]. Our results demonstrated that Kn can decrease the number of resting cell and increased the PI index, indicating that Kn promotes cell division and a smooth transition from the G0G1 phase into the S phase. Furthermore, our data suggest that Kn helps maintain the vitality and immune functions of spleen cells, thereby conferring protection against apoptosis and aging.
Under normal physiological conditions, the expression level of Bax is low in cells while that of Bcl-2 is high. In aging cells, the expression of Bcl-2 is decreased and the expression of Bax is elevated [13,17]. Our study showed that D-gal significantly decreased Bcl-2 expression in the aging group while Kn administration restored Bcl-2 expression. These results indicated that Kn can improve the PI index, reduce the proportion of resting cells, and protect against apoptosis by regulating the expression of Bcl-2 and Bax.
In summary, we have presented evidence that Kn helps regulate the expression of Bcl-2 and Bax, and effectively inhibits apoptosis of spleen lymphocytes while promoting lymphocyte proliferation. This could enhance immunity and have anti-aging effects. Furthermore, our data suggest that Kn not only inhibits age-related atrophy of the spleen and delays the aging of spleen lymphocytes, but also alters the ΔΨm and protects spleen cells from apoptosis. However, further studies are needed to elucidate the detailed molecular mechanisms underlying the changes of ΔΨm and other effects of Kn on the aging process.
Footnotes
There is no conflict of interest.
References
- 1.Alemi M, Sabouni F, Sanjarian F, Haghbeen K, Ansari S. Anti-inflammatory effect of seeds and callus of Nigella sativa L. extracts on mix glial cells with regard to their thymoquinone content. AAPS PharmSciTech. 2013;14:160–167. doi: 10.1208/s12249-012-9899-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aw D, Silva AB, Palmer DB. Is thymocyte development functional in the aged? Aging (Albany NY) 2009;1:146–153. doi: 10.18632/aging.100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basta-Kaim A, Szczęsny E, Leśkiewicz M, Głombik K, Slusarczyk J, Budziszewska B, Regulska M, Kubera M, Nowak W, Wędzony K, Lasoń W. Maternal immune activation leads to age-related behavioral and immunological changes in male rat offspring - the effect of antipsychotic drugs. Pharmacol Rep. 2012;64:1400–1410. doi: 10.1016/s1734-1140(12)70937-4. [DOI] [PubMed] [Google Scholar]
- 4.Boone N, Loriod B, Bergon A, Sbai O, Formisano-Tréziny C, Gabert J, Khrestchatisky M, Nguyen C, Féron F, Axelrod FB, Ibrahim EC. Olfactory stem cells, a new cellular model for studying molecular mechanisms underlying familial dysautonomia. PLoS One. 2010;5:e15590. doi: 10.1371/journal.pone.0015590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiu PC, Chan CC, Lin HM, Chiu HC. The clinical anti-aging effects of topical kinetin and niacinamide in Asians: a randomized, double-blind, placebo-controlled, split-face comparative trial. J Cosmet Dermatol. 2007;6:243–249. doi: 10.1111/j.1473-2165.2007.00342.x. [DOI] [PubMed] [Google Scholar]
- 6.Dkhar P, Sharma R. Late-onset dietary restriction modulates protein carbonylation and catalase in cerebral hemispheres of aged mice. Cell Mol Neurobiol. 2014;34:307–313. doi: 10.1007/s10571-013-0015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hošek J, Novotná R, Babula P, Vančo J, Trávníček Z. Zn(II)-chlorido complexes of phytohormone kinetin and its derivatives modulate expression of inflammatory mediators in THP-1 cells. PLoS One. 2013;8:e65214. doi: 10.1371/journal.pone.0065214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar D, Rizvi SI. Plasma paraoxonase 1 arylesterase activity in D-galactose-induced aged rat model: correlation with LDL oxidation and redox status. Aging Clin Exp Res. 2014;26:261–267. doi: 10.1007/s40520-013-0170-2. [DOI] [PubMed] [Google Scholar]
- 9.Lin HP, Lin CY, Hsiao PH, Wang HD, Sheng Jiang S, Hsu JM, Jim WT, Chen M, Kung HJ, Chuu CP. Difference in protein expression profile and chemotherapy drugs response of different progression stages of LNCaP sublines and other human prostate cancer cells. PLoS One. 2013;8:e82625. doi: 10.1371/journal.pone.0082625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin-Cano FE, Camello-Almaraz C, Acuña-Castroviejo D, Pozo MJ, Camello PJ. Age-related changes in mitochondrial function of mouse colonic smooth muscle: beneficial effects of melatonin. J Pineal Res. 2013 doi: 10.1111/jpi.12109. Epub ahead of print. doi: 10.1111/jpi.12109. [DOI] [PubMed] [Google Scholar]
- 11.Meneguello-Coutinho M, Caperuto E, Bacurau AV, Chamusca G, Uchida MC, Tibana RA, Pereira GB, Navalta JW, Wasinski F, Cavaglieri CR, Prestes J, Costa Rosa LF, Bacurau RF. Effects of dietary restriction or swimming on lymphocytes and macrophages functionality from old rats. Immunol Invest. 2014;43:113–122. doi: 10.3109/08820139.2013.847456. [DOI] [PubMed] [Google Scholar]
- 12.Mik V, Szüčová L, Smehilová M, Zatloukal M, Doležal K, Nisler J, Grúz J, Galuszka P, Strnad M, Spíchal L. N9-substituted derivatives of kinetin: effective antisenescence agents. Phytochemistry. 2011;72:821–831. doi: 10.1016/j.phytochem.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Minutoli L, Bitto A, Squadrito F, Marini H, Irrera N, Morgia G, Passantino A, Altavilla D. Serenoa Repens, lycopene and selenium: a triple therapeutic approach to manage benign prostatic hyperplasia. Curr Med Chem. 2013;20:1306–1312. doi: 10.2174/0929867311320100007. [DOI] [PubMed] [Google Scholar]
- 14.Mukherji R, Prabhune A. Novel glycolipids synthesized using plant essential oils and their application in quorum sensing inhibition and as antibiofilm agents. ScientificWorldJournal. 2014;2014:890709. doi: 10.1155/2014/890709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Philippe L, Tosca L, Zhang WL, Piquemal M, Ciapa B. Different routes lead to apoptosis in unfertilized sea urchin eggs. Apoptosis. 2014;19:436–450. doi: 10.1007/s10495-013-0950-3. [DOI] [PubMed] [Google Scholar]
- 16.Rice KM, Meduru S, Kakarla SK, Katta A, Mupparaju SP, Kidd B, Goebel LJ, Blough ER. Chronic paracetamol treatment influences indices of reactive oxygen species accumulation in the aging Fischer 344 X Brown Norway rat aorta. Ann Clin Lab Sci. 2012;42:152–161. [PubMed] [Google Scholar]
- 17.Rohn TT, Vyas V, Hernandez-Estrada T, Nichol KE, Christie LA, Head E. Lack of pathology in a triple transgenic mouse model of Alzheimer's disease after overexpression of the anti-apoptotic protein Bcl-2. J Neurosci. 2008;28:3051–3059. doi: 10.1523/JNEUROSCI.5620-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schöneich C, Dremina E, Galeva N, Sharov V. Apoptosis in differentiating C2C12 muscle cells selectively targets Bcl-2-deficient myotubes. Apoptosis. 2014;19:42–57. doi: 10.1007/s10495-013-0922-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song S, Gao P, Xiao H, Xu Y, Si LY. Klotho suppresses cardiomyocyte apoptosis in mice with stress-induced cardiac injury via downregulation of endoplasmic reticulum stress. PLoS One. 2013;8:e82968. doi: 10.1371/journal.pone.0082968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]