Abstract
A total of 156 Shiga-like toxin producing Escherichia coli (STEC) were isolated from fecal samples of Korean native (100/568, 18%) and Holstein dairy cattle (56/524, 11%) in Korea between September 2010 and July 2011. Fifty-two STEC isolates (33%) harbored both of shiga toxin1 (stx1) and shiga toxin2 (stx2) genes encoding enterohemolysin (EhxA) and autoagglutinating adhesion (Saa) were detected by PCR in 83 (53%) and 65 (42%) isolates, respectively. By serotyping, six STEC from native cattle and four STEC from dairy cattle were identified as O-serotypes (O26, O111, O104, and O157) that can cause human disease. Multilocus sequence typing and pulsed-field gel electrophoresis patterns highlighted the genetic diversity of the STEC strains and difference between strains collected during different years. Antimicrobial susceptibility tests showed that the multidrug resistance rate increased from 12% in 2010 to 42% in 2011. Differences between isolates collected in 2010 and 2011 may have resulted from seasonal variations or large-scale slaughtering in Korea performed to control a foot and mouth disease outbreak that occurred in early 2011. However, continuous epidemiologic studies will be needed to understand mechanisms. More public health efforts are required to minimize STEC infection transmitted via dairy products and the prevalence of these bacteria in dairy cattle.
Keywords: cattle, Escherichia coli, multidrug resistance, serotyping, Shiga toxin
Introduction
Shiga toxin-producing Escherichia (E.) coli (STEC) has been a main food-borne pathogen that causes illnesses ranging from mild diarrhea to bloody diarrhea, hemorrhagic colitis, and hemolytic-uremic syndrome (HUS) worldwide [27]. STEC strains produce Shiga toxins (Stxs) are essential virulence factors for STEC-associated diseases [23]. STEC strains may also express several other virulence factors that cause severe diseases such as intimin, STEC autoagglutinating adhesin (Saa), and enterohemolysin hlyA that are encoded by eaeA, saa, and ehxA, respectively [25,32,40]. Intimin and autoagglutinating adhesion are responsible for bacteria attachment to the intestinal surface while enterohemolysin is responsible for enterocyte damage [9,25]. There is a large number of serotypes associated with human diseases but only a few serotypes (O26, O91, O103, O111, and O157) cause severe cases of human illnesses [12,27,32].
Pulsed-field gel electrophoresis (PFGE) is a gold standard method of molecular subtyping for studying clonality among STEC strains [10,22]. Multilocus sequence typing (MLST) is a DNA sequence-based molecular subtyping method in which the sequences of gene fragments from a number of different housekeeping loci are assessed [35]. To date, bovine STEC epidemiological studies at a nationwide level and on a large scale have not been adequately performed in Korea [4,18]. Therefore, we used PFGE and MLST to study the general clonality of STEC strains at a nationwide level and determine the genetic relatedness of STEC isolates in Korea.
The usefulness of antimicrobial therapy for treating STEC infections is unclear but a recent study suggests that some antimicrobials may prevent the development of HUS if administered early in the course of infection [34]. Because STEC infections are not aggressively treated with antimicrobials, many isolates may yet be susceptible to these reagents. Therefore, antibiotic susceptibility patterns need to be continuously evaluated [33].
Since animals in good health, particularly healthy cattle, can harbor STEC, they are considered natural reservoirs of STEC. Consumption of undercooked ground beef or dairy products contaminated with pathogens is the main cause of STEC infection. Recently, the risk of STEC infection has gradually been increasing because of the increased consumption of meat worldwide [8,16].
National studies in Korea showed that enterohemorrhagic E. coli (EHEC) is present in workers in the farm industry as well as slaughter houses, especially among people who have more opportunities to come into contact with cattle [14,15]. Therefore, not only carcasses during slaughter but also cattle on farms during the pre-slaughter stage should be treated to prevent the spread of STEC. However, few investigations have studied bovine STEC on cattle farms in Korea. The present study was conducted to 1) investigate the prevalence of STEC in Korean native and Holstein dairy cattle in Korea, 2) characterize the virulence genes, serotypes, and antimicrobial susceptibility patterns of STEC, and 3) make genetic comparisons using MLST and PFGE.
Materials and Methods
Bacterial isolation
Han Woo (a breed of cattle native to Korea) and Holstein-Friesian (dairy cattle bred for the production of large quantities of milk) cattle 20 to 30 months old were selected from 54 farms (27 native cattle farms and 27 dairy cattle farms) of six regions of Korea (Gyeonggi, Chungcheong, Jeolla, Gyeongsang, Gangwon, and Jeju; Table 1). Rectal swab samples were aseptically obtained and immediately placed into the sterile collection tubes containing Amies transport medium (YUHAN LAB TECH, Korea) between September 2010 and July 2011. All farms did not break out foot and mouth disease (FMD) directly but were involved in the area of breaking out FMD except for ones Jeolla and Jeju. While sampling between 2010 and 2011, the farms collecting samples (native cattle farms/dairy cattle farms) were 4/3 in Gyeonggi, 4/3 in Chungcheong, 3/4 in Jeolla, 1/1 in Gyeongsang, 5/0 in Gangwon, and 2/0 in Jeju.
Table 1.
Prevalence of Shiga toxin-producing Escherichia (E.) coli (STEC) isolated from native and dairy cattle in Korea during 2010 and 2011
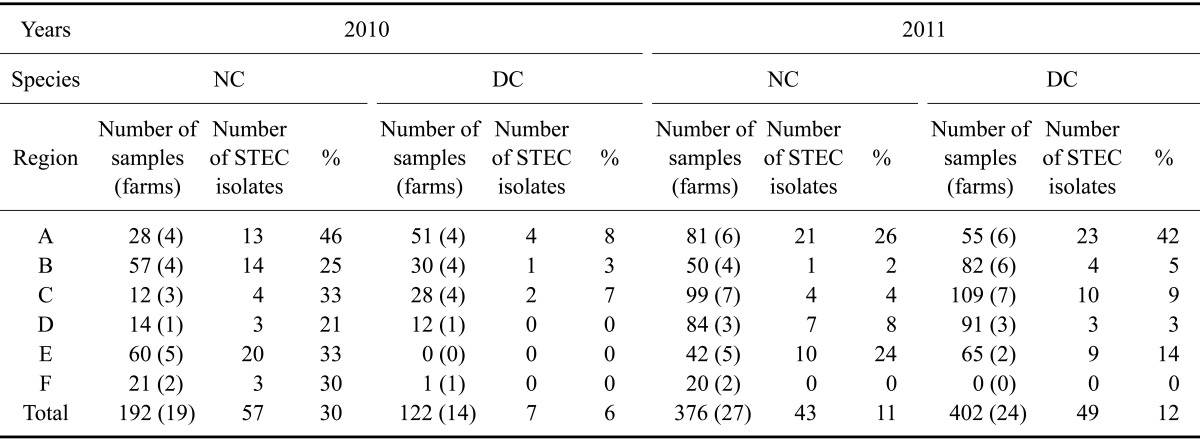
A: Gyeonggi, B: Chungcheong, C: Jeolla, D: Gyeongsang, E: Gangwon, F: Jeju. NC: native cattle, DC: dairy cattle.
Samples transported to the laboratory within 12 h were used to inoculate 3mL trypticase soy broth (Becton, Dickinson and Company, USA) supplemented with 20 mg/L novobiocin and incubated at 37℃ for 18 h. Next, 10 µL of the cultures were streaked onto MacConkey (MAC) agar (Becton, Dickinson and Company) or sorbitol-MacConkey (SMAC) agar (Becton, Dickinson and Company) and the plates were incubated at 37℃ for 24 h. After incubation, pink colonies on MAC agar and pink or white colonies on SMAC agar were selected. Glucose fermentation performed by the colonies was verified by about 30° slant culturing on Triple Sugar Iron Agar (Becton, Dickinson and Company). Identity of bacteria in the selected colonies was finally confirmed with a ViteK II microbial identification system (BioMérieux, France). DNA was extracted from the selected colonies using a genomic DNA extraction kit (Qiagen, Germany) and PCR amplification was performed to confirm the presence of the 16S rRNA gene that is conserved in E. coli. PCR protocols were described Sabat et al. [31].
Presence of the stx1 or stx2 genes encoding Shiga-like toxin (Stx) in the E. coli isolates was verified by PCR using two oligonucleotide probes described Paton et al. [24]. Expression of additional virulence genes (saa, eaeA, and ehxA) was also evaluated by the multiplex PCR as previously described by Paton et al. [24].
Reverse passive latex agglutination (RPLA) test
Stx1 and 2 produced by E. coli cultured from fecal samples were detected with an RPLA kit (Oxoid, UK). The E. coli isolates were used to inoculate brain heart infusion agar (Oxoid) and incubated at 37℃ for 18 to 20 h. After incubation, the colonies were suspended in 1 mL of a 0.85% sodium chloride solution containing 5,000 units/mL polymyxin B (Sigma-Aldrich, USA) that induced the extraction of stx1and 2. Toxin extraction was performed for 30 min at 37℃ with occasional shaking. After extraction, the culture was centrifuged at 1,200 × g for 20 min at 4℃ and the supernatant was used as a test sample. Twenty-five µL of diluents, latex and control reagent, were dispensed into each well of a V-bottom microplate (Sigma-Aldrich), and 25 µL of test sample supernatant were added to the first well in each column. Two-fold dilutions were made down each column, up to and including row 7. Twenty-five µL of the test latex stx1and 2 as well as the latex control were added to each well in the first, second, and third column, respectively. The contents of each well were mixed well and the plate was left undisturbed at room temperature for 20~24 h. Samples in the wells showing agglutination were classified as Stx-positive.
Serotyping
Determination of the O-antigen was carried out with heat-inactivated E. coli isolates using the slide agglutination method. A certain amount of bacterial growth was suspended in 3 mL 0.85% saline and heat to 100℃ for 1 h. The suspension was pelleted at 900 × g for 20 min and a supernatant was discarded. The precipitant was then suspended with 0.5 mL 0.85% saline and used as antigenic suspension. Antigenic suspension and a drop each of polyvalent serum (Denka Seiken, Japan) were mixed and tilted back and forth for 1 min on the slide glass. Only strong agglutination observed within 1 min in the reaction with each serum was regarded as positive. Positive samples were then tested with each monovalent serum consisting of the polyvalent serum. The mixture of 0.85% saline and serum was used as a negative control.
Determination of the H-antigen was carried out using the test tube method with the bacteria cultured in liquid media. E. coli isolates passed through the semi-liquid media with a Cragie's tube 3~5 times may be used for inoculation of the preparatory culture in the liquid medium. Then, a cell suspension was prepared by culturing in the liquid medium at 37℃ overnight and adding an equal amount of 0.85% saline containing 1 w/v% formalin. Three drops of each H-antisera and 0.5 mL of the cell suspension were added into the each tubes. A tube that did not contain the antisera was regarded as a negative control. After mixing thoroughly, the tubes were kept in a 50℃ water bath for 1h. Agglutination was observed in positive samples.
MLST
MLST was performed according to E. coli MLST scheme whose databases are managed by University College Cork (Ireland). Seven housekeeping genes (adk, fumC, gyrB, icd, mdh, purA, and recA) were amplified and the PCR products were sequenced to confirm the sequence type (ST). PCR protocol is that 2 min at 95°, 30 cycles of 1 min at 95°, 1 min at annealing temp, 2 min at 72° followed by 5 min at 72°. The PCR reaction contains 50 ng of chromosomal DNA, 20 pmol of each primer, 200 umol (10 µL of a 2 mM solution) of the dNPTs, 10 µL of 10 × PCR buffer, 5 units of Taq polymerase and water to 100 µL. Allelic profile of the reference E. coli strain is filed as GenBank Accession No. U00096.
PFGE
PFGE was performed to genetically compare the STEC isolates. In brief, bacterial cells from an overnight culture in 3 mL Tryptic Soy Broth (Becton, Dickinson and Company) were pelleted at 42,511 × g for 5 min. The pelleted cells were embedded in 1% SeaKem Gold agarose (Lonza, USA) plugs and lysed by proteinase K (Sigma-Aldrich). Lysed plugs were then digested for 24 h with 40 U of XbaI (New England BioLabs, USA) at 37℃ water bath. Digested plugs were placed on 1% SeaKem Glod agarose and electrophoresis was carried out at 6.0 V/cm for 19 h with an initial switch time value of 2.16 sec and final switch time of 54.17 sec in 0.5× Tris-Brate-EDTA (TBE) buffer at 14℃ [30]. PFGE patterns were analyzed using Bionumerics software (ver. 6.5; Applied Maths, USA). The degree of similarity was calculated using the unweighted pair group method with averaging (UPGMA) based on Dice similarity coefficiency with 2% of tolerance. More than three isolates with over 85% similarity were considered to be one cluster group.
Antimicrobial susceptibility test
A disk diffusion test was performed and verified by an agar dilution test according to the Clinical and Laboratory Standards Institute (CLSI) guidelines [5,6] with the following antibiotic disks (Becton, Dickinson and Company): carbenicillin (CB, 100 µg), cefazolin (CZ, 30 µg), amoxicillin/clavulanate (AMC, 20/10 µg), ampicillin (P, 10 µg), imipenem (IPM, 10 µg), chloramphenicol (C, 30 µg), tetracycline (TE, 30 µg), gentamicin (GM, 10 µg), amikacin (AN, 30 µg), streptomycin (S, 10 µg), neomycin (N, 30 µg), ciprofloxacin (C, 5 µg), and nalidixic acid (NA, 30 µg). Thirteen antibiotics belonging to different antimicrobial classes were tested using BD BBL Sensi-Disks (Becton, Dickinson and Company): carbenicillin, cefazolin, amoxicillin/clavulanate, ampicillin, imipenem, chloramphenicol, tetracycline, gentamicin, amikacin, streptomycin, neomycin, ciprofloxacin, and nalidixic acid. Colistin resistance was tested with the broth dilution technique. Multidrug-resistant (MDR) E. coli were defined as bacteria that were resistant to at least three different classes of antimicrobials.
Statistical analysis
Statistical differences between STEC strains isolated from native and dairy cattle were analyzed using a Chi-square test with PASW Statistics 18 (SPSS, USA). At the 95% confidence level, p values < 0.05 were considered statistically significant.
Results
Prevalence of STEC
A total of 1,092 fecal swab samples were collected in the present study. In 2010 and 2011, 192 and 376 samples were collected from native cattle, respectively, while 122 and 402 samples, respectively, were obtained from dairy cattle. E. coli isolates were classified as STEC when stx1 or stx2 genes were detected by multiplex PCR. STEC colonization rates of the native and dairy cows were 17.61% and 10.69%, respectively. The overall prevalence of STEC ranged from 20.38% (64/314) in 2010 to 11.82% (92/778) in 2011. STEC colonization rates in native cattle decreased significantly during the study period from 29.68% in 2010 to 11.43% in 2011 (p < 0.001). On the other hand, the colonization rates of dairy cows increased from 5.73% in 2010 to 12.19% in 2011 (p < 0.05). Evaluation of regional distribution showed that Gyeonggi and Gangwon had higher prevalence rates than other parts of the country regardless of the species and year except for dairy cattle in Gangwon surveyed in 2010 (Table 1).
Detection of stx1, stx2, and other virulence genes
The number of STEC isolates that carried only stx2 and not stx1 was highest in 2010 (Table 2). In contrast, the stx1+/stx2+ STEC rates increased among both native and dairy cows in 2011 (Table 2). saa and ehxA genes were generally prevalent. saa, ehxA, and eaeA genes were detected more frequently in isolates from dairy cattle than in those from native bovines (Table 3). From 2010 to 2011, there was little difference in the prevalence of all virulence genes in native cows. On the other hand, prevalence of the saa gene increased significantly in dairy cows while prevalence of the eaeA gene decreased. This is because the increased number of STEC isolates in 2011 compared to that obtained in 2010 enhanced the STEC population overall, and made the results of virulence gene survey in dairy cattle more reliable.
Table 2.
Genotypes and phenotype of STEC isolates from native and dairy cattle collected between 2010 and 2011
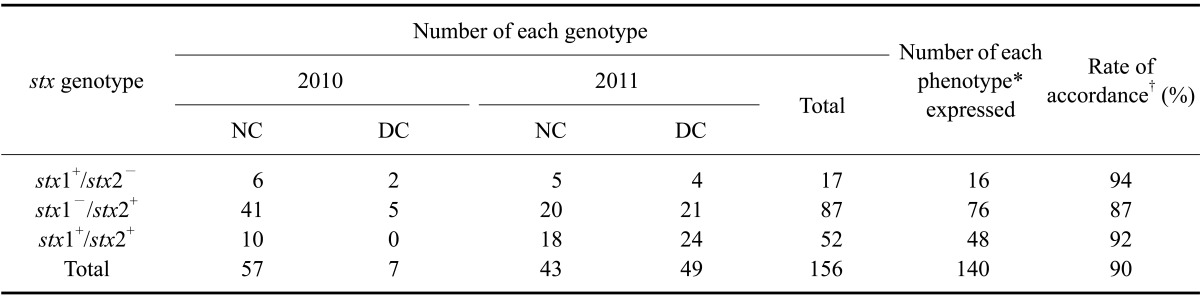
*Phenotype was determined by the reverse passive latex agglutination test. †Rate of accordance between the stx genotype and phenotype.
Table 3.
Characterization of virulence factor genes from STEC isolates from native and dairy cattle collected between 2010 and 2011
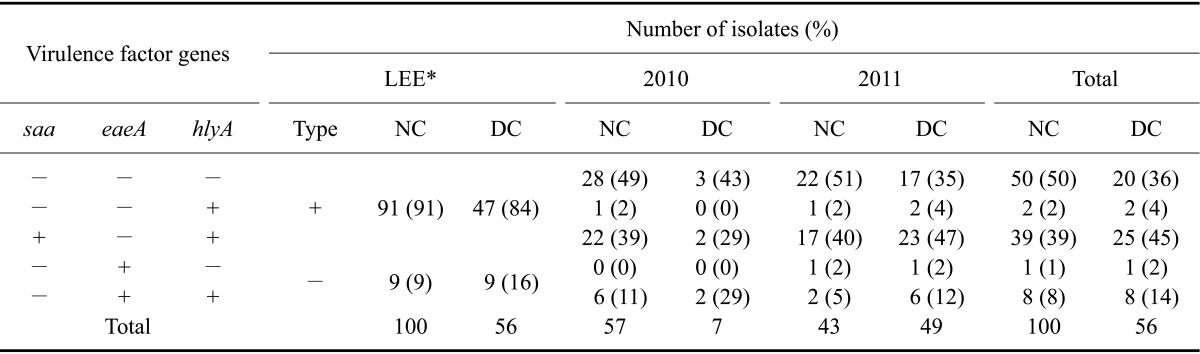
*LEE: locus for enterocyte effacement in the pathogenicity island encoded by eaeA.
Detection of SLT production
A correlation between the stx genotype and phenotype was observed in this study. Most STEC isolates bearing an stx gene produced of the corresponding toxin type that was detected with an RPLA test. The phenotypes of one (6%) stx1+/stx2- STEC isolate, 11 (13%) stx1-/stx2+ isolates, and four (8%) stx1+/stx2+ isolates could not be determined (Table 2).
Serotyping
In the present investigation, we focused on serotypes that have the greatest impact on public health and safety. In native cattle, STEC with three O26 isolates (5%), one O111 isolate (2%), and two O157 isolates (4%) serotypes were identified. All of these STEC isolates were obtained in 2010. From dairy cows, STEC with one O104 isolate (2%) and three O157 isolates (6%) serotypes were identified in 2011 (Table 4). Two stains of O8:H19 were obtained from native cattle in 2010 and one strain each of O8:H19 and O8:H21 was recovered from dairy cows in 2011. These serotypes have been associated with HUS in other countries [21]. No STEC with O157:H7 or O104:H4 serotypes were isolated in the present study.
Table 4.
Frequency of different STEC with the O-serotype from native and dairy cattle isolated between 2010 and 2011
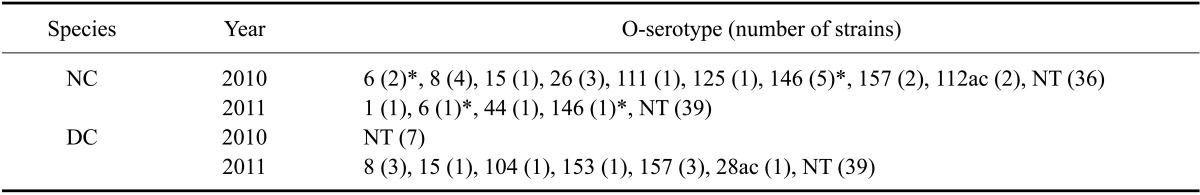
*O-serotypes were detected in both 2010 and 2011. NT: non-typable with antisera.
MLST
MLST analysis of isolates collected in 2010 revealed the presence of 45 different STs including 34 newly designated STs. The most commonly detected ST among native cows and dairy cows was ST223. The most prevalent ST was ST10 (5/64, 7.81%) followed by ST2101 (4/64, 6.25%) and ST297 (3/64, 4.69%). On the other hand, in 2011, due to increased STEC isolations of dairy cattle, various 32 STs were identified and different from six STs (except for ST223) among total seven STs from dairy cattle in 2010 samples. The most frequent ST was ST2385, a newly identified type that accounted for 22.45% (11/49); the remaining 22 STs accounted for 2~8%. For the native cattle strains, ST10 was predominant (25.58%, 11/43) and 19 remaining STs accounted for 2~9% (Table 5).
Table 5.
Multilocus sequence typing analysis of STEC isolates recovered from native and dairy cattle in 2010 and 2011
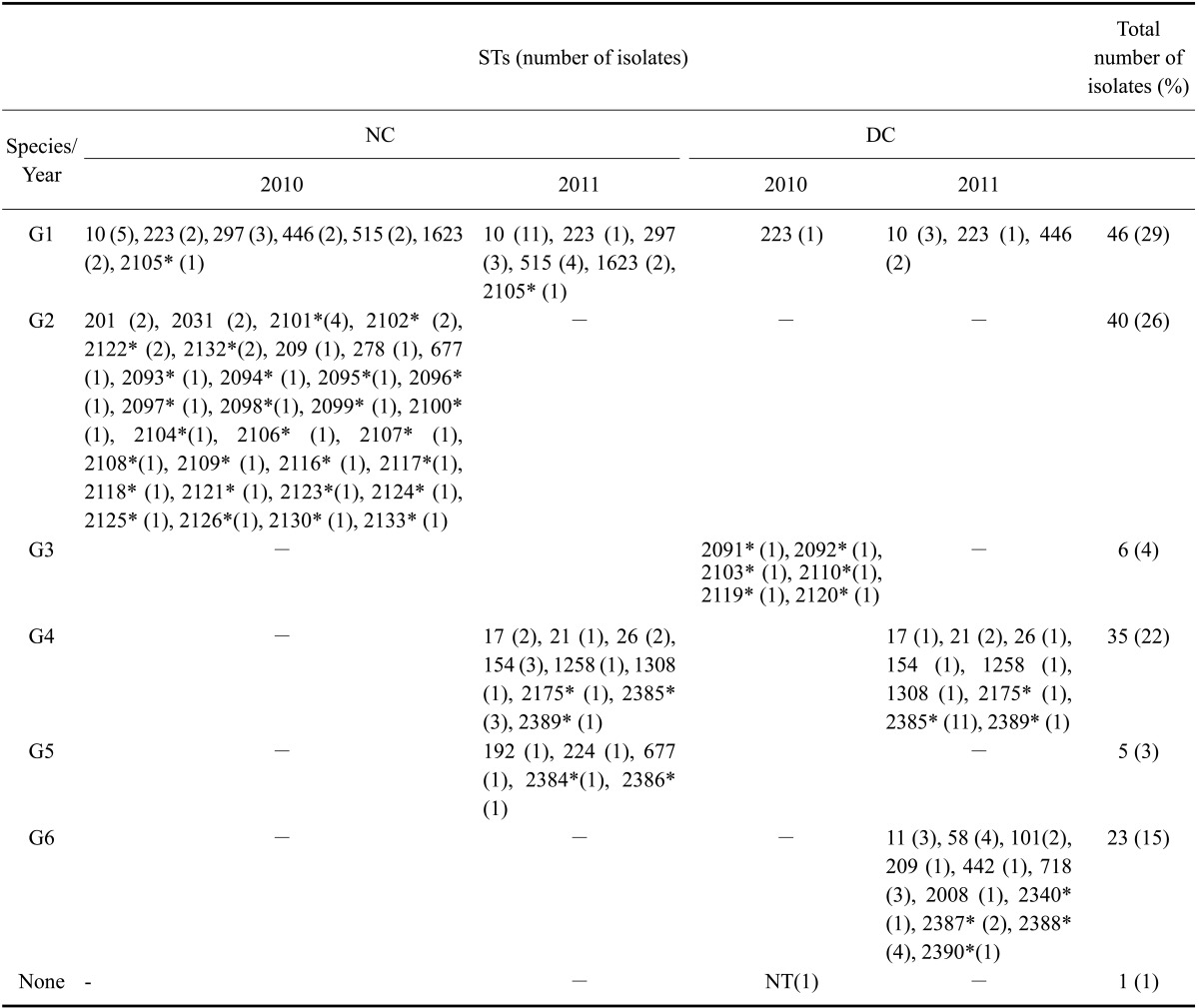
STEC were divided into six groups according to sequence typing (STs). G1: overlapping STs between 2010 and 2011, G2: STs found only among isolates from native cattle collected in 2010, G3: STs found only among isolates from dairy cattle obtained in 2010, G4: overlapping STs between native and dairy cattle in 2011, G5: STs discovered only among isolates from native cattle in 2011, G6: STs discovered only among isolates from dairy cattle in 2011. Sequence type of one STEC isolate obtained in 2010 could not be identified. *New alleles and STs.
PFGE
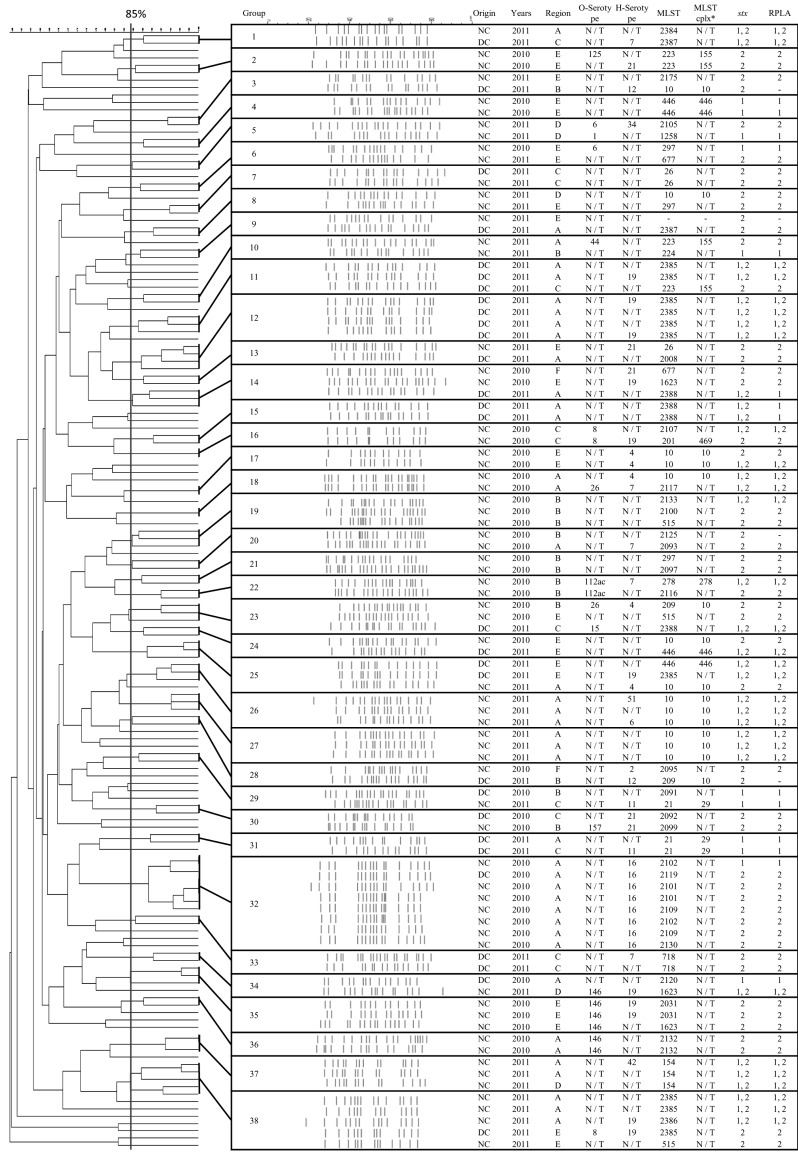
XbaI-digested DNA fragments from 156 STEC isolates revealed the presence of 38 genetically related groups with 85% similarity (Fig. 1). Individual PFGE patterns contained between 17 and 25 discernible DNA fragments ranging from 50 to 800 kb in size. There was no tendency for a specific group to be dominant throughout the country. Only seven groups (G6, G14, G23, G24, G28, G29, and G34) contained STEC isolates acquired in both 2010 and 2011. All other groups consisted of STEC isolates obtained during only one year (either 2010 or 2011). No differences in PFGE patterns were observed between dairy and native cows similar to the MLST findings, but PFGE patterns seemed to be affected by region. Eleven (84 .6%) out of 13 groups of PFGE patterns were identified in isolates acquired in 2010 from the same regions. Seven (38.9%) out of 18 groups were observed among STEC collected in 2011 from the same areas. The remaining groups contained STEC isolates from more than two different regions in which only Gyeonggi overlapped the various other areas except for Jeju.
Fig. 1.
Characteristics of 38 groups established based on PFGE (XbaI) patterns of 156 STEC isolated from native and dairy cattle in six regions of Korea between 2010 and 2011. A, Gyeonggi; B, Chungcheong; C, Jeolla; D, Gyeongsang; E, Gangwon; F, Jeju. *MLST cplx is the clonal complex of multilocus sequence typing.
Antimicrobial susceptibility test
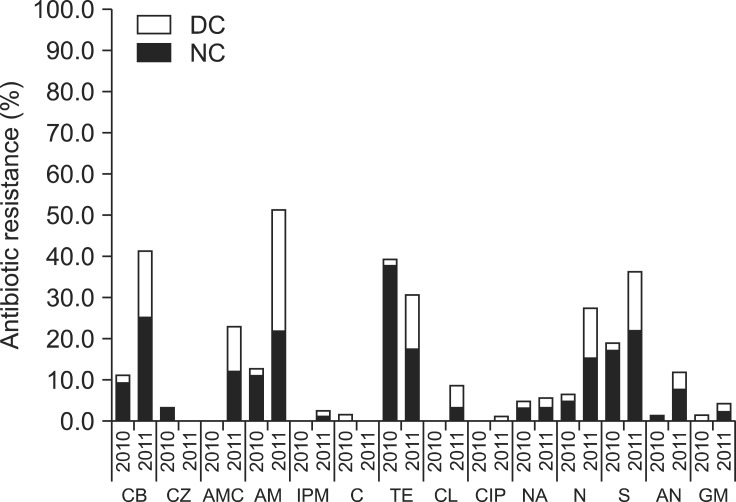
Results of antimicrobial susceptibility tests for isolates from 2010 and 2011 indicated that resistance to tetracycline, streptomycin, ampicillin, and carbenicillin were most frequent. A marked increase in antibiotic resistance rates was found for isolates obtained in 2011 (Fig. 2). Antibiotic resistance rates of STEC isolates that were resistant to more than one drug were 14.3% for dairy cows and 45.6% for native cattle in 2010, but grew the rate rapidly in 2011 (73.5% in dairy cows and 79.1% in native cattle). The rate of MDR (resistance to more than three antibiotics) also increased greatly over time from 14.29% to 51.16% in dairy cows and 12.28% to 28.57% in native cattle. Resistance rates against carbenicillin, amoxicillin/clavulanate, ampicillin, colistin, neomycin, amikacin, and streptomycin also significantly increased between 2010 and 2011. The analytical results of carbenicillin, amoxicillin/clavulanate, ampicillin, and neomycin were p < 0.001 and colistin, amikacin, and streptomycin were p < 0.05 that means the results were considered statistically significant.
Fig. 2.
Antimicrobial resistant rates for STEC isolated between 2010 and 2011. Black bars represent the antimicrobial resistant rates of native cattle isolates and white bars represent the antimicrobial resistant rates of dairy cattle isolates. DC: dairy cattle, NC: native cattle, CB: carbenicillin, CZ: cefazolin, AMC: amoxicillin/clavulanate, AM: ampicillin, IPM: imipenem, C: chloramphenicol, TE: tetracycline, CL: colistin, CIP: ciprofloxacin, NA: nalidixic acid, N: neomycin, S: streptomycin, AN: amikacin, GM: gentamicin.
Discussion
In this large-scale, nationwide study, the overall prevalence of STEC isolated from rectal swabs of healthy adult cows was 20.38% in 2010 and 11.82% in 2011. In previous studies, the STEC prevalence in Korea was 10.5% for diarrhea samples from calves [19] and 9.5% for samples from cattle feces [4]. These rates are lower than ours in 2010 but similar to those in 2011. The prevalence of STEC in cattle has also been reported in several studies from different countries. Compared to our results, the STEC prevalence for Vietnam (26%), Japan (37.5%), France (34%), and Spain (35%) were reported to be higher [3,11,29,37] and that for Germany (17.0%) was similar [20]. The STEC prevalence for the United States (8.4%) was lower than the prevalence found in the present study [38]. It is important to remember that the use of different detection methods, the rapid development of new methods, and differences in diet and cattle species make it difficult to compare the STEC prevalence rates reported for various geographic areas [17].
Although native cattle (17.61%) had a higher rate of STEC prevalence than dairy cattle (10.69%), the rate of increase among dairy cows was more than twice that observed in native cows for 2 years. This difference between cattle species could be affected by changes in the environment, management practices, and improvement of the feed given to each kind of cow [8]. In Korea, there was an outbreak of FMD from the end of 2010 to early 2011. Consequently, more studies are needed to determine if FMD might affect the prevalence of STEC among cattle.
When evaluating the regional distribution of STEC, detection rates in Gyeonggi and Gangwon were higher than those in the other regions regardless of the type of cow or the time of sample collection except for dairy cattle in Gangwon in 2010. There are some regional characteristics that caused high infection rates with STEC in cattle in Gyeonggi and Gangwon. First of all, Gyeonggi is immediately adjacent to the Seoul metropolitan area. As such, there is less space to raise many cattle in Gyeonggi than in the other areas we surveyed. This situation causes many farms to become more compact and concentrated in the neighboring areas. With a greater density of cattle, the environment becomes more contaminated with STEC and increases the possibility of STEC infections in animals from the region [11]. Second, the environment of Gangwon is the best preserved among the six regions in Korea. Consequently, many wild animals inhabit this region and have many opportunities to come into contact with cows. Wildlife sharing the surrounding farmland with cattle could potentially shed STEC along with the cattle, thereby contributing to the transmission of STEC to livestock herds [8].
PCR results demonstrated that stx1-/stx2+ was the predominant type among the STEC isolates from both kinds of cows in 2010. This result coincides with those from a previous report [3] showing that STEC harboring the stx2 gene is prevalent among adult cows. However, the number of stx1-/stx2+ STEC isolates collected in 2011 decreased while the number of stx1+/stx2+ STEC isolates increased. This may be due to seasonal variations during the sampling period or because of an FMD outbreak that occurred between the two sampling periods. These factors could have altered the populations of STEC organisms but further study is necessary.
An immunoassay for the in vitro detection of Shiga (Vero) toxins stx1 and 2 (VTEC-Screen; Seiken) was performed to compare with the results from the stx gene-specific PCR to detect STEC. Although PCR is suitable for the general identification of STEC, the RPLA assay has also a high degree of specificity for detecting Stx-positive stool samples. The RPLA assay evaluates the expression of stx genes rather than just checking for the existence of these genes in the bacteria chromosomal DNA [2]. The STEC-specific screen detected 95 (95%) out of 100 STEC isolates from native cattle and 45 (80.36%) out of 56 STEC isolates from dairy cows (Table 2). Sensitivity of the RPLA was less than that of PCR, which was similar to published results, and there were no false positives [2]. When the data were analyzed according to toxin type, there was a false negative stx1 sample and 15 false negative stx2 samples. Beutin et al. [2] noted that some stx2 and stx2 variant strains (i.e., Stxd-Ount and stx2e/stx2ev) produce only small amounts of toxin that are insufficient for detection by the RPLA assay. Therefore, additional experiments are required to determine whether suspected samples produce toxins. Additionally, there may be issues related to cross reactivity when performing the assay.
Pathogenic STEC strains not only produce Shiga toxins but can also manufacture other virulence factors that may cause more severe human illnesses [13]. The present study focused on intimin, auto-agglutinating adhesion, and plasmid-encoded enterohemolysin. These factors of STEC, which are considered to be highly virulent in humans, are necessary to generate the attaching and effacing lesions (A/E lesions) that enable adherence to enterocytes. Intimin encoded by eaeA, which is included in the "pathogenicity island" known as the locus for enterocyte effacement (LEE), is involved in the production of A/E lesions [40]. Therefore, the LEE, which is commonly identified by detection of the eaeA gene, appears to confer enhanced virulence. However, the LEE is not essential for pathogenesis because many cases of severe STEC disease, including HUS and thrombotic thrombocytopenic purpura, were caused by LEE-negative strains [26]. Paton suggested that saa is a marker of LEE-negative STEC used to designate an ill-defined subset similar to eaeA in LEE-positive STEC capable of causing life-threatening disease in humans [25]. In the present study, none of the STEC isolates possessed both eaeA and saa, and saa (41%) was detected more frequently than eaeA (12%) regardless of the collection period or species. This finding indicates that LEE-negative STEC strains are more prevalent than LEE-positive STEC in Korea.
It was previously shown that there is a correlation between the presence of saa and ehxA in certain STEC strains [25]. This result is in agreement with our finding. All of the isolates positive for saa were also positive for ehxA while 16 isolates positive for ehxA were negative for saa. Among the 16 isolates positive for ehxA, 13 were also positive for eaeA. Therefore, at least 13 ehxA-positive isolates were among the LEE-positive STEC strains. Considering the possible correlation between saa and ehxA, a plasmid-encoded repeats in toxin (RTX) toxin that carries the ehxA gene in the LEE-positive and -negative STEC strains is different [25]. According to the prevalence of the three virulence factors, more severe illness in humans is associated with STEC infection in dairy cattle since all virulence genes were found more frequently in isolates from dairy cows than native cows.
Detection of major STEC serotypes related to severe human diseases was performed. Only a few strains were found to possess the O26, O104, O111, and O157 serotypes. Among STEC obtained in 2010, three (5%) O26 isolates, one (2%) O111 isolate, and two (4%) O157 isolates were detected only in samples from native cattle. In 2011, one (2%) O104 isolate and three (6%) O157 isolates were detected only in samples from dairy cattle. In a previous study in Korea [4], the same O serotypes were found but the detection rates were different (O157, 42%; O26, 42%; and O111, 1%). This difference may be due to different sampling methods and animal species. Our results were similar with those from a study conducted in Japan (O157, 1.1%; O26, 9.8%; O111, 1.1%; O104, 2.2%) [17]. Along with the data for the virulence factor assay, we should take notice of the recent increases in the number of strains with disease-associated serotypes in dairy cattle.
STEC isolates acquired in 2010 and 2011 had different STs and several new STs were identified. This is because the majority of available databases contain information for E. coli strains from Europe, North America, Africa, and Asia [28] but only a few strains from Korea are included. In a previous investigation performed in Korea [18], MLST results for three pathogenic E. coli isolates showed that one strain was ST101 while the others had novel STs (ST1815 and ST1820). In the present study, we performed MLST on 156 STEC isolates from a large-scale sampling of healthy cows in Korea and discovered new STs. There was only one overlapping ST (ST223) between the native and dairy cows in 2010 but 11 STs overlapped in 2011. This finding could mean there was no preferable ST for the two breeds of cattle. As far as the regional aspect of STs, ST10 was detected throughout the country except for in the F region for over 2 years. Howeverit appeared only in the A and E regions narrowly in 2010 and was not the most frequently detected ST in 2011, it is reasonable to conclude that there is presently no predominant ST throughout Korea. Additionally, most identified STs were different from in 2010 with in 2011. This result could be due to seasonal variations or an FMD outbreak occurring over 2 years ago. We assume that seasonal differences including ambient temperature, rainfall, and other seasonally driven factors such as insect populations affect changes in STEC strain pools [1]. Another assumption is that the national large-scale slaughtering and burying of cattle to control the transmission of FMD as well as environmental changes on farms such as reinforcement of sanitation control and different raising systems prevented continuous infection with STEC strains and selected for other STEC strains.
In the present study, DNA from 156 STEC isolates was digested with XbaI and 38 clusters with 85% similarity were identified based on the PFGE patterns generated. There was no specific STEC isolated across the country in the clusters. Out of the 38 groups, only five (G6, G14, G23, G24, G28, G29, and G34; 18.4%) contained STEC strains collected during both 2010 and 2011. The other groups included STEC strains isolated in either 2010 or 2011. The low degree of similarity between STEC obtained in 2010 and strains acquired in 2011 is indicative of low genetic relatedness. This finding agrees with the MLST result demonstrating that there was not continuous infection by STEC strains all year round but PFGE profiles of infected STEC strains had changed.
In addition, no preference for STEC strain was found in a specific kind of cattle, but regional and spatial effects were important for the PFGE grouping. STEC from the same farm or region was mainly grouped together in the present study. Cobbold and Desmarchelier [7] previously suggested that contact between the animals and various materials, such as drinking and feeding troughs, enables STEC to be disseminated by horizontal transmission. Of all the regions, STEC patterns for isolates from Gyeonggi overlapped with those of STEC from other regions. This is because there is vigorous exchange of STEC between Gyeonggi (located in the capital area) and other areas. The current study used two methods to evaluated STEC epidemiology but could not compare the PFGE and MLST findings due to a number of new STs. However, both PFGE and MLST findings can be meaningful for assessing genetic relatedness among the STEC strains on a large national scale.
Results of the antimicrobial susceptibility tests in this investigation were similar to those from studies conducted in other countries demonstrating that streptomycin and tetracycline resistance were prevalent among isolates from cattle [21,23]. Furthermore, an earlier study performed in Korea indicated that most STEC isolates were resistant to carbenicillin and ampicillin [4,19]. Tetracycline, streptomycin, ampicillin, and carbenicillin resistance were frequent regardless of species in the current investigation. The prevalence of resistance to these antibiotics except for tetracycline increased in 2011. It is worth noting that the percentage of antibiotic resistance to not only one drug but also multiple drugs increased considerably in 2011 compared to the previous year. It was assumed that this result was also due to seasonal variations or FMD infection. Since antimicrobials are administered more frequently in summer than autumn, STEC isolates collected during the summer sampling in 2011 were more resistant to most antibiotics. To prevent secondary bacterial infection following viral diseases, many kinds of antibiotics might be excessively and indiscriminately administered. This would increase residual antibiotic levels in livestock and lead to the emergence of drug-resistant bacteria [36]. The increasing rate of antimicrobial resistance can cause public health problems. Therefore, continuous epidemiological evaluation will be needed.
To reduce the risk of STEC infection and improve food safety, we studied the characteristics of STEC isolated from farms in different parts of Korea. We discovered that the STEC pool had changed due to seasonal variations or FMD infection between 2010 and 2011. In addition, the risk of STEC infection increased more in dairy cows than native cattle, and the antibiotic resistance of STEC isolates also increased over the 2-year sampling period. This result might be attributed to seasonal variations during the sampling period or to FMD outbreaks that occurred between 2010 and 2011. Our findings indicated that attention should be paid to the STEC distribution among dairy cattle. In addition, continuous epidemiologic studies evaluating the factors influencing the changes in antibiotic resistance and the STEC pool over the past 2 years are needed.
Acknowledgments
This study was supported by the Animal and Plant Quarantine Agency (0468-20110013), and Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry, and Fisheries (IPET 311001-03-1-HD120), Korea. Additional support was provided by the Research Institute of Veterinary Science, Department of Veterinary Microbiology, College of Veterinary Medicine, and the BK21 Program for Veterinary Science, Seoul National University, Korea.
Footnotes
There is no conflict of interest.
References
- 1.Barkocy-Gallagher GA, Arthur TM, Rivera-Betancourt M, Nou X, Shackelford SD, Wheeler TL, Koohmaraie M. Seasonal prevalence of Shiga toxin-producing Escherichia coli including O157:H7 and non-O157:H7 serotypes, and Salmonella in commercial beef processing plants. J Food Prot. 2003;66:1978–1986. doi: 10.4315/0362-028x-66.11.1978. [DOI] [PubMed] [Google Scholar]
- 2.Beutin L, Zimmermann S, Gleier K. Evaluation of the VTEC-Screen "Seiken" test for detection of different types of Shiga toxin (verotoxin)-producing Escherichia coli (STEC) in human stool samples. Diagn Microbiol Infect Dis. 2002;42:1–8. doi: 10.1016/s0732-8893(01)00325-x. [DOI] [PubMed] [Google Scholar]
- 3.Blanco M, Blanco JE, Blanco J, Mora A, Prado C, Alonso MP, Mouriño M, Madrid C, Balsalobre C, Juárez A. Distribution and characterization of faecal verotoxin-producing Escherichia coli (VTEC) isolated from healthy cattle. Vet Microbiol. 1997;54:309–319. doi: 10.1016/s0378-1135(96)01292-8. [DOI] [PubMed] [Google Scholar]
- 4.Chae HS, Kim NH, Han HJ, Son HR, Kim CK, Kim SH, Lee JH, Kim JT. Characterization and isolation of shiga toxin-producing Escherichia coli from bovine feces and carcass. Korean J Vet Serv. 2009;32:241–249. [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Informational Supplement M31-S1. Wayne: Clinical and Laboratory Standards Institute; 2004. [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Seventeenth Informational Supplement. CLSI document M100-S17. Wayne: Clinical and Laboratory Standards Institute; 2007. [Google Scholar]
- 7.Cobbold R, Desmarchelier P. Horizontal transmission of Shiga toxin-producing Escherichia coli within groups of dairy calves. Appl Environ Microbiol. 2002;68:4148–4152. doi: 10.1128/AEM.68.8.4148-4152.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cobbold RN, Rice DH, Szymanski M, Call DR, Hancock DD. Comparison of shiga-toxigenic Escherichia coli prevalences among dairy, feedlot, and cow-calf herds in Washington State. Appl Environ Microbiol. 2004;70:4375–4378. doi: 10.1128/AEM.70.7.4375-4378.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donnenberg MS, Kaper JB, Finlay BB. Interactions between enteropathogenic Escherichia coli and host epithelial cells. Trends Microbiol. 1997;5:109–114. doi: 10.1016/S0966-842X(97)01000-7. [DOI] [PubMed] [Google Scholar]
- 10.Foley SL, Simjee S, Meng J, White DG, McDermott PF, Zhao S. Evaluation of molecular typing methods for Escherichia coli O157:H7 isolates from cattle, food, and humans. J Food Prot. 2004;67:651–657. doi: 10.4315/0362-028x-67.4.651. [DOI] [PubMed] [Google Scholar]
- 11.Fremaux B, Prigent-Combaret C, Vernozy-Rozand C. Long-term survival of Shiga toxin-producing Escherichia coli in cattle effluents and environment: an updated review. Vet Microbiol. 2008;132:1–18. doi: 10.1016/j.vetmic.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 12.Fukushima H, Seki R. High numbers of Shiga toxin-producing Escherichia coli found in bovine faeces collected at slaughter in Japan. FEMS Microbiol Lett. 2004;238:189–197. doi: 10.1016/j.femsle.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 13.Gyles CL. Shiga toxin-producing Escherichia coli: an overview. J Anim Sci. 2007;85(13 Suppl):E45–E62. doi: 10.2527/jas.2006-508. [DOI] [PubMed] [Google Scholar]
- 14.Hong S, Oh KH, Cho SH, Kim JC, Park MS, Lim HS, Lee BK. Asymptomatic healthy slaughterhouse workers in South Korea carrying Shiga toxin-producing Escherichia coli. FEMS Immunol Med Microbiol. 2009;56:41–47. doi: 10.1111/j.1574-695X.2009.00545.x. [DOI] [PubMed] [Google Scholar]
- 15.Hong S, Song SE, Oh KH, Kim SH, Yoo SJ, Lim HS, Park MS. Prevalence of farm and slaughterhouse vorkers carrying Shiga toxin-producing Escherichia coli in Korea. Osong Public Health Res Perspect. 2011;2:198–201. doi: 10.1016/j.phrp.2011.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hussein HS. Prevalence and pathogenicity of Shiga toxin-producing Escherichia coli in beef cattle and their products. J Anim Sci. 2007;85(13 Suppl):E63–E72. doi: 10.2527/jas.2006-421. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi H, Shimada J, Nakazawa M, Morozumi T, Pohjanvirta T, Pelkonen S, Yamamoto K. Prevalence and characteristics of shiga toxin-producing Escherichia coli from healthy cattle in Japan. Appl Environ Microbiol. 2001;67:484–489. doi: 10.1128/AEM.67.1.484-489.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koo HJ, Kwak HS, Yoon SH, Woo GJ. Phylogenetic group distribution and prevalence of virulence genes in Escherichia coli isolates from food samples in South Korea. World J Microbiol Biotechnol. 2012;28:1813–1816. doi: 10.1007/s11274-011-0954-5. [DOI] [PubMed] [Google Scholar]
- 19.Lim KG, Kang MI, Kim SK, Nam KW, Park HJ, Park JR, Cho KO, Lee BJ. Identification and characterization of Shiga toxin-producing Escherichia coli isolated from diarrhea in calves. Korean J Vet Res. 2006;46:135–142. [Google Scholar]
- 20.Montenegro MA, Bülte M, Trumpf T, Aleksić S, Reuter G, Bulling E, Helmuth R. Detection and characterization of fecal verotoxin-producing Escherichia coli from healthy cattle. J Clin Microbiol. 1990;28:1417–1421. doi: 10.1128/jcm.28.6.1417-1421.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mora A, Blanco JE, Blanco M, Alonso MP, Dhabi G, Echeita A, González EA, Bernárdez MI, Blanco J. Antimicrobial resistance of Shiga toxin (verotoxin)-producing Escherichia coli O157:H7 and non-O157 strains isolated from humans, cattle, sheep and food in Spain. Res Microbiol. 2005;156:793–806. doi: 10.1016/j.resmic.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Noller AC, McEllistrem MC, Stine OC, Morris JG, Jr, Boxrud DJ, Dixon B, Harrison LH. Multilocus sequence typing reveals a lack of diversity among Escherichia coli O157:H7 isolates that are distinct by pulsed-field gel electrophoresis. J Clin Microbiol. 2003;41:675–679. doi: 10.1128/JCM.41.2.675-679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Brien AD, Holmes RK. Shiga and Shiga-like toxins. Microbiol Rev. 1987;51:206–220. doi: 10.1128/mr.51.2.206-220.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paton AW, Paton JC. Direct detection and characterization of Shiga toxigenic Escherichia coli by multiplex PCR for stx1, stx2, eae, ehxA, and saa. J Clin Microbiol. 2002;40:271–274. doi: 10.1128/JCM.40.1.271-274.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paton AW, Srimanote P, Woodrow MC, Paton JC. Characterization of Saa, a novel autoagglutinating adhesin produced by locus of enterocyte effacement-negative Shiga-toxigenic Escherichia coli strains that are virulent for humans. Infect Immun. 2001;69:6999–7009. doi: 10.1128/IAI.69.11.6999-7009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paton AW, Woodrow MC, Doyle RM, Lanser JA, Paton JC. Molecular characterization of a Shiga toxigenic Escherichia coli O113:H21 strain lacking eae responsible for a cluster of cases of hemolytic-uremic syndrome. J Clin Microbiol. 1999;37:3357–3361. doi: 10.1128/jcm.37.10.3357-3361.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paton JC, Paton AW. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin microbiol rev. 1998;11:450–479. doi: 10.1128/cmr.11.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pitondo-Silva A, Minarini LA, Camargo IL, Darini AL. Clonal relationships determined by multilocus sequence typing among enteropathogenic Escherichia coli isolated in Brazil. Can J Microbiol. 2009;55:672–679. doi: 10.1139/w09-019. [DOI] [PubMed] [Google Scholar]
- 29.Pradel N, Livrelli V, De Champs C, Palcoux JB, Reynaud A, Scheutz F, Sirot J, Joly B, Forestier C. Prevalence and characterization of Shiga toxin-producing Escherichia coli isolated from cattle, food, and children during a one-year prospective study in France. J Clin Microbiol. 2000;38:1023–1031. doi: 10.1128/jcm.38.3.1023-1031.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ribot EM, Fair MA, Gautom R, Cameron DN, Hunter SB, Swaminathan B, Barrett TJ. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog Dis. 2006;3:59–67. doi: 10.1089/fpd.2006.3.59. [DOI] [PubMed] [Google Scholar]
- 31.Sabat G, Rose P, Hickey WJ, Harkin JM. Selective and sensitive method for PCR amplification of Escherichia coli 16S rRNA genes in soil. Appl Environ Microbiol. 2000;66:844–849. doi: 10.1128/aem.66.2.844-849.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt H, Beutin L, Karch H. Molecular analysis of the plasmid-encoded hemolysin of Escherichia coli O157:H7 strain EDL 933. Infect Immun. 1995;63:1055–1061. doi: 10.1128/iai.63.3.1055-1061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schroeder CM, Zhao C, DebRoy C, Torcolini J, Zhao S, White DG, Wagner DD, McDermott PF, Walker RD, Meng J. Antimicrobial resistance of Escherichia coli O157 isolated from humans, cattle, swine, and food. Appl Environ Microbiol. 2002;68:576–581. doi: 10.1128/AEM.68.2.576-581.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shiomi M, Togawa M, Fujita K, Murata R. Effect of early oral fluoroquinolones in hemorrhagic colitis due to Escherichia coli O157:H7. Pediatr Int. 1999;41:228–232. doi: 10.1046/j.1442-200x.1999.4121038.x. [DOI] [PubMed] [Google Scholar]
- 35.Urwin R, Maiden MC. Multi-locus sequence typing: a tool for global epidemiology. Trends Microbiol. 2003;11:479–487. doi: 10.1016/j.tim.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Vali L, Wisely KA, Pearce MC, Turner EJ, Knight HI, Smith AW, Amyes SG. High-level genotypic variation and antibiotic sensitivity among Escherichia coli O157 strains isolated from two Scottish beef cattle farms. Appl Environ Microbiol. 2004;70:5947–5954. doi: 10.1128/AEM.70.10.5947-5954.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vu-Khac H, Cornick NA. Prevalence and genetic profiles of Shiga toxin-producing Escherichia coli strains isolated from buffaloes, cattle, and goats in central Vietnam. Vet Microbiol. 2008;126:356–363. doi: 10.1016/j.vetmic.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 38.Wells JG, Shipman LD, Greene KD, Sowers EG, Green JH, Cameron DN, Downes FP, Martin ML, Griffin PM, Ostroff SM, Potter ME, Tauxe RV, Wachsmuth IK. Isolation of Escherichia coli serotype O157:H7 and other Shiga-like-toxin-producing E. coli from dairy cattle. J Clin Microbiol. 1991;29:985–989. doi: 10.1128/jcm.29.5.985-989.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wirth T, Falush D, Lan R, Colles F, Mensa P, Wieler LH, Karch H, Reeves PR, Maiden MC, Ochman H, Achtman M. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol Microbiol. 2006;60:1136–1151. doi: 10.1111/j.1365-2958.2006.05172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu J, Kaper JB. Cloning and characterization of the eae gene of enterohaemorrhagic Escherichia coli O157:H7. Mol Microbiol. 1992;6:411–417. doi: 10.1111/j.1365-2958.1992.tb01484.x. [DOI] [PubMed] [Google Scholar]